3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(11):2609-2619. doi:10.7150/ijms.111848 This issue Cite
Research Paper
Deciphering the Relationship Between Circulating Metabolites and Osteoarthritis: A Comprehensive Genetic Correlation and Mendelian Randomization Studies
1. Department of Orthopedics, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China.
2. Molecular Nutrition Branch, National Engineering Research Center of Rice and By-product Deep Processing, College of Food Science and Engineering, Central South University of Forestry and Technology, Changsha 410008, Hunan, China.
3. National Clinical Research Center for Geriatric Disorders, Department of Geriatrics, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China.
4. Teaching and Research Section of Clinical Nursing, Xiangya Hospital of Central South University, Changsha 410008, Hunan, China
#These authors contribute equally to this work and share the first authorship
Received 2025-2-10; Accepted 2025-4-21; Published 2025-5-16
Abstract
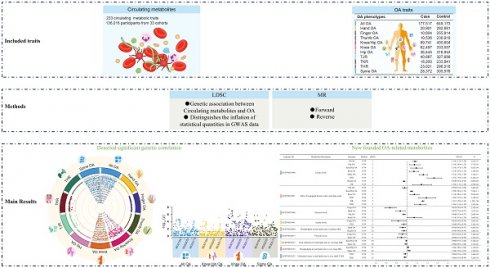
The causal impact of blood metabolites on OA has yet to be definitively established, further studies are needed to explore the specific roles of metabolites in OA. This is a genetic correlation and two-sample bidirectional mendelian randomization study. GWAS summary data of metabolites and OA were extracted from large-scale GWAS study based on Europeans and Asians. LDSC was conducted to estimate the genetic correlations between 233 circulating metabolites and 11 OA phenotypes, MR was then performed to explore the casual association. 41.20% of the metabolic traits showed genetic correlation with All OA, 15.88% with Knee/Hip OA, 51.50% with Knee OA, and 52.79% with Spine OA. No significant genetic correlations were detected between the metabolic traits and other OA phenotypes. Lactate levels was associated with increased odds of All OA (OR: 1.1558, P<0.001), Hip OA (OR: 1.1446, P=0.004), Knee/Hip OA (OR: 1.1820, P<0.001), Knee OA (OR: 1.1375, P=0.001), Spine OA (OR: 1.3179, P<0.001), THR (OR: 1.5290, P<0.001), and TJR (OR: 1.2827, P<0.001), except for Thumb OA (OR: 0.9429, P<0.001). Ratio of conjugated linoleic acid to total fatty acids was associated 6 OA phenotypes: Hip OA (OR: 0.9522, P=0.035), Knee/Hip OA (OR: 1.0890, P<0.001), Knee OA (OR: 1.1429, P<0.001), THR (OR: 1.3800, P<0.001), TJR (OR: 1.3102, P<0.001), and TKR (OR: 1.2555, P<0.001). Glycerol levels exhibited significant MR associations with four OA phenotypes: Finger OA (OR: 0.6669, P<0.001), Hand OA (OR: 0.8682, P=0.011), Hip OA (OR: 0.9395, P<0.001), and Knee OA (OR: 1.1409, P=0.036). This study underscores genetic and causal connections between specific metabolites and OA. These findings could inform future therapeutic metabolic pathways involved in OA.
Keywords: Circulating metabolites, OA, MR, LDSC, Genetic Correlation
Introduction
Osteoarthritis (OA) is a degenerative disease primarily characterized by joint pain and limited mobility, caused by various factors leading to the fibrosis, fissures, ulceration, and loss of joint cartilage [1]. The etiology of OA remains unclear, but its occurrence is associated with age, obesity, inflammation, trauma, and genetic factors [2]. The disease manifests pathologically through the degenerative destruction of joint cartilage, sclerosis or cystic changes in the subchondral bone, osteophyte formation at joint edges, synovial changes, and joint capsule contraction [3-5]. OA commonly affects the knees, hips, hands, ankles, and spine (including cervical and lumbar regions). Globally, the knee is the most commonly affected site, followed by the hands, other sites, and hips, accounting for approximately 60.6%, 23.7%, 10.2%, and 5.5% of all cases in 2019, respectively [6]. Recent data indicate that in 2020, approximately 595 million people worldwide suffered from OA, equivalent to 7.6% of the global population. For individuals over the age of 70, OA has become the seventh leading cause of years lived with disability (YLDs), up from sixth in 1990[7]. It is projected that by 2050, there will be 642 million people with knee OA, 279 million with hand OA, 62.6 million with hip OA, and 118 million with other types of OA [7]. Due to global population growth and increased life expectancy, OA, with its high prevalence and associated disability, has become a significant public health challenge. This condition is widespread worldwide and is expected to exert an increasingly profound impact as the population ages.
Metabolism is essential for the functionality of cartilage and synovial joints, and prior research has highlighted a critical role for metabolic processes in inflammatory joint diseases, notably OA [8-10]. OA is increasingly recognized as a metabolic-associated disorder, not only because it frequently co-occurs with various metabolic dysfunctions but also due to ongoing discoveries in metabolomics that have identified specific metabolites and metabolic pathways associated with OA [11, 12]. Aberrant immune metabolism may represent a key characteristic across many OA phenotypes [13]. Recent studies in metabolomics have pinpointed a range of circulating biomarkers in both humans and animal models, including amino acids, carbohydrates, and lipids [14-16]. These insights provide new avenues for exploring complex biological metabolic networks and their links to disease states. However, the causal impact of blood metabolites on OA has yet to be definitively established, owing to limitations in sample sizes and potential confounding factors.
Randomized Controlled Trials (RCTs) are considered the gold standard for causal inference in epidemiological studies. However, they are often difficult to conduct due to ethical constraints and high costs [17, 18]. Consequently, observational studies are frequently utilized for initial etiological investigations due to their simpler design and easier implementation. Nonetheless, these studies are limited in their capacity for causal inference due to potential confounders and issues of reverse causality [19]. Recent advancements in Mendelian randomization (MR) provide a robust method to overcome these limitations [20, 21]. MR, a type of instrumental variable analysis, is employed to test causal hypotheses in observational data [22]. This approach uses genetic variants as proxies for exposure factors, enabling random grouping and the collection of summary statistics on the associations between these variants and phenotypic outcomes in large populations. By estimating the strength of these associations through genetic epidemiological models, MR minimizes the influence of confounding factors [23]. Linkage Disequilibrium Score Regression (LDSC) is an efficient tool for analyzing genetic correlations [24]. LDSC differentiates the inflation in statistics due to polygenic effects from confounding due to population stratification or other factors, thereby facilitating the estimation of heritability and genetic correlations between traits based on single nucleotide polymorphisms (SNPs). Previous MR studies investigating the links between metabolites and OA did not include genetic correlation analysis and relied on small sample sizes, which constrained the applicability and statistical robustness of their findings [25]. Given this, we aim to conduct more stringent MR analyses using larger, more recent datasets to enhance the reliability and interpretive strength of our research findings.
In this study, we utilized MR to explore the genetic correlations and causal relationships between OA and circulating metabolic biomarkers, drawing upon extensive genome-wide association study data. Our analysis encompassed 233 metabolites and 11 OA phenotypes. By integrating both single-trait and pairwise LDSC, along with bidirectional two-sample MR, this research not only identifies crucial metabolites associated with the risk of OA but also probes their potential mechanisms and interactions. This could provide a scientific foundation for the development of novel preventative and therapeutic strategies, thereby making a positive impact on public health.
Methods
Data source and phenotype definition
Circulating metabolites exposures
Circulating metabolites were applied as exposures of MR analysis in this study. GWAS summary of 233 circulating metabolic traits was extracted from a large-scale genome-wide association study contains 136,016 participants from 33 cohorts [26]. Most of the cohorts consisted of individuals of European ancestry (6 Finnish and 21 non-Finnish), and six cohorts had individuals of Asian ancestry. Circulating metabolic biomarkers was all quantified by nuclear magnetic resonance spectroscopy using NMR metabolomics platform [27]. The NMR metabolomics platform provides data of lipoprotein subclasses and their lipid concentrations and compositions, including apoAI and apoB, cholesterol and triglyceride measures, albumin, various fatty acids and low-molecular-weight metabolites.
OA outcomes
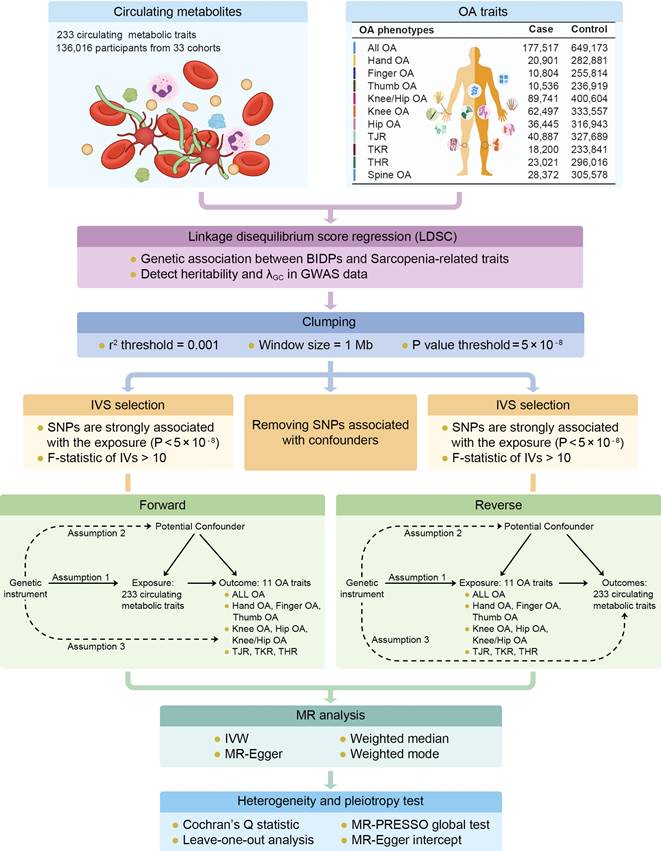
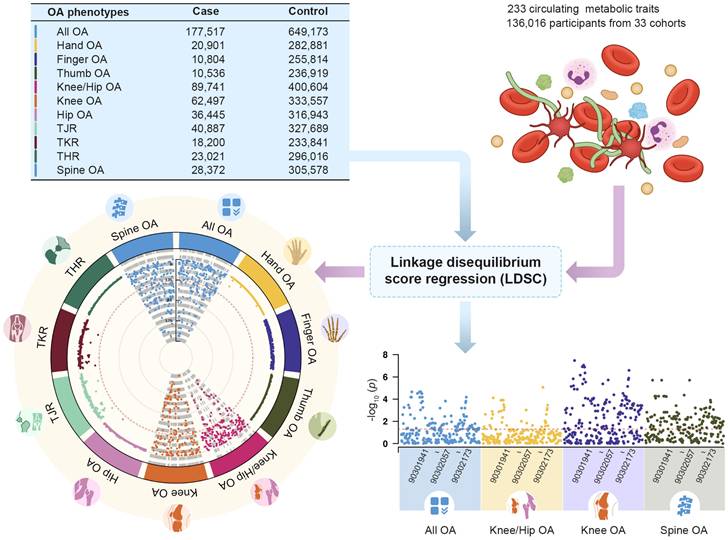
11 OA phenotypes were selected as outcomes of MR analysis in this study. GWAS summary of 11 OA phenotypes was obtained from large sample genome-wide association study across 826,690 individuals (177,517 with OA) [28]. Ethnicity including European (UK, Dutch, Icelandic, Estonian, Greece, European Americans) and Asian (Chinese and Japanese). OA was defined by either a) self-reported osteoarthritis, b) clinical diagnosed, c) ICD10 codes, d) radiographic as defined by the TREAT-OA consortium [29], depending on the data available in the cohort. 11 osteoarthritis phenotypes were descripted as follows: OA at any site (All OA), OA of the hip and/or knee (Knee/Hip OA), OA of the knee (Knee OA), OA of the hip (Hip OA), total joint replacement (TJR), total knee replacement (TKR), total hip replacement (THR), OA of the hand (Hand OA), OA of the finger (Finger OA), OA of the thumb (Thumb OA) and OA of the spine (Spine OA).
Statistics
Linkage disequilibrium score regression analysis
Linkage disequilibrium score regression (LDSC) was performed to estimate genome-wide genetic correlations between exposure and outcomes traits [30]. Single-trait LDSC was first used to estimate SNP-based heritability, mean χ2, genome inflation factor (λGC), and the intercept for each GWAS summary statistic. Polygenicity and confounding due to population stratification or cryptic relatedness can be assessed with λGC and intercept. Pairwise LDSC was conducted to estimate the genomic genetic correlations among the circulating metabolic traits and OA using the pre-computed LD scores of European ancestries from the 1000 Genomes Project Phase 3 (https://alkesgroup.broadinstitute.org/LDSCORE/). Benjamini-Hochberg procedure implemented in R 3.5.3 was used to obtain adjusted p values. P < 0.05 was considered as statistically significant in genetic correlation and MR analyses.
Two-sample bidirectional MR analysis
This is a 2-sample bidirectional Mendelian randomization using genetic variants to mimic the effect of circulating metabolic traits on OA traits. The analysis of bidirectional MR is divided into several parts including GWAS data extraction, selection of instrumental variables (threshold selection, clumping selection, pleiotropy selection, F-value selection), forward MR analysis, reverse MR analysis, etc. To ensure the validity of our Mendelian randomization (MR) analysis, we adhered to three core assumptions: Assumption 1: the instrumental variables must be strongly associated with the exposures; Assumption 2: the instrumental variables must be independent of the potential confounders of the association between the exposure and outcome; Assumption 3: the instrumental variables should not be associated with the outcomes directly (Figure 1). For Assumption 1, SNPs with P < 5 × 10-8 and F statistic > 10 were selected as instrumental variables (IVs) for MR analysis. In addition, a clumping process (r2 > 0.001, clumping distance = 10,000 kb) was conducted to assess the LD between the included SNPs. For Assumption 2, PhenoScanner [31]and PhenoScanner V2[32] was used to exclude SNPs strongly (P < 5 × 10-8) associated with confounding factors (Age, Sex, BMI, Smoking, Alcohol Consumption, Physical Activity, Diet, Thyroid Dysfunction, and Bone Mineral Density). For Assumption 3, IVs that are significantly associated with the outcome phenotype (P < 5 × 10-8) were excluded.
To conduct MR estimates, the random-effects model inverse-variance weighted (IVW) method was applied as the primary statistical analysis approach [33]. Random-effect IVW were conducted to reduce bias when heterogeneity exists [34]. The weighted median method, MR Egger regression method, weighted mode method and simple mode method were also performed as sensitivity analyses. MR-Egger intercept test was performed as an indicator of directional pleiotropy (P < 0.05 was considered statistically significant). MR-PRESSO test was also used to evaluate overall horizontal pleiotropy and detect SNP outliers. Cochran's Q statistic was used to check the heterogeneity among SNPs. The leave-one-out method was employed to evaluate whether single SNP could exert bias to the IVW estimate. The R packages “TwoSampleMR” (version 0.6.4) were used to conduct Mendelian randomization. Benjamini-Hochberg procedure implemented in R (version 4.3.2) was used to obtain adjusted p values, P < 0.05 was considered as statistically significant in genetic correlation and MR analyses.
Results
Single-trait and Pairwise LDSC result
Single-trait LDSC analysis showed that the average heritability of 233 metabolic traits calculated based on GWAS summary data was 0.0996, with a minimum value of 0.0147 and a maximum value of 0.1644. The average genomic control inflation factor (λGC) was 1.1641, ranging from a minimum of 1.0405 (Acetate levels) to a maximum of 1.2498 (Triglycerides to total lipids ratio in chylomicrons and extremely large VLDL) (Table S1). For 11 OA phenotypes, the average heritability was 0.0146, ranging from a minimum of 0.0035 to a maximum of 0.0245. The average λGC of 11 OA traits was 1.1808, ranging from a minimum of 1.0557 (Finger OA) to a maximum of 1.4037 (Knee/Hip OA) (Table S2). Pairwise LDSC analysis revealed that 41.20% of the metabolic traits showed a genetic correlation with All OA, 15.88% with Knee/Hip OA, 51.50% with Knee OA, and 52.79% with Spine OA (Figure 2). There were no significant genetic correlations observed between the metabolic traits and other OA phenotypes (Table S3).
Workflow of genetic correlation and two-sample bidirectional mendelian randomization. Assumption 1: the instrumental variables must be strongly associated with the exposures; Assumption 2: the instrumental variables must be independent of the potential confounders of the association between the exposure and outcome; Assumption 3: the instrumental variables should not be associated with the outcomes directly.
The genetic correlation between 233 circulating metabolic traits and 11 OA phenotypes.
Association between Circulating Metabolites and OA in loading extremity joint
8 circulating metabolites traits showed significant forward MR relationships with Knee/Hip OA. These traits were Ratio of conjugated linoleic acid to total fatty acids, Ratio of diacylglycerol to triglycerides, Isoleucine levels, Lactate levels, Phospholipids in large VLDL, Concentration of medium VLDL particles, Total cholesterol to total lipids ratio in very large HDL, and Phospholipids to total lipids ratio in very large HDL. Among them, Lactate levels had the highest absolute values of OR-1(OR: 1.1820, 95% CI: 1.0733 to 1.3017, P = 0.003) (Figure 3). 3 circulating metabolites traits showed significant associations with Knee OA, namely Ratio of conjugated linoleic acid to total fatty acids, Glycerol levels, and Lactate levels. Lactate levels exhibited the highest absolute values of OR-1 (OR: 1.1375, 95% CI: 1.0523 to 1.2296, P = 0.004). 7 circulating metabolites traits showed significant associations with Hip OA, including Ratio of conjugated linoleic acid to total fatty acids, Glycerol levels, Total cholesterol levels in HDL, Lactate levels, Total cholesterol to total lipids ratio in small LDL, Cholesteryl esters to total lipids ratio in very large HDL, and Phospholipids in very large HDL, Lactate levels obtained the highest absolute values of OR-1 (OR: 1.1446, 95% CI: 1.0447 to 1.2540, P = 0.012). In addition, 3 circulating metabolites traits were associated with THR, eight with TJR, and three with TKR (Table S4). Reverse MR analysis indicated a significant MR effect of Knee OA on Lactate levels (OR: 1.0646, P = 0.008). THR and TJR were both significantly associated with Ratio of conjugated linoleic acid to total fatty acids, with OR values of 1.0294 and 1.0485, respectively.
Association between Circulating Metabolites and OA in non-loading extremity joint
4 circulating metabolites traits showed significant forward MR relationships with Hand OA. These traits were Glycerol levels, Phospholipids to total lipids ratio in medium HDL, Cholesterol esters in very large HDL, and Triglycerides in very large HDL. Among them, Triglycerides in very large HDL had the highest OR-1 value (OR: 0.9730, 95% CI: 0.9492 to 0.9974, P = 0.039). 5 circulating metabolites traits showed significant MR relationships with Finger OA. These traits were Acetate levels, Ratio of 22:6 docosahexaenoic acid to total fatty acids, Glycerol levels, Free cholesterol in large HDL, and Pyruvate levels. Among them, Acetate levels had the highest OR-1 value (OR: 0.8266, 95% CI: 0.6914 to 0.9882, P = 0.040). 4 circulating metabolites showed significant associations with Thumb OA. These traits were Free cholesterol to total lipids ratio in IDL, Lactate levels, Total cholesterol to total lipids ratio in large HDL, and Phospholipids to total lipids ratio in medium HDL. Among them, Phospholipids to total lipids ratio in medium HDL had the highest absolute values of OR-1 (OR: 0.9421, 95% CI: 0.8966 to 0.9898, P = 0.030) (Table S4). Reverse MR analysis did not detect statistically significant results (Table S5).
Association between Circulating Metabolites and OA in spine
6 circulating metabolites traits showed significant forward MR relationships with Spine OA. These traits were 3-Hydroxybutyrate levels, Lactate levels, Ratio of polyunsaturated fatty acids to total fatty acids, Pyruvate levels, Total cholesterol to total lipids ratio in very large HDL, and Phospholipids to total lipids ratio in very large HDL. Among them, Lactate levels had the highest absolute values of OR-1 (OR: 1.3179, 95% CI: 1.2367 to 1.4044, P < 0.001). Both Pyruvate levels and Total cholesterol to total lipids ratio in very large HDL had OR effect values less than 1 for Spine OA (Table S4). Reverse MR analysis did not detect statistically significant results.
Circulating Metabolites exerting multi-dimensional effects on OA traits
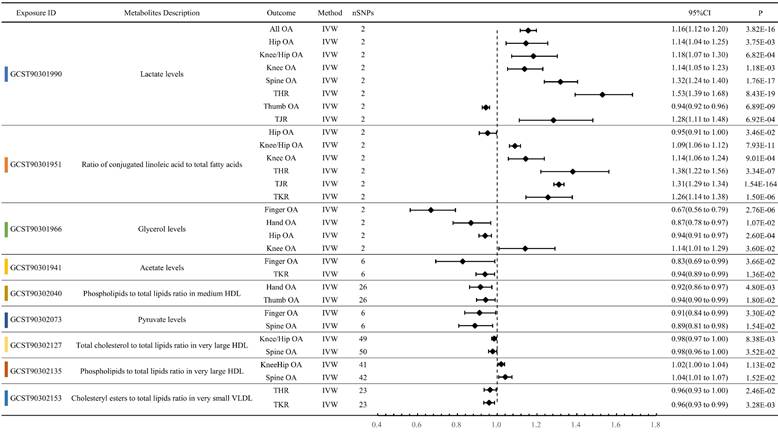
Lactate levels exhibited a significant forward MR relationship with eight OA phenotypes. Except for Thumb OA (OR: 0.9429, P < 0.001), the OR values for the other OA phenotypes were greater than 1. Specifically, they were All OA (OR: 1.1558, P < 0.001), Hip OA (OR: 1.1446, P = 0.004), Knee/Hip OA (OR: 1.1820, P < 0.001), Knee OA (OR: 1.1375, P = 0.001), Spine OA (OR: 1.3179, P < 0.001), THR (OR: 1.5290, P < 0.001), and TJR (OR: 1.2827, P < 0.001). Ratio of conjugated linoleic acid to total fatty acids also showed significant MR relationships with 6 OA phenotypes: Hip OA (OR: 0.9522, P = 0.035), Knee/Hip OA (OR: 1.0890, P < 0.001), Knee OA (OR: 1.1429, P < 0.001), THR (OR: 1.3800, P < 0.001), TJR (OR: 1.3102, P < 0.001), and TKR (OR: 1.2555, P < 0.001). Moreover, Glycerol levels exhibited significant MR associations with four OA phenotypes: Finger OA (OR: 0.6669, P < 0.001), Hand OA (OR: 0.8682, P = 0.011), Hip OA (OR: 0.9395, P < 0.001), and Knee OA (OR: 1.1409, P = 0.036) (Figure 4, Table S6).
Sensitivity Analysis
In 11 OA phenotypes, the metabolite with the largest absolute value of OR-1 is shown in Figure 3. The MR results of these phenotypes all exhibit statistical significance in the IVW method, but do not reach significance in other methods. Except for the effect of Phospholipids to total lipids ratio in medium HDL on ThumbOA, which also shows significance in Weighted median, Weighted mode, and Simple mode (Table S7). In the metabolites illustrated in Figure 4, most metabolites show statistical significance in the IVW method, but not in other MR methods. Except for Phospholipids to total lipids ratio in medium HDL, Pyruvate levels, Total cholesterol to total lipids ratio in very large HDL, Phospholipids to total lipids ratio in very large HDL, and Cholesteryl esters to total lipids ratio in very small VLDL, the significance of these metabolites is statistically significant in four or more MR methods. ALL sensitivity analysis result can be referenced in Table S8.
Representation of the metabolites with the highest absolute value of OR-1 among 11 OA trait.
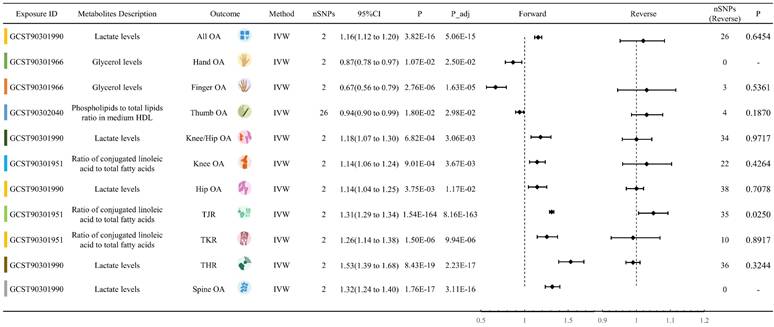
Forest plot of circulating metabolites exerting multi-dimensional effects on OA traits.
Discussion
This study employed MR to investigate the potential genetic correlations and causal relationships between circulating metabolic biomarkers and OA. OA is a prevalent degenerative joint disease that significantly impacts the quality of life for millions globally. Despite the incomplete understanding of its precise etiology, a growing body of epidemiological evidence indicates an association between metabolic disturbances and OA, particularly in younger demographics [35]. These disturbances include conditions such as obesity, hypertension, dyslipidemia, hyperglycemia, and insulin resistance, all of which can accelerate the onset and progression of OA across both weight-bearing and non-weight-bearing joints [36]. Our systematic analysis of 233 metabolites and 11 OA phenotypes revealed notable genetic and causal connections between various metabolites and distinct OA phenotypes. Results from paired LDSC analysis indicated significant genetic correlations between numerous metabolites and specific OA manifestations, including knee and spinal OA. Furthermore, our comprehensive MR analysis assessed the relationships between circulating metabolites and various types of OA, encompassing weight-bearing joints (knees and hips), non-weight-bearing joints (hands and fingers), and spinal OA. Our findings demonstrate that certain metabolites, such as lactate levels, the ratio of conjugated linoleic acid to total fatty acids, and glycerol levels, exhibit significant positive MR associations with various OA phenotypes. These results suggest that these metabolites might influence the progression of OA through specific biological pathways, offering insights that could inform future preventative and therapeutic strategies for OA.
This study identified a significant positive association between lactate levels and eight OA phenotypes through MR analysis. With the exception of Thumb OA (OR: 0.9429), lactate levels showed a consistent direction of effect for the remaining seven OA phenotypes, specifically indicating positive correlations with All OA, Hip OA, Knee/Hip OA, Knee OA, Spine OA, THR, and TJR. These findings suggest that elevated lactate levels are strongly linked to an increased risk of these OA phenotypes, underscoring the pathological significance of lactate as a metabolic factor in OA. Traditionally viewed as a metabolic waste product of glycolysis, recent studies have revealed that lactate serves not merely as an intermediary metabolite but also as a versatile immunomodulator that regulates inflammatory responses [37, 38]. In the synovium of chronic arthritis, lactate influences immune cell function through multiple mechanisms, including migration and cytokine production [39]. Lactate triggers a "stop migration" signal in T cells through the lactate transporters SLC5A12 in CD4+ T cells and SLC16A1 in CD8+ T cells, promoting their accumulation at inflammation sites [40, 41]. This lactate-mediated suppression of T cell mobility in inflamed tissues coincides with a reduction in glycolysis. Sodium lactate inhibits several glycolytic enzymes and decreases glucose flux expression in CD4+ T cells, leading to T cell accumulation at the site of inflammation. This process extends the duration of chronic inflammation by increasing the production of inflammatory cytokines and reducing cell lysis [42, 43]. OA is a chronic inflammatory joint disease, and lactate is known to exacerbate its progression through previously described pathways. Recent research has underscored the significant role of lactate in the pathogenesis of OA, revealing elevated levels of lactate in patients and linking these levels to various metabolic indicators [44]. Studies have demonstrated that lactate activates the HCAR1/PI3K signaling pathway, which upregulates the expression of NADPH oxidase 4 (NOX4). This upregulation leads to an increased production of reactive oxygen species (ROS), resulting in chondrocyte dysfunction. Furthermore, lactate is shown to promote the expression of degradative enzymes, reduce the synthesis of type II collagen, and induce the secretion of inflammatory factors, as well as chondrocyte hypertrophy and senescence. The application of the NOX4 inhibitor GLX351322 or the ROS scavenger N-acetylcysteine (NAC) has been found to mitigate the damage to chondrocytes induced by lactate [44]. Additionally, intra-articular injections of oxamate (an LDHA inhibitor that reduces the conversion of pyruvate to lactate) effectively decrease the expression of glycolysis-related proteins in a rat model of OA induced by anterior cruciate ligament transection (ACLT). This treatment significantly reduces chondrocyte apoptosis and alleviates pain and inflammation, while also inhibiting cartilage degeneration [45]. Both past studies and our findings highlight the role of lactate as a metabolic factor in OA progression, providing a theoretical foundation and potential targets for future metabolic treatment strategies.
Moreover, this study's findings reveal that the ratio of conjugated linoleic acid (CLA) to total fatty acids exhibits distinct association patterns across various OA phenotypes. Specifically, this ratio displays a protective effect in Hip OA (OR: 0.9522), suggesting that a higher proportion of CLA relative to total fatty acids may decrease the risk of Hip OA. Conversely, in Knee/Hip OA (OR: 1.0890), Knee OA (OR: 1.1429), THR (OR: 1.3800), TJR (OR: 1.3102), and TKR (OR: 1.2555), a higher ratio correlates with an increased disease risk, indicating that an elevated ratio of conjugated linoleic acid to total fatty acids may constitute a risk factor in these phenotypes. CLA, an unsaturated octadecadienoic acid containing conjugated double bonds, encompasses a mix of positional and geometric isomers of linoleic acid [46]. It is known for its physiological activities, which include anti-inflammatory, antihypertensive, anticancer, antioxidant, anti-atherosclerosis, and anti-obesity effects [47]. Theoretically, CLA includes 56 isomers, with 20 naturally occurring isomers identified through NMR, each exhibiting significantly different physiological functions [48]. Among these, the cis-9, trans-11 and trans-10, cis-12 isomers are the most abundant and are noted for their beneficial physiological activities [49-51]. CLA is typically recognized for its anti-inflammatory and antioxidant properties [52, 53], which may elucidate its protective role in Hip OA. However, in other OA phenotypes, increased levels of CLA might worsen disease risk through mechanisms such as altered lipid metabolism and heightened inflammatory responses. Particularly in severe OA phenotypes requiring joint replacement, the risk increase associated with high CLA ratios could be linked to its pro-inflammatory or detrimental metabolic effects within the local environment. This dual effect implies that the influence of CLA may vary based on the type and pathological state of OA. Additionally, these varying impacts of CLA could also be attributed to its different isomers [48]. Various CLA isomers might have distinct roles in lipid metabolism, inflammation regulation, and immune function, thus affecting the development and progression of OA. Future research should explore in greater depth the specific biological mechanisms of different CLA isomers across various OA phenotypes and assess their potential as therapeutic targets to better comprehend and manipulate their role in OA progression. This molecular-level understanding could help in precisely regulating CLA-related pathways, providing more targeted strategies for OA treatment. In the realm of experimental research, a study conducted by Shen CL and colleagues provides preliminary evidence concerning the potential role of CLA in OA treatment [54]. Theirs in vitro experiments assessed the impact of CLA, both alone and in combination with other polyunsaturated fatty acids (PUFAs), on the production of inflammatory mediators in human OA chondrocytes. The study found that CLA significantly decreased the production of prostaglandin E2 and nitric oxide, with the combination of CLA and eicosapentaenoic acid (EPA) proving particularly effective in reducing PGE2 levels. These findings indicate that CLA could play a role in OA treatment by modulating inflammatory mediators. However, given the relatively sparse research on CLA's effects on OA, further investigations are required to confirm these results and to explore the specific mechanisms through which CLA and its isomers act in various types and pathological states of OA. A deeper molecular understanding will facilitate the precise modulation of CLA-related pathways, providing more targeted therapeutic strategies for OA treatment.
This study also uncovered significant correlations between glycerol levels and various OA phenotypes: glycerol levels were inversely associated with both finger OA and hand OA, indicating a potential protective role for glycerol in these non-weight-bearing joints. Additionally, glycerol demonstrated a negative association with hip OA, suggesting it may protect joints subjected to lower mechanical stress. This protective effect may be due to glycerol's role in regulating lipid metabolism and anti-inflammatory pathways, which helps to maintain cell membrane stability and suppress the production of inflammatory mediators, thereby mitigating joint tissue damage and inflammation. Conversely, the positive correlation between glycerol levels and weight-bearing joints such as knee OA suggests a different mechanism might be at play. In such weight-bearing joints, increased levels of glycerol could be associated with disorders in lipid metabolism, leading to heightened local or systemic inflammatory states, thereby promoting the progression of OA. Furthermore, as an energy source, glycerol might cause imbalances in energy metabolism under conditions of excessive joint usage or increased mechanical stress, further aggravating degenerative changes in joint cartilage. These findings suggest potential therapeutic targets for different OA phenotypes. For example, modulating glycerol levels or its metabolic pathways could offer protective strategies for hand and finger joint OA, whereas strategies to decrease glycerol levels or adjust its related metabolic pathways may be beneficial for knee joint OA. Additionally, these results underscore the importance of personalized medicine, that is, developing treatment plans tailored to the specific OA phenotypes and metabolic characteristics of individual patients. These insights should be further explored in future research, particularly through clinical trials that assess the impact of modulating glycerol levels or its metabolic pathways on OA progression, as well as the safety and efficacy of such interventions.
While this study offers new insights into the associations between circulating metabolic biomarkers and OA, it is subject to several limitations. Firstly, the study used data from multiple ethnicities, but genetic differences across populations can impact associations between genetic variants and metabolic traits, potentially introducing bias in MR analysis. Secondly, the MR analysis in this study uses genetic instrumental variables to represent exposure factors. Due to the constraints of the sample size and the additive regression models used, only a small proportion of the variance in exposure factors is explained, making it difficult to detect subtle causal effects among complex traits. Lastly, the effect sizes derived from genetic correlation analyses represent estimates based on the current dataset and model, and should not be considered equivalent to or substitutes for effect sizes obtained from observational clinical studies. A more comprehensive understanding beneficial for clinical practice can only be achieved by integrating genetic correlation analysis with traditional epidemiological studies, real-world research, bibliometric reviews, or meta-analyses.
Conclusion
This study utilized MR and LDSC methods to identify significant genetic and causal links between circulating metabolites and various OA phenotypes. Lactate levels were positively associated with multiple OA phenotypes, suggesting their role in the processes of OA pathogenesis. The ratio of conjugated linoleic acid to total fatty acids and glycerol levels showed variable effects across different OA types, suggesting their potential as therapeutic targets. These findings emphasize the importance of metabolic profiling in OA management, supporting personalized medicine approaches. Future research should further explore these metabolites' mechanisms and validate the findings across diverse populations and clinical settings.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported in part by the High-Performance Computing Center of Central South University.
Fundings
This work was supported by the National Key R&D Program of China (2023YFC3603400, 2023YFB4606705, 2022YFF1100203), National Natural Science Foundation of China(82072506, 82272611, 92268115, 32372349), Hunan Provincial Science Fund for Distinguished Young Scholars (2024JJ2089), Hunan Young Talents of Science and Technology (2021RC3025), Provincial Clinical Medical Technology Innovation Project of Hunan (2023SK2024, 2020SK53709), Provincial Natural Science Foundation of Hunan (2020JJ3060), Natural Science Foundation of Hunan Province (2023JJ30949), National Clinical Research Center for Geriatric Disorders, Xiangya Hospital (2021KFJJ02, 2021LNJJ05), the Hunan Provincial Innovation Foundation for Postgraduate (CX20230308), the Independent Exploration and Innovation Project for Postgraduate Students of Central South University (2024ZZTS0163).
Availability of data and materials
GWAS summary data for 233 circulating metabolic traits was extracted from GWAS Catalog: https://www.ebi.ac.uk/gwas/home. GWAS summary data for 11 OA phenotypes was extracted from Genetics of Osteoarthritis (GO) Consortium GWAS: https://msk.hugeamp.org/downloads.html. This study relies on publicly accessible datasets from GWAS and does not involve the use of individual-level data.
Ethics approval and consent to participate
Our analysis used publicly available genome-wide association study (GWAS) summary statistics. No new data were collected, and no new ethical approval was needed.
Authors' contributions
Conceptualization, GY; Methodology, WFX and YPW; verification of the underlying data, HZL, WHL, YL; writing—original draft, GY, WQX; writing—review and editing, WFX and YSL. All the authors participated in planning, execution, and analysis and have read and approved the final submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (London, England). 2019;393:1745-59
2. Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. Jama. 2021;325:568-78
3. Bernabei I, So A, Busso N, Nasi S. Cartilage calcification in osteoarthritis: mechanisms and clinical relevance. Nature reviews Rheumatology. 2023;19:10-27
4. Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nature reviews Rheumatology. 2022;18:258-75
5. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H. et al. Osteoarthritis. Lancet (London, England). 2015;386:376-87
6. Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y. et al. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis & rheumatology (Hoboken, NJ). 2022;74:1172-83
7. Global regional, national burden of osteoarthritis 1990-2020, projections to 2050. a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Rheumatology. 2023;5:e508-e22
8. Choi WS, Lee G, Song WH, Koh JT, Yang J, Kwak JS. et al. The CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566:254-8
9. Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing research reviews. 2021;66:101249
10. Wu X, Liyanage C, Plan M, Stark T, McCubbin T, Barrero RA. et al. Dysregulated energy metabolism impairs chondrocyte function in osteoarthritis. Osteoarthritis and cartilage. 2023;31:613-26
11. Zhai G. Alteration of Metabolic Pathways in Osteoarthritis. Metabolites. 2019 9
12. Showiheen SAA, Sun AR, Wu X, Crawford R, Xiao Y, Wellard RM. et al. Application of Metabolomics to Osteoarthritis: from Basic Science to the Clinical Approach. Current rheumatology reports. 2019;21:26
13. Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nature reviews Rheumatology. 2017;13:302-11
14. Werdyani S, Liu M, Zhang H, Sun G, Furey A, Randell EW. et al. Endotypes of primary osteoarthritis identified by plasma metabolomics analysis. Rheumatology (Oxford, England). 2021;60:2735-44
15. Tu B, Fang R, Zhu Z, Chen G, Peng C, Ning R. Comprehensive analysis of arachidonic acid metabolism-related genes in diagnosis and synovial immune in osteoarthritis: based on bulk and single-cell RNA sequencing data. Inflammation research: official journal of the European Histamine Research Society [et al]. 2023;72:955-70
16. Tootsi K, Vilba K, Märtson A, Kals J, Paapstel K, Zilmer M. Metabolomic Signature of Amino Acids, Biogenic Amines and Lipids in Blood Serum of Patients with Severe Osteoarthritis. Metabolites. 2020 10
17. Jones DS, Podolsky SH. The history and fate of the gold standard. Lancet (London, England). 2015;385:1502-3
18. Pearce N, Vandenbroucke JP, Lawlor DA. Causal Inference in Environmental Epidemiology: Old and New Approaches. Epidemiology (Cambridge, Mass). 2019;30:311-6
19. D'Onofrio BM, Sjölander A, Lahey BB, Lichtenstein P, Öberg AS. Accounting for Confounding in Observational Studies. Annual review of clinical psychology. 2020;16:25-48
20. Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ (Clinical research ed). 2021;375:n2233
21. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. Journal of the American Society of Nephrology: JASN. 2016;27:3253-65
22. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA. et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. Jama. 2021;326:1614-21
23. Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. Jama. 2017;318:1925-6
24. Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature genetics. 2015;47:291-5
25. Gu Y, Jin Q, Hu J, Wang X, Yu W, Wang Z. et al. Causality of genetically determined metabolites and metabolic pathways on osteoarthritis: a two-sample mendelian randomization study. Journal of translational medicine. 2023;21:357
26. Karjalainen MK, Karthikeyan S, Oliver-Williams C, Sliz E, Allara E, Fung WT. et al. Genome-wide characterization of circulating metabolic biomarkers. Nature. 2024;628:130-8
27. Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. American journal of epidemiology. 2017;186:1084-96
28. Boer CG, Hatzikotoulas K, Southam L, Stefánsdóttir L, Zhang Y, Coutinho de Almeida R. et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. 2021;184:6003-5
29. Kerkhof HJ, Meulenbelt I, Akune T, Arden NK, Aromaa A, Bierma-Zeinstra SM. et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA consortium. Osteoarthritis and cartilage. 2011;19:254-64
30. Ni G, Moser G, Wray NR, Lee SH. Estimation of Genetic Correlation via Linkage Disequilibrium Score Regression and Genomic Restricted Maximum Likelihood. American journal of human genetics. 2018;102:1185-94
31. Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB. et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207-9
32. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J. et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851-3
33. Lee CH, Cook S, Lee JS, Han B. Comparison of Two Meta-Analysis Methods: Inverse-Variance-Weighted Average and Weighted Sum of Z-Scores. Genomics & informatics. 2016;14:173-80
34. Ye C-J, Liu D, Chen M-L, Kong L-J, Dou C, Wang Y-Y. et al. Mendelian randomization evidence for the causal effect of mental well-being on healthy aging. Nature Human Behaviour. 2024
35. Funck-Brentano T, Nethander M, Movérare-Skrtic S, Richette P, Ohlsson C. Causal Factors for Knee, Hip, and Hand Osteoarthritis: A Mendelian Randomization Study in the UK Biobank. Arthritis & rheumatology (Hoboken, NJ). 2019;71:1634-41
36. Wei G, Lu K, Umar M, Zhu Z, Lu WW, Speakman JR. et al. Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone research. 2023;11:63
37. Li X, Yang Y, Zhang B, Lin X, Fu X, An Y. et al. Lactate metabolism in human health and disease. Signal transduction and targeted therapy. 2022;7:305
38. Manosalva C, Quiroga J, Hidalgo AI, Alarcón P, Anseoleaga N, Hidalgo MA. et al. Role of Lactate in Inflammatory Processes: Friend or Foe. Frontiers in immunology. 2021;12:808799
39. Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nature reviews Immunology. 2021;21:151-61
40. Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D'Acquisto F. et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS biology. 2015;13:e1002202
41. Pucino V, Certo M, Bulusu V, Cucchi D, Goldmann K, Pontarini E. et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4(+) T Cell Metabolic Rewiring. Cell metabolism. 2019;30:1055-74.e8
42. Liu S, Liao S, Liang L, Deng J, Zhou Y. The relationship between CD4(+) T cell glycolysis and their functions. Trends in endocrinology and metabolism: TEM. 2023;34:345-60
43. Certo M, Llibre A, Lee W, Mauro C. Understanding lactate sensing and signalling. Trends in endocrinology and metabolism: TEM. 2022;33:722-35
44. Huang YF, Wang G, Ding L, Bai ZR, Leng Y, Tian JW. et al. Lactate-upregulated NADPH-dependent NOX4 expression via HCAR1/PI3K pathway contributes to ROS-induced osteoarthritis chondrocyte damage. Redox biology. 2023;67:102867
45. Wen ZH, Sung CS, Lin SC, Yao ZK, Lai YC, Liu YW. et al. Intra-Articular Lactate Dehydrogenase A Inhibitor Oxamate Reduces Experimental Osteoarthritis and Nociception in Rats via Possible Alteration of Glycolysis-Related Protein Expression in Cartilage Tissue. International journal of molecular sciences. 2023 24
46. Du M, Gong M, Wu G, Jin J, Wang X, Jin Q. Conjugated Linolenic Acid (CLnA) vs Conjugated Linoleic Acid (CLA): A Comprehensive Review of Potential Advantages in Molecular Characteristics, Health Benefits, and Production Techniques. Journal of agricultural and food chemistry. 2024;72:5503-25
47. Shokryzadan P, Rajion MA, Meng GY, Boo LJ, Ebrahimi M, Royan M. et al. Conjugated linoleic acid: A potent fatty acid linked to animal and human health. Critical reviews in food science and nutrition. 2017;57:2737-48
48. Li S, Xu L, Qing J, Wu X, Li H, Chen H. et al. Multiple biological activities and biosynthesis mechanisms of specific conjugated linoleic acid isomers and analytical methods for prospective application. Food chemistry. 2023;409:135257
49. Wang T, Lee HG. Advances in research on cis-9, trans-11 conjugated linoleic acid: a major functional conjugated linoleic acid isomer. Critical reviews in food science and nutrition. 2015;55:720-31
50. McCrorie TA, Keaveney EM, Wallace JM, Binns N, Livingstone MB. Human health effects of conjugated linoleic acid from milk and supplements. Nutrition research reviews. 2011;24:206-27
51. Wang K, Xin Z, Chen Z, Li H, Wang D, Yuan Y. Progress of Conjugated Linoleic Acid on Milk Fat Metabolism in Ruminants and Humans. Animals: an open access journal from MDPI. 2023 13
52. Xie W, Shi H, Zuo R, Zhou S, Ma N, Zhang H. et al. Conjugated Linoleic Acid Ameliorates Hydrogen Peroxide-Induced Mitophagy and Inflammation via the DRP1-mtDNA-STING Pathway in Bovine Hepatocytes. Journal of agricultural and food chemistry. 2024;72:2120-34
53. Viladomiu M, Hontecillas R, Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. European journal of pharmacology. 2016;785:87-95
54. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39:161-6
Author contact
![]() Corresponding authors: Yaping Wang, E-mail: wangyapingedu.cn; Wenfeng Xiao, E-mail: xiaowenfengedu.cn
Corresponding authors: Yaping Wang, E-mail: wangyapingedu.cn; Wenfeng Xiao, E-mail: xiaowenfengedu.cn

 Global reach, higher impact
Global reach, higher impact