3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(1):45-60. doi:10.7150/ijms.88216 This issue Cite
Review
Expression and regulation of HIF-1a in hypoxic pulmonary hypertension: Focus on pathological mechanism and Pharmacological Treatment
1. School of Integrated Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha 410208, Hunan, People's Republic of China.
2. Department of Respiratory Diseases, Medical School, Hunan University of Chinese Medicine, Changsha 410208, Hunan, People's Republic of China.
3. Hunan Provincial Key Laboratory of Vascular Biology and Translational Medicine, Changsha 410208, Hunan, People's Republic of China.
4. Department of Respiratory Medicine, First Affiliated Hospital, Hunan University of Chinese Medicine, Changsha 410021, Hunan, People's Republic of China.
5. The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha 410021, Hunan, People's Republic of China.
Received 2023-7-18; Accepted 2023-10-20; Published 2024-1-1
Abstract
Hypoxia inducible factor-1(HIF-1), a heterodimeric transcription factor, is composed of two subunits (HIF-1α and HIF-1β). It is considered as an important transcription factor for regulating oxygen changes in hypoxic environment, which can regulate the expression of various hypoxia-related target genes and play a role in acute and chronic hypoxia pulmonary vascular reactions. In this paper, the function and mechanism of HIF-1a expression and regulation in hypoxic pulmonary hypertension (HPH) were reviewed, and current candidate schemes for treating pulmonary hypertension by using HIF-1a as the target were introduced, so as to provide reference for studying the pathogenesis of HPH and screening effective treatment methods.
Keywords: Hypoxia-inducible factor-1a, Hypoxia induced pulmonary hypertension, Pulmonary vascular remodeling, Right ventricular remodeling
1. Introduction
The acute reduction of oxygen in the lung can lead to hypoxic pulmonary vasoconstriction (HPV). However, chronic hypoxic stimulation can cause to continuous contraction of pulmonary vessels, hypoxic pulmonary vascular remodeling (HPVR), and an increase of pulmonary circulatory resistance, resulting in the development of pulmonary hypertension (PAH). This change is largely irreversible1. The data of human subjects and animal models show that hypoxia-inducible factor-1 (HIF-1) plays a role in acute and chronic hypoxic pulmonary vascular reactions2. Combined with hypoxia response elements (HRE) in promoter region of target gene, the expression and regulation of HIF-1a enable organisms to cope with reduced partial pressure of oxygen in environment. However, prolonged activation of HIF-1a can give rise to changes in the structure of pulmonary blood vessels, thereby leading to the occurrence of PAH. PAH is characterized by an increase in pulmonary hypertension associated with pulmonary artery remodeling, accompanied by a reduction in the area of vascular lumen, and right heart hypertrophy; Eventually, the patient dies of right heart failure3. The apoptosis and proliferation imbalance of pulmonary artery smooth muscle cells (PASMCs) are considered to be the main link of hypoxic pulmonary hypertension (HPH), while HIF-1a plays an important role in the expression of various angiogenin and growth factors induced by hypoxia4. Semenza et al. found an oxygen-dependent nuclear transcription factor HIF-1 during the hypoxia treatment of hepatocellular carcinoma Hep3B in 19925. It is a DNA-binding protein that can regulate the transcription of erythropoietin (EPO) gene. Previous researches on HIF-1a mainly focus on tumors. At present, the correlation among hypoxia, HIF-1a and chronic respiratory diseases is one of the current research hotspots6. It is critical to identify new candidate schemes for treating HPH with HIF-1a as the target by understanding the function and mechanism of HIF-1a expression and regulation in HPH.
2. Structure and function of HIF-1a
HIF-1 including subunits HIF- 1α and HIF- 1β is a seterodimer transcription factor7, of which HIF- 1α is a functional one. As a regulatory and active subunit, it is only expressed in the nucleus under hypoxia8.O2 mostly regulates the activity of HIF-1 by this subunit. As aryl hydrocarbon receptor nuclear transporter (ARNT), HIF-1β is also a subunit of aromatic hydrocarbon receptor compound, which can be expressed under normoxia and hypoxia, and interact with HIF- 1α to form a dimer. However, which aspects HIF-1β plays its role in is not clear. Currently, it may be related to the active conformational transformation caused by the dimerization of HIF-1 and its stability9.
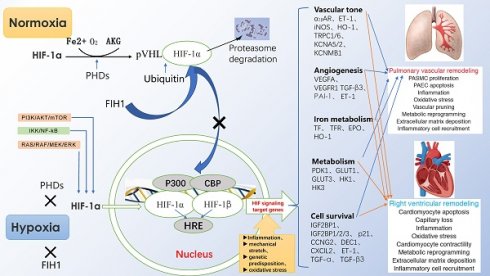
Under normoxia, HIF-1α hydroxylated by proline hydroxylase-domain protein (PHD) is quickly recognized by ubiquitin-E3 ligase compound, so that HIF-1α is rapidly degraded by ubiquitin proteasome10. However, under hypoxia, the effect of PHD is inhibited, so that HIF-1α can easily enter the nucleus to form a stable heterodimer structure with HIF-1β, and bind to the HRE of the gene. Among them, CBP/P300 protein is used as a bridge to connect RNA polymerase to induce the expression of the target gene. Activated HIF-1a can induce the expression of endothelin (ET), erythropoietin (EPO), vascular endothelial growth factor (VEGF) and inducible nitric oxide symthase (iNOS), and participate in the regulation of cell proliferation, angiogenesis, cell metabolism and inflammatory reaction (Fig. 1).
Regulation of HIF-1α expression, transcriptional activation pathways, and downstream target gene expression.
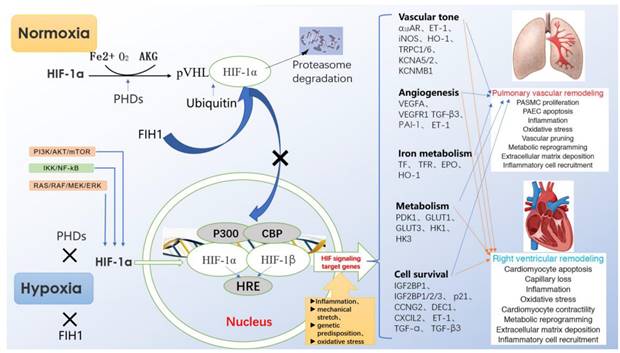
3. HIF-1a and HPH
HPH is a complex heart and lung disease caused by long-term hypoxia, which often occurs in chronic obstructive pulmonary diseases, interstitial pulmonary diseases and sleep apnea. Main clinical manifestations are exertional dyspnea, asthenia syncope, and sometimes angina pectoris, and clinicopathologic features are right heart overload, right ventricular hypertrophy, etc. Severe patients may have right heart failure, even die. Pathological mechanism of HPH is complex, which is mainly pulmonary vascular continuous contraction and irreversible remodeling under hypoxia11.
Under hypoxic condition, the synthesis of vasodilator and vasoconstrictor substances in the body decreases and increases respectively, resulting in a vasoconstrictive response; HIF-1a binds to the specific site of ET gene, which promotes the synthesis and secretion of ET by PAEC. ET-1 is a highly effective vasoconstrictor factor, and the imbalance of ET and NO expressions under hypoxia exposure is closely related to the occurrence of HPH. The changes of intracellular concentration of K+ and Ca2+ play an important role in regulating the contraction of PASMC. ET binds to ET-A on vascular smooth muscle cells and causes the contraction of PASMC through calcium dependent regulation and membrane depolarization12. Meanwhile, under long-term hypoxia exposure, ET can inhibit the binding of HIF-1a to the specific site of inducible nitric oxide synthase (iNOS) target genes, and the expression of iNOS mRNA, which causes a decrease in NO released from endothelial cells, and an imbalance between ET and NO production3. In addition, under hypoxia, the production of mitochondrial ROS also increases. ROS acts as a signaling molecule to activate HIF-1α and voltage gated calcium channel, and inhibit the expression of voltage gated potassium channel, which leads to an increase in intracellular concentration of Ca2+, and the contraction of smooth muscle cells13. Moreover, under continuous hypoxia, Rho kinase can be activated, and pulmonary vasoconstriction and HIF-1α expression can be enhanced14. However, under hypoxic stimulation, HIF-1a promotes an increase in the concentration of Ca2+ in PASMC. However, the specific mechanism of phenotypic changes remains to be explored.
The cellular structures and pathological processes involved in pulmonary vascular remodeling include the proliferation, migration, and dedifferentiation of pulmonary arterial smooth muscle cells (PASMCs); excessive proliferation and anti-apoptosis of pulmonary vascular endothelial cells (PAEC); proliferation and migration of fibroblasts; and macrophage aggregation. Further, the muscularization of non-muscular arteries, extracellular matrix deposition, and chronic inflammation are also observed, among other processes15, 16, 17, 18. In addition to pulmonary vascular remodeling, there is also obvious right ventricular remodeling (RVR). The following primarily introduces the impact of HIF-1α expression regulation on the above mechanisms.
3.1 HIF-1a and PASMCs
The proliferation and migration of PASMCs are closely related to pulmonary vascular remodeling. However, the regulation of HIF-1α under hypoxia is the key to affect angiogenesis and structural remodeling. Previous studies show that factors, such as vascular endothelial growth factor (VEGF), have a significant influence on the proliferation of vascular smooth muscle cells and the germination of new blood vessels19. It is found in the latest studies that a variety of cytokines, microRNA, non-coding RNA (ncRNA), glucose metabolism, oxidative stress, etc. can regulate the expression of HIF-1a20.
HIF-1α and heme oxygenase-1(HO-1) are important transcriptional regulatory factors in hypoxic cells and in maintaining cellular homeostasis. ZHANG et al.21 investigated the distribution of HIF-1α and HO-1 in the lungs of yaks. Immunohistochemistry and immunofluorescence results showed that HIF-1α and HO-1 are mainly concentrated in the middle layer of small pulmonary arteries and are significantly upregulated in hypoxic PASMCs. CD146 is significantly up-regulated in PASMCs, which is proportional to the severity of HPH. Destroying the interaction between CD146 and HIF-1α by gene ablation can weaken pulmonary vascular remodeling in chronic hypoxia model mice22. Another study indicates that HIF-1α mediates excessive proliferation, anti-apoptosis and calcification of PASMCs in pulmonary hypertension through the activation of Runt-related transcription factor 2 (RUNX2)23. Wang et al.24 found that the production of reactive oxygen species (ROS) increased and the expression of HIF- 1α was regulated under hypoxia, thus affecting the proliferation of PASMCs. Among them,2-methoxyestradiol can significantly improve the damage of mitochondria under hypoxia, and weaken the level of ROS in serum. It can also decrease HIF-1α in pulmonary tissue and vessels, inhibit pulmonary vascular remodeling and reduce pulmonary hypertension. Under hypoxia, the expression of KLF5 in PASMCs increases and both the expression of HIF-1α and the proliferation of PASMCs are promoted; silencing the expression of HIF-1α by small interfering RNA (siRNA) has no effect on the expression of KLF5, but weakens the proliferation ability of PASMCs25. The increased of SENP-1 expression can significantly up-regulate the HIF-1α expression and promote the proliferation of PASMCs under hypoxia26.
Various cytokines, microRNA and ncRNA participate in the regulation of HIF-1a expression under hypoxia. MiR-204 is primarily expressed in PASMCs, and its expression levels are downregulated in the chronic hypoxia and MCT-induced PH rat models27.Reduced miR-204 expression can activate HIF-1α, leading to PASMC proliferation and resistance to apoptosis28,while restoring miR-204 expression significantly reduces the severity of PH29. ET-1 and HIF-1α levels in serum of HPH patients are positively correlated with pulmonary artery systolic pressure (PASP)30. A study shows that HIF-1 regulates the expression of ET-1 by microRNA-543, and the reaction of pulmonary vessels to hypoxia31. Wang et al.[32]proved that mRNA levels of ET-1, HIF-1α and adrenomedullin (ADM) in neonatal rats during early hypoxia increased continuously, and mean pulmonary artery pressure and pulmonary vascular remodeling also rose. It is suggested that HIF-1α may regulate levels of ET-1 and ADM, thereby participating in the formation of neonatal HPH. Tang et al.[33]found that MicroRNA-143-5p regulated the function of PASMCs in HPH by targeting HIF-1α, and the over-expression of MicroRNA-143-5p significantly lowered the level of specific contraction marker protein and cell apoptosis of vascular smooth muscle, and improved the cell migration of PASMCs under hypoxia. Chen et al. observed34 that microRNA- 150 was down-regulated in hypoxic PASMCs and over-expressed HIF-1α weakened the inhibitory effect of miR-150 on the proliferation and migration of PASMCs. It can be concluded that miR-150 may inhibits the proliferation and migration of PASMCs by down-regulating HIF-1α. In addition, microRNA-195-5p secreted by apoptosis-resistant pulmonary microvascular endothelial cells (pecs/AR) has also been proved to promote the proliferation and migration of PASMCs in patients with PAH. Experiments have confirmed that MiRNA-195-5p, as a paracrine factor of the interaction between pecs/AR and PASMC, may play a role through HIF-1a/miR-195-5p/Smad7 pathway35. Under hypoxia, the down-regulation of lncRNA Rps41 expression in PASMCs leads to the increase of ILF3 and HIF-1α levels, and promotes the proliferation, migration and cell cycle process of PASMCs36.
Recent research reveals that hypoxia elevates a novel circular RNA, circ-myh8, which acts as a modular scaffold, recruiting histone acetyltransferase KAT7 to the promoter of the HIF-1α gene and subsequently inducing PASMC proliferation and cell cycle progression. The study posits that circ-myh8 and its associated pathways may serve as pivotal targets for the diagnosis and treatment of HPH37.
In HPH, PASMC transforms from normal oxidative phosphorylation of glucose to glycolytic reprogramming of glucose metabolism, which is extremely relevant to the abnormal activation of HIF-1α38. In the HPH animal model, it is found that the increase in the glycolysis and FDG uptake and expression level of glycolytic enzymes (GLUT1, PKM2, PFKFB3, HK1, etc.) in the lung and right ventricle is all connected to the increased expression of HIF-1α, indicating that HIF-1α is the key to promoting the glycolysis of PASMC under hypoxia39. Under hypoxia, the expression of glucose-6-phosphate dehydrogenase (G6PD) in PASMCs increases, which promotes expression of HIF-1a in PASMCs and lung tissues, and proliferation of PASMCs cells and remodeling of pulmonary vascular structure40, suggesting that the change in glucose metabolism may participate in the pathogenesis of HPH.
Xiao et al.[41]found that platelet-derived growth factor (PDGF) regulated HIF-1α expression through PI3K signal pathway, and promoted PASMCs proliferation. Ahmed et al.[42]revealed that oxidative stress might up-regulate transcription and translation of HIF-1α, which promoted PASMCs proliferation and participated in HPH formation. Moreover, Chen et al.[43]investigated that HIF-1α regulated mitochondrion division by directly up-regulating the expression of dynamic-related protein 1(Drp1), thus promoting the proliferation of PASMC and inhibiting its apoptosis under hypoxia. In lung sections or normal PASMCs, CoCl2 stabilizes HIF-1α, leading to DRP1-mediated mitochondrial fission44.These findings substantiate that the activation of HIF-1α mediates mitochondrial fission, thereby promoting PASMC proliferation45.
3.2 HIF-1a and PAECs
Endothelial cells, an indispensable barrier, can protect the vascular wall from damage caused by exogenous pathogens, etc.46 Recent studies show that endothelial dysfunction is an important sign of vascular remodeling47. As the earliest cell to perceive the change of oxygen content, PAEC is stimulated by hypoxia to stimulate its secretion function and regulate the proliferation process of itself and adjacent cells. In the pathogenesis of PH, PAECs may initially undergo apoptosis, but subsequently switch to an anti-apoptotic hyperproliferative state, which is related to the formation of plexiform lesions in the lungs of PAH patients and the pathological vascular remodeling in PH48,49.
Estradiol (17 β-Estradiol, E2) alleviates HPH through dependent effects of estrogen receptor (ER), including inhibiting the proliferation ofhypoxia induced endothelial cell. Andrea et al.[50]further confirmed that the protective effect of E2 in HPH was mediated by HIF-1α dependently increasing the expression of ERβ.In hypoxic PAEC, the stability of HIF-1α increases the ERβ of PAEC, while the down-regulation of HIF-1α decreases the abundance of ERβ;in addition, the down-regulation of ERβ also reduces the expression of proline hydroxylase domain 2(PHD2),a HIF inhibitor, while the activation of ERβ increases PHD2, and decreases HIF-1α and HIF-2α.It is indicated that the ERβ adjusts the shaft of PHD2/HIF-1α/HIF-2α under hypoxia. Wang Le et al.found that improving the expression of Hsp70 in PAEC of HPH newborn rats by adenovirus transfection promoted the degradation of HIF-1α, and down-regulated the expression of its downstream target genes ET-1 and iNOS, so as to reduce pulmonary artery pressure and alleviate pulmonary vascular remodeling.
In vivo, endothelial cells show different phenotypes according to local conditions. The interaction between endothelial cells and smooth muscle cells (SMCs) is the key mechanism of PH development51. Mesenchymal cells and SMCs are included in the reconstructed pulmonary vascular lumen, while cell markers of endothelial cells are not prominent. This phenomenon is called endothelial-to-mesenchymal transition (EndMT). Through EndMT, endothelial cells obtain the phenotype of mesenchymal cells and SMCs, but lose the phenotype of endothelial cells. Endothelial mesenchymal transforming cells can participate in vascular remodeling in PAH by directly transforming into SMCs with high proliferation and migration ability, and affecting the proliferation of vascular intima and media through paracrine52, 53. At the same time, EndMT can enhance the migration ability of PAEC and transform the slowly proliferating PAEC into a highly proliferating cell type, thereby resulting in the formation and development of occlusive endometrial lesions54. Studies have shown that[55]the co-localization of CD31 and its smooth muscle actin (α-SMA)of mesenchymal markers in the intimal layer α- of pulmonary arterioles in rats with chronic hypoxia is significantly down-regulated, and the expression of CD31 in cultured pulmonary microvascular endothelial cells (PMVECs) under hypoxia is significantly reduced; meanwhile, the expression of α-SMA and collagens Col1A1 and Col3A1 of other two mesenchymal markers is significantly increased; and inhibiting HIF-1α can effectively suppress the hypoxic induction of α-SMA, Col1A1 and transcription factor Twist1, and save the hypoxic inhibition of CD31 as well, indicating that in pulmonary artery remodeling, HIF-1α/Twist1 pathway significantly mediates the role of hypoxia induced EndMT for which HIF-1α is essential.
In recent years, it has been found that endothelial cell-specific molecule-1 (ESM-1) released by endothelial cells is a new regulatory factor related to vascular remodeling. ESM-1 is the downstream factor of VEGF. HIF-1α/VEGF can activate ESM-1, promote the adhesion between monocytes and endothelial cells, induce endothelial cell dysfunction and vascular remodeling, and may be involved in airway remodeling56. Moreover, IL-33, as a key inducing cytokine, is helpful for many pulmonary diseases. Liu et al.[57]found that the increase of IL-33 induced the proliferation, adhesion and angiogenesis of PAECs through the combination of its receptor ST2 and the activation HIF-1α/VEGF signaling pathway, and promoted vascular remodeling under hypoxia (Fig. 2).
3.3 HIF-1a and lung fibroblasts
Pulmonary vascular remodeling is mainly manifested by pulmonary vascular intimal hyperplasia, medial hypertrophy, adventitial fibrosis and inflammatory cell infiltration58. Studies have shown that the adventitia of pulmonary artery is the first structure to respond to vascular stress and injury with pathological changes59. Under the action of hypoxia, vasodilation and other factors, the pulmonary artery adventitia fibroblasts (PAAFs) are first activated, which is mainly manifested by significantly enhancing the proliferation and migration ability of the cells, and transforming into myofibroblasts, producing more extracellular matrix(ECM),growth factors, chemokines and inflammatory cytokines, regulating the growth of vascular wall cells, and participating in the process of pulmonary vascular remodeling60. In a study of PAH rats and COPD patients, it is found that inhibition of pyruvate dehydrogenase kinase (PDK), which is highly expressed in right ventricular fibroblasts, can accelerate degradation of HIF-1α, inhibit right ventricular fibrosis and hypertrophy, and improve right ventricular functions61.
Mechanism of HIF-1α in pathological process of hypoxia pulmonary hypertension.
HIf-1a tends to M1 or typically activated cells, this polarization leads to changes in the production and metabolism of inflammatory cytokines. In addition, metabolites and metabolic enzymes, including succinate, PDK1 and PKM2, can also play a role in the polarization of HIf-1a-dependent M1.
In terms of genes, HIF-1α can also express HRE on α-SMA gene by combining myofibroblasts, induce airway fibroblasts to differentiate into myofibroblasts, and participate in hypoxic pulmonary vascular remodeling induced by COPD62. A study shows63 that hypoxia induced PAAF is accompanied by a sharp decline in miR-29a-3p; after HIF-1α is knocked out, the increase in miR-29a-3p of adventitial cells of posterior vessels promotes the proliferation and migration of PAAF and the expression of α-SMA and extracellular matrix protein, significantly reduces pulmonary artery pressure, right ventricular hypertrophy index, and improves pulmonary vascular remodeling. It is indicated that HIF-1α plays an important role in regulating the proliferation of PAAF, but its mechanism is still unclear. In addition, by adopting RNA interference technique to inhibit HIF-1 specifically, it is found that the migration reaction of PAAF is related to HIF-1α64. Interestingly, a recent study has discovered that even under normoxic conditions, human PAAF demonstrates a reduction in HIF-1α hydroxylation and an augmentation in the expression of HIF target genes. The study suggests that merely inhibiting HIF is insufficient to reverse the “persistently activated” phenotype observed in both human and bovine PAAF65.
In summary, hypoxia-induced fibroblast differentiation into myofibroblasts, mediated by the HIF-1α signaling pathway, is a component of hypoxia-induced PAAF proliferation. HIF-1α can serve as a therapeutic target to reduce the formation of these activated fibroblasts, thus reducing the formation of new intima and vascular remodeling during the HPH process. The specific mechanism of this process still requires further investigation.
3.4 HIF-1a and pulmonary macrophages
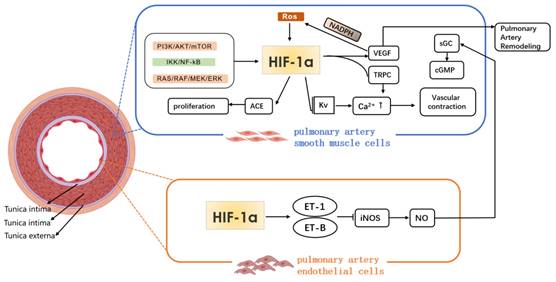
The role of immune inflammatory reaction in PAH vascular remodeling has attracted more and more attention. Macrophages with high heterogeneity and plasticity derived from monocytes are an important member of the reaction, and important inflammatory cells that cause pulmonary vascular remodeling. Under various conditions, they differentiate into multiple subtypes, and mediate a variety of biological effects, that is, the macrophage polarization66.
Different microenvironments, such as hypoxia, inflammation and toxicant, can polarize macrophages into type M1 or M2, thus affecting the process of PAH67. Kojima et al.68 used targeted therapy technique to selectively knock out the gene HIF-1a of myeloid cells of mouse C57BL/6, extracted the peripheral blood macrophages of the gene-knockout and wild mice, and adopted cobalt chloride to induce the expression of HIF-1a. From the levels of mRNA and protein, it is confirmed that the HIF-1a expression of peripheral blood macrophages of the gene-knockout one is significantly lower than that of the wild one, and the protein expression is basically missing. Cramer et al.[69]revealed the relationship among the hypoxia signal pathway, metabolism and polarization state of macrophages. Various HIF subtypes may regulate the polarization state of macrophages (and vice versa), thus affecting the inflammatory results (Fig. 3).
Tannahill et al.[70]regarded succinate as HIF-1a-dependent inflammatory signal: the succinate produced by macrophages was necessary for the stability of HIF-1a to generate cytokine IL-1b. In recent years, Palson-McDermott et al.[71]found that as an additional HIF-dependent regulator, PKM2 was transferred to the nucleus in the form of non-enzymatic activity, and interacted with HIF-1a to up-regulate IL-1b. In addition, such as glycolytic regulator pyruvate dehydrogenase kinase 1(PDK1), it is also conducive to HIF-1a-induced glycolysis of macrophages72. To conclude, HIF-1a subtype related to macrophage metabolism significantly regulates macrophage.
3.5 HIF-1a and chronic inflammation
In chronic respiratory diseases, inflammatory cells and factors are important factors to induce airway remodeling. Under hypoxia, HIF-1α can promote inflammation with more inflammatory factors produced, such as interleukin (IL-1, IL-9, IL-13), tumor necrosis factor-alpha (TNF- α), which promotes excess secretion of mucus and induce airway remodeling in COPD. The addition of HIF-1α also helps release IL-1β, IL-8, monocyte chemoattractant protein-1 and other inflammatory factors. Knocking out HIF-1α or inhibiting phosphatidylinositol 3-kinase (PI3K)/protein serine-threonine kinase (Akt)/HIF-1α can reduce the production of inflammatory factors induced by hypoxia73. On the contrary, inflammatory cells and factors can also stimulate the expression of HIF-1. Neutrophils express HIF-1 to maintain the survival of neutrophils, and macrophages also express it. What is the difference is that subtypes of HIF-1 can induce different polarization states of macrophages74. Inflammatory factors, such as interferon (IFN-γ), mainly activate transcription of HIF-1α through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). After IL-1β activates NF-κB signal to induce the expression of HIF-1α, HIF-1α can combine with the promoter of gene Muc5ac, promote mucus production and participate in airway remodeling75. Research shows that HIF-1α under the conditions of hypoxia and inflammation can activate NF-κB, and promotes immune inflammatory reaction, leading to the occurrence and development of HPH. Therefore, HIF-1α significantly promote the inflammatory reaction of HPH76.
To sum up, hypoxic pathway is closely related to chronic inflammatory process. Hypoxia can activate inflammatory pathway to induce inflammation and airway remodeling, and inflammatory microenvironment can also up-regulate HIF-1a to exacerbate hypoxia. The specific mechanism remains to be explored.
3.6 HIF-1a and right ventricular remodeling
HPH is a complex cardiopulmonary disease, beyond instigating pulmonary vascular remodeling, it further precipitates right ventricular remodeling. Clinically, right ventricular (RV) function emerges as a pivotal determinant of patients' long-term survival, with chronic hypoxia-induced right ventricular remodeling (RVR) characteristically correlating with a deteriorated prognosis in HPH. The RVR process is generally bifurcated into 'compensatory' and 'decompensatory' phenotypes. The former is chiefly characterized by right ventricular hypertrophy (RVH) and is accompanied by minimal RV dilation and fibrosis, indicative of RV functional compensation. In contrast, the latter phenotype, 'decompensatory' RVR, is typified by cardiomyocyte apoptosis, fibrosis, progressive dilation, and a decrease in RV capillary density, collectively contributing to diminished exercise capacity and cardiac output77. HIF-1α exerts a pivotal regulatory influence on the pathogenic mechanisms underpinning the progression of pulmonary arterial hypertension and ventricular remodeling in congenital heart disease78, however, the mechanisms facilitating RVR occurrence within a chronic hypoxic environment remains unclear.
Recent clinical and experimental research has identified several key structural and molecular determinants associated with "compensatory" or "decompensatory" RVR. These factors encompass capillary rarefaction, metabolic transition from oxidative metabolism to glycolysis, excessive sympathetic nervous system activity, and upregulation of fibrotic pathways79.
Reduced microvascular density is considered a requisite factor in "decompensatory" RVR, and previous studies have observed a relative decrease in capillary density in RV samples from PH patients and animal models of PH. Holscher et al. demonstrates[80]that cardiac-specific HIF-1 overexpression exacerbates pressure overload-induced myocardial remodeling and heart failure. They found increased expression of HIF-1α in the hearts of late-stage heart failure patients, suggesting that prolonged chronic upregulation of HIF-1α has a deteriorating effect on the heart. However, Wei et al.'s study[81]indicate that HIF-1 gene knockout exacerbates pressure overload-induced myocardial fibrosis, cardiomyocyte hypertrophy, reduced myocardial capillary density, and cardiomyocyte apoptosis, promoting the occurrence of heart failure, suggesting a protective role of HIF-1 in the hearts of pressure-overloaded mice. Smith et al.[82]explored the impact of intracellular HIF-1α expression on HPH and observed that chronic hypoxia reduced stroke volume and cardiac output in wild-type mouse hearts, but not in HIF-1α-deficient mouse hearts. Simultaneously, the absence of HIF-1α in smooth muscle cells attenuated the rise in RV systolic pressure induced by chronic hypoxia, without improving right ventricular hypertrophy. Conversely, HIF-1α deficiency in cardiomyocytes exacerbated right ventricular remodeling, underscoring the protective role of HIF-1α in chronic hypoxia-induced RVR.
The results of the aforementioned studies indicate that HIF-1α plays a protective role in pressure overload-induced RVR. However, as pressure overload-induced RVR progresses to heart failure, increased HIF-1α expression exacerbates the condition. Therefore, there is a discrepancy in the role of HIF-1α in myocardial cells at different stages of HPH. Further elucidating the cellular and molecular mechanisms of HIF-1α in the pathogenesis of HPH will provide new evidence for the prevention and treatment of right ventricular remodeling and heart failure induced by pressure overload.
Additionally, research has suggested a potential linkage between mitochondrial metabolism, ischemia, and "decompensatory" RVR. Bekeredjian et al.'s study83, utilizing mice with cardiac-specific HIF-1α overexpression, provides evidence that elevated HIF-1α levels can result in reduced myocardial contractile function. During chronic hypoxia, the production of reactive oxygen species (ROS) can enhance the stability and increased expression of HIF-1α, further indicating that prolonged stable activation of HIF-1 has a worsening effect in chronic heart failure.
4. HIF-1a as a target for the treatment of HPH
In recent years, more and more drugs directly inhibiting the HIF-1a pathway have been found to improve HPH. However, these drugs have not been clinically approved yet84. HIF-1a regulates the occurrence of HPH under hypoxia. Since its regulatory mechanism was discovered, drug research and development for target HIF-1a has not stopped so far.
4.1 Treatment of HPH by using natural drugs to regulate HIF-1a
Due to their diverse components, natural drugs have multiple therapeutic effects on various diseases. According to the differences of active components and chemical structures, they are divided into flavonoids, saponins, anthraquinones, alkaloids, astragalus, saponins, etc. The content of HIF-1a in the body is also affected by regulating its synthesis, degradation, etc. (Table 1), thus effectively treating cancer, inflammation, etc.85
Resveratrol, a stilbene polyphenol with antioxidant, neuroprotective, cardioprotective, anti-inflammatory and anticancer effects, has been proven to inhibit HPH by down-regulating the expression of HIF-1 through MAPK/ERK1 and PI3K/AKT signaling pathways86. Tagitinin C inhibits the metabolic disorder caused by the increase of HIF-1 under chronic hypoxia and the Warburg effect of PASMC by reducing the content of the key enzyme PDK1 that increases the Warburg effect87. Gong Xiaonan's team has proved through experiments that Astragaloside IV may pass signal pathway of Calpain-1/HIF-1α to improve MCT-induced oxidative stress in PAH rats88.
Chrysin (CH) is a flavonoid compound with a large number of pharmacological activities. A study shows that CH significantly improves hemodynamic parameters of HPH rat model, such as right ventricular hypertrophy index. Its mechanism may be related to the down-regulation of HIF-1α, BMP4, TRPC1 and TRPC expressions in PASMCs, thus regulating Ca2+ to play a role of HPH protection89.
Tetramethylpyrazine (TMP), an effective component of traditional Chinese medicine Chuanxiong, has many biological activities, including improving microcirculation, protecting coronary artery, clearing free radicals, and anti-tumor90, 91. Li Youwei's team found92 that after TMP intervention, the level of rat HIF-1α, VEGF was significantly lower than that of the model group, indicating that TMP inhibited the increase of HIF-1α and VEGF levels induced by hypobaric hypoxia, thus reducing the proliferation of PASMC, and alleviating the occurrence and development of vascular remodeling and pulmonary hypertension.
Lycium barbarum polysaccharides (LBP) is an effective component extracted from Lycium barbarum93, which has the functions of controlling blood sugar, aging, oxidation and hypoxia resistances94. Zhu Y anni's team confirmed that levels of mRNA and protein of silent mating type information regulation 2 homolog 1 (SIRT1) of PASMCs treated with LBP under hypoxia decreased, and the levels of matrix metalloproteinase 9 (MMP-9), mRNA and protein of HIF-1α increased. It is suggested that LBP can stimulate the expression of gene SIRT1, resist the decrease of SIRT1 level after hypoxic treatment, and inhibit the increase of HIF-1α and MMP-9 under hypoxia, which may play a role in alleviating pulmonary vascular remodeling and pulmonary hypertension95.
Natural drugs that can inhibit HIF-1α in HPH.
| Compounds | Original Plants | Chemical Structure | Experimental Models | Targets & Mechanisms | Reference |
|---|---|---|---|---|---|
| Resveratrol | Veratrum grandiflorum O, skins of grape (50- 100 μg/mL), peanuts, bilberries, blueberries and cranberries, et al | 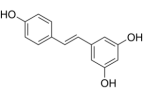 | Male Sprague-Dawley rats, weighing 260 ± 9.6 g | Down-regulate the expression of HIF-1a through MAPK/ERK1 and PI3K/AKT signaling pathways | [86] |
| Tagitinin C | Tithonia diversifolia, Helianthus annuus | 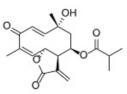 | PASMC | Reduce the content of the key enzyme PDK1 that increases the Warburg effect and HIF-1 | [87] |
| Astragaloside IV | Astragalus propinquus Schischkin | 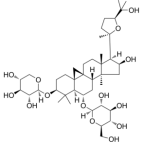 | Male Sprague-Dawley rats, weighing200 ± 10g | Pass signal pathway of Calpain-1/HIF-1α to improve oxidative stress | [88] |
| Chrysin | citrus fruits, honey, propolis | 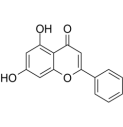 | PASMC | Down-regulation of HIF-1α, BMP4, TRPC1 and TRPC expressions in PASMCs | [89] |
| Tetramethylpyrazine | Ligusticum chuanxiong Hort | 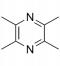 | Male Sprague-Dawley rats, weighing 250 ± 20g | Inhibit the increase of HIF-1α and VEGF levels | [91] |
| Lycium Burbarum polysaccharides | Lycium barbarum L | None | PASMC | Resist the decrease of SIRT1 level after hypoxic treatment, inhibit the increase of HIF -1α and MMP-9 under hypoxia | [93] |
| Salidroside | Rhodiola rosea L. | 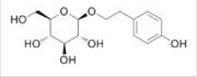 | Male/Femal Wistar rats, weighing 150±10g | Indirectly inhibit HIF-1α and up- regulate the role of VEGF of downstream target gene | [96] |
| Genistein, Daidzein | soybean | 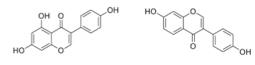 | Human alveolar epithelial cells | Affect the expression of annexin A1, a formyl peptide receptor agonist in hypoxic alveolar epithelial cells through pathways of HIF -1α and NF- κB | [95] |
| Baicalin | Scutellaria radix (Huang Qin) | 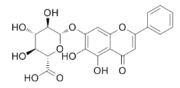 | PASMC | Inhibit the expression of HIF- 1α and aromatic hydrocarbon receptor, reduce the proliferation and phenotype transformation of PASMC | [96] |
| Echinacoside | Cistanche tubulosa | 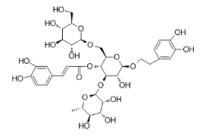 | PASMC | Inhibit the proliferation of PASMC induced in hypoxia by reducing the expression of HIF -1a | [103] |
| Caffeic acid phenethyl ester promotes | propolis | 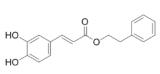 | PASMC | Inhibit the hypoxia and the expression of PDGF -BB- induced HIF -1α by reducing the activation of AKT/ERK pathway | [105] |
| Icariin | Epimedii | 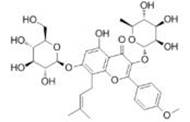 | Male C57 mice, weighing 20-25g | Inhibit the signal pathway of HIF-1α/TNF -α/NF-κB | [107] |
| Magnesium lithospermate B | Salviae miltiorrhizae | 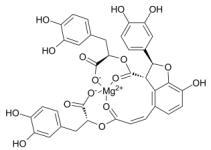 | Male Sprague- Dawley (SD) rats, weighing 120-160 g | Down-regulate the expression of HIF- 1, NF-κB, monocyte chemotactic protein-1, (CDK4) of the HPH rat model | [108] |
Salidroside, a traditional Chinese medicine, is the active ingredient of rhodiola which has an anti-hypoxia effect and can be used for the treatment of high-altitude reaction96. Zhou Zhengguang's team confirmed that salidroside down-regulated the expression of HIF-1α in HPH rat model, indirectly inhibiting HIF-1α and up-regulating the role of VEGF of downstream target gene; it also reduced the damage of hypoxia to endothelial cells, improved the dynamic imbalance of pulmonary artery vasoconstriction and relaxation factors, and weakened the proliferation of pulmonary vascular smooth muscle; it also effectively inhibited the pulmonary vascular remodeling, and the development of HPH97.
Phytoestrogen, genistein (Gen) and daidzein (DD) are the main active components of soybean abnormal flavonoids, which have antioxidant and anti-inflammatory effects98, 99. Previous studies show that phytoestrogens can significantly lower the thickening of pulmonary vascular wall caused by hypoxia, and reduce HPH100. Yang Juan et al. found[101]that phytoestrogens might affect the expression of annexin A1 (AnxA1), a formyl peptide receptor agonist in hypoxic alveolar epithelial cells through pathways of HIF-1α and NF-κB; and phytoestrogens inhibited the expression of AnxA1 in hypoxic alveolar epithelial cells, thereby regulating the infiltration of inflammatory cells around the pulmonary vessels, and improving HPH.
Baicalin is a natural flavone with anti-thrombotic, anti-hyperlipidemic and anti-inflammatory effects. Huang S102 found that baicalin inhibited the expression of HIF-1α and aromatic hydrocarbon receptor (AhR), and reduced the proliferation and phenotype transformation of PASMC. Therefore, baicalin is expected to become a new drug for the treatment of PAH.
Echinaceoside (ECH) is a natural derivative compound with a structure of polyhydroxyphenol, which exists in many plants, such as cistanche deserticola, and has the functions of anti-oxidation, anti-inflammation, neuroprotection, liver protection and scavenging activity of nitric oxide free radical103. Gai XY et al.104 confirmed that ECH partially inhibited the proliferation of PASMC induced by hypoxia by reducing the expression of HIF-1a.
Caffeic acid phenylethyl ester (CAPE) is the main active ingredient in propolis, because it inhibits the activity of NF-κB; its anti-inflammatory effect has been widely known105. CAPE inhibits the hypoxia and the expression of PDGF-BB-induced HIF-1α by reducing the activation of AKT/ERK pathway, thus inhibiting the proliferation of PASMCs. In addition to NF-κB, HIF-1α is considered as an alternative target of CAPE. Thus, CAPE may be a promising therapeutic agent for PAH106.
Icariin (Ica) has pharmacological effects, including regulating immunity, anti-oxidation and anti-inflammation. It can inhibit the signal pathway of HIF-1α/TNF-a/NF-κB, alleviate pulmonary vascular remodeling and improve pulmonary artery and right ventricular hemodynamic abnormalities107.
Magnesium lhospermate B (MLB) is the main component of water extract of Salvia przewalskii Maxim, which has therapeutic effects on angina, cardiovascular injury, anti-inflammation, anti-oxidation, anti-apoptosis, etc. Wang et al.108 found that the expression of HIF-1α, proliferating cell nuclear antigen (PCNA), NF-κB, monocyte chemotactic protein-1 (MCP-1) and cyclin dependent kinase 4 (CDK4) of the HPH rat model after MLB treatment decreased, which ultimately inhibited the remodeling of pulmonary microvascular, and reduced mPAP and right ventricular hypertrophy index in HPH rat.
4.2 Chemotherapy scheme using HIF-1a as a target
At present, drug research and development using HIF-1a as a target are mainly aimed at the treatment of malignant tumors. However, inhibiting angiogenesis in the tumor microenvironment under hypoxia for HPH treatment has been drawn less attention.
Bortezomib can reduce the expression of HIF-1α under hypoxia. A further study shows that it can inhibit the proliferation of PASMC under hypoxia109. Etakalin hydrochloride can reduce the expression of HIF-1 in PASMC and pulmonary vessels under hypoxia, inhibit the proliferation of PASMC and promote apoptosis, thereby decreasing pulmonary artery pressure110. Braga CL et al. confirmed that the combined treatment of niclosamide and sildenafil/niclosamide reduced the expression of downstream target genes of signal transduction and transcription activating factor-3 (STAT3) in PEAC and PAAF. STAT3 is one of the main intracellular transcription factors involved in HPH vascular remodeling. This process is related to the fact that the combined treatment of niclosamide and sildenafil/niclosamide reduces the expression of STAT3 downstream targets of HIF-1, etc. in lung tissue111.
Celastramycin as a benzoyl pyrrole compound is originally found in bacterial extracts. It can reduce HIF-1α and NF-κB in PASMCs levels, thereby lowering the secretion of inflammatory factors and inhibiting the proliferation of PAH-PASMCs in a dose-dependent manner112.
Topotecan (TPT) can significantly inhibit hypoxic-induced PASMCs in normal rats. The up-regulation of HIF-1α and TRPC1/4/6 of rat'pulmonary artery in PAH model can effectively reduce the remodeling of its pulmonary artery in hypoxic-induced PAH model, improve its hemodynamics, and reduce right ventricular hypertrophy and ventricular wall thickening113.
The latest research has discovered that novel derivatives of bosentan, namely 17d, 16j, and 16h, endothelin receptor antagonists, can dose-dependently decrease HIF-1α levels in PAH rats114. A novel lysosomal autophagy inhibitor (ROC-325) can downregulate HIF-1αprotein expression in PASMCs, activate the eNOS-NO signaling pathway, and inhibit autophagy, thus suppressing pulmonary vascular remodeling, inducing pulmonary vasodilation, and alleviating the occurrence and progression of pulmonary arterial hypertension115.
5. Conclusion and prospects
HPH is resulted from the interaction of PASMCs, vascular endothelial cells, fibroblasts and macrophages under hypoxia, and the joint participation of various factors.HIF-1a as an important transcription and regulation factor that regulates angiogenesis, glucose metabolism and hematopoiesis, plays a key regulatory role in the occurrence of HPH.Although a large number of studies have proved that HIF-1a promotes the development of airway remodeling in chronic respiratory diseases, the relevant mechanism is not clear. Therefore, inhibiting pulmonary vascular remodeling by regulating HIF-1a is an effective target for the treatment of HPH.
It is shown that many natural drugs can affect the content of HIF-1a, and reduce the side effects of other drugs when used in combination with other marketed drugs as well. The pharmacological effects of these natural drugs are primarily achieved by inhibiting the proliferation of PASMCs, promoting PASMC apoptosis, regulating vasoconstrictive factors, suppressing oxidative stress, alleviating inflammatory responses, and modulating autophagy. These natural medicines mainly exert their therapeutic actions by curbing PASMC proliferation, facilitating PASMC apoptosis, adjusting vasoconstrictor agents, counteracting oxidative stress, mitigating inflammation, and regulating autophagy. At present, well-known monomer components affecting HIF-1a mainly include flavonoids, terpenes and glycosides. Therefore, still more active monomers exist in natural drugs and traditional Chinese medicine.
The evidence collected so far shows that chemical drugs and natural drugs can treat various diseases (especially tumors) by regulating HIF-1a85. However, most experimental results only come from animal or in vitro cell experiments. Limited and single HPH animal models may not be able to verify the efficacy of natural drugs. Nowadays, most studies only focus on the inhibitory effect of natural drugs compound on HIF-1a. However, few studies deeply explore their effects on the specific process of HIF-1a synthesis. In addition, how natural compounds regulate key genes, proteins, and even microRNA and lncRNA still need to be highly concerned. Extensive pharmacological experiments show that microRNAs also regulate proliferation, migration, and apoptosis-related signaling pathways by activating specific targets. Therefore, in the future research, it is suggested to pay more attention to the promotion of natural drugs on HIF-1a, and further explore its mechanism, pharmacokinetics and safety evaluation by combining in vivo and in vitro research methods. It is believed that with the in-depth study of HIF-1a regulation mechanism, this review can provide new ideas for HPH intervention.
Abbreviations
HIF: hypoxia inducible factor; PAH: pulmonary hypertension; HPH: hypoxia induced pulmonary hypertension; HPV: hypoxic pulmonary vasoconstriction; HPVR: hypoxic pulmonary vascular remodeling; HRE: hypoxia response element; PASMCs: pulmonary artery smooth muscle cells; EPO: erythropoietin; ARNT: aryl hydrocarbon receptor nuclear translacator; PHD: prolyl hydroxylase-domain protein; ROS: reactive oxygen species; VEGF: vascular endothelial growth factor; VSMC: vascular smooth muscle cell; EndMT: endothelial-to-mesenchymal transition; TGF-β: transforming growth factor beta; ET: endothelin; iNOS: inducible nitric oxide synthase; PAEC: pulmonary artery endothelial cell; PCNA: Proliferating Cell Nuclear Antigen; ncRNA: non-coding RNA; RUNX2: Runt-related Transcription Factor 2; KLF5: Krüppel-like factor 5; SENP-1: SUMO-specific protease-1; ADM: adrenomedullin; FDG: Fludeoxyglucose; GLUT1: Glucose transporter 1; PKM2: Pyruvate kinase isozyme type M2; G6PD: Glucose6 Phosphate Dehydrogenase; PDGF: platelet-derived growth factor; Drp1: dynamin-related protein 1; AKG: Alpha-ketoglutarate; PFKFB3: 6-phosphofructokinase-2isoenzyme 3; PI3K: Phosphatidylinositol-4:5-bisphosphate 3-kinase; Akt: protein kinase B; E2: 17β-Estradiol; SMC: smooth muscle cell; α-SMA: α-smooth muscle actin; ESM-1: endothelial-cell-specific molecule-1; IL: interleukin; PAAF: pulmonary artery adventitia fibroblast; ECM: extracellular matrix; PDK: pyruvate dehydrogenase kinase; LPS: Lipopolysaccharide; PDK1: pyruvate dehydrogenase kinase 1; TNF-α: tumor necrosis factor-alpha; IFN-γ: interferon; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; CH: Chrysin; BMP-4: bone morphogenetic protein-4; TRPC: transient receptor potential cation channel; TMP: tetramethylpyrazine; LBP: Lycium Burbarum polysaccharides; SIRT1: silent mating type information regulation 2 homolog 1; MMP-9: matrix metalloproteinase 9; mPAP: mean pulmonary arterial pressure; DD: daidzein; AnxA1: Annexin A1; ECH: Echinacoside; CAPE: Caffeic Acid Phenethyl Ester; Ica: icariin; TLR4: Toll-like receptor 4; STAT3: signal transducer and activator of transcription 3; TPT: Topotecan.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82200066, 81570052 and 81270118). This research is also financially supported by the Natural Science Foundation of Hunan Province (No. 2021JJ30017,and 2022JJ40308), the Postdoctoral Foundation of China (No. 2021M690982), the Postgraduate Scientific Research Innovation Project of Hunan Province (No. CX20220770 and 2022CX196), the Scientific Research Project of Changsha Science and Technology Bureau (No. kq2202272), Open Fund Project for the Cultivation Base of National Key Laboratory Jointly Constructed by the Ministry and the Ministry of Traditional Chinese Medicine Powder and Innovative Drugs (No. 21PTKF1001 and 21PTKF1004), Key Projects of Hunan Provincial Health Commission (202213055529) and the Key Project of the Educational Department of Hunan Province (No. 21A0226). Traditional Chinese Medicine scientific Project of Hunan Provincial (No. C2022001).
Author contributions
Jia-Jing Wan searched the literature and drafted the manuscript. Ai-Guo Dai conceived and designed the review. Jian Yi, Chao Zhang examined the literature and made the figures. Fei-Ying Wang, Ai-Guo Dai made a critical revision of the review. All authors contributed to the article and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol (1985). 2014Apr1;116(7):867-74 doi: 10.1152/japplphysiol.00643.2013
2. Sala MA, Chen C, Zhang Q, Do-Umehara HC, Wu W, Misharin AV, Waypa GB, Fang D, Budinger GRS, Liu S, Chandel NS, Schumacker PT, Sznajder JI, Liu J. JNK2 up-regulates hypoxia-inducible factors and contributes to hypoxia-induced erythropoiesis and pulmonary hypertension. J Biol Chem. 2018Jan5;293(1):271-284 doi: 10.1074/jbc.RA117.000440
3. Pullamsetti SS, Mamazhakypov A, Weissmann N, Seeger W, Savai R. Hypoxia-inducible factor signaling in pulmonary hypertension. J Clin Invest. 2020Nov2;130(11):5638-5651 doi: 10. 1172/JCI137558
4. Chuan-Chuan L. et al. HIF-1 regulates hypoxic pulmonary hypertension. J Progress in Physiological Sciences. 2018;49(06):423-427
5. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992Dec;12(12):5447-54.doi 10.1128/mcb.12. 12.5447-5454.1992
6. Guan R, Wang J, Li D, Li Z, Liu H, Ding M, Cai Z, Liang X, Yang Q, Long Z, Chen L, Liu W, Sun D, Yao H, Lu W. Hydrogen sulfide inhibits cigarette smoke-induced inflammation and injury in alveolar epithelial cells by suppressing PHD2/HIF- 1α/MAPK signaling pathway. Int Immunopharmacol. 2020Apr;81:105979. doi:10.1016/j.intimp.2019.105979
7. Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res. 2001May;49(5):614-7.doi 10. 1203/00006450-200105000-00002
8. Chan XY, Volkova E, Eoh J, Black R, Fang L, Gorashi R, Song J, Wang J, Elliott MB, Barreto-Ortiz SF, Chen J, Lin BL, Santhanam L, Cheng L, Lee FS, Prchal JT, Gerecht S. HIF2A gain-of-function mutation modulates the stiffness of smooth muscle cells and compromises vascular mechanics. iScience. 2021Mar2;24(4):102246.doi 10.1016/j.isci.2021.102246
9. Bing-hao J. et al. Research progress of transcription factor HIF-1. Journal of Science of Teachers′ College and University. 2017;37(08):70-73
10. Påhlman S, Mohlin S. Hypoxia and hypoxia-inducible factors in neuroblastoma. Cell Tissue Res. 2018May;372(2):269-275.doi 10. 1007/s00441-017-2701- 1
11. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S; ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment ofpulmonary hypertension. Eur Respir J. 2023Jan6;61(1):2200879. doi:10.1183/13993003.00879-2022
12. Norton CE, Sheak JR, Yan S, Weise-Cross L, Jernigan NL, Walker BR, Resta TC. Augmented Pulmonary Vasoconstrictor Reactivity after Chronic Hypoxia Requires Src Kinase and Epidermal Growth Factor Receptor Signaling. Am J Respir Cell Mol Biol. 2020Jan;62(1):61-73 doi:10. 1165/rcmb.2018-0106OC
13. Jernigan NL, Resta TC, Gonzalez Bosc LV. Altered Redox Balance in the Development of Chronic Hypoxia-induced Pulmonary Hypertension. Adv Exp Med Biol. 2017;967:83-103 doi: 10.1007/978-3-319-63245-2_7
14. Bousseau S, Sobrano Fais R, Gu S, Frump A, Lahm T. Pathophysiology and new advances in pulmonary hypertension. BMJ Med. 2023Mar23;2(1):e000137. doi: 10. 1136/bmjmed-2022-000137
15. Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014Jun20;115(1):165-75 doi: 10. 1161/CIRCRESAHA. 113.301141
16. Singh N, Dorfmüller P, Shlobin OA, Ventetuolo CE. Group 3 Pulmonary Hypertension: From Bench to Bedside. Circ Res. 2022Apr29;130(9):1404-1422 doi: 10. 1161/CIRCRESAHA. 121.319970
17. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012Dec;122(12):4306-13.doi 10. 1172/JCI60658
18. Gallardo-Vara E, Ntokou A, Dave JM, Jovin DG, Saddouk FZ, Greif DM. Vascular pathobiology of pulmonary hypertension. J Heart Lung Transplant. 2023May;42(5):544-552 doi: 10.1016/j.healun.2022.12.012
19. Veith C, Schermuly RT, Brandes RP, Weissmann N. Molecular mechanisms of hypoxia- inducible factor-induced pulmonary arterial smooth muscle cell alterations in pulmonary hypertension. J Physiol. 2016Mar1;594(5):1167-77 doi: 10. 1113/JP270689
20. Semenza GL. Hypoxia-inducible factors: roles in cardiovascular disease progression, prevention, and treatment. Cardiovasc Res. 2023Mar31;119(2):371-380 doi:10.1093/cvr/cvac089 10.1093/cvr/cvac089
21. Zhang H, He H, Cui Y, Yu S, Li S, Afedo SY, Wang Y, Bai X, He J. Regulatory effects of HIF-1α and HO-1 in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells in yak. Cell Signal. 2021Nov;87:110140.doi 10.1016/j.cellsig.2021.110140
22. Luo Y, Teng X, Zhang L, Chen J, Liu Z, Chen X, Zhao S, Yang S, Feng J, Yan X. CD146- HIF- 1α hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nat Commun. 2019Aug7;10(1):3551.doi 10.1038/s41467-019-11500-6
23. Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, Meloche J, Pflieger A, Breuils-Bonnet S, Nadeau V, Paradis R, Tremblay E, Girerd B, Hautefort A, Montani D, Fadel E, Dorfmuller P, Humbert M, Perros F, Paulin R, Provencher S, Bonnet S. Role for Runt- related Transcription Factor 2 in Proliferative and Calcified Vascular Lesions in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2016Nov15;194(10):1273-1285 doi: 10. 1164/rccm.201512-2380OC
24. Wang L, Zheng Q, Yuan Y, Li Y, Gong X. Effects of 17β-estradiol and 2-methoxyestradiol on the oxidative stress-hypoxia inducible factor- 1 pathway in hypoxic pulmonary hypertensive rats. Exp Ther Med. 2017May;13(5):2537-2543 doi: 10.3892/etm.2017.4243
25. Li X, He Y, Xu Y, Huang X, Liu J, Xie M, Liu X. KLF5 mediates vascular remodeling via HIF- 1α in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016Feb15;310(4):L299-310 doi:10. 1152/ajplung.00189.2015
26. Zhou F, Dai A, Jiang Y, Tan X, Zhang X. SENP-1 enhances hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells by regulating hypoxia-inducible factor-1α. Mol Med Rep. 2016Apr;13(4):3482-90 doi: 10.3892/mmr.2016.4969
27. Santos-Ferreira CA, Abreu MT, Marques CI, Gonçalves LM, Baptista R, Girão HM. Micro-RNA Analysis in Pulmonary Arterial Hypertension: Current Knowledge and Challenges. JACC Basic Transl Sci. 2020Nov23;5(11):1149-1162 doi: 10.1016/j.jacbts.2020.07.008
28. Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014Feb18;129(7):786-97 doi:10.1161/CIRCULATIONAHA.113.006167
29. Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, Meloche J, Pflieger A, Breuils-Bonnet S, Nadeau V, Paradis R, Tremblay E, Girerd B, Hautefort A, Montani D, Fadel E, Dorfmuller P, Humbert M, Perros F, Paulin R, Provencher S, Bonnet S. Role for Runt-related Transcription Factor 2 in Proliferative and Calcified Vascular Lesions in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2016Nov15;194(10):1273-1285 doi: 10.1164/rccm.201512-2380OC
30. Zhou Y, Wang L, Li MX. Serum levels of HIF-1α, ET-1 and Ca(2+) in neonates with hypoxic pulmonary hypertension. Zhongguo Dang Dai Er Ke Za Zhi. 2011Mar;13(3):181-4 Chinese
31. Wang CC, Ying L, Barnes EA, Adams ES, Kim FY, Engel KW, Alvira CM, Cornfield DN. Pulmonary artery smooth muscle cell HIF- 1α regulates endothelin expression via microRNA-543. Am J Physiol Lung Cell Mol Physiol. 2018Sep1;315(3):L422-L431.doi 10. 1152/ajplung.00475.2017
32. Wang L, Zhou Y, Li M, Zhu Y. Expression of hypoxia-inducible factor-1α, endothelin-1 and adrenomedullin in newborn rats with hypoxia-induced pulmonary hypertension. Exp Ther Med. 2014Jul;8(1):335-339 doi:10.3892/etm.2014.1728
33. Tang BI, Tang MM, Xu QM, Guo JL, Xuan L, Zhou J, Wang XJ, Zhang H, Kang PF. MicroRNA-143-5p modulates pulmonary artery smooth muscle cells functions in hypoxic pulmonary hypertension through targeting HIF-1α. J Biosci. 2020;45:37. PMID: 32098916
34. Chen M, Shen C, Zhang Y, Shu H. MicroRNA- 150 attenuates hypoxia-induced excessive proliferation and migration of pulmonary arterial smooth muscle cells through reducing HIF-1α expression. Biomed Pharmacother. 2017Sep;93:861-868.doi 10. 1016/j.biopha.2017.07.028
35. Zeng Z, Yao J, Li Y, Xue Y, Zou Y, Shu Z, Jiao Z. Anti-apoptosis endothelial cell-secreted microRNA- 195-5p promotes pulmonary arterial smooth muscle cell proliferation and migration in pulmonary arterial hypertension. J Cell Biochem. 2018Feb;119(2):2144-2155.doi 10. 1002/jcb.26376
36. Liu Y, Zhang H, Li Y, Yan L, Du W, Wang S, Zheng X, Zhang M, Zhang J, Qi J, Sun H, Zhang L, Li G, Zhu D. Long Noncoding RNA Rps4l Mediates the Proliferation of Hypoxic Pulmonary Artery Smooth Muscle Cells. Hypertension. 2020Oct;76(4):1124-1133 doi:10.1161/HYPERTENSIONAHA.120.14644
37. Xing Y, Qi J, Cheng X, Song X, Zhang J, Li S, Zhao X, Gong T, Yang J, Zhao C, Xin W, Zhu D, Zheng X. Circ-myh8 Promotes Pulmonary Hypertension by Recruiting KAT7 to Govern Hypoxia-Inducible Factor-1α Expression. J Am Heart Assoc. 2023Apr4;12(7):e028299. doi: 10.1161/JAHA.122.028299
38. Akagi S, Nakamura K, Kondo M, Hirohata S, Udono H, Nishida M, Saito Y, Yoshida M, Miyoshi T, Ito H. Evidence for Hypoxia-Induced Shift in ATP Production from Glycolysis to Mitochondrial Respiration in Pulmonary Artery Smooth Muscle Cells in Pulmonary Arterial Hypertension. J Clin Med. 2023Jul31;12(15):5028. doi:10.3390/jcm12155028
39. Xu W, Janocha AJ, Erzurum SC. Metabolism in Pulmonary Hypertension. Annu Rev Physiol. 2021Feb10;83:551-576 doi:10. 1146/annurev-physiol-031620-123956
40. Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015Feb1;308(3):L287-300 doi:10. 1152/ajplung.00229.2014
41. Xiao Y, Peng H, Hong C, Chen Z, Deng X, Wang A, Yang F, Yang L, Chen C, Qin X. PDGF Promotes the Warburg Effect in Pulmonary Arterial Smooth Muscle Cells via Activation of the PI3K/AKT/mTOR/HIF-1α Signaling Pathway. Cell Physiol Biochem. 2017;42(4):1603-1613 doi:10.1159/000479401
42. Ahmed LA, Rizk SM, El-Maraghy SA. Pinocembrin ex vivo preconditioning improves the therapeutic efficacy of endothelial progenitor cells in monocrotaline-induced pulmonary hypertension in rats. Biochem Pharmacol. 2017Aug15;138:193-204.doi 10.1016/j.bcp.2017.04.024
43. Chen X, Yao JM, Fang X, Zhang C, Yang YS, Hu CP, Chen Q, Zhong GW. Hypoxia promotes pulmonary vascular remodeling via HIF- 1α to regulate mitochondrial dynamics. J Geriatr Cardiol. 2019Dec;16(12):855-871 doi: 10.11909/j.issn.1671-5411.2019.12.003
44. Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012May25;110(11):1484-97 doi: 10.1161/CIRCRESAHA.111.263848
45. Zeidan EM, Hossain MA, El-Daly M, Abourehab MAS, Khalifa MMA, Taye A. Mitochondrial Regulation of the Hypoxia-Inducible Factor in the Development of Pulmonary Hypertension. J Clin Med. 2022Sep3;11(17):5219. doi: 10.3390/jcm11175219
46. Yu B, Wang X, Song Y, Xie G, Jiao S, Shi L, Cao X, Han X, Qu A. The role of hypoxia- inducible factors in cardiovascular diseases. Pharmacol Ther. 2022Oct;238:108186. doi: 10. 1016/j.pharmthera.2022.108186
47. Jing-yuan M. et al. Pulmonary vascular remodeling in pulmonary hypertension. Med Postgra. 2021;34(09):985-990.DOI 10. 16571/j.cnki.1008-8199.2021.09.017
48. Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005Jul;19(9):1178-80 doi:10.1096/fj.04-3261fje
49. Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007Sep;293(3):L548-54 doi: 10.1152/ajplung.00428.2006
50. Frump AL, Selej M. et al. Hypoxia Upregulates Estrogen Receptor β in Pulmonary Artery Endothelial Cells in a HIF- 1α-Dependent Manner. Am J Respir Cell Mol Biol. 2018Jul;59(1):114-126 doi: 10. 1165/rcmb.2017-0167OC
51. Spiekerkoetter E, Goncharova EA, Guignabert C, Stenmark K, Kwapiszewska G, Rabinovitch M, Voelkel N, Bogaard HJ, Graham B, Pullamsetti SS, Kuebler WM. Hot topics in the mechanisms of pulmonary arterial hypertension disease: cancer-like pathobiology, the role of the adventitia, systemic involvement, and right ventricular failure. Pulm Circ. 2019Nov20;9(4):2045894019889775.doi 10. 1177/2045894019889775
52. Suzuki T, Carrier EJ, Talati MH, Rathinasabapathy A, Chen X, Nishimura R, Tada Y, Tatsumi K, West J. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2018Jan1;314(1):L118-L126 doi: 10. 1152/ajplung.00296.2017
53. Yu-hao Z. et al. Progress in the study of epithelial mesenchymal transition involved in the process of pulmonary fibrosis. Journal of Medical Postgraduates. 2021;34(05):519-523
54. Lei J. et al. Recent advances of HIF-2α in pulmonary hypertension. Acta Pharmaceutica Sinica. 2022;57(02):277-286 DOI:10. 16438/j.0513-4870.2021-0632
55. Zhang B, Niu W, Dong HY, Liu ML, Luo Y, Li ZC. Hypoxia induces endothelial mesenchymal transition in pulmonary vascular remodeling. Int J Mol Med. 2018Jul;42(1):270-278 doi: 10.3892/ijmm.2018.3584
56. Sun H, Zhang H, Li K, Wu H, Zhan X, Fang F, Qin Y, Wei Y. ESM- 1 promotes adhesion between monocytes and endothelial cells unde intermittent hypoxia. J Cell Physiol. 2019Feb;234(2):1512-1521 doi: 10.1002/jcp.27016
57. Liu J, Wang W, Wang L, Chen S, Tian B, Huang K, Corrigan CJ, Ying S, Wang W, Wang C. IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF- 1α and VEGF Expression in Vascular Endothelial Cells. EBioMedicine. 2018Jul;33:196-210 doi: 10. 1016/j.ebiom.2018.06.003
58. Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Ur Respir J. 2019Jan24;53(1):1801887. doi:10.1183/13993003.01887-2018
59. Meyrick B, Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am J Pathol. 1979Jul;96(1):51-70
60. Liyao P. et al. Effects of CDDO-Me on hypoxia-induced activation of human pulmonary adventitia fibroblasts. Journal of Nanjing Medical University (Natural Sciences). 2021;41(09):1273-1280
61. Tian L, Wu D, Dasgupta A, Chen KH, Mewburn J, Potus F, Lima PDA, Hong Z, Zhao YY, Hindmarch CCT, Kutty S, Provencher S, Bonnet S, Sutendra G, Archer SL. Epigenetic Metabolic Reprogramming of Right Ventricular Fibroblasts in Pulmonary Arterial Hypertension: A Pyruvate Dehydrogenase Kinase-Dependent Shift in Mitochondrial Metabolism Promotes Right Ventricular Fibrosis. Circ Res. 2020Jun5;126(12):1723-1745.doi 10. 1161/CIRCRESAHA. 120.316443
62. Xu H, Ling M, Xue J, Dai X, Sun Q, Chen C, Liu Y, Zhou L, Liu J, Luo F, Bian Q, Liu Q. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium- fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics. 2018Oct29;8(19):5419-5433 doi:10.7150/thno.27876
63. Luo Y, Dong HY, Zhang B, Feng Z, Liu Y, Gao YQ, Dong MQ, Li ZC. miR-29a-3p attenuates hypoxic pulmonary hypertension by inhibiting pulmonary adventitial fibroblast activation. Hypertension. 2015Feb;65(2):414-20 doi:10. 1161/HYPERTENSIONAHA. 114.04600
64. Eul B, Rose F, Krick S, Savai R, Goyal P, Klepetko W, Grimminger F, Weissmann N, Seeger W, Hänze J. Impact of HIF-1alpha and HIF-2alpha on proliferation and migration of human pulmonary artery fibroblasts in hypoxia. FASEB J. 2006Jan;20(1):163-5 doi:10.1096/fj.05- 4104fje
65. Hu CJ, Laux A, Gandjeva A, Wang L, Li M, Brown RD, Riddle S, Kheyfets VO, Tuder RM, Zhang H, Stenmark KR. The Effect of Hypoxia-inducible Factor Inhibition on the Phenotype of Fibroblasts in Human and Bovine Pulmonary Hypertension. Am J Respir Cell Mol Biol. 2023Jul;69(1):73-86.doi 10.1165/rcmb.2022-0114OC
66. Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020Jan24;15:123-147 doi:10. 1146/annurev- pathmechdis-012418-012718
67. Kang S W. et al. Research Progress of Effect of Macrophage Metabolic Reprogramming in the Pathogenesis and Treatment of Pulmonary Hypertension[J]. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease. 2019(17):1353-1356.
68. Kojima H. et al. Hypoxia- inducible factor-1α deletion in myeloid lineage attenuates hypoxia-induced pulmonary hypertension. Physiol Rep. 2019Apr;7(7):e14025. doi:10. 14814/phy2.14025
69. Watts ER, Walmsley SR. Inflammation and Hypoxia:HIF and PHD Isoform Selectivity. Trends Mol Med. 2019Jan;25(1):33-46 doi:10. 1016/j.molmed.2018. 10.006
70. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL- 1β through HIF- 1α. Nature. 2013Apr11;496(7444):238-42.doi 10. 1038/nature11986
71. Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR, Domingo-Fernandez R, Johnston DGW, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O'Neill LAJ. Pyruvate Kinase M2 Regulates Hif-1α Activity and IL-1β Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015Feb3;21(2):347.doi 10.1016/j.cmet.2015.01.017
72. Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, Miyazawa H, Yamaguchi Y, Miura M, Jenkins DM, Choi H, Kim JW, Asagiri M, Cowburn AS, Abe H, Soma K, Koyama K, Katoh M, Sayama K, Goda N, Johnson RS, Manabe I, Nagai R, Komuro I. HIF-1α-PDK1 axis- induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016May18;7:11635. doi:10.1038/ncomms11635
73. Huang X, He Z, Jiang X, Hou M, Tang Z, Zhen X, Liang Y, Ma J. Folic Acid Represses Hypoxia-Induced Inflammation in THP- 1 Cells through Inhibition of the PI3K/Akt/HIF- 1α Pathway. PLoS One. 2016Mar14;11(3):e0151553. doi:10.1371/journal.pone.0151553. PMID: 26974319; PMCID: PMC4790958
74. Shukla SD, Walters EH, Simpson JL, Keely S, Wark PAB, O'Toole RF, Hansbro PM. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology. 2020Jan;25(1):53-63 doi:10.1111/resp. 13722
75. Wu S, Li H, Yu L, Wang N, Li X, Chen W. IL- 1β upregulates Muc5ac expression via NF-κB-induced HIF-1α in asthma. Immunol Lett. 2017Dec;192:20-26.doi 10. 1016/j.imlet.2017.10.006
76. van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008Jun15;412(3):477-84.doi 10.1042/BJ20080476
77. Keranov S, Dörr O, Jafari L, Liebetrau C, Keller T, Troidl C, Kriechbaum S, Voss S, Richter M, Tello K, Gall H, Ghofrani HA, Mayer E, Seeger W, Hamm CW, Nef H. SPARCL1 as a biomarker of maladaptive right ventricular remodelling in pulmonary hypertension. Biomarkers. 2020May;25(3):290-295 doi: 10.1080/1354750X.2020.1745889
78. Liu J, Wang W, Wang L, Chen S, Tian B, Huang K, Corrigan CJ, Ying S, Wang W, Wang C. IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF-1α and VEGF Expression in Vascular Endothelial Cells. EBioMedicine. 2018Jul;33:196-210.doi 10.1016/j.ebiom.2018.06.003
79. Deng H, Tian X, Sun H, Liu H, Lu M, Wang H. Calpain-1 mediates vascular remodelling and fibrosis via HIF-1α in hypoxia-induced pulmonary hypertension. J Cell Mol Med. 2022May;26(10):2819-2830 doi: 10.1111/jcmm.17295
80. Hölscher M, Schäfer K, Krull S, Farhat K, Hesse A, Silter M, Lin Y, Pichler BJ, Thistlethwaite P, El-Armouche A, Maier LS, Katschinski DM, Zieseniss A. Unfavourable consequences of chronic cardiac HIF-1α stabilization. Cardiovasc Res. 2012Apr1;94(1):77-86 doi: 10.1093/cvr/cvs014
81. Wei H, Bedja D, Koitabashi N, Xing D, Chen J, Fox-Talbot K, Rouf R, Chen S, Steenbergen C, Harmon JW, Dietz HC, Gabrielson KL, Kass DA, Semenza GL. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-β signaling. Proc Natl Acad Sci USA. 2012Apr3;109(14):E841-50 doi: 10.1073/pnas.1202081109
82. Smith KA, Waypa GB, Dudley VJ, Budinger GRS, Abdala-Valencia H, Bartom E, Schumacker PT. Role of Hypoxia-Inducible Factors in Regulating Right Ventricular Function and Remodeling during Chronic Hypoxia-induced Pulmonary Hypertension. Am J Respir Cell Mol Biol. 2020Nov;63(5):652-664.doi 10.1165/rcmb.2020-0023OC
83. Bekeredjian R, Walton CB, MacCannell KA, Ecker J, Kruse F, Outten JT, Sutcliffe D, Gerard RD, Bruick RK, Shohet RV. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS One. 2010Jul21;5(7):e11693. doi: 10.1371/journal.pone.0011693
84. Fallah J, Rini BI. HIF Inhibitors: Status of Current Clinical Development. Curr Oncol Rep. 2019Jan22;21(1):6. doi: 10. 1007/s11912-019-0752-z
85. Li RL, He LY, Zhang Q, Liu J, Lu F, Duan HX, Fan LH, Peng W, Huang YL, Wu CJ. HIF-1α is a Potential Molecular Target for Herbal Medicine to Treat Diseases. Drug Des Devel Ther. 2020Nov16;14:4915-4949.doi 10.2147/DDDT.S274980
86. Mirhadi E, Roufogalis BD, Banach M, Barati M, Sahebkar A. Resveratrol: Mechanistic and therapeutic perspectives in pulmonary arterial hypertension. Pharmacol Res. 2021Jan;163:105287. doi:10.1016/j.phrs.2020. 105287
87. Arai MA, Sakuraba K, Makita Y, Hara Y, Ishibashi M. Evaluation of Naturally Occurring HIF- 1 Inhibitors for Pulmonary Arterial Hypertension. Chembiochem. 2021Sep14;22(18):2799-2084.doi 10.1002/cbic.202100223
88. Xiaonan G. et al. Astragaloside IV ameliorates oxidative stress in monocrotaline-induced pulmonary hypertension rats through Calpain- 1/HIF-1a. Pharmacology and Clinics of Chinese Materia Medica. 2021;37(02):33-38
89. Dong F, Zhang J, Zhu S, Lan T, Yang J, Li L. Chrysin Alleviates Chronic Hypoxia-Induced Pulmonary Hypertension by Reducing Intracellular Calcium Concentration in Pulmonary Arterial Smooth Muscle Cells. J Cardiovasc Pharmacol. 2019Nov;74(5):426-435.doi 10.1097/FJC.0000000000000726
90. Xuemei Y. Research Progress on Pharmacological Effect of Tetramethylpyrazine. Chinese Journal of Biochemical and Pharmaceuticals. 2010;31(03):215-217
91. Cao J, Miao Q, Miao S, Bi L, Zhang S, Yang Q, Zhou X, Zhang M, Xie Y, Zhang J, Wang S. Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma. Int Immunopharmacol. 2015May;26(1):212-20.doi 10.1016/j.intimp.2015.03.028
92. Youwei L. et al. Preventive Effect and Underlying Mechanism of Tetramethylpyrazine on Hypobaric and Hypoxic Pulmonary Hypertension in Rats. China Pharmacist. 2017;20(04):607-611
93. Xiaoli L. et al. Effects of Lycium barbarum polysaccharides on tolerance to normobaric hypoxia in mice. Acta Nutrimenta Sinica. 2000(04):337-340
94. Wei S. et al. Effects of Lycium barbarum polysaccharides on hypoxia tolerance and fatigue resistance in mice. Journal of Xinxiang Medical College. 2011;28(03):298-300
95. Yanni Z. et al. Lycium barbarum polysaccharides enhances SIRT1 expression and decreases MMP-9 and HIF-1α expressions in hypoxic pulmonary vascular smooth muscle cells. Chinese Journal of Cellular and Molecular Immunology. 2016;32(07):906-910 +916
96. Gupta V, Lahiri SS, Sultana S, Tulsawani RK, Kumar R. Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C-H-R) exposure and post-stress recovery. Food Chem Toxicol. 2010Apr;48(4):1019-25 doi: 10. 1016/j.fct.2010.01.012
97. Zheng-guang Z. et al. Salidroside regulates hypoxia-mediated pulmonary arterial hypertension via targeting VEGF /HIF- 1α signaling pathway in rats. Guangxi Medical Journal. 2021;43(23):2834-2837 +2866
98. Xiaoli L. et al. Preventive effect of phytoestrogens on chronic hypoxic pulmonary hypertension in rats. Chinese Pharmacological Bulletin. 2004(08):897-900
99. Zhang M, Wu Y, Wang M, Wang Y, Tausif R, Yang Y. Genistein rescues hypoxia-induced pulmonary arterial hypertension through estrogen receptor and β-adrenoceptor signaling. J Nutr Biochem. 2018Aug;58:110-118.doi 10. 1016/j.jnutbio.2018.04.016
100. Xiaoli L. et al. Intervention effect of genistein on chronic hypoxic pulmonary vascular remodeling. Chinese Pharmacological Bulletin. 2004(11):1257- 1261.
101. Yang Juan, Li Xiaoxu2. et al. Phytoestrogen attenuates hypoxia-induced expression of annexin A1 in alveolar epithelial cells through down-regulating NF-κB and HIF-1α. Journal of Army Medical University. 2013;35(19):2019-2023.DOI 10. 16016/j. 1000-5404.2013.19.034
102. Huang S, Chen P, Shui X, He Y, Wang H, Zheng J, Zhang L, Li J, Xue Y, Chen C, Lei W. Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J Pharm Pharmacol. 2014Oct;66(10):1469-77 doi: 10. 1111/jphp. 12273
103. Yang X, Li F, Yang Y, Shen J, Zou R, Zhu P, Zhang C, Yang Z, Li P. Efficacy and safety of echinacoside in a rat osteopenia model. Evid Based Complement Alternat Med. 2013;2013:926928. doi: 10. 1155/2013/926928
104. Gai XY, Tang F, Ma J, Zeng KW, Wang SL, Wang YP, Wuren TN, Lu DX, Zhou Y, Ge RL. Antiproliferative effect of echinacoside on rat pulmonary artery smooth muscle cells under hypoxia. J Pharmacol Sci. 2014;126(2):155-63 doi: 10. 1254/jphs. 14072fp
105. Cho MS, Park WS, Jung WK, Qian ZJ, Lee DS, Choi JS, Lee DY, Park SG, Seo SK, Kim HJ, Won JY, Yu BC, Choi IW. Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-κB signaling in activated HMC- 1 human mast cells. Pharm Biol. 2014Jul;52(7):926-32 doi: 10.3109/13880209.2013.865243
106. Cheng CC, Chi PL, Shen MC, Shu CW, Wann SR, Liu CP, Tseng CJ, Huang WC. Caffeic Acid Phenethyl Ester Rescues Pulmonary Arterial Hypertension through the Inhibition of AKT/ERK-Dependent PDGF/HIF- 1α In Vitro and In Vivo. Int J Mol Sci. 2019Mar22;20(6):1468. doi: 10.3390/ijms20061468
107. Ming-ming L. et al. Effects of icariin on hypoxia-induced pulmonary hypertension in mice. Chin J New Drugs Clin Rem. 2020;39(04):235-237
108. Wang Y, Duo D, Yan Y, He R, Wu X. Magnesium lithospermate B ameliorates hypobaric hypoxia-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition and its potential targets. Biomed Pharmacother. 2020Oct;130:110560.doi 10. 1016/j.biopha.2020.110560
109. Zhang J, Lu W, Chen Y, Jiang Q, Yang K, Li M, Wang Z, Duan X, Xu L, Tang H, Sun D, Wang J. Bortezomib alleviates experimental pulmonary hypertension by regulating intracellular calcium homeostasis in PASMCs. Am J Physiol Cell Physiol. 2016Sep1;311(3):C482-97 doi:10. 1152/ajpcell.00324.2015
110. Xu Q, Wu X, Li Y, Kong H, Jin Y, Xie W, Wang H. Iptakalim induces mitochondria- dependent apoptosis in hypoxic rat pulmonary arterial smooth muscle cells. Biomed Pharmacother. 2016Dec;84:773-779.doi 10. 1016/j.biopha.2016.09.031
111. Braga CL, Felix NS, Teixeira DE, Vieira JB, Silva-Aguiar RP, Bose RM, Antunes MA, Rocha NN, Caruso-Neves C, Cruz FF, Rocco PRM, Silva PL. Niclosamide attenuates lung vascular remodeling in experimental pulmonary arterial hypertension. Eur J Pharmacol. 2020Nov15;887:173438. doi:10.1016/j.ejphar.2020.173438
112. Kurosawa R, Satoh K, Kikuchi N, Kikuchi H, Saigusa D, Al-Mamun ME, Siddique MAH, Omura J, Satoh T, Sunamura S, Nogi M, Numano K, Miyata S, Uruno A, Kano K, Matsumoto Y, Doi T, Aoki J, Oshima Y, Yamamoto M, Shimokawa H. Identification of Celastramycin as a Novel Therapeutic Agent for Pulmonary Arterial Hypertension. Circ Res. 2019Jul19;125(3):309-327 doi:10. 1161/CIRCRESAHA. 119.315229
113. Jiang Y, Zhou Y, Peng G, Liu N, Tian H, Pan D, Liu L, Yang X, Li C, Li W, Chen L, Ran P, Dai A. Topotecan prevents hypoxia-induced pulmonary arterial hypertension and inhibits hypoxia-inducible factor-1α and TRPC channels. Int J Biochem Cell Biol. 2018Nov;104:161-170.doi 10. 1016/j.biocel.2018.09.010
114. Panchal J, Jaiswal S, Jain S, Kumawat J, Sharma A, Jain P, Jain S, Verma K, Dwivedi J, Sharma S. Development of novel bosentan analogues as endothelin receptor antagonists for pulmonary arterial hypertension. Eur J Med Chem. 2023Nov5;259:115681.doi 10.1016/j.ejmech.2023.115681
115. Bao C, Liang S, Han Y, Yang Z, Liu S, Sun Y, Zheng S, Li Y, Wang T, Gu Y, Wu K, Black SM, Wang J, Nawrocki ST, Carew JS, Yuan JX, Tang H. The Novel Lysosomal Autophagy Inhibitor (ROC-325) Ameliorates Experimental Pulmonary Hypertension. Hypertension. 2023Jan;80(1):70-83 doi: 10.1161/HYPERTENSIONAHA.122.19397
Author contact
![]() Corresponding author: Department of Respiratory Diseases, School of Medicine, Hunan University of Chinese Medicine, Changsha 410208, Hunan, People's Republic of China. E-mail address: daiaiguoedu.cn (A.-G. Dai).
Corresponding author: Department of Respiratory Diseases, School of Medicine, Hunan University of Chinese Medicine, Changsha 410208, Hunan, People's Republic of China. E-mail address: daiaiguoedu.cn (A.-G. Dai).

 Global reach, higher impact
Global reach, higher impact