Impact Factor
ISSN: 1449-1907
Int J Med Sci 2026; 23(3):1121-1143. doi:10.7150/ijms.126361 This issue Cite
Review
Reviewing the Peripheral and Central Mechanisms of Visceral Hypersensitivity in Intestinal Disorders
1. Physiology and Pathophysiology Department, School of Basic Medicine, Qingdao University, Qingdao, Shandong, China.
2. Department of gastroenterology, Affiliated Qingdao Third People's Hospital, Qingdao University, Qingdao, Shandong, China.
3. Special Medicine Department, School of Basic Medicine, Qingdao University, Qingdao 266071, China.
4. Department of cardiology, Affiliated Qingdao Third People's Hospital, Qingdao University, Qingdao, Shandong, China.
† These two authors contributed equally to this work and were considered co-first authors.
Received 2025-10-7; Accepted 2026-2-4; Published 2026-2-18
Abstract
Visceral hypersensitivity (VH) is a condition where the internal organs have an enhanced sensitization to normal physiological stimuli or mild pathological stimuli, leading to chronic visceral pain or other discomforts, which is a typical characteristic of some intestinal disorders, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). VH might be caused by gene, psychological disorders, social stress factors, gut microbiota, and some other factors, but the exact mechanisms are not yet clear. This review focuses on recent developments in the effect of intestinal cells on sensitization of nociceptors, high excitability of brain nuclei regulating visceral pain, and the novel roles of gut microbiota in VH. It is hoped to synthesize research advancements to demonstrate the possible peripheral and intracerebral processes of hypersensitization. Additionally, more animal experiments and clinical studies are still needed to improve our understanding about VH to reduce the suffering of patients with IBS and IBD.
Keywords: visceral hypersensitivity, dorsal root ganglion, nociceptor, gut microbiota
1. Introduction
Visceral hypersensitivity (VH) denotes a heightened responsiveness and sensitivity of internal organs to different mechanical, chemical, and thermal stimuli, and often leads to typical symptoms including abdominal pain, bloating, altered bowel habits, and general discomfort in the abdominal region [1]. Until now, this type of abdominal pain is difficult to manage, posing a challenge for patients and clinicians. The symptoms of VH can negatively impact daily activities, work performance, emotional well-being, and overall life satisfaction. VH is now recognized as a major pathophysiological mechanism contributing to abdominal pain in patients with functional or inflammatory bowel diseases, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) [2]. As known, IBD, including ulcerative colitis (UC) and Crohn's disease (CD), is characterized by marked mucosal inflammation, whereas IBS mainly demonstrates altered gastrointestinal environment, persistent low-grade inflammation, abnormal neuro-immune interactions, and dysbiosis [3, 4]. Although IBS and IBD are two different diseases, IBD patients usually manifest IBS-like symptoms during remission, which is termed “irritable inflammatory bowel syndrome” for persistent hypersensitivity [5]. Moreover, studies exhibited many overlapping influencing factors of IBS-like symptoms in IBS and IBD, such as sensitized sensory nervous system, enhanced immune responses, and impaired intestinal barrier [2].
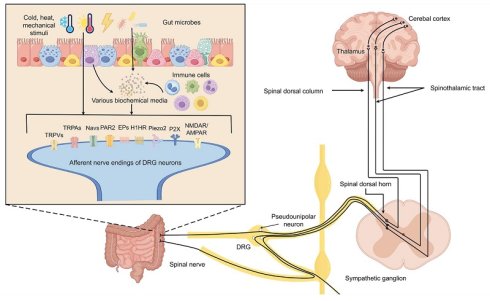
It is known that VH might be caused by the sensitization of the afferent neural pathways located at different levels. Visceral nociceptors are mostly present at the nerve terminals of dorsal root ganglia (DRG) neurons, and can be directly activated by a wide variety of stimuli [6]. For example, the transient receptor potential (TRP) can sense thermal, chemical, and mechanical stimuli. The voltage-gated sodium channels (Nav1.7, Nav1.8, and Nav1.9), Piezo1, Piezo2 can sense mechanical stimuli [7, 8]. The purinergic receptors (P2X and P2Y), toll-like receptor 4 (TLR4), protease-activated receptor 2 (PAR2) and 5- hydroxytryptamine (5-HT) receptor can sense chemical stimuli [8, 9]. The activated nociceptors potentiated action potential firing of DRG neurons, which project axons to the dorsal horn of the spinal cord. Then, the major nociceptive signals are sent to the contralateral ventral posterolateral nucleus of the thalamus via the spinothalamic tract. Neurons in thalamus relay the sensory information (pain) to the primary somatosensory cortex, posterior parietal cortex, and the limbic system for perceiving the pain signaling, processing emotional response, and making responding decisions [10]. Based on the pathway for the transmission of visceral pain signals, VH arises primarily in the following processes: 1) sensitization of sensory neurons (predominantly involving changes in nociceptor function), 2) alterations in the excitability of spinal afferent pathways (with a focus on spinal dorsal horn neurons), and 3) sensitization of central pain-processing neurons. The exact causes of neural sensitization are not fully clear, but a complex interplay of intestinal cell function, gut microbiota, genetics, psychological factors, and environmental triggers might be involved.
Current narrative reviews on VH mainly focus on IBS. These reviews discuss advances in physiological mechanisms, covering potential drug targets like TRPV1, ASICs, voltage-gated sodium channels, ATP, PAR-2, cannabinoid, prostaglandin, tachykinin, and 5HT3 receptors [11]. Research also shows that increased excitatory synaptic plasticity and changes in brain neural circuits contribute to VH [12, 13]. Furthermore, the dynamic "microbiome shifts," characterized by the enrichment or depletion of specific bacterial taxa in IBS, and their significant impact on disease progression and pathology are explained [14]. Recently, some articles have examined IBS-type symptoms in IBD, including low-grade residual inflammation, altered intestinal permeability, and VH, among others [15]. The causes of VH in IBD are briefly outlined, such as highly sensitive or overexpressed TRPs, low-grade inflammation, alterations in the brain-gut axis, and post-inflammatory microbiota and metabolites [16-18]. Thus, previous reviews primarily address pathophysiological mechanisms of VH, like abnormal nociceptors, dysbiosis, and enhanced sensory centers. However, few articles simultaneously discuss both IBS and IBD.
Based on the studies of VH in IBS and IBD, it is found that the peripheral nociceptors and central sensory neurons were strongly affected by molecules or transmitters from surrounding or functionally related cells, even the gut microbiota. For better understanding VH, this review presents the mechanism from three tightly interlinked aspects: (1) the active roles of intestinal cells (e.g., epithelial, enteroendocrine, immune cells) as translators of luminal stimuli into signals that prime peripheral nociceptor sensitization; (2) the specific circuits underlying the heightened excitability of defined brain nuclei, particularly those within the limbic and hypothalamic areas, which are responsible for maintaining the visceral pain state; and (3) the effects of gut microbiota dynamically influencing both peripheral intestinal afferent nerve and central sensory signals processing via the "microbiota-gut-brain" axis. This review integrates these components into a continuous narrative, tracing the pathogenic process from initial peripheral signal transduction in the gut to advanced central pain processing. We aim to provide a refined and coherent framework for understanding VH. Such an integrated perspective not only advances our mechanistic insight into the pathology of disorders like IBS and IBD but also illuminates novel potential targets for future therapeutic strategies.
2. Methods
2.1 Review design and guideline adherence
This narrative review designed to synthesize and critically evaluate current knowledge on the mechanisms of VH. To ensure methodological transparency and quality, the review was conducted and reported following the recommendations of the Scale for the Assessment of Narrative Review Articles (SANRA) [19].
2.2 Search strategy and information sources
A structured, broad literature search was performed to identify relevant studies. The search strategy was informed by key concepts related to our review's aim, loosely guided by a PICO framework:
Population/Problem: Patients with VH disorders (e.g., IBS, IBD with persistent pain) and relevant animal models.
Intervention/Exposure/Mechanism: Various biological mechanisms involve the interactions of intestinal epithelial cells, immune cells, the enteric and central nervous systems, and the gut microbiota.
Context: Pathophysiology and mechanistic research.
Electronic searches were conducted in the following databases: PubMed and Web of Science Core Collection. The search covered publications from inception until January, 2026. A combination of Medical Subject Headings (MeSH) terms and keywords was used, including but not limited to: “visceral hypersensitivity”, “visceral pain”, “irritable bowel syndrome”, “functional bowel disease”, “inflammatory bowel disease”, “intestinal barrier”, “enteroendocrine cell”, “mast cell”, “microbiota-gut-brain axis”, “TRPV1”, “central sensitization”, “stress”, “CRF”. Search strings were adapted for each database. The reference lists of key articles and recent reviews were also manually scanned to identify additional pertinent studies.
2.3 Eligibility criteria
Studies were considered for inclusion based on the following criteria:
Included: Original research articles (clinical, translational, and basic science), high-impact review articles, and meta-analyses published in peer-reviewed journals. We prioritized studies that elucidated mechanistic pathways in visceral pain perception, including human studies, rodent models, and seminal in vitro findings that inform biological plausibility. The focus was on literature published within the last two decades, with particular emphasis on high-impact findings and consensus views established in the field.
Excluded: Editorials, letters, conference abstracts without full data, studies exclusively focused on somatic pain pathways without visceral relevance, and articles not available in English.
2.4 Selection and synthesis process
The initial pool of records retrieved from database searches was deduplicated. The lead author screened titles and abstracts against the eligibility criteria to identify potentially relevant papers. Full-text versions of these articles were then obtained and assessed for final inclusion. Given the narrative and integrative nature of this review aimed at constructing a coherent model, the selection of representative literature was based on scientific rigor, novelty, and contribution to the overarching narrative of the gut-brain axis in VH. Conflicting results are recognized and explored in the text. The data from the included studies were thematically extracted and narratively combined to form the conceptual model in this review.
Confidence and consistency of evidence for key pathways
| Pathway/ Mechanism | Representative studies and dominant Evidence Source | Confidence in Human Pathophysiology | Consistency Across Studies |
|---|---|---|---|
| Enterocytes and L cells release ATP/UTP/ADP upon activation, which act on P2X3/7 and P2Y receptors to promote VH | James R F Hockley et al. (animal models) [20] Annemie Deiteren et al. (animal models) [21] Olena A Petrushenko et al. (ex vivo studies) [22] Alexander Eser et al. (human ex vivo studies) [23] Van B Lu et al. (animal models) [24] | Low-Medium | High |
| Enterocyt and L cells, upon activation, release Glu to promote VH | Michelle Y Meng et al. (animal models) [25] Qingqing Qi et al. (animal models and human studies) [26] | Low-Medium | High |
| Activated enterocytes release BDNF to promote VH | Peng Wang et al. (animal models and human ex vivo studies) [27] Yu Zhang et al. (human ex vivo studies) [28] | Low-Medium | High |
| CRF in the intestine can promote VH by acting on CRFR1, while acting on CRFR2 inhibits VH. | K Saito Nakaya et al. (animal models) [29] Seth Sweetser et al. (Human RCT) [30] Eloísa Salvo-Romero et al. (human ex vivo studies) [31] | High | Medium |
| 5-HT (primarily derived from ECs) participates in VH by acting on 5-HT3R | Cesare Cremon et al. (human ex vivo studies) [32] James R Bayrer et al. (animal models) [33] Rui Fu et al. (human ex vivo studies) [34] Satish S C Rao et al. (human RCT) [35] Ghazaleh Mohammadian et al. (human ex vivo studies) [36] | High | Medium |
| Piezo1 and Piezo2 promote VH by acting on EC and DRG | Constanza Alcaino et al. (animal models) [37] Erika Sugisawa et al. (animal models) [38] Zili Xie et al. (animal models) [8] | Low | High |
| The GUY2C pathway regulates VH | Joshua R Barton et al. (Ex vivo and animal models) [39] Kunwar Shailubhai et al. (animal models) [40] | Low | High |
| IL-33 acts on MCs to promote VH | Zhiping Wei et al. (animal models) [41] | Low | Low |
| IL-1β participates in VH by promoting intestinal inflammation and disrupting the intestinal barrier. | Tsukasa Nozu et al. (animal models) [42] | Low | High |
| IL-6 promotes VH | Sven Benson et al. (human RCT) [43] Maria M Buckley et al. (animal models) [44] Emmeline Salameh et al. (animal models) [45] | Medium-High | High |
| TNF-α promotes VH | Katie H Barker et al. (animal models) [46] Sagi Gudes et al. (animal models) [47] Sven Benson et al. (human RCT) [43] | Medium-High | High |
| NGF from sources such as MC and EGC promotes VH by facilitating the growth of nerve endings or sensitizing neurons | Giovanni Dothel et al. (human ex vivo studies) [48] Yan Chen et al. (animal models) [49] | Medium | High |
| Tryptases promote VH by acting on PAR2 through mechanisms such as disrupting the intestinal barrier. | Silvia Amadesi et al. (animal models) [50] Claire Jacob et al. (human ex vivo studies) [51] Tamira K Klooker et al. (human RCT) [52] | High | Medium |
| PGE2 derived from cells such as MC and EGC promotes VH | Gintautas Grabauskas et al. (animal models) [53] Jun Gao et al. (animal models) [54] Karem Awad et al. (human ex vivo studies) [55] Wilmarie Morales-Soto et al. (animal models) [56] | Low-Medium | High |
| Histamine promotes VH by acting on H1HR and H4HR. | Annemie Deiteren et al. (animal models) [57] D Balemans et al. (human ex vivo studies) [58] Lisse Decraecker et al. (human RCT) [59] | High | High |
| Proke2 promotes VH | Robert P Watson et al. (animal models) [60] | Low | Low |
| EGC interacts with other immune cells to promote VH | Felipe Meira de-Faria et al. (human ex vivo studies) [61] Vladimir Grubišić et al. (animal models) [62] Ninotchska M Delvalle et al. (animal models) [63] | Low-Medium | High |
| The activation of ACC (glutamatergic and microglia, etc.) promotes VH | Ling Yang et al. (human cross-sectional studies) [64] Zhijun Cao et al. (animal models) [65] | Medium | High |
| The activation of the CL-ACC circuit promotes VH | Qiya Xu et al. (animal models) [66] Ying Xiao et al. (animal models) [67] | Low | High |
| The activation of MT-ACC promotes VH | Jun Wang et al. (animal models) [68] Ying Li et al. (animal models) [69] | Low | High |
| Activation of CRF neurons in PVN promotes VH | Gongliang Zhang et al. (animal models) [70] Si Ting Huang et al. (animal models) [71] Catherine S Hubbard et al. (human RCT) [72] | Medium-High | High |
| The activation of PVN-LSV promotes VH | Yong Chang Li et al. (animal models) [73] | Low | Low |
| The activation of PVN-VTA (CRF) promotes VH | Ning Ning Ji et al. (animal models) [74] | Low | Low |
| The activation of PVN-PFC (oxytocin) inhibits VH | Yaling Liu et al. (animal models) [75] | Low | Low |
| The inhibition of GABAergic projections and activation of glutamatergic projections from BNST-PVN promote VH | Si Ting Huang et al. (animal models) [76] Yu Song et al. (animal models) [77] | Low | Low |
| Activation of NMDA receptors in ARC promotes VH | Hang Zheng et al. (animal models) [78] | Low | Low |
| Downregulation of GRK6 in ARC leads to upregulation of NF-κB, which promotes VH | Xin Li et al. (animal models) [79] | Low | Low |
| Upregulation of glutamate neurons 5-HT2B receptors in Re promotes VH | Di Li et al. (animal models) [80] | Low | Low |
| Activation of the insular cortex promotes VH | Fu-Chao Zhang et al. (animal models) [81] | Low | Low |
| Estrogen in RVM promotes VH through GPER | Yingfu Jiao et al. (animal models) [82] | Low | Low |
| The activation of VTA promotes VH | Meng-Ge Li et al. (animal models) [83] | Low | Low |
| B.longum inhibits VH | A.P.Allen et al. (human clinical controlled trail) [84] Chunhua Zhou et al. (animal models) [85] | Medium | High |
| BCM and LCM inhibit VH | Shuangshuang Guo et al. (ex vivo studies) [86] Erin D Lewis et al. (human RCT) [87] Christopher J Martoni et al. (human RCT) [88] Huan Wang et al. (animal models) [89] Afifa Ait-Belgnaoui et al. (animal models) [90] | High | High |
| Ruminococcus gnavus promotes VH | Lixiang Zhai et al. (animal models) [91] Lijuan Han et al. (human cohort studies) [92] Fernanda Cristofori et al. (human RCT) [93] | High | High |
| Clostridium promotes VH by increasing BA metabolism | Antal Bajor et al. (human cross-sectional studies) [94] Ling Zhao et al. (human case-control studies) [95] Wen Ting Li et al. (animal models) [96] | Medium | High |
| Adherent-Invasive E. coli promotes VH | Amandine Lashermes et al. (animal models) [97] Arlette Darfeuille-Michaud et al. (human ex vivo studies) [98] Franck Carbonnel et al. (human RCT) [99] Moira Paroni et al. (human ex vivo studies) [100] | High | High |
| SCFAs regulate VH (conflicting results) | Qing Hua Sun et al. (human case-control studies) [101] C Tana et al. (human case-control studies) [102] Andrea S Shin et al. (human case-control studies) [103] Yi Xing et al. (Ex vivo studies) [104] Dabo Xu et al. (animal models) [105] Tsukasa Nozu et al. (animal models) [106] Yingjie Li et al. (animal models) [107] Erfeng Li et al. (human RCT) [108] | High | Low |
| Microbial metabolite dopamine regulates VH (conflicting results) | Cezary Chojnacki et al. (human ex vivo studies) [109] Ammar Hassanzadeh Keshteli et al. (human case-control studies) [110] | Medium | Low |
| F.varium promotes VH by increasing Piezo2 | Haonan Zheng et al. (animal models) [111] | Low | Low |
Grading Criteria: Confidence: High = Supported by multiple, robust human clinical studies; Medium = Supported by strong animal data and preliminary/mechanistic human evidence; Low = Primarily based on preclinical data with minimal direct human evidence.
Consistency: High = Findings are replicated across independent studies; Medium = General agreement exists with some conflicting reports; Low = Evidence is limited or findings are contradictory. Detailed mechanistic descriptions and key references are provided in the main text.
2.5 Evidence synthesis and grading
As part of our commitment to SANRA's emphasis on explaining the level of evidence, we implemented supplementary evidence grading framework. Key mechanistic statements in the text are tagged by primary evidence source (Human Clinical Study, Animal Model, Ex Vivo/In Vitro). Moreover, Table 1 provides a summary of the confidence and consistency of evidence for key pathways. This method improves the critical evaluation of the compiled literature.
3. Intestinal cells sensitizing sensory neurons
VH mainly arises from the abnormal activation of intestinal nociceptors. Some researches indicated that intestinal cells, such as intestinal epithelial cells (IECs), intestinal immune cells, and enteric glial cells (EGCs), directly sensitized nociceptors via different functional mediators.
3.1 IECs
The major cell types of the intestinal epithelium are enterocytes, enteroendocrine cells (EECs), Paneth cells, tuft cells, and goblet cells. In recent years, extensive researches have elucidated the role of the IECs on the DRG neurons in modulating visceral pain.
3.1.1 Enterocytes
Enterocytes, as the main cell type in the intestine, play a key role in absorbing nutrients and secreting functional molecules like ATP, Glutamate (Glu), brain-derived neurotrophic factor (BDNF), IL-33, trypsin-3, and corticotropin-releasing factor (CRF).
ATP serves not only as the direct source of energy at the cellular level, but also as a signaling molecule to modulate the visceral sensation. After activated by heat, low osmolarity, mechanical stress, metabolites of arachidonic acid, and proinflammatory mediators via the TRPV4, enterocytes generate more ATP [112]. Subsequently, excess ATP binds to P2X and/or P2Y purinoceptors to activate the nociceptors. For example, the P2X3 receptor subtype mediates the onset of pain sensitization by sensitizing TRPV1 expressed on DRG neurons or upregulation of β2 adrenergic signaling in primary sensory neurons, the P2X7 receptor subtype leads to increased release of IL-1β from immunocytes to indirectly induce VH, and the activation of P2Y receptors by ATP, UTP or ADP enhance the excitability of colonic pain sensation via the NaV1.9 channel co-expressed with P2Y on DRG neurons [20-22, 113].
Except ATP, the enterocyte activated by TRPV4 releases the excitatory neurotransmitter Glu. On the one hand, Glu directly excites the DRG neurons to participate in visceral pain [12, 25], on the other hand, Glu indirectly contributes to the visceral hypersensitive response through promoting the release of substance P (SP) and calcitonin gene-related peptide (CGRP) from DRG neurons and BDNF from enterocytes [12, 26-28, 114].
Furthermore, the enterocytes were found to secret more proteases and CRF in IBS patients and rodent IBS models. The proteases, predominantly trypsin-3, activate PAR2 of the enteric sensory neurons to induce VH [115]. CRF indirectly contributes to VH by binding to CRF receptor 1 (CRFR1) on immune cells to promote inflammation via TLR4 and IL-1 pathways [116, 117]. In both IBD patients and mouse models, the enterocytes secrete abundant IL-33, which subsequently acts on tumorigenicity 2 receptor (ST2) of mast cells. Activated mast cells disrupt the intestinal barrier and promote inflammatory responses, further contributing to the development of VH [41].
3.1.2 EECs
Another important cell type of the intestine is EECs. Among various EECs, enterochromaffin cells (ECs), neuropod cells and L cells are believed to play more important role in VH.
3.1.2.1 ECs
ECs are responsible for secreting approximately 95% of 5-HT in the body, which significantly influence intestinal movement and secretion [118]. There are ongoing debates regarding the number of ECs in the patients with VH. Most research reported that patients with IBS showed a significant increase in ECs counts, and there was a significantly greater ECs number in patients with diarrhea-predominant IBS compared with patients with constipation-predominant IBS [32]. A handful of studies reported that all IBS subtypes demonstrated a reduction in ECs [119], and there is still a small portion of research indicated that the quantity of ECs remains unchanged in IBS patients [36]. Therefore, it is suggested that VH primarily attributed to alterations in ECs signaling rather than cell amount.
Studies suggested that individuals with IBS have notably elevated levels of 5-HT and 5-HT3R in the intestinal mucosal tissues compared to healthy people, and this abnormal overexpression was closely linked to the development of VH [34]. Although the processes that elevate 5-HT release are still not completely defined, changes in intestinal pressure could play a role. Notably, mechanically gated ion channels of the Piezo family are expressed in intestinal epithelial cells [37, 38]. Piezo1 and Piezo2 activate p38 signaling pathways, leading to increased expression of tryptophan hydroxylase 1 (TPH1), a key synthase for 5-HT in ECs, and thereby affecting 5-HT production [120]. James et al. has proved that ECs establish synapses with intestinal mucosal afferent neurons to transmit visceral pain signals via the neurotransmitter 5-HT, which means more 5-HT releasing, higher visceral sensitivity [33]. Besides, 5-HT might participate in VH by sensitizing TRPV1 and TRPV4 channels on DRG neurons [121, 122]. All of the above suggests the pathway involving ECs, 5-HT, and mucosal afferent nerves (DRG) might play an important role in VH.
3.1.2.2 Neuropod cells
Neuropod cells, as a specialized subtype of EECs with highly expressed Guanylyl Cyclase C (GUCY2C), modulates the DRG-neuron excitability via synapses [39]. Clinical studies indicated that patients with IBS exhibit reduced levels of endogenous GUCY2C ligands, such as urinary guanosine [123]. It is consistent with the animal experiment, which showed that blocking GUCY2C signaling leads to VH, and, conversely, activation of GUCY2C signaling alleviates visceral pain in colitis model mice [40]. Although the detailed mechanism is still not clear, the basic process of GUCY2C signaling pathway has been explored. Ligand-activating GUCY2C converts guanosine triphosphate into cyclic guanosine monophosphate (cGMP), then cGMP acts as a second messenger to phosphate protein kinases, which leads to inhibit visceral pain transmission either by suppressing the release of excitatory neurotransmitters or by promoting the release of inhibitory factors [124].
3.1.2.3 L cells
Another EECs L cells, were detected to secrete ATP, Glu, glucagon-like peptide 1, PYY and so on [24]. ATP and Glu, like those released from enterocytes, can respectively act on P2X/P2Y receptors and glutamate receptors (N-methyl-D-aspartate receptors (NMDAR) and α-amino-5-hydroxy-3-methyl-4-isoxazolepropionic acid receptors (AMPAR)) located on neuronal endings, contributing to the onset of VH [125].
Table 2 summarizes intestinal epithelial cell-derived substances participate in VH by sensitizing nociceptors.
3.2 Immune cells
It is proved that VH of IBS and IBD patients is closely related to the activation of intestinal immune system, including enhanced infiltration of immunocytes, the low-grade inflammation, and so on. IBD is often characterized by infiltration of neutrophils, macrophages, innate lymphoid cells, mast cells, as well as Th1 (CD) and Th17 cells. Among these, neutrophils, macrophages, and mast cells play critical roles in the development of VH [127, 128]. In contrast, mast cell and their close proximity to colonic nerves are more prominent in IBS, which is particularly evident in Diarrhea-predominant IBS-D and Post-infectious IBS (PI-IBS) [129]. These immune cells contribute to VH primarily through two pathways: by releasing immune mediators that directly interact with nociceptive afferent neurons, and by altering intestinal permeability, thereby promoting VH [130, 131].
Intestinal epithelial cell-derived substances participate in visceral hypersensitivity by sensitizing nociceptors
| Mediator | Receptors | Source/ Location | Specific Mechanism | Primary Associated Phenotypes | Citation |
|---|---|---|---|---|---|
| ATP | P2X3 P2X7 | Enterocyte L cell | Enhancing calcium responses sensitizes TRPV1 on DRG neurons Regulating the release of IL-1β | IBS IBD(UC) PI-IBS | [21, 22, 24, 113, 126] |
| UTP | P2Y2, P2Y4 | Enterocyte | Enhance action potentials induced by electrical stimulation, promoting cell membrane depolarization | - | [20] |
| ADP | P2Y1, P2Y12, P2Y13 | Enterocyte | Increase action potential discharge | - | [20] |
| Glu | NMDAR AMPAR | Enterocyte L cells | Stimulate DRG neurons; Promotes the release of SP and CGRP from DRG neurons; Promotes the release of BDNF from enterocytes. | IBS | [12, 28, 114, 125] |
| trypsin-3 | PAR2 | Enterocyte | Signal to human submucosal enteric neurons | IBS, IBD | [115] |
| CRF | CRFR1 CRFR2 | Enterocyte | Activation of CRFR1 leads to increased intestinal permeability, while activation of CRFR2 exerts an antagonistic effect. Indirectly promotes the inflammatory response | IBS | [116, 117] |
| IL-33 | ST2 | Enterocyte | Activating mast cells and disrupting the intestinal barrier. | IBD(UC) | [41] |
| 5-HT | 5-HT3R | ECs | Activating submucosal neurons Sensitizing TRPV1 and TRPV4 on DRG neurons. | IBS | [33, 121, 122] |
| Piezo2 | - | Enterocyte ECs DRG | Promoting the release of 5-HT from ECs; Mediating VH through TRPV1 on DRG neurons. | IBS, IBD IBS, IBD | [37, 38, 120] [8] |
3.2.1 MCs
Studies have found that the number of mast cells (MCs) is increased in the intestines of both IBD and IBS patients, which may be closely related to the occurrence of VH [132, 133]. MCs, as major effector cells of innate immunity and regulators of adaptive immunity, secrete a wide array of inflammatory cytokines including IL-1β, IL-4, IL-5, IL-6, TNF-α and IFN-γ, as well as diverse inflammatory mediators such as histamine, tryptase and prostaglandin E2 (PGE2) [134].
The inflammatory cytokines not only act directly on the afferent nerve endings of DRG neurons to sensitize nociceptor ion channels, but also disrupt the intestinal barrier to facilitate the translocation of harmful substances across the barrier and exacerbate intestinal inflammation, which has been widely recognized as an important inducer of VH [135].
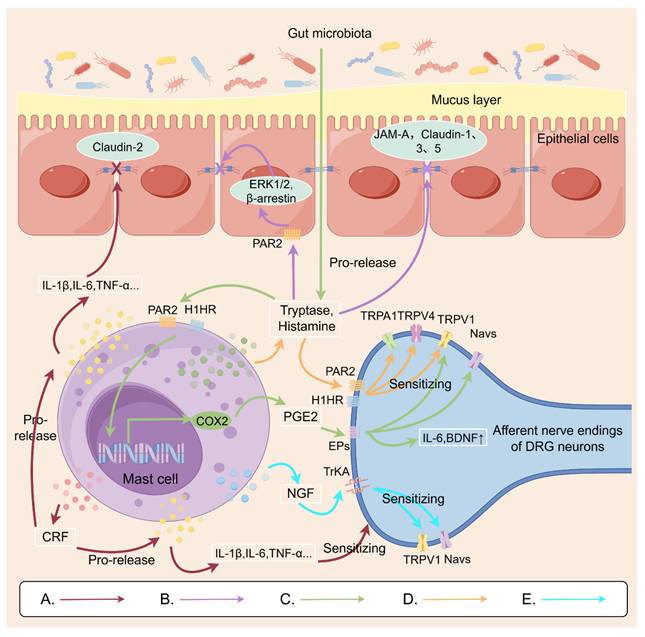
Many studies have showed that histamine released from MCs could induce VH via histamine receptor 1 (H1HR). D. Balemans et al. further explored that incubation of IBS colon biopsy supernatants or histamine-supplemented supernatants of healthy subjects with isolated mouse DRG neurons overnight enhanced neuronal Ca2+ responses by sensitizing TRPV1, TRPA1, and TRPV4 ion channels on DRG neurons, reducing the threshold for painful stimuli and inducing VH [58, 136]. Additionally, the role of TRPV4 in histamine induced neuronal sensitization is more highlighted, because a MAPKK-dependent increase of TRPV4 expression on plasma membranes of colonic sensory neuron was closely related to H1HR relocation [58, 121].
Tryptases secreted from MCs can bind to the PAR2 on the afferent nerve endings of DRG neurons, potentially sensitizing TRPV1 of DRG neurons in a protein kinase C (PKC)-dependent manner to increase neuronal excitability [50]. Likewise, the PAR2 of IECs is also recognized by tryptases, which triggers the release of intestinal inflammatory mediators [137]. Furthermore, tryptases are found to cleave tight junction proteins, such as junctional adhesion molecule-A (JAM-A), claudin-1, claudin-3 and claudin-5, leading to intestinal barrier dysfunction [138, 139]. All of these effects of tryptases contribute to VH.
PGE2 is another important inflammatory mediator released from MCs. In patients with IBS-D, lipopolysaccharide acting in concert with trypsin was found to stimulate mucosal mast cells to release PGE2 [54]. PGE2 binds to E-type prostanoid receptor 2 located on the afferent nerve endings of nociceptive DRG neurons, activating TRPV1 and various sodium channels [53]. Moreover, a mouse model study has demonstrated that in patients with IBS-D, substances such as lipopolysaccharide and trypsin can stimulate mast cells to release PGE2, downregulate the serotonin reuptake transporter, and elevate mucosal 5-HT levels, thereby contributing to the development of VH [54].
Surprisingly, some neurotrophin and neuropeptides secreted by MCs are involved in the modulation of nociceptors. Nerve growth factor (NGF) also activates TrkA receptors on nerve endings to promote Nav1.7 currents and sensitize TRPV1 channels [140]. Simultaneously, the neuropeptide CRF modulates the macrophages to release inflammatory cytokines such as IL-1 and IL-6, which would reduce the expression of the tight junction protein claudin-2 in the colonic mucosa leading to increasing intestinal permeability and inflammatory response [116, 141]. Therefore, it is believed that the effects of neurotrophin and neuropeptides on neurons, macrophage and IECs would further contributes to VH.
Figure 1 demonstrates the possible mechanisms of MCs involved in VH, which might be a novel promising therapeutic target for IBS or IBD.
3.2.2 Other immunocytes
In IBD patients, there is an increased infiltration of neutrophils and macrophages, which were proved to secret IL-1β, IL-6, TNF-α, PGE2, prokineticin 2 (PROK2) and insulin-like growth factor I (IGF-1). IL-1β, IL-6 and TNF-α lower the thresholds of Nav channels or enhance the expression of TRPV1 channels in the DRG neurons [46, 47, 142-145]. The PROK2, an inflammatory cytokine-like molecule, increases intracellular calcium levels in enteric and dorsal root ganglia neurons, which plays a role in IBD caused by trinitrobenzene sulfonic acid (TNBS) [60]. IGF-1 enhances TRPV1-mediated membrane currents in DRG neurons and promotes TRPV1 translocation to the cell membrane [146]. In addition, patients with IBD and corresponding animal models exhibit elevated expression of transient receptor potential melastatin (TRPM) channels 2, 3, and 8. These channels play a key role in sensitizing DRG neurons and driving VH [147]. However, how immune cells influence TRPM expression in IBD remains unclear.
From these contents, VH is greatly influenced by immunocytes, especially in IBD. The effectors secreted from immunocytes mainly increase intestinal permeability, expression of nociceptors, concentration of 5-HT, and so on. For better understand the potential contribution of immunocytes on VH, Table 3 summarizes the main mechanism of inflammatory effectors in the IBS or IBD.
Possible mechanisms by which mast cells are involved in visceral hypersensitivity. A: Mast cells secrete the neuropeptide CRF, which modulates macrophage release of inflammatory cytokines. This action can affect the colonic mucosa, reducing expression of the tight junction protein claudin-2. It can also act on DRG nerve endings, increasing sensitivity. B: Mast cells release trypsin, which binds to PAR2 receptors on intestinal epithelial cells. This activates the ERK 1/2 and β-arrestin signaling pathway, affecting the distribution of tight junction proteins on the cell surface. This leads to impaired intestinal barrier function, further contributing to visceral hypersensitivity. C: Histamine and trypsin from the gut microbiota activate mast cells to release PEG2. PEG2 binds to EP receptors on DRG nerve endings, sensitizing TRPV1 and sodium channels. D: Metabolites from gut microbiota induce mast cell degranulation, releasing tryptase and histamine that act on DRG nerve endings to sensitize TRPV1/TRPV4/TRPA1 receptors. E: Mast cell-secreted NGF acts on TrKA in DRG neurons, thereby sensitizing TRPV1 and sodium channels. This Figure was created by Figdraw.
3.3 EGCs
In the enteric nervous system, there are plenty of EGCs, which not only support the growth of neurons but also regulate the neural activity. As known, EGCs release NGF to support the growth, survival, and maintenance of neurons [152]. However, NGF levels significantly increase during inflammation, and NGF receptor TrkA are extensively coexpressed with TRPV1 in visceral afferents. The abnormal increased NGF not only promotes nerve ending growth, but also potentiates TRPV1 signaling, which might induce visceral hyperalgesia and hypersensitivity [153]. Additionally, EGCs activated by inflammatory cytokines in IBD release PGE2, which further sensitizes TRPV1 on afferent nerve endings of DRG neurons via EP4 receptor [56]. It is also found that more SP is released in a PLCγ1-dependent manner from EGCs in the IBS model mice. SP, as the first discovered member of the tachykinin family, has long been considered an effector of pain, acting on nociceptive nerve endings and participating in the development of VH [27]. Therefore, it indicate that EGCs directly modulate sensibility of sensory neurons via various mechanisms.
Furthermore, Vladimir et al. demonstrated that in dextran sulfate sodium induced colitis, EGCs stimulated by IL-1β release TNF-α to activate macrophages, which could act on the terminals of DRG neurons inducing visceral nociceptor sensitization [62]. Additionally, ECGs were found to regulate other types of immune cells, including MCs, antigen-presenting cells, and lymphocytes, which enhanced neuroinflammation, TRPV1-positive neuronal varicosities, and Glia-immunocytes interactions in the IBS to participate in VH [61, 63].
Various immune effectors participate in visceral hypersensitivity
| Mediator | Source | Receptors on DRG sensory neurons | Possible mechanisms | Primary Associated Phenotypes | Citation |
|---|---|---|---|---|---|
| IL-1β | MC neutrophil macrophage | IL-1R | Promoting intestinal inflammation and disrupting intestinal homeostasis | IBD, IBS | [42, 148, 149] |
| Sensitizing TRPV1 | IBD | [145] | |||
| IL-6 | MC neutrophils macrophage | IL-6R | Participates in VH by interacting with CRF, increasing intestinal permeability | IBS | [43, 44, 142, 149] |
| Lower the thresholds of Nav channels | IBD | ||||
| TNF-α | MC neutrophil macrophage | TNFR | p38MAPK-dependent manner enhances Nav 1.8, Nav 1.9 | IBD | [47, 149] |
| p38 MAPK-dependent way to sensitize TRPV1 | IBS, IBD | [46] | |||
| NGF | MC | TrkA | Enhancement of Nav 1.7 Sensitization of TRPV1 | IBS-D IBD | [140] [49] |
| NTRK1 | Enhancement of GAP43 transcription to promote neuronal growth | IBD | [48] | ||
| Tryptases | MC | PAR2 | Sensitization of TRPV1 in a PKC-dependent manner | IBD | [50] |
| Disruption of the intestinal barrier | IBS, IBD | [51, 138, 139] | |||
| Promotes the release of PGE2 | IBS | [53] | |||
| PGE2 | MC Neutrophil Macrophage | EPs | Enhancement of TRPV1 and sodium channels | IBS-D | [53, 135] |
| Promoting VH by elevating mucosal 5-HT levels | IBS-D | [54] | |||
| Increasing intestinal permeability | IBS-M | [55] | |||
| CRF | MC Eosinophil Enterocyte | CRFR1 | Autocrine-promoted MC activates degranulation to release various mediators; Increasing intestinal permeability. | IBS | [31, 116, 141] |
| Histamine | MC | H1HR H4HR | Sensitizes TRPV1, TRPA1, TRPV4 | IBS IBD (UC) | [57, 58] |
| IGF-1 | Macrophage | IGF-1R | Sensitization of TRPV1 by SNARE-dependent cytotoxicity | IBD | [150] |
| PROK2 | Neutrophil Macrophage | PKR1 PKR2 | Increases intracellular calcium levels in cultured enteric and DRG neurons Stimulates intrinsic neuronally mediated ion transport in ileal mucosa | IBD (UC) | [60] |
| Unclear | Unclear | TRPM2 TRPM3 TRPM8 | Increase expression of TRPM2, TRPM3, TRPM8 in the DRG neurons | IBD | [147, 151] |
Therefore, the complex functions of EGCs make themselves play an important role in the development of VH.
4. Brain nuclei and circuits involved in VH
While the drivers of VH often originate in the periphery, the pain experience is ultimately mediated and amplified by the central nervous system. As known, special brain nuclei are responsible for processing information of various visceral stimulation and producing pain perceptions. There are other different nuclei and neural circuits involved in modulation of visceral pain and generation of negative emotions. Therefore, exploring the central neuromodulation mechanism of VH in patients with intestinal disorders may provide suitable targets for the treatment of the visceral pain of such diseases [154].
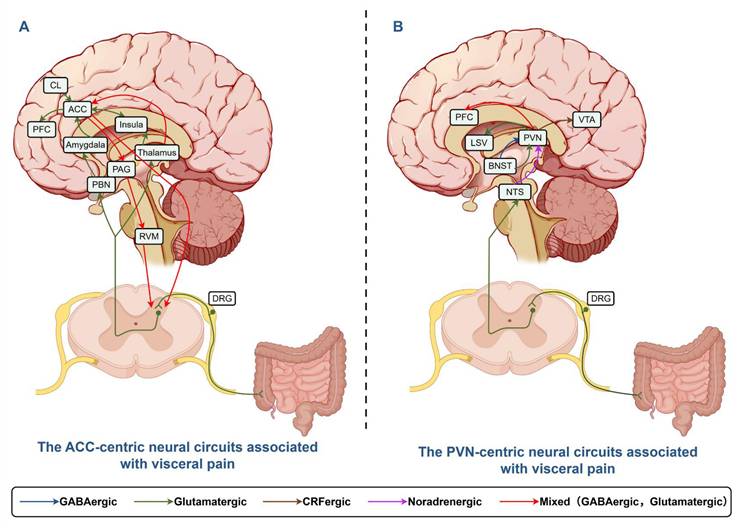
The anterior cingulate cortex (ACC) and the paraventricular nucleus of hypothalamus (PVN) of the hypothalamus are extensively involved in the modulation of both ascending pain transmission and descending pain inhibition. Moreover, these regions serve as critical hubs in the neural circuitry responsible for processing emotions and pain signals. This review primarily focuses on potential alterations in these nuclei—particularly the ACC and PVN—in patients with intestinal disorders, as well as their functional roles in such conditions [155].
4.1 ACC
The ACC, as a crucial area within the limbic system, is involved in the processing of sensory and emotional components of chronic pain. Functional magnetic resonance imaging studies have shown heightened ACC activation in individuals with IBS [156]. Studies showed that chemical and electrical stimulation on ACC enhanced the visceral motor response to colorectal distention (CRD) and nociceptive sensitization in normal or IBS rats, while ACC impairment lessens the response [65]. The IBS patients also demonstrated higher activation levels of ACC and greater intensity of pain than healthy control [157]. During IBD, ACC exhibits more activation of neuron or microglia in animal model, and shows higher low-frequency fluctuation values and degree centrality values in patients, indicating abnormal brain metabolism within the ACC of IBD patients [64].
These findings collectively demonstrate that the ACC plays an essential role in processing the affective dimension, anticipation, and memory formation of pain. Meanwhile, ACC has a wide range of fiber connections with different brain nuclei (including the substantia nigra, hippocampus, and insular cortex), which are involved in the modulation of emotions and sensations linked to pain [158, 159].
4.1.1 CL-ACC circuit
The central lateral nucleus (CL), positioned between the insular cortex and the striatum with broad links to the cerebral cortex, is essential in the transmission of visceral pain. Xu's experiments utilized neonatal maternal deprivation (NMD)-induced VH mice to evaluate the functional role of nerve fiber projections from CL to ACC in visceral pain processing [66]. The results demonstrated that c-Fos expression was significantly increased in the CL of NMD mice, indicating that visceral pain can activate the CL. The activation of glutamatergic neurons in the CL can lead to the activation of those in the ACC, potentially contributing to the development of persistent internal pain. For VH, overexpression of NMDAR and hyperactivation of calcium-calmodulin-dependent protein kinase II α (CaMKIIα) in the postsynaptic density region of the ACC may sensitize the CL-ACC neural circuitry in NMD mice. This was further supported by other experiments showing that an inhibitor targeting NMDAR in glutamatergic neurons of the ACC might be a new point for relieving visceral pain in IBS [67].
4.1.2 MT-ACC circuit
Medial thalamus (MT), the primary relay station for transmitting noxious information to the ACC, has been shown to modulate visceral pain via the MT-ACC pathway [68]. The increase in local field potential of rats with VH indicates enhanced synaptic activity at the MT-ACC interface. θ burst stimulation could induce long-term potentiation (LTP) at MT-ACC synapses of normal rats. These findings suggest that electrically induced LTP and VH may share a common mechanism. To test this hypothesis, repeated application of θ-mode stimulation at MT in normal rats enhanced CRD-induced ACC responses and increased visceral pain. The results imply that the MT-ACC pathway might be an important regulating mechanism of visceral pain [69].
4.2 PVN and its associated neural circuits
The PVN serves as a critical neuroendocrine hub, releasing several neuropeptides including CRF, arginine vasopressin, oxytocin, and thyrotropin-releasing hormone [72]. Its best-characterized role lies in initiating the hypothalamic-pituitary-adrenal (HPA) axis: CRF from the PVN stimulates pituitary adrenocorticotropic hormone secretion, which in turn promotes glucocorticoid release from the adrenal cortex [160]. Zhang et al. revealed that administration of CRF-RNAi effectively prevented VH in rats [70]. Mechanistic studies indicate that increased CRF elevated glucocorticoid secretion, which might lead to the overexpression of proinflammatory cytokines such as TNF-α, IL-1β, TLR4 et al. [70, 71]. Prolonged excessive glucocorticoid exposure was found to cause impairment of hippocampal function, which weakened the inhibitory effect of hippocampus on the HPA axis, leading to sustained release of CRF [161]. This vicious CRF- glucocorticoid circle might be an important promoter of VH. These discoveries emphasis the key role of PVN in the regulation of visceral sensory. The PVN also has widely bidirectional fiber connection with brain nuclei, such as lateral septal nucleus (LSV), ventral tegmental area (VTA), BNST, prefrontal cortex (PFC), arcuate nucleus (ARC), which are involved in different functional modulations, including sensory modulation.
4.2.1 PVN-LSV circuit
The LSV is capable of modulating affective behavior and visceral pain. For example, activating the PVN-LSV glutamatergic projections intensifies visceral pain in NMD mice, reversely inhibiting the circuits relieves the visceral pain [73].
4.2.2 PVN-VTA circuit
The VTA is an important center processing reward, addiction, learning, sleep-wakefulness cycles, and so on. The projections of CRF neurons to the VTA are found to be involved in colonic distension-induced pain [74]. In IBS model mice, the active CRF neurons significantly increased c-Fos expression and calcium ion activity of VTA glutamatergic neurons, and selective NMDA receptor 2A inhibitor administrated in VTA decreased the number of visceral pain-induced c-Fos positive neurons and attenuated visceral pain [83]. Therefore, the paraventricular nucleus of the thalamus (PVT)-VTA circuit might be another molecular target involved in chronic visceral pains.
4.2.3 PVN-PFC circuit
The PFC is the anterior portion of the frontal lobe of the cerebral cortex. The PFC is not only important in executive functions such as planning, problem solving, and social control, but also pain processing, which is dependent on neural circuits with hypothalamus, hippocampus, periaqueductal gray (PAG), amygdala, and other pain-related areas of brain. Recent studies have demonstrated that PVN oxytocin signaling boosts PFC neuronal activity in response to acute pain stimulation. However, in the context of chronic visceral pain, PVN oxytocin signaling to the PFC alleviates both the emotional and sensory dimensions of pain induced by mechanical stimuli and suppresses the development of VH [75].
4.2.4 BNST-PVN circuit
As a part of the extended amygdala, the BNST plays a critical role in stress response, fear memory, and social behavior. It has been reported that the anteroventral BNST (avBNST) directly projects GABAergic and glutamatergic neural fibers to the PVN. The avBNST GABAergic neurons inhibits activity of PVN CRF neuron to alleviate visceral pain. Conversely, glutamatergic inputs from the avBNST to the PVN exert an excitatory effect. In mice prone to VH, an imbalance between two types of inputs was noted, leading to heightened excitability of PVN CRF neurons and increased CRF release, which triggers the HPA axis [71, 76, 77]. Therefore, stimulating glutamatergic neurons or suppressing GABAergic neurons within the avBNST-PVN neural pathway promotes VH.
The neural circuits of the ACC and PVN involved in the context of visceral pain were summarized and illustrated in Figure 2.
4.3 ARC
The ARC is a critical hypothalamus area involved in energy homeostasis and mediating pain sensation. In the ARC of chronic pancreatitis (CP) model rats, expression of cystathionine β-synthetase (CBS) and PKC were significantly upregulated, accompany with higher phosphorylation level of the NMDA receptor GluN2B subunit [78]. Microinjection of the CBS inhibitor aminooxyacetic acid into the ARC of CP rats reversed the upregulation of PKC and alleviated VH. The results indicate that CP causes an increase in CBS expression in the ARC, which may play a role in VH by activating NMDA receptor.
NF-κB is another important molecule in the ARC to affect the processing of visceral pain. The selective NF-κB inhibitor pyrrolidine dithiocarbamate alleviated visceral pain in NMD rats [79]. G Protein-Coupled Receptor Kinase 6 (GRK6) is a kind of endogenous protein suppressing NF-κB expression, and was found to be low expressed in the ARC of NMD rats, which is believed to be an important modulator of VH.
The ACC-centric and PVN-centric neural circuits associated with visceral pain. A: Afferent neurons convey signals to DRG and spinal dorsal horn nuclei, projecting directly to thalamus or indirectly to ACC via PBN/Amygdala. ACC modulates spinal neurons through PAG/RVM, integrating BLA/CL/LC/NTS inputs for visceral pain and emotion regulation. B: Afferents transmit signals to DRG, projecting to PVN. BNST sends glutamatergic/GABAergic signals to PVN, which projects to LSV/VTA/PFC for visceral pain modulation. This Figure was created by Figdraw.
4.4 Rostral ventromedial medulla
The rostral ventromedial medulla (RVM) is a relay station connecting some brain regions like the PAG and amygdala to the dorsal horn of the spinal cord. The RVM dynamically balances pain signals through two types of neurons: ON cells promoting the transmission of pain signals in the spinal cord and OFF cells inhibiting the transmission. ON cells specifically express G protein-coupled estrogen receptor (GPER), which is recognized by estrogen to significantly enhance ON cells activation via the Ca²⁺/PKC pathway. Once activated, these GABAergic ON neurons reduce the inhibitory tone of spinal interneurons, thereby facilitating ascending pain transmission [162]. Estrogen also inhibits opioid signaling in ON cells, leading to increased pain and reducing the analgesic effects of morphine [82]. In contrast, testosterone exerts sex-dependent effects in the RVM: it downregulates serotonin transporter expression while upregulating 5-HT2A receptor mRNA. These changes are associated with reduced hyperalgesia in female preclinical models, but not in males [163]. Collectively, estrogen-mediated facilitation via GPER and opioid interference, together with testosterone-modulated serotonergic signaling, provide a mechanistic basis for the pronounced female predominance observed in functional visceral pain disorders.
4.5 Other nuclei
The reuniens (Re) is a component of the ventral midline thalamus, and is associated with diverse cognitive functions, such as working memory and attentional processes. A significant increase of c-Fos-positive neurons, primarily the glutamatergic neurons, was observed following CRD stimulation in the Re of mice with NMD-induced VH, accompanied by an upregulation of 5-HT2B receptor levels in glutamatergic neurons within Re. Then, blocking 5-HT2B receptors in Re led to decreased c-Fos expression and eased visceral pain [80]. The findings suggest that enhancing 5-HT2B receptor levels in glutamatergic neurons of Re might be a key factor contributing to VH.
The insula cortex integrates autonomic activity from the viscera and has been referred to as the "visceral brain." In mice with an NMD-induced IBS model or a TNBS-induced IBD model, there was an increase in c-Fos expression in the insula cortex and a boost in excitatory synaptic transmission. Compared with healthy controls, the insula cortex of IBS patients exhibited persistent activation both at rest and during CRD stimulation [155]. Further studies show that glutamatergic neurons of the insula cortex are significantly increased by CRD in the neonatal colonic inflammation (NCI)-induced IBS model, and neural signals transmitted from PVT glutamatergic neurons to insula cortex glutamatergic neurons promoted colonic pain in NCI mice [81]. These findings suggest that the insula cortex might be involved in the formation of visceral pain.
4.6 Developmental windows program stress-responsive brain nuclei and VH
It has been known that the structure and function of mammal brain undergo tremendous changes with development. Based on the brain's plasticity and adaptive capacity, the timing of initial VH is a key determinant of pathogenesis and long-term outcomes. Comparing with the results of stress in adult, NMD primarily simulates severe psychosocial trauma during early life, and particularly activates stress-related regions like PFC, amygdala, hypothalamus, and hippocampus, even the HPA axis. Phenotypic features generated by NMS models include: permanent alterations in central stress circuits (e.g., amygdala, PFC), hyperactivity of the HPA axis with elevated baseline CRF levels, long-term gut dysfunction (permeability, microbiome alterations), more severe and treatment-resistant VH in adulthood. In contrast, adult-onset models typically induce physiological and psychological responses, and produce transient hypersensitivity that resolves more readily, highlighting distinct underlying neuroplasticity mechanisms. These studies suggest that early intervention in children with functional abdominal pain or a history of adversity may prevent the development of chronic, severe clinical symptoms in adulthood [164].
5. Gut microbes participate in VH
The human gastrointestinal tract is colonized by a diverse array of microorganisms, encompassing bacteria, viruses, fungi, and protozoa, with bacteria being the most predominant component. Gut microbes are involved in numerous physiological processes, including nutrition, metabolism, defense, and immunity. The microbiota serves not merely as a local factor but as the core biological interface connecting the peripheral gut and the central brain. It drives a complete sensitization loop between the periphery and the central nervous system, thereby forming a “brain-gut” sensitization system. Dysbiosis of the intestinal flora is frequently observed in patients with VH, characterized by altered abundance of specific bacterial populations and an imbalance between beneficial and opportunistic pathogenic bacteria [165].
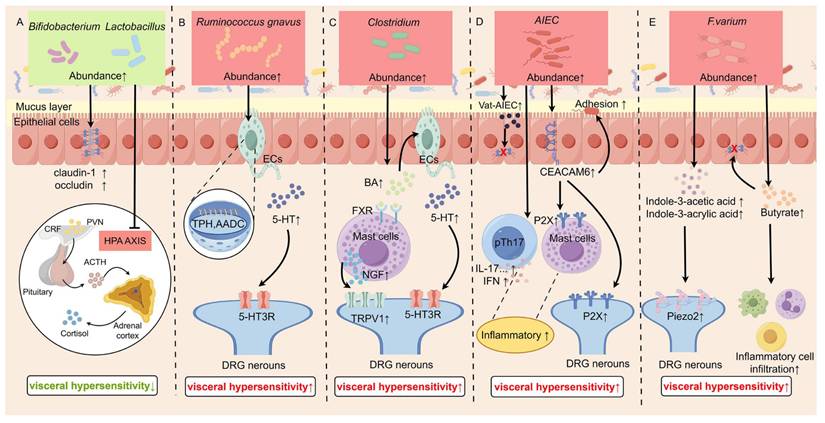
Mechanisms by which specific gut microbiota modulate visceral hypersensitivity. A: Probiotics (Bifidobacterium longum, Lactobacillus) alleviate visceral hypersensitivity by enhancing the intestinal barrier and dampening HPA axis activity. B: Ruminococcus gnavus promotes visceral hypersensitivity by increasing intestinal 5-HT synthesis. C: Clostridium species exacerbate sensitivity via a bile acid-FXR-NGF axis that upregulates TRPV1 expression on sensory neurons. D: AIEC induces hypersensitivity through barrier disruption and activation of mast cells/macrophages via proteases and CEACAM6. E: F. varium elevates visceral sensitivity by upregulating Piezo2, impairing the barrier, and promoting inflammatory cell infiltration. This Figure was created by Figdraw.
5.1 Bifidobacterium and Lactobacillus participate in VH by affecting the intestinal barrier
Bifidobacterium and Lactobacillus are gram-positive bacterial genus, and common probiotics in the human gastrointestinal tract. The strictly anaerobic Bifidobacterium belongs to the phylum Actinobacteria, and primarily resides in the colon. Lactobacillus, a member of the family Lactobacillaceae, is either partially anaerobic or microaerophilic and predominantly colonizes the small intestine and stomach. Studies about VH have noted the involvement of the bacteria in both genus, such as Bifidobacterium longum (B. longum), Bifidobacterium infantis (BCM), and Lactobacillus acidophilus (LCM) [88].
B. longum is one of the major probiotics colonizing the human gastrointestinal tract. In IBS patients, the colonization of B. longum in the intestines was significantly reduced compared to that in normal subjects [166]. Administration of B. longum not only reduced depression scores and alleviated stress in IBS patients, but also reversed the reduction in crypt depth and villus length, decreased intestinal permeability, and restored the proliferation of intestinal mucosal IECs [84, 85]. BCM and LCM were found to normalize the protein expression of claudin-1 and occluding, thereby protecting the intestinal barrier and preventing inflammation [86, 87, 89]. Furthermore, it's shown that the combination of Lactobacillus and Bifidobacterium reduced CRF secretion and HPA axis activity, contributing to recover of VH, anxiety, and depression [90]. Therefore, Bifidobacterium and Lactobacillus exert the function of “probiotics” via various ways, Figure 3A shows their mechanisms for relieving VH.
5.2 Ruminococcus gnavus participates in VH by affecting peripheral 5-HT levels
Ruminococcus gnavus is Gram-positive, anaerobic bacterium that exhibits spherical or spheroidal morphologies, and is involved in digestion and immune regulation [93]. It was demonstrated that Ruminococcus gnavus was enriched in IBD patients and increased with disease activity [167].
Ruminococcus gnavus was found to increased serum 5-HT levels, which was drawn by analyzing the correlation between intestinal flora composition and serum 5-HT levels in IBS-D patients [91]. Increased Ruminococcus gnavus promotes the conversion of phenylalanine and tryptophan into phenethylamine and tryptamine, which in turn activate protein kinase A (PKA) and CaMKII, leading to an increase in TPH1 and aromatic L-amino acid decarboxylase (AADC), and subsequently causing 5-HT biosynthesis. Then, 5-HT contributes to VH development by acting on the afferent nerve endings of nociceptive DRG neurons (Figure 3B) [92].
5.3 Clostridium participates in VH by increasing the bile acids excretion
Clostridium, an anaerobic Gram-positive bacterium of the Firmicutes phylum, participates in various metabolic activities, such as carbohydrate fermentation, protein breakdown, and bile acid (BA) metabolism. About the BA metabolism, experiments in pseudo-germ-free mice colonized with Clostridium revealed upregulated expression of Cyp7a1 and Cyp8b1, key enzymes in BA synthesis, thereby enhancing hepatic BA production. Concurrently, reduced ileal Fgf15 expression attenuated farnesoid X receptor (FXR)-mediated BA reabsorption, while increased taurine-conjugated BAs in the intestinal lumen inhibited FXR activation, further diminishing BA reuptake [168].
Researches indicated a positive relationship between elevated levels of fecal BA and the severity of abdominal pain in IBS patients and visceral pain model rats [94, 169], highlighting the critical role of BAs in the pathogenesis of VH. Regarding the mechanisms underlying BA-induced visceral sensitivity, two principal pathways have been proposed. One explanation is that BA activates the FXR on MCs, leading to increased release of NGF. This, in turn, enhances the expression of TRPV1 in DRG neurons, ultimately promoting VH [96]. An alternative mechanism suggests that BA can trigger the release of 5-HT from EECs through activation of the Takeda G protein-coupled receptor 5 [170].
Therefore, there is a strong association between Clostridium-rich microbiota, elevated BA indices, and VH, and Figure 3C shows the mechanisms in a diagram.
5.4 Adherent-Invasive E. coli participate in VH by increasing intestinal permeability and P2X receptor expression
Adherent-Invasive Escherichia coli (AIEC) is a subtype of diarrhea-inducing E. coli isolated from the enteropathogenic E. coli group. This strain exhibits the ability to adhere to IECs and proliferate on the surface of IECs, leading to microvillous lesions. Researches showed that patients with CD have a higher occurrence of AIEC in the ileal mucosa, along with unusual expression of carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 5 and CEACAM6 in the ileum [98]. Individuals with IBS frequently exhibit increased levels of AIEC.
The potential mechanism of VH induced by AIEC LF82 is complicated (Figure 3D). One reason is that the protein hydrolase Vat-AIEC secreted by AIEC promotes mucin degradation and disrupts the intestinal barrier, thereby increasing intestinal permeability [171]. Another reason is the level of CEACAM6 receptor is elevated in patients with IBS and IBD, facilitating the adhesion of AIEC to IECs. Additionally, AIEC enhances the expression of P2XRs in the colon, which are involved in the formation and transmission of visceral pain [97]. Furthermore, AIEC was found to activate pTh17 cells in vivo or in vitro, leading to the production of pro-inflammatory cytokines that promote the development of VH [100].
5.5 F. varium participates in VH by elevating Piezo2 expression
Chen et al. showed that fecal microbiota transplantation (FMT) from IBS-D patients into germ-free mice induced VH and intestinal motility dysfunction. These mice also exhibited significantly increased Piezo2 expression in the colon and DRG. Through fecal 16S rRNA sequencing, Fusobacterium varium (F. varium) was identified as a key bacterial genus. F. varium is suggested to elevate Piezo2 levels and increase production of the metabolites indole-3-acetic acid and indole-3-acrylic acid, which can bind to Piezo2. Knocking down Piezo2 alleviated the IBS-D-like symptoms induced by the microbiota transplant [111]. Furthermore, F. varium, isolated from the colonic mucosa of patients with UC, successfully induced symptom and pathological changes of VH in mice via the metabolic production butyrate, which might disrupt the intestinal barrier and induce release of cytokines [172]. Therefore, it indicates that the increased abundance of F. varium induces VH by upregulating Piezo2 expression and producing specific ligands (or metabolites) that bind to and activate the target effectors (Figure 3E).
Certainly, there are many other microorganisms involved in the regulation of visceral sensory, but the detailed functioning manners are still unclear.
Figure 3 illustrates the impact of alterations in gut microbiota abundance on visceral sensitivity and their potential underlying mechanisms.
5.6 Microbial metabolites modulate VH
Gut microbiota-derived metabolites are key regulators of visceral sensitivity, achieved through their interactions with the host's nervous, immune, and endocrine systems. A balanced metabolic profile supports homeostasis, whereas dysbiosis-induced shifts can promote VH.
5.6.1 Short-chain fatty acids (SCFAs) in VH
SCFAs—primarily acetate, propionate, and butyrate—are key metabolites produced from dietary fiber fermentation. Although recognized as contributors to conditions like IBS, their precise roles remain incompletely understood, and clinical associations across studies are inconsistent, presenting an ongoing controversy [108].
Clinical and microbial observations highlight specific patterns. IBS-D patients exhibit elevated fecal propionate and reduced acetate levels, changes positively correlated with an increase in Prevotella 9 and Escherichia-Shigella abundance, suggesting a link to hypersensitivity [101]. More broadly, IBS patients often show higher overall SCFA levels and an increased abundance of producer bacteria like Veillonella and Lactobacillus [102]. Notably, the correlation between gut bacterial abundance and SCFA concentration is strongest in IBS-D, with specific taxa such as Bacteroides plebeius, Prevotella sp, CAG:1031, and Bifidobacterium pseudocatenulatum being positively associated [103]. In summary, these findings solidify the connection between a dysbiotic microbiota, altered SCFA profiles, and the pathophysiology of IBS, particularly the diarrhea-predominant subtype.
Mechanistically, SCFAs influence visceral sensitivity through multiple pathways. Propionate, for instance, can directly activate the vagus nerve via the GPR41 receptor or indirectly stimulate it by promoting 5-HT release from enterochromaffin cells. SCFAs also modulate ion channel function and regulate the release of inflammatory mediators [173]. Thus, SCFAs act at the intersection of neural, endocrine, and immune signaling to modulate gut-brain communication.
The role of butyrate is complex and context-dependent. Some studies indicate it promotes hypersensitivity by upregulating neuropeptides (e.g., substance P, CGRP) in dorsal root ganglion neurons [104] or by activating the MAPK-ERK1/2 pathway [105]. Conversely, other research demonstrates that butyrate can alleviate hypersensitivity via anti-inflammatory pathways such as AMPK and PPAR-γ [106]. This “double-edged sword” effect appears to be dose-dependent: low physiological concentrations support barrier integrity and exert anti-inflammatory effects, while high concentrations overstimulate neuronal receptors, increasing excitability. This concept is supported by animal studies where VH coincided with elevated Clostridium sensu stricto 1 and fecal butyrate, both of which decreased after probiotic treatment [107]. Therefore, the net effect of butyrate is not inherently beneficial or detrimental but is determined by its local concentration and the physiological context, underscoring the nuanced role of microbial metabolites in health and disease.
5.6.2 Dopamine and gut microbiota in VH
Dopamine is a key catecholamine neurotransmitter, with roughly half of its total production occurring in the intestine. Specific gut bacteria, including Proteus, Bacillus subtilis, and Corynebacterium, are known to produce dopamine. Animal studies support this gut-brain link; FMT from specific pathogen-free mice into germ-free mice alleviated anxiety-like behavior and significantly altered dopamine metabolism. However, clinical findings in IBS patients are inconsistent. One study reported elevated dopamine levels in IBS-C patients compared to healthy controls, with no significant change in IBS-D patients [109]. Conversely, another study found lower dopamine levels in the serum and urine of IBS patients overall [110]. Despite these discrepancies, the collective evidence indicates an association between altered dopamine signaling and IBS, highlighting this pathway as a potential target for therapeutic strategies.
Table 4 summarizes the common gut microbiota alterations in IBS and IBD.
Common alterations of gut microbes in IBS and IBD
| Type of disease | Gut microbes | Change in abundance | Mechanisms leading to visceral hypersensitivity | Citation |
|---|---|---|---|---|
| IBS | Lactobacillus | lower | Affecting the intestinal barrier | [174] |
| IBS | Lactobacillus | Higher | Increases lactic acid levels, leading to increased synthesis of SCFAs | [102] |
| IBS | Veillonella | Higher | Increase SCFA levels | [102] |
| IBS | Hungatella | Higher | unknown | [174] |
| IBS | Ruminococcus gnavus | Higher | Promotes peripheral 5-HT synthesis | [174] |
| IBS | Clostridium leptum | Higher | Promotes BA synthesis | [95] |
| IBS | Clostridium sensu stricto 1 | Higher | Increase butyrate levels | [107] |
| IBS | F.varium | Higher | Increase the Piezo2 level | [111] |
| IBS | Prevotella 9 | Higher | Increase levels of propionic acid | [101] |
| IBD (UC) | Bifidobacterium | lower | Affecting the intestinal barrier | [175] |
| IBD (UC&CD) | Ruminococcus | Higher | intestinal inflammation | [176] |
| IBD (UC&CD) | Escherichia coli | Higher | Increased intestinal permeability and P2X receptor expression | [177] |
6. Comparative pathophysiology: distinct drivers of VH in IBS and IBD
Although visceral pain is a common hallmark in both IBS and IBD, the underlying pathways leading to hypersensitivity differ fundamentally. Clarifying these distinctions is crucial for both mechanistic research and clinical management.
6.1 Etiology and inflammatory microenvironment
The primary drivers of VH in IBD and IBS originate from distinct pathological processes. In IBD, hypersensitivity is primarily driven by tissue damage and inflammation. Active intestinal inflammation acts as a prerequisite, wherein pro-inflammatory cytokines (e.g., TNF-α, IL-1β) and infiltrating immune cells directly stimulate and sensitize peripheral nociceptive neurons [178]. By contrast, in IBS the process is best characterized as functional and perceptual dysregulation, centered on aberrant visceral perception arising from brain-gut axis dysfunction. This persistent hypersensitive state stems largely from the central nervous system's misinterpretation of normal gut signals. The dysregulation can be initiated by diverse triggers, such as post-infectious immune activation, gut microbiota dysbiosis, or early-life stress. Although overt inflammation is absent, low-grade immune activation and increased intestinal permeability are often observed [179].
6.2 Neuro-immune pathways
The mechanisms and primary drivers of neuro-immune communication differ substantially between IBD and IBS. In IBD, the neuro-immune communication is initiated by the inflammatory response. The abnormal activation of innate and adaptive immunity produces a large amount of inflammatory mediators, which act on enteric nerve endings, driving intense peripheral sensitization [180]. In contrast, IBS involves a bidirectional interaction between neural system and immune reaction. At the gut level, low-grade immune activation promotes neuronal sensitization via mast cell-neuron axis or mediator such as PAR2, PGE2, histamine and so on [135]. Concurrently, central factors including long-term stress, anxiety and depression induce functional and structural plasticity particularly in emotion-sensory integration brain regions such as ACC and PVN. These central changes can, in turn, amplify peripheral immune responses and intestinal permeability. This bidirectional dysregulation creates a persistent vicious cycle that underpins the chronicity of IBS symptoms [181].
6.3 Peripheral vs. central contributions
The relative contributions of peripheral and central sensitization to visceral pain differ markedly between IBD and IBS. In IBD, pain during active inflammation is thought to be primarily driven by peripheral sensitization. This process is characterized by a direct lowering of nociceptor thresholds at the site of inflammation, mediated by local inflammatory mediators [182]. As the disease progresses to a chronic stage, persistent peripheral nociceptive signaling can induce secondary central sensitization [183]. Conversely, in IBS, although peripheral triggers exist, evidence indicates that central sensitization plays a more significant and enduring role [184]. This conclusion is supported by functional neuroimaging showing enhanced reactivity in the limbic system and is further corroborated by strong clinical associations with psychiatric comorbidities and stress reactivity, suggesting dysregulation of the HPA axis and descending pain modulatory systems [185].
6.4 Clinical and therapeutic implications
The pathophysiological divergence of VH in IBD and IBS carry direct clinical relevance for diagnosis and management. In terms of diagnosis, pain in IBD correlates with the degree of endoscopic inflammation and serum/fecal inflammatory markers (such as C-reactive protein and fecal calprotectin) [186]. In contrast, the diagnosis of IBS is based on symptomatic criteria, primarily the Rome criteria, after excluding organic diseases; the biomarkers (e.g., fecal calprotectin) are mainly used to rule out IBD [154]. Regarding treatment, the cornerstone of IBD management is the suppression of immune inflammation (e.g., using biologics), and pain often resolves with the control of inflammation. Treatment for IBS, however, focuses on modulating the brain-gut axis, including the use of visceral analgesics (e.g., opioid receptor modulators), a low-FODMAP diet, neuromodulators (e.g., tricyclic antidepressants), and cognitive-behavioral therapy [2, 154, 187].
7. Conclusion and Future Perspectives
This article reviews recent studies investigating the pathogenesis of VH, a common symptom in IBS and IBD. It examines the following aspects: 1) the peripheral neural circuits underlying visceral pain, with a focus on the role of intestinal components, including IECs, immune cells, and EGCs in driving peripheral sensitization of nociceptors on DRG neurons; 2) the sensitization of brain nuclei and associated neural circuits; 3) the mechanisms by which microbes contribute to both peripheral and central pathways leading to VH. These three aspects dynamically interact through neural, endocrine, and immune pathways. The gut microbiota affects the functional states of both the intestine and the brain. In turn, the brain modulates gastrointestinal secretion, immunity, and motility, thereby influencing the diversity and composition of the gut microbial community. This complex interaction constitutes the microbiota-gut-brain axis—a highly coordinated and sophisticated system.
There has been a notable shift in therapeutic paradigms from merely alleviating symptoms to implementing targeted interventions that address the underlying pathophysiology of these conditions. This transition is clearly reflected in the growing clinical adoption of various central modulators, such as low-dose amitriptyline and gabapentin, which specifically target central sensitization. Additionally, mast cell stabilizers like sodium cromoglycate are being utilized to tackle immune activation, while 5-HT receptor antagonists, such as alosetron, are employed to modulate enteroendocrine function. Furthermore, microbiota-directed strategies, such as low FODMAP diets and the use of Bifidobacterium longum probiotics, are gaining traction as effective treatment options. The mechanism-targeted therapeutic strategies for VH phenotypes are listed in Table 5, including molecular targets, representative drugs, proposed mechanism, target phenotype, biomarker and clinical evidence status. However, despite these promising developments, significant translational barriers remain. A lack of non-invasive and objective diagnostic tools, together with over-reliance on subjective patient feedback or invasive interventions like colorectal distension, poses challenges to standardization in treatment protocols. Moreover, the inherent heterogeneity of these diseases complicates the ability to target specific mechanisms effectively, as patients may present with varying contributions from intestinal immunity, microbiota, central regulation, or neuroendocrine pathways. Therefore, real-world implementation of these advanced therapeutic strategies necessitates overcoming these obstacles to facilitate the selection of personalized therapies that cater to the unique needs of each patient.
Mechanism-targeted therapeutic strategies for visceral hypersensitivity phenotypes
| Molecular Targets | Representative drugs | Proposed Mechanism in VH | Target Phenotype / Biomarker | Clinical Evidence Status |
|---|---|---|---|---|
| Histamine H1 Receptor | Ebastine | Blocks histamine-mediated activation and sensitization of visceral afferent nerves | IBS patients with evidence of mast cell infiltration and mediator release (e.g., elevated mucosal tryptase). | Phase II Trials [59] |
| Ketotifen | Stabilizes mast cells, reduced number and decreased activity of mast cells in the intestinal mucosa, especially in the terminal ileum. | Phase II Trials [188] | ||
| Guanylate Cyclase-C (GC-C) | Linaclotide, Plecanatide | Increases extracellular cGMP, stimulating fluid secretion and reducing nociceptor activity | IBS-C; Patients with constipation-predominant symptoms. | Phase III Trials [189, 190] |
| 5-HT3 Receptor | Alosetron, Ondansetron | Antagonizes serotonin-induced activation of visceral sensory pathways, slows colonic transit, allows increased water reabsorption and stool formation. | IBS-D patients; potentially those with elevated mucosal 5-HT availability. | Approved [191] |
| Tryptase (Mast Cell) | Future target | Inhibits tryptase, a key mast cell mediator that activates PAR2 on sensory neurons, disrupting a primary pathway of neuro-immune activation. | A mast-cell-dominant subtype of IBS or IBD-with-IBS symptoms. | Preclinical evidence [192] |
| Bile Acid Modulators | Colesevelam | Colesevelam binds excess BA in the colon, reducing their secretagogue and pro-motility effects, thereby improving stool consistency and avoiding steatorrhoea in patients with IBS-D. It increases the delivery of total and secondary bile acids to stool, enhances hepatic bile acid synthesis, and upregulates colonic mucosal expression of genes involved in regulating BA, farnesoid X (FXR), and GPBAR1 receptors. | IBS-D patients with bile acid malabsorption (BAM). | Phase II Trials [193] |
| P2X3 Antagonist | Gefapixant,Eliapixant | Antagonizing the binding of ATP to P2X3 receptors in the intestine reduces the transmission of visceral discomfort signals to the central nervous system. | - | Due to taste-related side effects of the drug, its clinical development has been discontinued, necessitating the development of a new generation of medications. [194] |
| Microbial Modulation Therapy | Low FODMAP diet | Reduce short-chain carbohydrates that are difficult for the small intestine to absorb and prone to fermentation, thereby lowering intestinal osmotic pressure and gas production. | For all subtypes of IBS patients | Phase II Trials [195] |
| Microbial Modulation Therapy | Supplement specific probiotics | Competing with and excluding harmful bacteria to reduce their survival space, strengthening the intestinal barrier and regulating immunity. | IBS: For patients seeking complementary therapies to alleviate symptoms IBD(UC): Maintenance during the remission period | [196] |
| Probiotic Lactobacillus BD7807 | Enhanced intestinal barrier function by increasing MUC2 protein, antimicrobial peptide and tight junction gene expression. Increased abundance of beneficial gut bacteria and elevated levels of SCFAs in the gut. | Phase II clinical trial [197] | ||
| Lactobacillus plantarum 299V | Inhibits the adhesion of pathogenic bacteria, reduces colonic gas production, and modulates immune responses. | Phase II clinical trial [198] | ||
| Bifidobacterium bifidum MIMBb75 | Enhance intestinal barrier function, downregulate intestinal TRPV1 expression and activation and modulate immune responses. | Phase III clinical trial [199] |
Further research holds substantial promise in these domains: The construction of a cross-species transcriptomic atlas through single-cell analysis of DRG neurons offers key molecular insights. These include nociceptor signatures, species variations, and gene expression correlates of pain and inflammation, which collectively form a consensus framework for exploring the mechanisms of VH. In-depth characterization of neuronal subtypes thereby supports precise etiological identification [200]; identifying multi-omics-derived biomarkers to stratify patients by pathophysiological subtype, enabling mechanism-based targeting; developing novel therapeutics against validated molecular targets; and optimizing combination strategies (pharmacological, dietary, psychosocial) to leverage synergistic effects. VH management will likely remain chronic, but the definitive endpoint—accurate diagnosis, prevention, and personalized care—remains achievable with continued innovation. Collectively, these advancements will shift the paradigm of VH from a poorly defined symptom cluster to a precisely characterized, mechanism-driven clinical entity.
Abbreviations
5-HT: 5-hydroxytryptamine
AADC: aromatic L-amino acid decarboxylase
ACC: anterior cingulate cortex
AIEC: Adherent-Invasive Escherichia coli
AMPAR: α-amino-5-hydroxy-3-methyl-4-isoxazolepropionic acid receptor
avBNST: anteroventral BNST
B. longum: Bifidobacterium longum
BA: bile acids
BCM: Bifidobacterium infantis
BDNF: brain-derived neurotrophic factor
BNST: bed nucleus of the stria terminalis
CaMKIIα: calcium-calmodulin-dependent protein kinase II α
CBS: cystathionine β-synthetase
CD: Crohn's disease
CEACAM: carcinoembryonic antigen-related cell adhesion molecules
CGRP: calcitonin gene-related peptide
cGMP: cyclic guanosine monophosphate
CL: central lateral nucleus
CP: chronic pancreatitis
CRD: colorectal distension
CRF: corticotropin-releasing factor
CRFR1: corticotropin-releasing factor receptor 1
DRG: dorsal root ganglion
ECs: enterochromaffin cells
EECs: enteroendocrine cells
EGCs: enteric glial cells
FMT: fecal microbiota transplantation
FXR: farnesoid X receptor
Glu: glutamate
GPER: G protein-coupled estrogen receptor
GUCY2C: Guanylyl Cyclase C
H1HR: histamine receptor
HPA: hypothalamic-pituitary-adrenal
IBD: inflammatory bowel disease
IBS: irritable bowel syndrome
IEC: intestinal epithelial cell
IGF-1: insulin-like growth factor-1
IL: intralaminar nucleus
JAM-A: junctional adhesion molecule-A
LCM: Lactobacillus acidophilus
LSV: lateral septal nucleus
LTP: long-term potentiation
MCs: mast cells
MT: medial thalamus
NA: norepinephrine
NCI: neonatal colonic inflammation
NGF: nerve growth factor
NMD: neonatal maternal deprivation
NMDAR: N-methyl-D-aspartate receptors
NTRK1: neurotrophic tyrosine kinase receptor type 1
P2RX3, P2RX4, P2RX6 and P2RX7: Purinergic receptors P2X 3, 4, 6 and 7
PAG: periaqueductal gray
PAR2: protease-activated receptor 2
PBN: parabrachial nucleus
PFC: prefrontal cortex
PGE2: prostaglandin E2
PKA: protein kinase A
PKC: protein kinase C
PROK2: prokineticin 2
PVN: paraventricular nucleus
PVT: paraventricular nucleus of the thalamus
Re: reuniens
RVM: rostral ventromedial medulla
SANRA: scale for the assessment of narrative review articles
SNARE: soluble N-ethylmaleimide-sensitive factor attachment protein receptor
SP: substance P
TLR4: toll-like receptor 4
TNBS: trinitrobenzene sulfonic acid
TRP: transient receptor potential
TRPM: transient receptor potential melastatin
UC: ulcerative colitis
VTA: ventral tegmental area
VH: visceral hypersensitivity
Acknowledgements
Funding
The authors thank the financial support of the Shandong Provincial Natural Science Foundation (ZR2025MS458); National Undergraduate Training Programs for Innovation and Entrepreneurship (202411065320); ShanDong Undergraduate Training Programs for Innovation and Entrepreneurship (S202311065054, S202411065020); Qingdao Medical and Health Research Guidance Project (2022-WJZD108, 2023-WJZD106).
Author contributions
Wenhui Tang: Writing - original draft. Jiarui Wang: Writing - original draft. Wenlei Wang: Writing - original draft. Jiamiao Xue: Writing - original draft. Yuyan Wang: Writing - original draft. Fuhao Jiang: Writing - original draft. Dimkpa christabel kechiyerunda: Writing - review & editing. Shengli Gao: Writing - original draft. Tao Yuan: Funding acquisition, Validation, Supervision. Feifei Guo: Writing - review & editing, Validation, Supervision, Conceptualization. All authors had access to the data.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L. et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308-15
2. Spiller R, Major G. IBS and IBD - separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol. 2016;13:613-21
3. Stanisic V, Quigley EM. The overlap between IBS and IBD: what is it and what does it mean? Expert Rev Gastroenterol Hepatol. 2014;8:139-45
4. Yuan Y, Wang X, Huang S, Wang H, Shen G. Low-level inflammation, immunity, and brain-gut axis in IBS: unraveling the complex relationships. Gut Microbes. 2023;15:2263209
5. Wellens J, Sabino J, Vanuytsel T, Tack J, Vermeire S. Recent advances in clinical practice: mastering the challenge-managing IBS symptoms in IBD. Gut. 2025;74:312-21
6. Ceuleers H, Van Spaendonk H, Hanning N, Heirbaut J, Lambeir AM, Joossens J. et al. Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: The role of proteases. World J Gastroenterol. 2016;22:10275-86
7. Feng B, Guo T. Visceral pain from colon and rectum: the mechanotransduction and biomechanics. J Neural Transm (Vienna). 2020;127:415-29
8. Xie Z, Feng J, Hibberd TJ, Chen BN, Zhao Y, Zang K. et al. Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity. Neuron. 2023;111:526-38.e4
9. Loyd DR, Henry MA, Hargreaves KM. Serotonergic neuromodulation of peripheral nociceptors. Semin Cell Dev Biol. 2013;24:51-7
10. Grundy L, Erickson A, Brierley SM. Visceral Pain. Annu Rev Physiol. 2019;81:261-84
11. Akbar A, Walters JR, Ghosh S. Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther. 2009;30:423-35
12. Cheng F, Du L, Kim JJ, Zhu F, He H, Dai N. NMDA and AMPA receptor physiology and role in visceral hypersensitivity: a review. Eur J Gastroenterol Hepatol. 2022;34:471-7
13. Chang X, Zhang H, Chen S. Neural circuits regulating visceral pain. Commun Biol. 2024;7:457
14. Aggeletopoulou I, Triantos C. Microbiome Shifts and Their Impact on Gut Physiology in Irritable Bowel Syndrome. Int J Mol Sci. 2024 25
15. Butt MF, Reghefaoui MH, Benedict AS, Reghefaoui M, Al-Jabir H, Shaikh A. et al. Irritable Bowel Syndrome in Inflammatory Bowel Disease: An Evidence-Based Practical Review. J Clin Med. 2025 15
16. Jiang Y, Wang X, Zhou Y, Feng Y, Gu H, Liu X. et al. Transient receptor potential channels as emerging therapeutic targets: mechanisms and therapeutic insights in inflammatory bowel disease. Biochem Pharmacol. 2026;245:117693
17. Wils P, Caron B, D'Amico F, Danese S, Peyrin-Biroulet L. Abdominal Pain in Inflammatory Bowel Diseases: A Clinical Challenge. J Clin Med. 2022 11
18. Shute A, Bihan DG, Lewis IA, Nasser Y. Metabolomics: The Key to Unraveling the Role of the Microbiome in Visceral Pain Neurotransmission. Front Neurosci. 2022;16:917197
19. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5
20. Hockley JR, Tranter MM, McGuire C, Boundouki G, Cibert-Goton V, Thaha MA. et al. P2Y Receptors Sensitize Mouse and Human Colonic Nociceptors. J Neurosci. 2016;36:2364-76
21. Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP. et al. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One. 2015;10:e0123810
22. Petrushenko OA, Stratiievska AO, Petrushenko MO, Lukyanetz EA. Resensitization of TRPV1 channels after the P2 receptor activation in sensory neurons of spinal ganglia in rats. Front Cell Neurosci. 2023;17:1192780
23. Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M. et al. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn's Disease: A Randomized Placebo-controlled, Double-blind, Phase IIa Study. Inflamm Bowel Dis. 2015;21:2247-53
24. Lu VB, Rievaj J, O'Flaherty EA, Smith CA, Pais R, Pattison LA. et al. Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat Commun. 2019;10:1029
25. Meng MY, Paine LW, Sagnat D, Bello I, Oldroyd S, Javid F. et al. TRPV4 stimulates colonic afferents through mucosal release of ATP and glutamate. Br J Pharmacol. 2025;182:1324-40
26. Qi Q, Chen F, Zhang W, Wang P, Li Y, Zuo X. Colonic N-methyl-d-aspartate receptor contributes to visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2017;32:828-36
27. Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T. et al. Author Correction: BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2021;11:19139
28. Zhang Y, Qin G, Liu DR, Wang Y, Yao SK. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2019;25:269-81
29. Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008;20:1147-56
30. Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R. et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299-306
31. Salvo-Romero E, Martínez C, Lobo B, Rodiño-Janeiro BK, Pigrau M, Sánchez-Chardi AD. et al. Overexpression of corticotropin-releasing factor in intestinal mucosal eosinophils is associated with clinical severity in Diarrhea-Predominant Irritable Bowel Syndrome. Sci Rep. 2020;10:20706
32. Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R. et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290-8
33. Bayrer JR, Castro J, Venkataraman A, Touhara KK, Rossen ND, Morrie RD. et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature. 2023;616:137-42
34. Fu R, Chen M, Chen Y, Mao G, Liu S. Expression and clinical significance of 5-HT and 5-HT(3)R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exp Ther Med. 2019;17:3077-82
35. Rao SSC, Coss-Adame E, Yan Y, Erdogan A, Valestin J, Ayyala DN. Sensory Adaptation Training or Escitalopram for IBS With Constipation and Rectal Hypersensitivity: A Randomized Controlled Trial. Clin Transl Gastroenterol. 2021;12:e00381
36. Mohammadian G, Dlugosz A, Shetye J, Lindberg G. Quantification of mucosal EEC in jejunum. A comparative study of IBS patients and healthy controls. Scand J Gastroenterol. 2020;55:543-8
37. Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR. et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115:E7632-e41
38. Sugisawa E, Takayama Y, Takemura N, Kondo T, Hatakeyama S, Kumagai Y. et al. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell. 2020;182:609-24.e21
39. Barton JR, Londregan AK, Alexander TD, Entezari AA, Bar-Ad S, Cheng L. et al. Intestinal neuropod cell GUCY2C regulates visceral pain. J Clin Invest. 2023 133
40. Shailubhai K, Palejwala V, Arjunan KP, Saykhedkar S, Nefsky B, Foss JA. et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 2015;6:213-22
41. Wei Z, Tang X, Yi C, Ocansey DKW, Mao F, Mao Z. HucMSC-Ex alleviates DSS-induced colitis in mice by decreasing mast cell activation via the IL-33/ST2 axis. Am J Transl Res. 2024;16:2727-44
42. Nozu T, Arie H, Miyagishi S, Ishioh M, Takakusaki K, Okumura T. Tranilast alleviates visceral hypersensitivity and colonic hyperpermeability by suppressing NLRP3 inflammasome activation in irritable bowel syndrome rat models. Int Immunopharmacol. 2024;133:112099
43. Benson S, Labrenz F, Kotulla S, Brotte L, Rödder P, Tebbe B. et al. Amplified gut feelings under inflammation and depressed mood: A randomized fMRI trial on interoceptive pain in healthy volunteers. Brain Behav Immun. 2023;112:132-7
44. Buckley MM, O'Halloran KD, Rae MG, Dinan TG, O'Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235-50
45. Salameh E, Meleine M, Gourcerol G, do Rego JC, do Rego JL, Legrand R. et al. Chronic colitis-induced visceral pain is associated with increased anxiety during quiescent phase. Am J Physiol Gastrointest Liver Physiol. 2019;316:G692-g700
46. Barker KH, Higham JP, Pattison LA, Taylor TS, Chessell IP, Welsh F. et al. Sensitization of colonic nociceptors by TNFα is dependent on TNFR1 expression and p38 MAPK activity. J Physiol. 2022;600:3819-36
47. Gudes S, Barkai O, Caspi Y, Katz B, Lev S, Binshtok AM. The role of slow and persistent TTX-resistant sodium currents in acute tumor necrosis factor-α-mediated increase in nociceptors excitability. J Neurophysiol. 2015;113:601-19
48. Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L. et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002-11.e4
49. Chen Y, Cheng J, Zhang Y, Chen JDZ, Seralu FM. Electroacupuncture at ST36 Relieves Visceral Hypersensitivity via the NGF/TrkA/TRPV1 Peripheral Afferent Pathway in a Rodent Model of Post-Inflammation Rectal Hypersensitivity. J Inflamm Res. 2021;14:325-39
50. Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M. et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300-12
51. Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS. et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936-48
52. Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S. et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213-21
53. Grabauskas G, Wu X, Gao J, Li JY, Turgeon DK, Owyang C. Prostaglandin E(2), Produced by Mast Cells in Colon Tissues From Patients With Irritable Bowel Syndrome, Contributes to Visceral Hypersensitivity in Mice. Gastroenterology. 2020;158:2195-207.e6
54. Gao J, Xiong T, Grabauskas G, Owyang C. Mucosal Serotonin Reuptake Transporter Expression in Irritable Bowel Syndrome Is Modulated by Gut Microbiota Via Mast Cell-Prostaglandin E2. Gastroenterology. 2022;162:1962-74.e6
55. Awad K, Barmeyer C, Bojarski C, Nagel O, Lee IM, Schweiger MR. et al. Impaired Intestinal Permeability of Tricellular Tight Junctions in Patients with Irritable Bowel Syndrome with Mixed Bowel Habits (IBS-M). Cells. 2023 12
56. Morales-Soto W, Gonzales J, Jackson WF, Gulbransen BD. Enteric glia promote visceral hypersensitivity during inflammation through intercellular signaling with gut nociceptors. Sci Signal. 2023;16:eadg1668
57. Deiteren A, De Man JG, Ruyssers NE, Moreels TG, Pelckmans PA, De Winter BY. Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut. 2014;63:1873-82
58. Balemans D, Aguilera-Lizarraga J, Florens MV, Jain P, Denadai-Souza A, Viola MF. et al. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2019;316:G338-g49
59. Decraecker L, De Looze D, Hirsch DP, De Schepper H, Arts J, Caenepeel P. et al. Treatment of non-constipated irritable bowel syndrome with the histamine 1 receptor antagonist ebastine: a randomised, double-blind, placebo-controlled trial. Gut. 2024;73:459-69
60. Watson RP, Lilley E, Panesar M, Bhalay G, Langridge S, Tian SS. et al. Increased prokineticin 2 expression in gut inflammation: role in visceral pain and intestinal ion transport. Neurogastroenterol Motil. 2012;24:65-75 e12
61. Meira de-Faria F, Casado-Bedmar M, Mårten Lindqvist C, Jones MP, Walter SA, Keita Å V. Altered interaction between enteric glial cells and mast cells in the colon of women with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14130
62. Grubišić V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E. et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020;32:108100
63. Delvalle NM, Dharshika C, Morales-Soto W, Fried DE, Gaudette L, Gulbransen BD. Communication Between Enteric Neurons, Glia, and Nociceptors Underlies the Effects of Tachykinins on Neuroinflammation. Cell Mol Gastroenterol Hepatol. 2018;6:321-44
64. Yang L, Zhang L, Liu Y, Liu J, Li K, Cai J. The different impacts of pain-related negative emotion and trait negative emotion on brain function in patients with inflammatory bowel disease. Sci Rep. 2024;14:23897
65. Cao Z, Wu X, Chen S, Fan J, Zhang R, Owyang C. et al. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008;134:535-43
66. Xu QY, Zhang HL, Du H, Li YC, Ji FH, Li R. et al. Identification of a Glutamatergic Claustrum-Anterior Cingulate Cortex Circuit for Visceral Pain Processing. J Neurosci. 2022;42:8154-68
67. Xiao Y, Xie L, Xu QY, Chen L, Chen H, Xu GY. et al. Transcranial direct current stimulation relieves visceral hypersensitivity via normalizing GluN2B expression and neural activity in anterior cingulate cortex. J Neurophysiol. 2021;125:1787-97
68. Wang J, Zhang X, Cao B, Liu J, Li Y. Facilitation of synaptic transmission in the anterior cingulate cortex in viscerally hypersensitive rats. Cereb Cortex. 2015;25:859-68
69. Li Y. Synaptic Plasticity and Synchrony in the Anterior Cingulate Cortex Circuitry: A Neural Network Approach to Causality of Chronic Visceral Pain and Associated Cognitive Deficits. Adv Neurobiol. 2018;21:219-45
70. Zhang G, Yu L, Chen ZY, Zhu JS, Hua R, Qin X. et al. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav Immun. 2016;55:93-104
71. Huang ST, Chen BB, Song ZJ, Tang HL, Hua R, Zhang YM. Unraveling the role of Epac1-SOCS3 signaling in the development of neonatal-CRD-induced visceral hypersensitivity in rats. CNS Neurosci Ther. 2022;28:1393-408
72. Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE. et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491-500
73. Li YC, Wang Q, Li MG, Hu SF, Xu GY. A paraventricular hypothalamic nucleus input to ventral of lateral septal nucleus controls chronic visceral pain. Pain. 2023;164:625-37
74. Ji NN, Kang J, Hua R, Zhang YM. Involvement of dopamine system in the regulation of the brain corticotropin-releasing hormone in paraventricular nucleus in a rat model of chronic visceral pain. Neurol Res. 2018;40:650-7
75. Liu Y, Li A, Bair-Marshall C, Xu H, Jee HJ, Zhu E. et al. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron. 2023;111:1795-811.e7
76. Huang ST, Song ZJ, Liu Y, Luo WC, Yin Q, Zhang YM. BNST(AV) (GABA)-PVN(CRF) Circuit Regulates Visceral Hypersensitivity Induced by Maternal Separation in Vgat-Cre Mice. Front Pharmacol. 2021;12:615202
77. Song Y, Meng QX, Wu K, Hua R, Song ZJ, Song Y. et al. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology. 2020;117:104690
78. Zheng H, Zhu HY, Zhang XY, Wang M, Xiao Y, Xu GY. et al. Upregulation of cystathionine β-synthetase in the arcuate nucleus produces pain hypersensitivity via PKC upregulation and GluN2B phosphorylation in rats with chronic pancreatitis. Sheng Li Xue Bao. 2016;68:575-84
79. Li X, Xu YC, Tian YQ, Zhang PA, Hu SF, Wang LH. et al. Downregulation of GRK6 in arcuate nucleus promotes chronic visceral hypersensitivity via NF-κB upregulation in adult rats with neonatal maternal deprivation. Mol Pain. 2020;16:1744806920930858
80. Li D, Du H, Qu ST, Wu JL, Li YC, Xu QY. et al. Thalamic Nucleus Reuniens Glutamatergic Neurons Mediate Colorectal Visceral Pain in Mice via 5-HT(2B) Receptors. Neurosci Bull. 2024;40:1421-33
81. Zhang FC, Wei YX, Weng RX, Xu QY, Li R, Yu Y. et al. Paraventricular thalamus-insular cortex circuit mediates colorectal visceral pain induced by neonatal colonic inflammation in mice. CNS Neurosci Ther. 2024;30:e14534
82. Jiao Y, Gao P, Dong L, Ding X, Meng Y, Qian J. et al. Molecular identification of bulbospinal ON neurons by GPER, which drives pain and morphine tolerance. J Clin Invest. 2023 133
83. Li MG, Qu ST, Yu Y, Xu Z, Zhang FC, Li YC. et al. Upregulation of NR2A in Glutamatergic VTA Neurons Contributes to Chronic Visceral Pain in Male Mice. Neurosci Bull. 2025;41:2113-26
84. Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G. et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6:e939
85. Zhou C, Fang X, Xu J, Gao J, Zhang L, Zhao J. et al. Bifidobacterium longum alleviates irritable bowel syndrome-related visceral hypersensitivity and microbiota dysbiosis via Paneth cell regulation. Gut Microbes. 2020;12:1782156
86. Guo S, Gillingham T, Guo Y, Meng D, Zhu W, Walker WA. et al. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus Protect Intestinal Epithelial Barrier Function. J Pediatr Gastroenterol Nutr. 2017;64:404-12
87. Lewis ED, Antony JM, Crowley DC, Piano A, Bhardwaj R, Tompkins TA. et al. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients. 2020 12
88. Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients. 2020 12
89. Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y. et al. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One. 2014;9:e90153
90. Ait-Belgnaoui A, Payard I, Rolland C, Harkat C, Braniste V, Théodorou V. et al. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J Neurogastroenterol Motil. 2018;24:138-46
91. Zhai L, Huang C, Ning Z, Zhang Y, Zhuang M, Yang W. et al. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe. 2023;31:33-44.e5
92. Han L, Zhao L, Zhou Y, Yang C, Xiong T, Lu L. et al. Altered metabolome and microbiome features provide clues in understanding irritable bowel syndrome and depression comorbidity. Isme j. 2022;16:983-96
93. Cristofori F, Calabrese FM, Iacobellis I, Santamaria M, Celano G, Ferrocino I. et al. Calcium butyrate efficacy in pediatric irritable bowel syndrome: Randomized placebo-controlled multiomics-based clinical trial. J Pediatr Gastroenterol Nutr. 2025;81:551-61
94. Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64:84-92
95. Zhao L, Yang W, Chen Y, Huang F, Lu L, Lin C. et al. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J Clin Invest. 2020;130:438-50
96. Li WT, Luo QQ, Wang B, Chen X, Yan XJ, Qiu HY. et al. Bile acids induce visceral hypersensitivity via mucosal mast cell-to-nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. Faseb j. 2019;33:2435-50
97. Lashermes A, Boudieu L, Barbier J, Sion B, Gelot A, Barnich N. et al. Adherent-Invasive E. coli enhances colonic hypersensitivity and P2X receptors expression during post-infectious period. Gut Microbes. 2018;9:26-37
98. Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N. et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-21
99. Carbonnel F, Barnich N, Lepage P, Hébuterne X, Michiels C, Gilletta C. et al. A randomized controlled trial of antibiotics targeting adherent and invasive Escherichia coli versus placebo in Crohn's disease: the TEOREM trial. J Crohns Colitis. 2025 19
100. Paroni M, Leccese G, Ranzani V, Moschetti G, Chiara M, Perillo F. et al. An Intestinal Th17 Subset is Associated with Inflammation in Crohn's Disease and Activated by Adherent-invasive Escherichia coli. J Crohns Colitis. 2023;17:1988-2001
101. Sun QH, Liu ZJ, Zhang L, Wei H, Song LJ, Zhu SW. et al. Sex-based differences in fecal short-chain fatty acid and gut microbiota in irritable bowel syndrome patients. J Dig Dis. 2021;22:246-55
102. Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512-9 e114-5
103. Shin AS, Xing Y, Waseem MR, Siwiec R, James-Stevenson T, Rogers N. et al. Microbiota and short chain fatty acid relationships underlie clinical heterogeneity and identify key microbial targets in irritable bowel syndrome (IBS). Sci Rep. 2025;15:35375
104. Xing Y, Liu Z, Wang LH, Huang F, Wang HJ, Li ZZ. Butyrate sensitizes the release of substance P and calcitonin gene-related peptide evoked by capsaicin from primary cultured rat dorsal root ganglion neurons. Neuro Endocrinol Lett. 2006;27:695-701
105. Xu D, Wu X, Grabauskas G, Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62:1466-74
106. Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome. Sci Rep. 2019;9:19603
107. Li YJ, Li J, Dai C. Butyrate promotes visceral hypersensitivity in IBS model via mast cell-derived DRG neuron lincRNA-01028-PKC-TRPV1 pathway. mBio. 2024;15:e0153324
108. Li E, Wang J, Guo B, Zhang W. Effects of short-chain fatty acid-producing probiotic metabolites on symptom relief and intestinal barrier function in patients with irritable bowel syndrome: a double-blind, randomized controlled trial. Front Cell Infect Microbiol. 2025;15:1616066
109. Chojnacki C, Błońska A, Kaczka A, Chojnacki J, Stępień A, Gąsiorowska A. Evaluation of serotonin and dopamine secretion and metabolism in patients with irritable bowel syndrome. Pol Arch Intern Med. 2018;128:711-3
110. Keshteli AH, Madsen KL, Mandal R, Boeckxstaens GE, Bercik P, De Palma G. et al. Comparison of the metabolomic profiles of irritable bowel syndrome patients with ulcerative colitis patients and healthy controls: new insights into pathophysiology and potential biomarkers. Aliment Pharmacol Ther. 2019;49:723-32
111. Zheng H, Chen Y, Lu S, Liu Z, Ma Y, Zhang C. et al. Mechanosensory Piezo2 regulated by gut microbiota participates in the development of visceral hypersensitivity and intestinal dysmotility. Gut Microbes. 2025;17:2497399
112. Mihara H, Boudaka A, Tominaga M, Sugiyama T. Transient Receptor Potential Vanilloid 4 Regulation of Adenosine Triphosphate Release by the Adenosine Triphosphate Transporter Vesicular Nucleotide Transporter, a Novel Therapeutic Target for Gastrointestinal Baroreception and Chronic Inflammation. Digestion. 2020;101:6-11
113. Keating C, Pelegrin P, Martínez CM, Grundy D. P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol. 2011;187:1467-74
114. Salter MW, Pitcher GM. Dysregulated Src upregulation of NMDA receptor activity: a common link in chronic pain and schizophrenia. Febs j. 2012;279:2-11
115. Rolland-Fourcade C, Denadai-Souza A, Cirillo C, Lopez C, Jaramillo JO, Desormeaux C. et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut. 2017;66:1767-78
116. Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Altered colonic sensory and barrier functions by CRF: roles of TLR4 and IL-1. J Endocrinol. 2018;239:241-52
117. Chatoo M, Li Y, Ma Z, Coote J, Du J, Chen X. Involvement of Corticotropin-Releasing Factor and Receptors in Immune Cells in Irritable Bowel Syndrome. Front Endocrinol (Lausanne). 2018;9:21
118. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31:187-99
119. El-Salhy M, Gilja OH. Abnormalities in ileal stem, neurogenin 3, and enteroendocrine cells in patients with irritable bowel syndrome. BMC Gastroenterol. 2017;17:90
120. Zhu Z, Chen X, Chen S, Hu C, Guo R, Wu Y. et al. Examination of the mechanism of Piezo ion channel in 5-HT synthesis in the enterochromaffin cell and its association with gut motility. Front Endocrinol (Lausanne). 2023;14:1193556
121. Cenac N, Altier C, Motta JP, d'Aldebert E, Galeano S, Zamponi GW. et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481-8
122. Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521-30
123. Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut. 2018;67:1543-52
124. Londregan A, Alexander TD, Covarrubias M, Waldman SA. Fundamental Neurochemistry Review: The role of enteroendocrine cells in visceral pain. J Neurochem. 2023;167:719-32
125. Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X. et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018 361
126. Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096-104
127. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B. et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74
128. Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A. et al. Crohn's disease. Nat Rev Dis Primers. 2020;6:22
129. Camilleri M, Boeckxstaens G. Irritable bowel syndrome: treatment based on pathophysiology and biomarkers. Gut. 2023;72:590-9
130. Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555-65
131. Wallrapp A, Chiu IM. Neuroimmune Interactions in the Intestine. Annu Rev Immunol. 2024;42:489-519
132. Alghamdi KS, Kassar RH, Farrash WF, Obaid AA, Idris S, Siddig A. et al. Key Disease-Related Genes and Immune Cell Infiltration Landscape in Inflammatory Bowel Disease: A Bioinformatics Investigation. Int J Mol Sci. 2024 25
133. Nishida Y, Murase K, Isomoto H, Furusu H, Mizuta Y, Riddell RH. et al. Different distribution of mast cells and macrophages in colonic mucosa of patients with collagenous colitis and inflammatory bowel disease. Hepatogastroenterology. 2002;49:678-82
134. Vanuytsel T, Bercik P, Boeckxstaens G. Understanding neuroimmune interactions in disorders of gut-brain interaction: from functional to immune-mediated disorders. Gut. 2023;72:787-98
135. Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil. 2022;34:e14339
136. Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA. et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016;150:875-87.e9
137. Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK. et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903-15
138. Groschwitz KR, Wu D, Osterfeld H, Ahrens R, Hogan SP. Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2013;304:G479-89
139. Wilcz-Villega EM, McClean S, O'Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108:1140-51
140. Xu XJ, Liu L, Yao SK. Nerve growth factor and diarrhea-predominant irritable bowel syndrome (IBS-D): a potential therapeutic target? J Zhejiang Univ Sci B. 2016;17:1-9
141. Nozu T, Okumura T. Pathophysiological Commonality Between Irritable Bowel Syndrome and Metabolic Syndrome: Role of Corticotropin-releasing Factor-Toll-like Receptor 4-Proinflammatory Cytokine Signaling. J Neurogastroenterol Motil. 2022;28:173-84
142. Atmaramani RR, Black BJ, de la Peña JB, Campbell ZT, Pancrazio JJ. Conserved Expression of Nav1.7 and Nav1.8 Contribute to the Spontaneous and Thermally Evoked Excitability in IL-6 and NGF-Sensitized Adult Dorsal Root Ganglion Neurons In Vitro. Bioengineering (Basel). 2020; 7
143. Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L. et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062-73
144. Ebbinghaus M, Uhlig B, Richter F, von Banchet GS, Gajda M, Bräuer R. et al. The role of interleukin-1β in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum. 2012;64:3897-907
145. Eijkelkamp N, Heijnen CJ, Elsenbruch S, Holtmann G, Schedlowski M, Kavelaars A. G protein-coupled receptor kinase 6 controls post-inflammatory visceral hyperalgesia. Brain Behav Immun. 2009;23:18-26
146. Forster R, Sarginson A, Velichkova A, Hogg C, Dorning A, Horne AW. et al. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. Faseb j. 2019;33:11210-22
147. Silverman HA, Chen A, Kravatz NL, Chavan SS, Chang EH. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front Immunol. 2020;11:590261
148. Aggeletopoulou I, Kalafateli M, Tsounis EP, Triantos C. Exploring the role of IL-1β in inflammatory bowel disease pathogenesis. Front Med (Lausanne). 2024;11:1307394
149. Libero ML, Lucarini E, Recinella L, Ciampi C, Veschi S, Piro A. et al. Anti-inflammatory and anti-hyperalgesic effects induced by an aqueous aged black garlic extract in rodent models of ulcerative colitis and colitis-associated visceral pain. Phytother Res. 2024;38:4177-88
150. Camprubí-Robles M, Planells-Cases R, Ferrer-Montiel A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. Faseb j. 2009;23:3722-33
151. King JW, Bennett ASW, Wood HM, Baker CC, Alsaadi H, Topley M. et al. Expression and function of transient receptor potential melastatin 3 in the spinal afferent innervation of the mouse colon. Am J Physiol Gastrointest Liver Physiol. 2024;326:G176-g86
152. Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571-87
153. Fujikawa Y, Tominaga K. Enhanced neuron-glia network in the submucosa and increased neuron outgrowth into the mucosa are associated with distinctive expressions of neuronal factors in the colon of rat IBS model. Neurogastroenterol Motil. 2023;35:e14595
154. Camilleri M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. Jama. 2021;325:865-77
155. Nisticò V, Rossi RE, D'Arrigo AM, Priori A, Gambini O, Demartini B. Functional Neuroimaging in Irritable Bowel Syndrome: A Systematic Review Highlights Common Brain Alterations With Functional Movement Disorders. J Neurogastroenterol Motil. 2022;28:185-203
156. Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl 1):S50-s63
157. Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y. et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842-8
158. Valentinova K, Acuña MA, Ntamati NR, Nevian NE, Nevian T. An amygdala-to-cingulate cortex circuit for conflicting choices in chronic pain. Cell Rep. 2023;42:113125
159. Lee JA, Chen Q, Zhuo M. Synaptic Plasticity in the Pain-Related Cingulate and Insular Cortex. Biomedicines. 2022 10
160. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383-95
161. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R. et al. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6:603-21
162. Gao T, Dong L, Qian J, Ding X, Zheng Y, Wu M. et al. G-Protein-Coupled Estrogen Receptor (GPER) in the Rostral Ventromedial Medulla Is Essential for Mobilizing Descending Inhibition of Itch. J Neurosci. 2021;41:7727-41
163. Plumb AN, Lesnak JB, Rasmussen L, Sluka KA. Female specific interactions of serotonin and testosterone in the rostral ventromedial medulla after activity-induced muscle pain. J Pain. 2025;26:104723
164. Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501-6
165. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019-40
166. Bahaka D, Neut C, Khattabi A, Monget D, Gavini F. Phenotypic and genomic analyses of human strains belonging or related to Bifidobacterium longum, Bifidobacterium infantis, and Bifidobacterium breve. Int J Syst Bacteriol. 1993;43:565-73
167. Henke MT, Brown EM, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Capsular polysaccharide correlates with immune response to the human gut microbe Ruminococcus gnavus. Proc Natl Acad Sci U S A. 2021 118
168. Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-35
169. Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135:2075-83
170. Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fénelon VS, Zizzari P, Quarta C, Bellocchio L. et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021;33:1483-92.e10
171. Gibold L, Garenaux E, Dalmasso G, Gallucci C, Cia D, Mottet-Auselo B. et al. The Vat-AIEC protease promotes crossing of the intestinal mucus layer by Crohn's disease-associated Escherichia coli. Cell Microbiol. 2016;18:617-31
172. Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52:79-83
173. Takeda M, Sashide Y, Utugi S. Neurophysiological Basis of Short-Chain Fatty Acid Action in Pain Modulation: Therapeutic Implications. Int J Mol Sci. 2025 26
174. Zhu S, Liu S, Li H, Zhang Z, Zhang Q, Chen L. et al. Identification of Gut Microbiota and Metabolites Signature in Patients With Irritable Bowel Syndrome. Front Cell Infect Microbiol. 2019;9:346
175. Vich Vila A, Imhann F, Collij V, Jankipersadsing SA, Gurry T, Mujagic Z. et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci Transl Med. 2018 10
176. Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655-62
177. Pittayanon R, Lau JT, Leontiadis GI, Tse F, Yuan Y, Surette M. et al. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology. 2020;158:930-46.e1
178. Tan WW, Liu ZX, Liu XY, Zhang WB, Zheng L, Zhang YL. et al. Abdominal Pain in Inflammatory Bowel Disease-Epidemiology, Pathophysiology, and Management: A Narrative Review. Pain Ther. 2024;13:1447-69
179. Paine P. Review article: current and future treatment approaches for pain in IBS. Aliment Pharmacol Ther. 2021;54(Suppl 1):S75-s88
180. Brinkman DJ, Ten Hove AS, Vervoordeldonk MJ, Luyer MD, de Jonge WJ. Neuroimmune Interactions in the Gut and Their Significance for Intestinal Immunity. Cells. 2019 8
181. Meerveld BG, Johnson AC. Mechanisms of Stress-induced Visceral Pain. J Neurogastroenterol Motil. 2018;24:7-18
182. Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:778-88
183. Falling C, Stebbings S, Baxter GD, Siegel CA, Gearry RB, Nijs J. et al. Symptoms of central sensitization in patients with inflammatory bowel diseases: a case-control study examining the role of musculoskeletal pain and psychological factors. Scand J Pain. 2021;21:283-95
184. Midenfjord I, Grinsvall C, Koj P, Carnerup I, Törnblom H, Simrén M. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol Motil. 2021;33:e14156
185. Jing C, Liu T, Li Q, Zhang C, Sun B, Yang X. et al. Study of dynamic brain function in irritable bowel syndrome via Hidden Markov Modeling. Front Neurosci. 2024;18:1515540
186. Sakurai T, Saruta M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion. 2023;104:30-41
187. Stallmach A, Atreya R, Grunert PC, Stallhofer J, de Laffolie J, Schmidt C. Treatment Strategies in Inflammatory Bowel Diseases. Dtsch Arztebl Int. 2023;120:768-78
188. Wang J, Wang Y, Zhou H, Gu W, Wang X, Yang J. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur J Gastroenterol Hepatol. 2020;32:706-12
189. Kalola UK, Patel P, Chowdhury YS. Linaclotide. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC. 2025
190. Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD. et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714-24 quiz p.25
191. Butt I, Kasmin F. Alosetron. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC. 2025
192. Annaházi A, Gecse K, Dabek M, Ait-Belgnaoui A, Rosztóczy A, Róka R. et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain. 2009;144:209-17
193. Vijayvargiya P, Camilleri M, Carlson P, Nair A, Nord SL, Ryks M. et al. Effects of Colesevelam on Bowel Symptoms, Biomarkers, and Colonic Mucosal Gene Expression in Patients With Bile Acid Diarrhea in a Randomized Trial. Clin Gastroenterol Hepatol. 2020;18:2962-70.e6
194. Huerta M, Marcos-Frutos D, Nava J, García-Ramos A, Tejada M, Roza C. P2X3 and P2X2/3 receptors inhibition produces a consistent analgesic efficacy: A systematic review and meta-analysis of preclinical studies. Eur J Pharmacol. 2024;984:177052
195. Kasti AN, Katsas K, Pavlidis DE, Stylianakis E, Petsis KI, Lambrinou S. et al. Clinical Trial: A Mediterranean Low-FODMAP Diet Alleviates Symptoms of Non-Constipation IBS-Randomized Controlled Study and Volatomics Analysis. Nutrients. 2025 17
196. Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR. et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-61 quiz 6, 62
197. Li T, Wu J, Xia S, Yang H, Mu H, Zhu Y. et al. Lactiplantibacillus plantarum BD7807 ameliorates high-fat diet-induced lipid metabolic disorders and intestinal dysfunction via SCFAs-GPR43 pathway. Food Res Int. 2025;220:117108
198. Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012-8
199. Andresen V, Gschossmann J, Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol Hepatol. 2020;5:658-66
200. Jung M, Dourado M, Maksymetz J, Jacobson A, Laufer BI, Baca M. et al. Cross-species transcriptomic atlas of dorsal root ganglia reveals species-specific programs for sensory function. Nat Commun. 2023;14:366
Author contact
![]() Corresponding authors: Feifei Guo, E-mail: feifeiguoedu.cn; Tao Yuan, E-mail: bymqycom; Shengli Gao, E-mail: shengli-gaoedu.cn.
Corresponding authors: Feifei Guo, E-mail: feifeiguoedu.cn; Tao Yuan, E-mail: bymqycom; Shengli Gao, E-mail: shengli-gaoedu.cn.

 Global reach, higher impact
Global reach, higher impact