3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2026; 23(1):63-75. doi:10.7150/ijms.123494 This issue Cite
Review
Tumor-Promoting Gut Microbes in Colorectal Cancer: Mechanisms and Translational Perspectives
Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
# Contributed equally.
Received 2025-8-10; Accepted 2025-10-21; Published 2026-1-1
Abstract
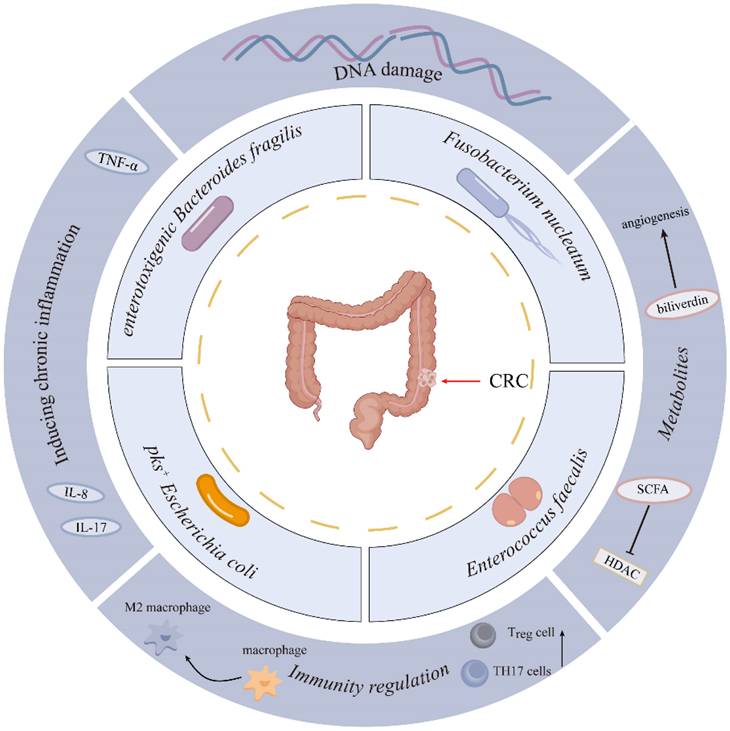
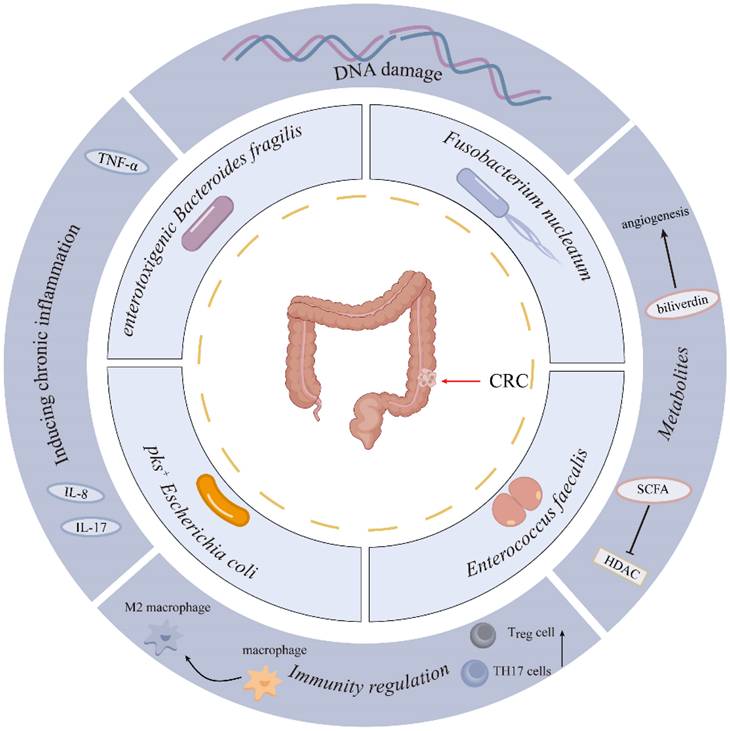
Colorectal cancer (CRC) represents a predominant global malignancy, characterized by increasing incidence and mortality rates. Recent investigations have underscored the gut microbiota as a pivotal element in the pathogenesis and progression of CRC. This review synthesizes current evidence regarding the association between gut microbial dysbiosis and CRC, with a particular emphasis on pathogenic bacteria such as Fusobacterium nucleatum, enterotoxigenic Bacteroides fragilis, pks⁺ Escherichia coli, and Enterococcus faecalis, among others. The mechanisms through which these microbes contribute to tumorigenesis include the induction of DNA damage, the promotion of chronic inflammation, and the induction of immunosuppression, and the production of oncogenic metabolites. Additionally, the review examines the clinical implications of gut microbiota, highlighting their potential as non-invasive biomarkers for early CRC detection and their impact on the efficacy and toxicity of chemotherapy, radiotherapy, and immunotherapy. Furthermore, emerging microbiota-targeted interventions, such as fecal microbiota transplantation, dietary modification, and probiotics, are evaluated for their therapeutic potential. Despite substantial progress, challenges remain in standardizing microbial markers and optimizing individualized microbiota modulation strategies. Future studies integrating multi-omics and machine learning approaches may pave the way for microbiome-based precision medicine in CRC.
Keywords: colorectal cancer, gut microbes, therapeutic strategies, tumor immunology
1. Background
Cancer is one of the major global public health challenges. The incidence of cancer has been rising due to environmental changes, dietary habits, lifestyle factors, and population aging. This increase poses a significant threat to human health and results in substantial economic losses[1]. Colorectal cancer is a common and highly malignant tumor, ranking third globally in incidence and second in mortality among all cancer types. The latest statistics estimate that, in 2022, there were approximately 1.9 million new cases of colorectal cancer and 0.9 million related deaths worldwide[2]. The development of colorectal cancer is influenced by multiple factors, including age, gender, lifestyle, obesity, diet, and environmental conditions[3-7]. Recent research has increasingly highlighted the role of gut microbiota in the onset and progression of colorectal cancer[8].
Gut microbes are microbial communities that reside in the human gut, along with the gut environment, forming the gut microbiome. Literature reports that the human gut hosts trillions of microbial cells from over a thousand species, including bacteria, fungi, archaea, protists, and viruses, with bacteria being the most abundant[9]. The vast and complex gut microbiota contains a collective microbial genome far larger than the human genome, encoding over 3 million genes, often referred to as the “second genome” of the human body[10, 11]. Certain strains of gut microbiota play key roles in digestion, the production of beneficial metabolites, immunity regulation, and defense against pathogenic microorganisms. An imbalance in the gut microbiome can lead to digestive disorders, including ulcerative colitis, Crohn's disease, and irritable bowel syndrome[12-14]. The majority of human gut microbiota resides in the colon, the most common site for digestive tract tumors. Studies have shown that colorectal cancer patients exhibit significant alterations in their gut microbiota[15]. Compared to healthy individuals, these patients have marked differences in species composition and microbial abundance, including an increased abundance of cancer-associated microbes and a decrease in the abundance of protective microbes[16]. These suggest that imbalances in gut microbiota composition may be closely associated with colorectal cancer development. However, it remains unclear whether the alterations in gut microbiota are a cause or a consequence of colorectal carcinogenesis. The interaction between gut microbiota and colorectal cancer has become a prominent research topic in recent years.
Advances in genome sequencing and bioinformatics, particularly the development of 16S rRNA gene sequencing and metagenomic sequencing technologies, have revolutionized scientific research. These innovations have significantly enhanced our ability to study complex gut microbiota, improving the identification of intestinal microorganisms and enabling deeper exploration of the relationship between gut microbiota and tumors. This paper reviews recent research on gut microbiota and colorectal cancer, analyzing future research directions to offer new insights for colorectal cancer treatment.
2. Intestinal flora associated with colorectal cancer development
Increasing studies have shown a close link between gut microbiota and colorectal carcinogenesis. However, the specific microbial species driving colorectal carcinogenesis, and their causal relationships with CRC initiation/progression, remain to be fully delineated. Advances in genomics and bioinformatics have significantly enhanced the study of bacterial flora. Recent studies highlight the roles of Fusobacterium nucleatum (F. nucleatum), enterotoxigenic Bacteroides fragilis (B. fragilis), pks+ Escherichia coli (E. coli), and Enterococcus faecalis (E. faecalis) in colorectal cancer development[17-19] (Figure 1). This section summarizes recent studies on the intestinal flora associated with colorectal cancer (Table 1).
2.1 Fusobacterium nucleatum
Fusobacterium nucleatum is a Gram-negative anaerobic bacterium that primarily colonizes the oral cavity and acts as a conditionally pathogenic organism. Early studies on F. nucleatum—a common member of the oral microbiota—focused on its role in oral diseases, particularly its pro-inflammatory effects and impact on immune cell function, which are closely linked to periodontitis and oral tumor progression. However, with the progress of research, the contribution of F. nucleatum to colorectal cancer has attracted attention. A 2012 study first observed that F. nucleatum signals were enriched in tumor tissues compared to adjacent normal tissues[29], and its abundance increased as colorectal cancer progressed from early to advanced stages[30]. The abundance of F. nucleatum was significantly correlated with colorectal cancer prognosis, with higher levels associated with poorer outcomes[31, 32]. Specifically, F. nucleatum-high cases showed a 58% increased risk of CRC-specific mortality compared with F. nucleatum-negative cases[33]. F. nucleatum accumulates in CRC tissues via binding of its Fap2 protein to Gal-GalNAc residues on tumor cell surfaces[34]. F. nucleatum proteins FadA promote colorectal cancer by binding to E-cadherin, respectively, activating β-catenin signaling and enhancing tumor proliferation[35, 36]. F. nucleatum has also been shown to activate the NF-κB pathway in colorectal cancer cells via the ALPK1 receptor, inducing ICAM1 expression and enhancing cancer cell invasiveness and metastasis[37]. F. nucleatum can promote colorectal cancer liver metastasis via the miR-5692a/IL-8 axis by inducing epithelial-mesenchymal transition[38]. F. nucleatum upregulates integrin α5 (ITGA5) in colorectal cancer by activating E-cadherin/KLF4 signaling in a Ca²⁺-dependent manner. This process enhances tumor growth and metastasis, which can be attenuated by targeting ITGA5 or KLF4[39].
2.2 Enterotoxigenic Bacteroides fragilis
Bacteroides fragilis is a common Gram-negative bacillus, classified into enterotoxigenic and non-toxin-producing strains. Enterotoxigenic B. fragilis secretes a 20-kDa metalloprotease toxin, known as B. fragilis toxin. This strain can disrupt the intestinal barrier, promoting inflammation and disease progression[40, 41]. A strong association between enterotoxigenic B. fragilis and colorectal cancer has also been reported[42-44]. When B. fragilis colonizes the colon, it produces large amounts of toxins that damage the intestinal mucosa and activate STAT3 in the epithelial cells. STAT3 activation is closely linked to inflammation, cell proliferation, angiogenesis, and cancer development and metastasis. Long-term activation of STAT3 by enterotoxigenic B. fragilis maintains a pro-carcinogenic inflammatory environment in colorectal cells, significantly increasing their likelihood of becoming cancerous[45]. Enterotoxigenic B. fragilis secretes toxins that cleave E-cadherin on colonic cells, disrupting epithelial cell connections. This damage promotes bacterial translocation and activates the Wnt/β-catenin signaling pathway, which contributes to colorectal carcinogenesis[46]. Furthermore, enterotoxigenic B. fragilis activates Toll-like receptor 4 (TLR4) in colorectal cancer cells, upregulating JMJD2B expression through the TLR4-NFAT5-dependent signaling pathway. This results in high NONAG expression and the acquisition of tumor stem cell characteristics[47].
2.3 pks+ Escherichia coli
Escherichia coli, a common commensal bacterium in the human intestinal tract, includes certain strains capable of causing disease under specific conditions. Analysis of colorectal cancer tissue samples has revealed a significant enrichment of E. coli within tumor tissues, with its abundance correlating with cancer stage and prognosis. Notably, some E. coli strains harbor polyketide synthase (pks) gene islands, which encode colibactin—a genotoxic small molecule that interacts with DNA, inducing damage through its molecular warhead structure[25, 48]. The prevalence of pks⁺ E. coli is higher in CRC patients compared to healthy individuals, suggesting a potential role in tumorigenesis[49]. Emerging evidence indicates that pks⁺ E. coli promotes CRC development by inducing DNA damage, cell cycle arrest, chromosomal aberrations, and cellular senescence in colorectal cells[50, 51].
Microbial Species Contributing to Colorectal Carcinogenesis.
Pathogenic Gut Bacteria in Colorectal Cancer Development
| Classification | Bacteria | Influence | Mechanisms |
|---|---|---|---|
| Fusobacteriota | Fusobacterium nucleatum | Adheres to the epithelium; modulates immune response; promotes inflammation | Activates β-catenin signaling via FadA binding to E-cadherin, leading to upregulation of Cyclin D1[20]; Suppresses immune cytotoxicity via Fap2-TIGIT interaction; Induces a stem-like phenotype and chemoresistance[21] |
| Bacteroidota | Enterotoxigenic Bacteroides fragilis | Produces B. fragilis toxin (BFT); Disrupts epithelial barrier; Triggers Th17 inflammation | BFT cleaves E-cadherin and activates β-catenin signaling[22]; Induces IL-17-mediated inflammation[23]; Activates STAT3 in epithelial cells, leading to upregulation of ZEB2[24] |
| Proteobacteria | pks+ Escherichia coli | Produces colibactin; Induces DNA double-strand breaks | Colibactin alkylates host DNA[25], causes genomic instability and specific mutation signatures; Promotes carcinogenesis |
| Firmicutes | Enterococcus faecalis | Produces reactive oxygen species (ROS); Induces DNA damage and macrophage activation | Secretes superoxide that causes DNA strand breaks; Promotes tumor-associated inflammation via COX-2/PGE2 |
| Campylobacterota | Campylobacter jejuni | Adheres to mucosa; Secretes cytolethal distending toxin | CDT induces DNA damage and cell cycle arrest; Disrupts epithelial integrity; Promotes IL-8-driven inflammatory response |
| Firmicutes | Peptostreptococcus anaerobius | Alters lipid metabolism; Activates TLR2/4 signaling | Activates PI3K-Akt pathway via α2/β1 integrin, leading to increased cell proliferation[26]; Enhances ROS production and cholesterol biosynthesis[27]; Facilitates tumor-promoting microenvironment[28] |
2.4 Enterococcus faecalis
Enterococcus faecalis is the predominant enterococcal species in the human gut, colonizing from the neonatal period and playing a crucial role in intestinal development. In neonates, E. faecalis exhibits anti-inflammatory properties and supports colonic maturation by inducing intestinal epithelial cells to secrete IL-10, thereby suppressing inflammation and reducing IL-8 production. Due to its immunomodulatory effects, E. faecalis has been utilized in the treatment of chronic sinusitis, bronchitis, and acute diarrhea in children. However, its role in colorectal cancer remains controversial[52]. Some studies suggest that E. faecalis may exert protective effects against CRC, as evidenced by the E. faecalis EC-12 strain's ability to inhibit β-catenin signaling and suppress tumorigenesis in colorectal cells[53]. Conversely, other studies report a higher abundance of E. faecalis in CRC patients compared to healthy individuals, implicating a potential pro-tumorigenic role[54]. E. faecalis promotes cell proliferation and angiogenesis in CRC via producing biliverdin. Biliverdin can significantly increase the expression levels of IL-8 and VEGFA by regulating the PI3K/AKT/mTOR signaling pathway[55]. Moreover, E. faecalis has been shown to generate reactive oxygen species, leading to colonic DNA damage, genetic instability, and CRC progression[56]. Further research is needed to elucidate the precise role of E. faecalis in CRC development.
2.5 Other strains of bacteria
In addition to Fusobacterium nucleatum, enterotoxigenic Bacteroides fragilis, pks+ Escherichia coli, and Enterococcus faecalis, other bacterial strains are also associated with colorectal carcinogenesis, such as Campylobacter jejuni. This bacterium produces cytolethal distending toxin (CDT), which are homologous to DNA enzymes and can induce DNA double-strand breaks, leading to gene mutations and chromosomal aberrations that promote colorectal cancer development[57]. Furthermore, the use of rapamycin inhibits the tumor-promoting activity of Campylobacter jejuni[58]. Peptostreptococcus anaerobius interacts with α2/β1 integrins on colorectal cancer cells via the surface protein PCWBR2. This interaction selectively enriches on the mucosal surface of colorectal cancer, activating the PI3K-Akt signaling pathway and significantly enhancing the proliferative capacity of colorectal cancer cells[26]. The composition of the intestinal flora is diverse, and the interactions between different strains and between strains and the human body are complex. Further studies are needed to elucidate the relationship between common strains and colorectal carcinogenesis.
3. Mechanisms of colorectal cancer occurrence and development caused by intestinal flora
The development of colorectal cancer is a multifactorial process influenced by genetic, environmental, dietary, and lifestyle factors. Its initiation and progression result from the complex interplay of these elements. The critical role of the gut microbiota in CRC pathogenesis is well-established; however, the precise mechanisms through which microbial communities contribute to colorectal carcinogenesis remain incompletely understood and are an active area of investigation. This section provides an overview of the key mechanisms implicated in CRC development based on current research findings (Figure 2).
Key Mechanisms of Gut Microbiota Implicated in CRC Development.
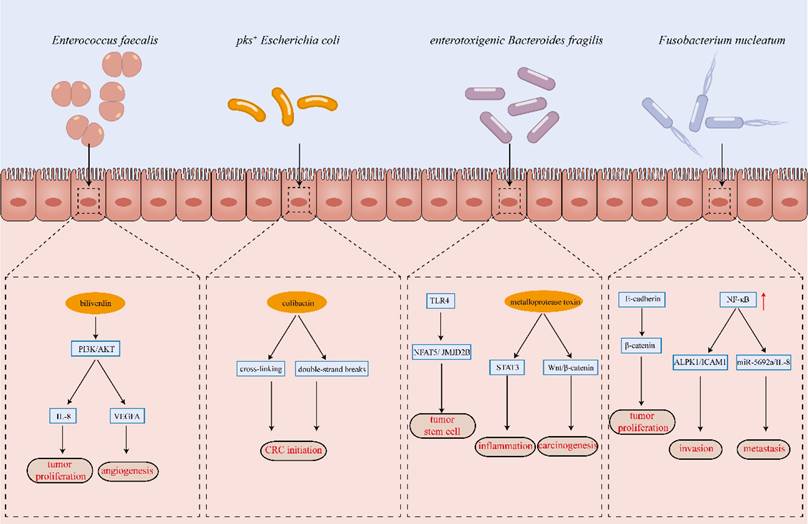
3.1 Direct action leading to DNA damage
Colorectal cancer is driven by the accumulation of mutations in proto-oncogenes and oncogenes, with the "adenoma-carcinoma sequence" model describing chromosomal instability as a key feature of disease progression. In this model, genetic mutations lead to hyperplasia and dysplasia of the colonic epithelium, ultimately resulting in malignant transformation. Emerging evidence suggests that specific bacterial strains within the gut microbiota contribute to CRC development by inducing DNA damage in colorectal epithelial cells[59]. For instance, co-culture of Fusobacterium nucleatum with CRC cells leads to significant DNA damage and upregulation of the DNA repair factor Chk2[60]. Similarly, Escherichia coli harboring pks⁺ alkylate adenine residues in DNA, causing double-strand breaks and cross-linking[61]. Whole-genome sequencing of colonic organoids exposed to colibactin has revealed distinct mutational signatures, further supporting the role of pks⁺ E. coli in colorectal carcinogenesis[62]. Additionally, both pks⁺ E. coli and enterotoxigenic Bacteroides fragilis induce 8-oxoguanine DNA lesions, which are closely linked to CRC initiation[63]. Toxins secreted by enterotoxigenic B. fragilis upregulate spermine oxidase in colonic cells, promoting ROS production and subsequent DNA damage[64]. Likewise, Campylobacter jejuni produces toxins with DNase activity, leading to DNA double-strand breaks, gene mutations, and chromosomal aberrations, thereby contributing to CRC progression[65].
3.2 Inducing chronic inflammation
Chronic inflammation is a well-established risk factor for tumorigenesis, particularly in colorectal cancer. Persistent intestinal inflammation and poorly controlled inflammatory bowel disease significantly elevate the risk of colorectal carcinogenesis. Pro-inflammatory cytokines such as TNF-α, IL-8, and IL-17 play a crucial role in CRC development and progression[66]. Gut microorganisms contribute to intestinal inflammation by interacting with pattern recognition receptors via surface-associated molecular signatures, triggering the secretion of inflammatory mediators through innate immune signaling pathways. Enterotoxigenic Bacteroides fragilis promotes colonic inflammation by activating STAT3 in colonic epithelial cells and inducing IL-17 production. Additionally, its toxin activates the NF-κB pathway via E-cadherin in intestinal epithelial cells, leading to excessive IL-8 secretion and inflammation[67]. Similarly, Peptostreptococcus anaerobius has been shown to disrupt the intestinal barrier, and promote macrophage pyroptosis and IL-1β secretion via the TLR2/4-NF-κB-NLRP3 signaling pathway[68]. While intestinal inflammation is a natural component of tissue repair following microbial dysbiosis, chronic and unresolved inflammation can create a pro-tumorigenic environment, thereby increasing the risk of CRC development.
3.3 Influence of intestinal metabolites
The gut microbiota colonizes the human intestine and generates a diverse array of metabolites that directly interact with the host, playing a critical role in colorectal cancer initiation and progression[69]. Enterococcus faecalis produces biliverdin, which alleviates cell cycle arrest in CRC cells, thereby promoting proliferation and colony formation. Additionally, biliverdin induces angiogenesis and accelerates tumor progression by activating the PI3K/AKT/mTOR signaling pathway, leading to the upregulation of IL-8 and VEGFA in CRC cells[55]. Bile acids, another key class of microbial metabolites, are synthesized as primary bile acids in hepatocytes and subsequently converted into secondary bile acids by the intestinal microbiota, particularly under a high-fat diet[70]. Secondary bile acids, including deoxycholic acid, lithocholic acid, taurolithocholic acid, and their derivatives, exhibit pro-tumorigenic effects by inducing ROS formation, causing DNA damage and gene mutations, disrupting mitosis, and activating the EGFR and NF-κB pathways[71]. Additionally, sulfate-reducing bacteria in the gut metabolize intestinal sulfate into hydrogen sulfide, which induces DNA damage, oxidative stress, inflammation, and colonic mucosal hyperproliferation, thereby promoting CRC development[72]. In contrast, dietary fiber metabolism by gut microbiota produces short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, which exert beneficial effects. Among these, butyrate possesses potent anti-inflammatory and antitumor properties by inhibiting histone deacetylase (HDAC), a key regulator of oncogenic gene expression. A decline in butyrate-producing bacteria, such as Clostridium butyricum and Faecalibacterium prausnitzii, has been associated with an increased risk of CRC[73].
3.4 Regulation of the body's immunity
The immune system plays a crucial role in defending against pathogens and surveilling malignant cells, eliminating cancerous tissues. However, tumor cells can evade immune detection by altering their molecular phenotypes, secreting immunosuppressive cytokines, and recruiting regulatory immune cells. Intestinal microbiota influence cytokine expression and activate specific immune cell populations, thereby modulating both local and systemic immune responses to tumors. The abundance of F. nucleatum is inversely correlated with CD3+ T cell infiltration in colorectal tumors[74]. Through activation of the NF-κB pathway, F. nucleatum upregulates miR-1322 in colorectal cancer cells, leading to increased CCL20 secretion and the induction of M2 macrophage polarization[75]. M2 macrophages, which suppress T cell-mediated antitumor immunity via Arg-1 expression, are closely linked to tumor proliferation, metastasis, and angiogenesis[76]. In colorectal cancer patients with microsatellite instability, F. nucleatum abundance is strongly associated with immune responses. Tumors with high F. nucleatum levels exhibit increased proliferation, invasiveness, and distinct immune microenvironment alterations, including reduced FoxP3+ T-cell infiltration and enhanced M2 macrophage polarization, which collectively impair immunotherapy efficacy[77]. Enterotoxigenic Bacteroides fragilis activates STAT3 signaling in colonic mucosal immune cells, upregulating IL-17A and promoting the infiltration of pro-tumorigenic Th17 cells[23]. Moreover, Streptococcus bovis stimulates colorectal cancer cells to secrete cytokines such as IL-6, Scyb1, Ptgs2, IL-1β, TNF, and CCL2, thereby recruiting CD11b+ TLR-4+ immune cells to the tumor site, establishing an immunosuppressive microenvironment that fosters colorectal cancer progression[78] . Peptostreptococcus anaerobius promotes colorectal cancer progression and resistance to anti-PD-1 therapy by activating integrin α2β1-NF-κB signaling to recruit CXCR2+ myeloid-derived suppressor cells (MDSCs) and directly enhancing MDSC immunosuppressive activity via lytC_22-Slamf4 interactions[28].
4. Clinical application and treatment strategies
4.1 Early screening and diagnosis of colorectal cancer
Colorectal cancer is often asymptomatic in its early stages, leading to late-stage diagnoses and poor prognoses. Early screening is crucial for timely intervention, significantly reducing mortality and improving patient survival rates[79]. Currently, the fecal occult blood test (FOBT) and colonoscopy are the primary screening methods for CRC, and their combined use has been shown to reduce CRC-related mortality by 16%[80]. However, FOBT has limited specificity, necessitating confirmatory colonoscopy for positive cases. Although colonoscopy remains the gold standard due to its high detection accuracy, its invasiveness, high cost, and associated risks, such as perforation and hemorrhage, limit its widespread acceptance and feasibility for large-scale population screening. Therefore, there is an urgent need for a non-invasive, highly sensitive, and specific screening method to enhance early CRC detection and improve clinical outcomes.
The intestinal flora in colorectal cancer patients differs significantly from that in healthy individuals, with a notable increase in strains associated with carcinogenesis and a decrease in protective strains. This suggests that intestinal flora could serve as an early marker for colorectal cancer screening[81]. Recent studies have explored the use of intestinal microorganisms to distinguish colorectal cancer patients from healthy individuals. F. nucleatum is significantly enriched in colorectal cancer tissues, detected in 74% of cases, whereas its abundance in peri-tumoral tissues is much lower—about 1/250 of that in cancerous tissues[82]. In colorectal cancer patients, detection rates of pks+ Escherichia coli and enterotoxigenic Bacteroides fragilis are also significantly higher than in healthy individuals, suggesting that F. nucleatum, pks+ Escherichia coli, and enterotoxigenic Bacteroides fragilis could serve as potential biomarkers for colorectal cancer screening[83]. In colorectal cancer screening, testing for F. nucleatum abundance combined with fecal immunochemical test (FIT) offers similar specificity but a 26% increase in sensitivity compared to FIT alone. A recent meta-analysis found that F. nucleatum had a sensitivity of 71%, specificity of 76%, and an AUC of 0.80 for diagnosing colorectal cancer, indicating its potential as a biomarker for non-invasive screening[84].
Beyond the microbiome itself, microbial metabolites also hold promise as potential biomarkers for colorectal cancer screening. Notably, short-chain fatty acids, which possess anticancer properties, are significantly reduced in the feces of CRC patients compared to healthy individuals[85]. Moreover, alterations in the levels of amino acids such as proline and cysteine have been observed in CRC samples[86]. A fecal metabolomic analysis using gas chromatography-mass spectrometry further revealed decreased levels of fructose, linoleic acid, and niacin, alongside elevated concentrations of proline and uridine in CRC patients[87]. These findings highlight the potential of fecal metabolites as non-invasive biomarkers for CRC detection, offering new avenues for early diagnosis and screening.
In conclusion, the composition of gut microbiota differs significantly between colorectal cancer patients and healthy individuals, with distinct pathogenic bacterial signatures associated with disease progression. Integrating gut microbial screening—a rapid and non-invasive diagnostic approach—with existing colorectal cancer screening methods can improve both sensitivity and specificity, offering a promising strategy for early detection and intervention.
4.2 Influence of intestinal flora on clinical treatment efficacy
Colorectal cancer is currently managed through a multimodal approach that includes surgical intervention, chemotherapy, radiotherapy, immunotherapy, and other treatment modalities[88-91]. Given the significant relationship between gut microbiota and colorectal cancer development, as well as their impact on treatment outcomes, there has been growing interest in researching this connection. Numerous studies have demonstrated that gut microbiota can influence the efficacy of chemotherapy, radiotherapy, and immunotherapy in the treatment of colorectal cancer[92, 93].
4.2.1 Chemotherapy
Intestinal flora can influence the efficacy of chemotherapeutic drugs by regulating their metabolism in colorectal cancer cells. In colorectal cancer patients treated with 5-fluorouracil after radical surgery, the abundance of Fusobacterium nucleatum is correlated with chemoresistance. Further studies demonstrated that Fusobacterium nucleatum upregulated the expression of BIRC3 via the TLR4/NF-κB signaling pathway, which directly inhibited apoptosis by suppressing the cysteine asparaginase cascade, thereby contributing to drug resistance in colorectal cancer cells[94]. Fusobacterium nucleatum can also inhibit the expression of miR-18a and miR-4802 via the TLR4/MYD88 innate immune signaling pathway, leading to increased expression of ULK1 and ATG7, which activate autophagy and contribute to drug resistance to oxaliplatin and 5-fluorouracil in colorectal cancer cells[95]. In addition to influencing drug resistance by affecting drug metabolism and response, some bacterial strains can directly alter chemotherapeutic drugs, rendering them inactive and reducing their antitumor efficacy. Literature reports indicate that in the presence of specific γ-Proteobacteria or Escherichia coli in tumors, gemcitabine is converted into its inactive form by cytidine deaminase, reducing its anticancer efficacy[96].
Intestinal flora not only influences chemotherapy efficacy but also plays a significant role in chemotherapy-related adverse effects. Approximately 30% of chemotherapy patients experience chemotherapy-related pain. Chemotherapy-induced peripheral neuropathy causes neuropathic pain that can persist for months or even years, limiting chemotherapy dosages and hindering optimal therapeutic outcomes. Intestinal flora plays a crucial role in chemotherapy-induced mechanical pain hypersensitivity; oxaliplatin-induced hypersensitivity was reduced in germ-free mice and in mice pretreated with antibiotics. This is mainly due to the interaction between bacterial LPS and TLR4 on macrophages, which stimulates the secretion of inflammatory factors in response to oxaliplatin, leading to mechanical pain hypersensitivity[97, 98]. Irinotecan, a DNA topoisomerase I inhibitor, blocks DNA replication and RNA synthesis. It is a first-line treatment for advanced colorectal cancer but causes serious gastrointestinal side effects, including mucositis and delayed diarrhea. In vivo, irinotecan is converted to SN38, which inhibits DNA topoisomerase I and tumor proliferation. SN38 is then cleared via the gastrointestinal tract by binding to glucuronic acid, forming the inactive SN38-G. However, β-glucuronidase produced by intestinal commensal bacteria removes glucuronic acid from SN38-G, reactivating SN38 and causing intestinal epithelial damage and hemorrhagic diarrhea. In the human gut, β-glucuronidase is primarily expressed by Enterococcus faecalis. Diarrhea caused by irinotecan can be prevented by selectively inhibiting this enzyme, allowing for dose intensification and improving irinotecan effectiveness[99].
4.2.2 Radiotherapy
Radiotherapy can rapidly and persistently alter the composition of the intestinal flora, increasing the abundance of Bacteroides and decreasing Clostridium in the intestines of mice treated with systemic radiotherapy compared to controls[100]. A study found that vancomycin treatment, which alters the Gram-positive flora in the intestinal microbiota, significantly enhanced both the direct and distant antitumor effects of radiotherapy by remodeling the tumor microenvironment and promoting antigen presentation in the draining lymph nodes[101]. Beyond influencing tumor sensitivity to radiotherapy, intestinal flora also impacts radiotherapy toxicity. Germ-free mice receiving lethal whole-body irradiation showed reduced endothelial and lymphocyte apoptosis in the small intestinal villi compared to conventionally reared mice, and were significantly more resistant to radiation enteritis. This resistance was linked to the Fiaf factor, a fibrinogen/angiopoietin-like protein typically secreted by small intestinal villous epithelial cells, but inhibited by intestinal flora. Numerous studies have shown that modulating the specific microbial composition of the gut through flora transplantation can either exacerbate or alleviate intestinal radiation damage[102, 103].
4.2.3 Immunotherapy
The advent of immunotherapy has revolutionized cancer treatment, yielding remarkable therapeutic success across multiple malignancies. Immunotherapy is primarily indicated for patients with microsatellite instability-high (MSI-H) and mismatch repair-deficient (dMMR) CRC. With its growing application, the impact of gut microbiota on immunotherapy efficacy has garnered increasing attention. Clinical studies have demonstrated a strong correlation between gut microbiome composition and the response to immune checkpoint inhibitors (ICIs)[104, 105]. Preclinical models further support these findings, showing that increasing the abundance of Lactobacillus rhamnosus GG in the gut enhances dendritic cell and CD8+ T-cell infiltration into colorectal tumors. This bacterium activates the cGAS/STING signaling pathway in dendritic cells, induces IFN-γ secretion, and potentiates the efficacy of PD-1 blockade therapy[106]. Additionally, Bifidobacterium pseudolongum, Lactobacillus johnsonii, and Olsenella have been shown to improve ICI response in various murine cancer models, with the gut microbiota-derived metabolite inosine playing a pivotal role in immune activation[107]. Fusobacterium nucleatum promotes colorectal cancer by inducing an ALPK1-dependent pro-inflammatory response and upregulating PD-L1 expression[108]. Moreover, a clinical study investigating regorafenib combined with toripalimab in metastatic colorectal cancer revealed that patients with high F. nucleatum abundance had a lower immunotherapy response rate and shorter median progression-free survival compared to those with lower F. nucleatum levels[109].
4.3 Interventions for intestinal flora
Intestinal flora significantly impacts the efficacy of chemotherapy, radiotherapy, and immunotherapy in colorectal cancer. Modulating the composition of the gut flora can influence the effectiveness of CRC therapies, making this approach a potential therapeutic strategy[110].
Fecal microbiota transplantation (FMT) involves transferring the gut microbial community from a donor to a patient[111]. This approach reduces competitive inhibition in the recipient's microbiome while enhancing overall diversity and stability, offering advantages over targeting the abundance of a single microbial species. FMT is widely used to treat Clostridioides difficile (C. difficile) infections and inflammatory bowel disease[112, 113]. While FMT has shown promise in enhancing the efficacy of ICI in melanoma patients[114], conclusive data on FMT's efficacy in colorectal cancer clinical trials is lacking. Some preclinical studies demonstrate that transplanting feces from colorectal cancer patients into mice elevated intestinal inflammatory factors and increased the occurrence of high-grade dysplasia and polyps, indicating that the microbiota of colorectal cancer patients promotes carcinogenesis in animal models[115]. Conversely, transplanting gut microbiota from healthy mice increased resistance to carcinogen-induced colorectal cancer in recipient mice[116]. Although FMT's safety and efficacy in treating C. difficile infections are well-documented, potential risks such as pathogen transmission remain, and its use in immunocompromised colorectal cancer patients is still controversial.
Diet is one of the most significant factors affecting gut microbial composition, with a direct correlation between gut flora and dietary substrates. The gut microbiota rapidly alters in response to dietary modifications[117]. Dietary fiber modifies gut microbiota composition by enhancing the abundance of probiotics like Bifidobacteria and Lactobacillus, while also increasing butyrate levels, which possess anticancer properties, via microbial fermentation[118]. In contrast, red meat and processed meat consumption is linked to a higher risk of colorectal cancer, classified as carcinogenic, and reducing red meat and processed meat intake can significantly lower colorectal cancer incidence[119, 120]. Modulating patients' intestinal flora through dietary interventions seems to be a safe, feasible, and cost-effective approach[121]. However, if a healthy diet cannot be sustained following dietary changes, gut microbiota composition may revert to its original state. Changing long-term dietary habits is challenging and difficult to monitor, highlighting the need for scientifically sound and easily implementable dietary regimens.
Probiotics are microorganisms that confer health benefits, and when administered in adequate amounts, they can restore the balance of intestinal flora and enhance overall health. Preclinical studies indicate that genera such as Bifidobacterium and Lactobacillus spp. can exert tumor-suppressive effects by inhibiting cell proliferation, inducing apoptosis in tumor cells, enhancing antitumor immunity, and producing anticancer compounds[122]. However, questions regarding which probiotic strains to use for treating colorectal cancer, the optimal ratios of each strain, the appropriate dosages, and potential pitfalls remain unresolved.
Prebiotics (e.g., inulin, fructo-oligosaccharides) represent another critical intervention, as they selectively stimulate the growth of beneficial taxa (e.g., Bifidobacterium spp.) to restore gut microbial homeostasis. Recent trials have shown prebiotic supplementation reduces CRC-associated inflammation markers[123], supporting their potential as adjuvant therapies.
5. Summary and Outlook
The critical role of intestinal flora in colorectal carcinogenesis, progression, and treatment has been recognized and validated by previous studies. The identification of colorectal cancer-associated pathogenic bacteria and metabolic markers, along with the elucidation of flora-host interaction mechanisms, contributes to early screening, diagnosis, and treatment of colorectal cancer, offering novel insights into innovative diagnostic and therapeutic approaches.
Differences in gut flora between colorectal cancer patients and healthy individuals highlight their potential as screening markers, and advances in microbiological testing technology have facilitated their clinical application. Although some studies have shown promising results by combining gut flora testing with existing colorectal cancer screening techniques, leading to significant improvements in sensitivity and specificity, challenges related to reproducibility and standardization persist. These challenges arise from the complexity of the intestinal microbiota and the influence of environmental and genetic factors. Designing an optimal combination of biomarkers and validating them across diverse populations to develop a safe and cost-effective clinical screening technique remains a significant research challenge.
Numerous methods can influence the composition of gut flora, many of which have been explored in clinical trials, including probiotics, prebiotics, antibiotics, FMT, dietary modifications, and physical activity. However, the optimal approach to manipulate the microbiota remains to be determined. It remains unclear which method—fecal microbiota transplantation, dietary modification, or probiotic intervention—is superior. The criteria for utilizing FMT and probiotic supplementation in colorectal cancer patients, along with potential contraindications, are still under investigation. Further research is necessary to identify which strains are effective for clinical use, the optimal administration rates, and appropriate dosages. Individual differences in probiotic colonization within the intestinal mucosa exist, highlighting the need for comprehensive analysis of a patient's microbiome, metabolome, and dietary factors. This approach can facilitate the design of individualized treatments through microbial modification.
Although numerous studies have demonstrated an association between intestinal flora and colorectal cancer, research on fungi, viruses, and protozoa—non-bacterial components of the intestinal microbiota—remains limited. This gap is primarily due to the low abundance of these non-bacterial components in the intestinal tract and the challenges associated with their detection and investigation. The gut microecology represents a complex and balanced system in which each component interacts with one another. Focusing exclusively on the bacterial components may lead to a one-sided understanding, leaving many questions regarding the roles of non-bacterial components unresolved.
Given the vast number and complex, variable composition of intestinal flora, elucidating the relationship between gut microbiota and the occurrence and progression of colorectal cancer remains a challenging task. Advances in sequencing genomics, bioinformatics analysis technologies, and cultivation techniques may lead to breakthroughs in future research. Integrative multi-omics approaches (e.g., metagenomics + metabolomics + transcriptomics) can be used to construct robust gut microbial diagnostic models for early CRC, while machine learning algorithms can optimize personalized FMT regimens by predicting patient response based on baseline microbial composition[124, 125].
Abbreviations
B. fragilis: Bacteroides fragilis; C. difficile: Clostridioides difficile; CRC: colorectal cancer; CDT: cytolethal distending toxin; E. faecalis: Enterococcus faecalis; E. coli: Escherichia coli; FIT: fecal immunochemical test; FMT: fecal microbiota transplantation; FOBT: fecal occult blood test; F. nucleatum: Fusobacterium nucleatum; HDAC: histone deacetylase; ICIs: immune checkpoint inhibitors; ITGA5: integrin α5; MyD88: myeloid differentiation primary response 88; MDSCs: myeloid-derived suppressor cells; MSI-H: microsatellite instability-high; dMMR: mismatch repair-deficient; pks: polyketide synthase; ROS: reactive oxygen species; SCFAs: short-chain fatty acids; TLR4: Toll-like receptor 4.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China under Grant No. 82130092 and No. 82373522.
Author contributions
Yulong Yu: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing
Weiheng Zhao: Writing - Original Draft, Writing - Review & Editing, Supervision.
Mu Yang: Investigation, Writing - Review & Editing.
Bili Wu: Investigation, Writing - Review & Editing.
Xianglin Yuan: Writing - Review & Editing, Conceptualization, Supervision, Funding Acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63
3. Global regional, national burden of colorectal cancer its risk factors, 1990-2019. a systematic analysis for the Global Burden of Disease Study 2019. The lancet Gastroenterology & hepatology. 2022;7:627-47
4. Wele P, Wu X, Shi H. Sex-Dependent Differences in Colorectal Cancer: With a Focus on Obesity. Cells. 2022 11
5. Han Y, Pu Y, Liu X, Liu Z, Chen Y, Tang L. et al. YTHDF1 regulates GID8-mediated glutamine metabolism to promote colorectal cancer progression in m6A-dependent manner. Cancer Lett. 2024;601:217186
6. Yuan Q, Liu J, Wang X, Du C, Zhang Y, Lin L. et al. Deciphering the impact of dietary habits and behavioral patterns on colorectal cancer. Int J Surg. 2025;111:2603-12
7. Abdulla A, Sadida HQ, Jerobin J, Elfaki I, Mir R, Mirza S. et al. Unraveling molecular interconnections and identifying potential therapeutic targets of significance in obesity-cancer link. Journal of the National Cancer Center. 2025;5:8-27
8. Eng C, Yoshino T, Ruíz-García E, Mostafa N, Cann CG, O'Brian B. et al. Colorectal cancer. Lancet. 2024;404:294-310
9. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260-70
10. Ding T, Liu C, Li Z. The mycobiome in human cancer: analytical challenges, molecular mechanisms, and therapeutic implications. Mol Cancer. 2025;24:18
11. Lee KA, Luong MK, Shaw H, Nathan P, Bataille V, Spector TD. The gut microbiome: what the oncologist ought to know. Br J Cancer. 2021;125:1197-209
12. Petracco G, Faimann I, Reichmann F. Inflammatory bowel disease and neuropsychiatric disorders: Mechanisms and emerging therapeutics targeting the microbiota-gut-brain axis. Pharmacol Ther. 2025;269:108831
13. Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-60
14. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145:16-27
15. El Tekle G, Andreeva N, Garrett WS. The Role of the Microbiome in the Etiopathogenesis of Colon Cancer. Annu Rev Physiol. 2024;86:453-78
16. Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25:679-89
17. Montalban-Arques A, Scharl M. Intestinal microbiota and colorectal carcinoma: Implications for pathogenesis, diagnosis, and therapy. EBioMedicine. 2019;48:648-55
18. Galasso L, Termite F, Mignini I, Esposto G, Borriello R, Vitale F. et al. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers (Basel). 2025 17
19. Joo JE, Chu YL, Georgeson P, Walker R, Mahmood K, Clendenning M. et al. Intratumoral presence of the genotoxic gut bacteria pks(+) E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer. Br J Cancer. 2024;130:728-40
20. Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D. et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019 20
21. Wang Q, Hu T, Zhang Q, Zhang Y, Dong X, Jin Y. et al. Fusobacterium nucleatum promotes colorectal cancer through neogenesis of tumor stem cells. J Clin Invest. 2025 135
22. Li S, Liu J, Zheng X, Ren L, Yang Y, Li W. et al. Tumorigenic bacteria in colorectal cancer: mechanisms and treatments. Cancer biology & medicine. 2021;19:147-62
23. Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-22
24. Yang J, Wang X, Hu T, Huang H, Chen G, Jin B. et al. Entero-toxigenic Bacteroides fragilis contributes to intestinal barrier injury and colorectal cancer progression by mediating the BFT/STAT3/ZEB2 pathway. Cell cycle (Georgetown, Tex). 2024;23:70-82
25. Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A. et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019 363
26. Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nature microbiology. 2019;4:2319-30
27. Tsoi H, Chu ESH, Zhang X, Sheng J, Nakatsu G, Ng SC. et al. Peptostreptococcus anaerobius Induces Intracellular Cholesterol Biosynthesis in Colon Cells to Induce Proliferation and Causes Dysplasia in Mice. Gastroenterology. 2017;152:1419-33.e5
28. Liu Y, Wong CC, Ding Y, Gao M, Wen J, Lau HC. et al. Peptostreptococcus anaerobius mediates anti-PD1 therapy resistance and exacerbates colorectal cancer via myeloid-derived suppressor cells in mice. Nature microbiology. 2024;9:1467-82
29. Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292-8
30. Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nature medicine. 2019;25:968-76
31. Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida T, Fujimoto M, Watanabe M. et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2018;53:517-24
32. Yamane T, Kanamori Y, Sawayama H, Yano H, Nita A, Ohta Y. et al. Iron accelerates Fusobacterium nucleatum-induced CCL8 expression in macrophages and is associated with colorectal cancer progression. JCI insight. 2022 7
33. Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973-80
34. Abed J, Emgård JE, Zamir G, Faroja M, Almogy G, Grenov A. et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215-25
35. Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D. et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 2019 20
36. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195-206
37. Zhang Y, Zhang L, Zheng S, Li M, Xu C, Jia D. et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-kappaB/ICAM1 axis. Gut Microbes. 2022;14:2038852
38. Yu Y, Yin H, Wu B, Zhao W, Wang Y, Aili A. et al. Fusobacterium nucleatum promotes colorectal cancer liver metastasis via miR-5692a/IL-8 axis by inducing epithelial-mesenchymal transition. J Biomed Sci. 2025;32:5
39. Yan X, Qu X, Wang J, Lu L, Wu W, Mao J. et al. Fusobacterium nucleatum Promotes the Growth and Metastasis of Colorectal Cancer by Activating E-Cadherin/Krüppel-Like Factor 4/Integrin α5 Signaling in a Calcium-Dependent Manner. MedComm. 2025;6:e70137
40. Yan Y, Tian L, Zhao Y, Xuan B, Xu X, Ding J. et al. Bacteroides fragilis Toxin Suppresses METTL3-Mediated m6A Modification in Macrophage to Promote Inflammatory Bowel Disease. J Crohns Colitis. 2025 19
41. Cao Y, Wang Z, Yan Y, Ji L, He J, Xuan B. et al. Enterotoxigenic Bacteroidesfragilis Promotes Intestinal Inflammation and Malignancy by Inhibiting Exosome-Packaged miR-149-3p. Gastroenterology. 2021;161:1552-66.e12
42. Xia S, Ma L, Li H, Li Y, Yu L. Prevalence of enterotoxigenic Bacteroides fragilis in patients with colorectal cancer: a systematic review and meta-analysis. Frontiers in cellular and infection microbiology. 2025;15:1525609
43. Butt J, Jenab M, Werner J, Fedirko V, Weiderpass E, Dahm CC. et al. Association of Pre-diagnostic Antibody Responses to Escherichia coli and Bacteroides fragilis Toxin Proteins with Colorectal Cancer in a European Cohort. Gut Microbes. 2021;13:1-14
44. Wu Z, Yu M, Zeng Y, Huang Y, Zheng W. LRP11-AS1 mediates enterotoxigenic Bacteroides fragilis-related carcinogenesis in colorectal Cancer via the miR-149-3p/CDK4 pathway. Cancer Gene Ther. 2025;32:184-97
45. Cheng WT, Kantilal HK, Davamani F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays J Med Sci. 2020;27:9-21
46. Purcell RV, Permain J, Keenan JI. Enterotoxigenic Bacteroides fragilis activates IL-8 expression through Stat3 in colorectal cancer cells. Gut Pathog. 2022;14:16
47. Liu QQ, Li CM, Fu LN, Wang HL, Tan J, Wang YQ. et al. Enterotoxigenic Bacteroides fragilis induces the stemness in colorectal cancer via upregulating histone demethylase JMJD2B. Gut Microbes. 2020;12:1788900
48. Wernke KM, Xue M, Tirla A, Kim CS, Crawford JM, Herzon SB. Structure and bioactivity of colibactin. Bioorg Med Chem Lett. 2020;30:127280
49. Miyasaka T, Yamada T, Uehara K, Sonoda H, Matsuda A, Shinji S. et al. Pks-positive Escherichia coli in tumor tissue and surrounding normal mucosal tissue of colorectal cancer patients. Cancer Sci. 2024;115:1184-95
50. Dubinsky V, Dotan I, Gophna U. Carriage of Colibactin-producing Bacteria and Colorectal Cancer Risk. Trends Microbiol. 2020;28:874-6
51. de Souza JB, de Almeida Campos LA, Palácio SB, Brelaz-de-Castro MCA, Cavalcanti IMF. Prevalence and implications of pKs-positive Escherichia coli in colorectal cancer. Life Sci. 2024;341:122462
52. De Almeida CV, Lulli M, di Pilato V, Schiavone N, Russo E, Nannini G. et al. Differential Responses of Colorectal Cancer Cell Lines to Enterococcus faecalis' Strains Isolated from Healthy Donors and Colorectal Cancer Patients. Journal of clinical medicine. 2019 8
53. Miyamoto S, Komiya M, Fujii G, Hamoya T, Nakanishi R, Fujimoto K. et al. Preventive Effects of Heat-Killed Enterococcus faecalis Strain EC-12 on Mouse Intestinal Tumor Development. Int J Mol Sci. 2017 18
54. Geravand M, Fallah P, Yaghoobi MH, Soleimanifar F, Farid M, Zinatizadeh N. et al. INVESTIGATION OF ENTEROCOCCUS FAECALIS POPULATION IN PATIENTS WITH POLYP AND COLORECTAL CANCER IN COMPARISON OF HEALTHY INDIVIDUALS. Arq Gastroenterol. 2019;56:141-5
55. Zhang L, Liu J, Deng M, Chen X, Jiang L, Zhang J. et al. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: biliverdin. J Transl Med. 2023;21:72
56. de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap Adv Gastroenterol. 2018 11
57. He Z, Yu J, Gong J, Wu J, Zong X, Luo Z. et al. Campylobacter jejuni-derived cytolethal distending toxin promotes colorectal cancer metastasis. Cell Host Microbe. 2024;32:2080-91.e6
58. He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S. et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68:289-300
59. Garvey M. Intestinal Dysbiosis: Microbial Imbalance Impacts on Colorectal Cancer Initiation, Progression and Disease Mitigation. Biomedicines. 2024 12
60. Guo P, Tian Z, Kong X, Yang L, Shan X, Dong B. et al. FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J Exp Clin Cancer Res. 2020;39:202
61. Xue M, Kim CS, Healy AR, Wernke KM, Wang Z, Frischling MC. et al. Structure elucidation of colibactin and its DNA cross-links. Science. 2019 365
62. Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J. et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580:269-73
63. Irrazabal T, Thakur BK, Kang M, Malaise Y, Streutker C, Wong EOY. et al. Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer. Nat Commun. 2020;11:1802
64. Lopez LR, Bleich RM, Arthur JC. Microbiota Effects on Carcinogenesis: Initiation, Promotion, and Progression. Annu Rev Med. 2021;72:243-61
65. Lee SJ, Oh HB, Yoon SI. Crystal Structure of the Recombination Mediator Protein RecO from Campylobacter jejuni and Its Interaction with DNA and a Zinc Ion. Int J Mol Sci. 2022 23
66. Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653-67
67. Lee CG, Hwang S, Gwon SY, Park C, Jo M, Hong JE. et al. Bacteroides fragilis Toxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 by the E-Cadherin/beta-Catenin/NF-kappaB Dependent Pathway. Biomedicines. 2022 10
68. Shen XH, Guan J, Lu DP, Hong SC, Yu L, Chen X. Peptostreptococcus Anaerobius enhances dextran sulfate sodium-induced colitis by promoting nf-κB-NLRP3-Dependent macrophage pyroptosis. Virulence. 2024;15:2435391
69. Qu R, Zhang Y, Ma Y, Zhou X, Sun L, Jiang C. et al. Role of the Gut Microbiota and Its Metabolites in Tumorigenesis or Development of Colorectal Cancer. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2023;10:e2205563
70. Zhuang T, Wang X, Wang Z, Gu L, Yue D, Wang Z. et al. Biological functions and pharmacological behaviors of bile acids in metabolic diseases. J Adv Res. 2024
71. Yang S, Wang Y, Sheng L, Cui W, Ma C. The effect of fecal bile acids on the incidence and risk-stratification of colorectal cancer: an updated systematic review and meta-analysis. Sci Rep. 2025;15:740
72. Jiang ZL, Liu Y, Zhang CH, Chu T, Yang YL, Zhu YW. et al. Emerging roles of hydrogen sulfide in colorectal cancer. Chem Biol Interact. 2024;403:111226
73. Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M. et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13:1-28
74. Borowsky J, Haruki K, Lau MC, Dias Costa A, Väyrynen JP, Ugai T. et al. Association of Fusobacterium nucleatum with Specific T-cell Subsets in the Colorectal Carcinoma Microenvironment. Clinical cancer research: an official journal of the American Association for Cancer Research. 2021;27:2816-26
75. Xu C, Fan L, Lin Y, Shen W, Qi Y, Zhang Y. et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut microbes. 2021;13:1980347
76. Cheruku S, Rao V, Pandey R, Rao Chamallamudi M, Velayutham R, Kumar N. Tumor-associated macrophages employ immunoediting mechanisms in colorectal tumor progression: Current research in Macrophage repolarization immunotherapy. Int Immunopharmacol. 2023;116:109569
77. Lee JA, Yoo SY, Oh HJ, Jeong S, Cho NY, Kang GH. et al. Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status. Cancer Immunol Immunother. 2021;70:47-59
78. Deng Q, Wang C, Yu K, Wang Y, Yang Q, Zhang J. et al. Streptococcus bovis Contributes to the Development of Colorectal Cancer via Recruiting CD11b(+)TLR-4(+) Cells. Med Sci Monit. 2020;26:e921886
79. Kwan ASH, Uwishema O, Mshaymesh S, Choudhary K, Salem FK, Sengar AS. et al. Advances in the diagnosis of colorectal cancer: the application of molecular biomarkers and imaging techniques: a literature review. Annals of medicine and surgery (2012). 2025;87:192-203
80. Wu D, Song QY, Dai BS, Li J, Wang XX, Liu JY. et al. Colorectal cancer early screening: Dilemmas and solutions. World J Gastroenterol. 2025;31:98760
81. Zalila-Kolsi I, Dhieb D, Osman HA, Mekideche H. The Gut Microbiota and Colorectal Cancer: Understanding the Link and Exploring Therapeutic Interventions. Biology. 2025 14
82. Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J. et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311-8
83. Khodaverdi N, Zeighami H, Jalilvand A, Haghi F, Hesami N. High frequency of enterotoxigenic Bacteroides fragilis and Enterococcus faecalis in the paraffin-embedded tissues of Iranian colorectal cancer patients. BMC Cancer. 2021;21:1353
84. Zhang X, Zhu X, Cao Y, Fang JY, Hong J, Chen H. Fecal Fusobacterium nucleatum for the diagnosis of colorectal tumor: A systematic review and meta-analysis. Cancer medicine. 2019;8:480-91
85. Zhang H, Tian Y, Xu C, Chen M, Xiang Z, Gu L. et al. Crosstalk between gut microbiotas and fatty acid metabolism in colorectal cancer. Cell Death Discov. 2025;11:78
86. Lin Z, Yang S, Qiu Q, Cui G, Zhang Y, Yao M. et al. Hypoxia-induced cysteine metabolism reprogramming is crucial for the tumorigenesis of colorectal cancer. Redox biology. 2024;75:103286
87. Phua LC, Chue XP, Koh PK, Cheah PY, Ho HK, Chan EC. Non-invasive fecal metabonomic detection of colorectal cancer. Cancer Biol Ther. 2014;15:389-97
88. Liu C, Lan Y, Wang H, Zhang Y. Current status of multiple markers in precision immunotherapy for colorectal cancer. Cancer biology & medicine. 2025
89. Tang Z, Zhou G, Xu Y, Zhang Y. Survival analysis and prediction of early-onset colorectal cancer patients post-chemotherapy: an analysis based on the SEER database. Int J Colorectal Dis. 2025;40:74
90. Sekido Y, Ogino T, Takeda M, Hata T, Hamabe A, Miyoshi N. et al. Surgery for Colorectal Cancer Associated with Crohn's Disease-Toward a Medical Treatment Strategy Based on the Differences Between Japan and Western Countries. Cancers (Basel). 2025 17
91. Tiwari A, Lee SL, MacCabe T, Woyton M, West CT, Micklethwaite R. et al. Intraoperative electron radiotherapy (IOERT) in colorectal cancer: Updated systematic review of techniques, oncological outcomes and complications. Eur J Surg Oncol. 2025;51:109724
92. Liu J, Liu C, Yue J. Radiotherapy and the gut microbiome: facts and fiction. Radiat Oncol. 2021;16:9
93. Gazzaniga FS, Kasper DL. The gut microbiome and cancer response to immune checkpoint inhibitors. J Clin Invest. 2025 135
94. Zhang S, Yang Y, Weng W, Guo B, Cai G, Ma Y. et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. 2019;38:14
95. Yu T, Guo F, Yu Y, Sun T, Ma D, Han J. et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548-63 e16
96. Iosifidou N, Anagnostopoulou E, Botou M, Kalfa E, Tatsaki E, Frillingos S. Elucidation of the Gemcitabine Transporters of Escherichia coli K-12 and Gamma-Proteobacteria Linked to Gemcitabine-Related Chemoresistance. Int J Mol Sci. 2024 25
97. Corriero A, Giglio M, Inchingolo F, Moschetta A, Varrassi G, Puntillo F. Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review. Pain and therapy. 2024;13:33-51
98. Shen S, Lim G, You Z, Ding W, Huang P, Ran C. et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 2017;20:1213-6
99. Gao R, Yue B, Lv C, Geng X, Yu Z, Wang H. et al. Targeted inhibition of Gus-expressing Enterococcus faecalis to promote intestinal stem cell and epithelial renovation contributes to the relief of irinotecan chemotoxicity by dehydrodiisoeugenol. Acta pharmaceutica Sinica B. 2024;14:5286-304
100. Lu Q, Liang Y, Tian S, Jin J, Zhao Y, Fan H. Radiation-Induced Intestinal Injury: Injury Mechanism and Potential Treatment Strategies. Toxics. 2023 11
101. Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gómez L, Verginadis I, Bittinger K. et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest. 2020;130:466-79
102. Lu L, Li F, Gao Y, Kang S, Li J, Guo J. Microbiome in radiotherapy: an emerging approach to enhance treatment efficacy and reduce tissue injury. Mol Med. 2024;30:105
103. Tu Y, Luo L, Zhou Q, Ni J, Tang Q. Fecal Microbiota Transplantation Repairs Radiation Enteritis Through Modulating the Gut Microbiota-Mediated Tryptophan Metabolism. Radiat Res. 2024;201:572-85
104. Raziq MF, Khan N, Manzoor H, Tariq HMA, Rafiq M, Rasool S. et al. Prioritizing gut microbial SNPs linked to immunotherapy outcomes in NSCLC patients by integrative bioinformatics analysis. J Transl Med. 2025;23:343
105. Ciernikova S, Sevcikova A, Novisedlakova M, Mego M. Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors. Cancers (Basel). 2024 16
106. Si W, Liang H, Bugno J, Xu Q, Ding X, Yang K. et al. Lactobacillus rhamnosus GG induces cGAS/STING- dependent type I interferon and improves response to immune checkpoint blockade. Gut. 2022;71:521-33
107. Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481-9
108. Duizer C, Salomons M, van Gogh M, Gräve S, Schaafsma FA, Stok MJ. et al. Fusobacterium nucleatum upregulates the immune inhibitory receptor PD-L1 in colorectal cancer cells via the activation of ALPK1. Gut Microbes. 2025;17:2458203
109. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y. et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2:100383
110. Hu Y, Zhou P, Deng K, Zhou Y, Hu K. Targeting the gut microbiota: a new strategy for colorectal cancer treatment. Journal of translational medicine. 2024;22:915
111. Moutsoglou D, Ramakrishnan P, Vaughn BP. Microbiota transplant therapy in inflammatory bowel disease: advances and mechanistic insights. Gut Microbes. 2025;17:2477255
112. Ianiro G, Bibbò S, Porcari S, Settanni CR, Giambò F, Curta AR. et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center. Gut microbes. 2021;13:1994834
113. Allegretti JR, Axelrad J, Dalal RS, Kelly CR, Grinspan A, Fischer M. Outcomes After Fecal Microbiota Transplantation in Combination With Bezlotoxumab for Inflammatory Bowel Disease and Recurrent Clostridioides difficile Infection. The American journal of gastroenterology. 2024;119:1433-6
114. Routy B, Lenehan JG, Miller WH Jr, Jamal R, Messaoudene M, Daisley BA. et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nature medicine. 2023;29:2121-32
115. Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W. et al. Gavage of Fecal Samples From Patients With Colorectal Cancer Promotes Intestinal Carcinogenesis in Germ-Free and Conventional Mice. Gastroenterology. 2017;153:1621-33.e6
116. Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K. et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell. 2017;171:1015-28.e13
117. Feng Y, Jin Q, Liu X, Lin T, Johnson A, Huang H. Advances in understanding dietary fiber: Classification, structural characterization, modification, and gut microbiome interactions. Comprehensive reviews in food science and food safety. 2025;24:e70092
118. Wang W, Fan Z, Yan Q, Pan T, Luo J, Wei Y. et al. Gut microbiota determines the fate of dietary fiber-targeted interventions in host health. Gut Microbes. 2024;16:2416915
119. Liao Z, Wu W, Xia S, Yu L, Xu Z, Li Y. Associations between the consumption of red meat and processed meat and the incidence of colorectal cancer in Asia: a meta-analysis. Frontiers in medicine. 2025;12:1555717
120. Niedermaier T, Gredner T, Hoffmeister M, Mons U, Brenner H. Impact of Reducing Intake of Red and Processed Meat on Colorectal Cancer Incidence in Germany 2020 to 2050-A Simulation Study. Nutrients. 2023 15
121. Koletic C, Mrad A, Martin A, Devkota S. Diet's impact on gut microbial assemblage in health and disease. J Clin Invest. 2025 135
122. McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20:e77-e91
123. Meng S, Liu C, Zhang K, Li J, Wang D, Zhao J. et al. A Prebiotic-Supplemented Formula Improves Gut Microbiota and Intestinal Inflammatory Microenvironment in Patients with Colorectal Adenoma: A Double-Blind, Placebo-Controlled Trial. The Journal of nutrition. 2025
124. Zou S, Yang C, Zhang J, Zhong D, Meng M, Zhang L. et al. Multi-omic profiling reveals associations between the gut microbiome, host genome and transcriptome in patients with colorectal cancer. Journal of translational medicine. 2024;22:175
125. Jayakrishnan TT, Sangwan N, Barot SV, Farha N, Mariam A, Xiang S. et al. Multi-omics machine learning to study host-microbiome interactions in early-onset colorectal cancer. NPJ precision oncology. 2024;8:146
Author contact
![]() Corresponding author: Xianglin Yuan, Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China; email: yuanxianglinedu.cn; telephone number: 86-13667241722.
Corresponding author: Xianglin Yuan, Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China; email: yuanxianglinedu.cn; telephone number: 86-13667241722.

 Global reach, higher impact
Global reach, higher impact