3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(16):4227-4235. doi:10.7150/ijms.121138 This issue Cite
Research Paper
Association Between Cardiopulmonary Fitness and Degenerative Lumbar Spine Disease in Patients with Heart Failure: a Retrospective Study Using CPET
1. Department of Orthopedics, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan (R.O.C.).
2. Department of Rehabilitation Medicine, Cishan Hospital, Ministry of Health and Welfare, Kaohsiung, Taiwan (R.O.C.).
3. Department of Physical Medicine and Rehabilitation, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan (R.O.C.).
4. Department of Physical Medicine and Rehabilitation, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (R.O.C.).
5. Department of Physical Medicine and Rehabilitation, Kaohsiung Municipal Siaogang Hospital, Kaohsiung, Taiwan (R.O.C.).
6. Cardiovascular Center, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (R.O.C.).
7. Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (R.O.C.).
8. Institute of Allied Health Sciences, College of Medicine, National Chen Kung University, Tainan, Taiwan (R.O.C.).
9. School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan (R.O.C.).
10. Department of Physical Therapy, School of Medical and Health Science, Fooyin University, Kaohsiung, Taiwan (R.O.C.).
† Zong-Han Lin and Sheng-Hui Tuan contributed equally to this work and share first authorship.
Received 2025-7-7; Accepted 2025-9-20; Published 2025-10-1
Abstract
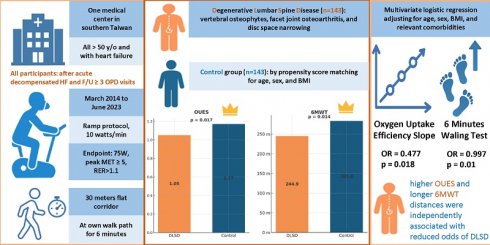
Heart failure (HF) is a complex clinical syndrome characterized by impaired exercise capacity and reduced quality of life. While musculoskeletal conditions such as degenerative lumbar spine disease (DLSD) are common in older adults, their contribution to exercise intolerance in HF patients remains under-investigated. This retrospective cohort study evaluated the relationship between DLSD and functional capacity in HF patients using cardiopulmonary exercise testing (CPET) and the six-minute walk test (6MWT). We included 286 HF patients who underwent CPET following hospitalization for acute decompensated HF. Based on imaging findings, patients were divided into DLSD (n = 143) and non-DLSD (n = 143) groups after propensity score matching for age, sex, and BMI. The DLSD group exhibited significantly poorer exercise tolerance, with lower oxygen uptake efficiency slope (OUES) (1.05 ± 0.44 vs. 1.17 ± 0.45; p = 0.017) and shorter 6MWT distances (244.9 ± 130.36 vs. 283.36 ± 132.22 m; p = 0.014). Multivariate logistic regression adjusting for age, sex, BMI, and comorbidities revealed that higher OUES and longer 6MWT distances were independently associated with reduced odds of DLSD (OUES: OR = 0.477; 95% CI: 0.259-0.879; p = 0.018; 6MWT: OR = 0.997; 95% CI: 0.995-0.999; p = 0.01). These findings suggest that DLSD may exacerbate exercise intolerance in HF and highlight the value of CPET and 6MWT in identifying high-risk subgroups. Early recognition of DLSD may facilitate tailored rehabilitation strategies to improve clinical outcomes in patients with HF.
Keywords: cardiopulmonary exercise testing, degenerative lumbar spine disease, heart failure, oxygen uptake efficiency slope, six-minute walk test
1. Introduction
Heart failure (HF) is a multifaceted clinical condition, denoting a heart abnormality that hinders sufficient blood supply to meet the body's demands. It imposes a significant global burden, affecting approximately 64 million individuals worldwide[1, 2]. HF is characterized by cardiac insufficiency and typical symptoms such as dyspnea, limited exercise tolerance, and reduced peripheral perfusion[3-5]. Exercise intolerance in HF is multifactorial, involving impaired cardiac and pulmonary reserves, diminished skeletal muscle perfusion, and reduced muscular functionality[6]. Evaluating this intolerance is crucial, given its profound impact on patients' quality of life and mortality risk, especially in the context of extended survival due to advances in HF treatment[6].
To assess functional impairment and stratify prognosis, several tools are commonly employed in clinical practice, including the New York Heart Association (NYHA) functional classification[7], the six-minute walk test (6MWT)[8, 9], and cardiopulmonary exercise test (CPET)[10]. Among these, CPET is regarded as the gold standard for evaluating aerobic capacity and predicting event-free survival across all HF phenotypes[11]. Peak oxygen consumption (peak VO₂) is a well-established CPET parameter, yet many patients, particularly those with preserved (HFpEF) or mildly reduced ejection fraction (HFmrEF), often cannot reach maximal effort levels due to fatigue, dyspnea, or hemodynamic instability[12]. Consequently, submaximal indices such as the anaerobic threshold (AT)[13], ventilatory efficiency (VE/VCO₂ slope)[14], and oxygen uptake efficiency slope (OUES)[15] have gained recognition as robust prognostic markers that are less effort-dependent and more broadly applicable.
Approximately 84% of adults have encountered lower back pain (LBP) at some stage in their lives[16, 17]. Among the significant contributors to LBP is degenerative lumbar spine disease (DLSD), whose prevalence increases with age[18], potentially leading to spinal or hip pain and spinal stenosis in severe cases[19, 20]. Research on DLSD risk factors is limited[21]. Previous studies have suggested associations with factors, including sex, obesity, age[22], and biomechanics[20, 23]. Biochemical stressors (e.g., the intestinal microbiota) may adversely impact the spinal structure cells and tissues[24, 25]. Emerging evidence also points to cardiovascular disease (CVD) as a potential risk factor for DLSD[26], with mechanisms involving atherosclerosis[27], abdominal aorta calcification[28, 29], and peripheral vascular obstruction[30]. These vascular changes may reduce spinal perfusion, leading to disc degeneration and facet joint osteoarthritis (OA) via both macrovascular and microvascular ischemia[29].
Importantly, the relationship between HF and DLSD may be bidirectional. Vascular dysfunction in HF, including reduced cardiac output and impaired peripheral perfusion, may compromise spinal blood supply and accelerate degeneration[29]. Conversely, DLSD can lead to chronic pain and reduced mobility, further contributing to physical inactivity and cardiovascular deconditioning[1]. Notably, back pain is prevalent in 67.71% of older adults with HF[31], which has been associated with increased cardiovascular mortality[32]. DLSD often coexists with OA, another condition linked to higher risks of myocardial infarction and HF[33]. These overlapping mechanisms, such as inflammation, vascular stiffness, and inactivity, underscore the need to consider spinal health in the comprehensive care of HF patients. Despite the high prevalence of low back pain among patients with heart failure, the specific contribution of degenerative lumbar spine disease and how it alters cardiopulmonary exercise performance remains poorly defined. This study aims to bridge this knowledge gap by using CPET to objectively evaluate the exercise performance of HF patients with DLSD, correlating real-time functional capacity and spine pathology. This study seeks to improve understanding of how DLSD influences exercise capacity in HF patients and emphasize the importance of early diagnosis and intervention.
2. Materials and Methods
2.1 Study design and participants
This was a retrospective cohort study conducted at a tertiary medical center in Southern Taiwan and included patients with HF who underwent their first CPET between March 2014 and June 2023. Eligible participants were those who had been previously hospitalized at our institution for acute decompensated HF and had subsequently attended at least three outpatient follow-up visits in the departments of cardiology, cardiovascular surgery, or rehabilitation. Patients with HF with reduced ejection fraction (HFrEF), HFmrEF, and HFpEF were all included. The diagnosis of HF was based on the criteria outlined by the American Heart Association[34], which incorporate clinical signs and symptoms of congestion (e.g., dyspnea, peripheral edema), elevated natriuretic peptide levels, and objective evidence of structural or functional cardiac abnormalities, including variations in ejection fraction. All HF diagnoses were confirmed by experienced cardiologists following a comprehensive clinical evaluation. Only data from each patient's first CPET were included in the analysis. Inclusion criteria were: (1) age ≥50 years and (2) a confirmed diagnosis of HF (3) possess a comprehensive record of a standard 12-lead electrocardiogram (ECG). Exclusion criteria included: (1) missing clinical or CPET data; (2) inability to complete CPET due to frailty or physical limitations; (3) prolonged bedridden status exceeding three months; (4) cognitive impairment or neuromuscular disorders affecting participation in rehabilitation; (5) ventilator dependence; or (6) severe pulmonary disease requiring oxygen therapy. The study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital (IRB No. VGHKS17-CT11-11). Given the retrospective nature of the study, the requirement for informed consent was waived; however, all patients had undergone CPET as part of routine clinical care and were informed of its purpose at the time of testing.
2.2. Cardiopulmonary exercise testing
All cardiopulmonary exercise tests (CPETs) were performed using a symptom-limited, progressive ramp protocol on a leg ergometer (MetaLyzer 3B system, Cortex Biophysik GmbH Co., Leipzig, Germany), which included an ECG monitor, gas analyzer, flow module, and leg dynamometer. The workload was incrementally increased at a rate of 10 watts per minute. Each test was supervised by a board-certified physiatrist with over 10 years of clinical experience (K.-L. Lin) and conducted by physical therapists with more than 5 years of experience in cardiac rehabilitation (W.-Y. Huang). Continuous 12-lead ECG monitoring was conducted throughout the test, along with real-time measurements of oxygen consumption (VO₂), carbon dioxide production (VCO₂), minute ventilation (VE), respiratory rate, and other derived variables such as the respiratory exchange ratio (RER), OUES, and VE/VCO₂ slope. CPET was terminated upon the appearance of intolerable symptoms such as severe dyspnea, chest pain, dizziness, extreme fatigue, or instability, or if patients reached a submaximal endpoint following the criteria set by the American College of Sports Medicine[35]. This endpoint was defined as achieving ≥75 watts workload, a peak VO₂ of ≥5 metabolic equivalents (METs), a peak heart rate ≥70% of the age-predicted maximum, or an RER ≥1.1. These modified criteria, originally adapted by Lin et al.[36], have been effectively implemented at our institution for patients with acute myocardial infarction and HF, demonstrating their clinical applicability and safety in this population.
The AT is determined by a noticeable increase in the VCO2-VO2 slope[37]. Peak VO2 represents the highest value of oxygen uptake recorded during the test. OUES was calculated using the linear regression formula VO₂ = a·log(VE) + b, where the slope "a" represents OUES[38]. The VE/VCO₂ slope was calculated from exercise onset to a point just beyond the AT[39]. In addition, the 6MWT was administered according to the American Thoracic Society guidelines[40]. Participants were instructed to walk back and forth along a 30-meter flat corridor for 6 minutes at their own pace while attempting to cover as much distance as possible. Standardized encouragement was provided at regular intervals, and total walking distance in meters was recorded as the 6MWT result.
2.3 Definition of outcomes and covariates
Demographic and clinical variables, including age, height, weight, and comorbidities, were extracted from patients' medical records prior to their initial CPET. Comorbidities were identified based on International Classification of Diseases, 10th Revision (ICD-10) codes, narrative clinical documentation, medication records, and relevant imaging findings.
DLSD-related conditions, such as vertebral osteophytes, facet joint OA (FOA), and disc space narrowing due to intervertebral disc degeneration, were identified through a comprehensive review of each patient's medical chart and imaging studies. The diagnostic criteria for DLSD were based on prior systematic reviews that established strong correlations between clinical symptoms and radiographic findings[41]. Diagnosis was confirmed using plain radiography and computed tomography (CT). X-ray findings considered diagnostic included the presence of vertebral osteophytes, disc space narrowing, and FOA. CT imaging was used to further assess spinal canal stenosis and the extent of intervertebral disc degeneration[19, 42]. All imaging data were independently reviewed by at least two physicians, one radiologist (Dr. M.Y. Tsai, acknowledged contributor) and one rehabilitation specialist (S.F. Sun), to ensure diagnostic accuracy and consistency. Patients diagnosed with DLSD were assigned to the DLSD group (as experimental group), while those without DLSD formed the control group. To reduce baseline differences between groups, propensity score matching was performed in a 1:1 ratio based on age at index, sex, and body mass index (BMI).
2.4 Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 27.0 (Released 2020; IBM Corp., Armonk, NY, USA). Categorical variables were compared using the chi-square test, while continuous variables were analyzed using independent t-tests to evaluate between-group demographic differences. To enhance comparability and control for potential confounding factors, propensity score matching was conducted using a nearest-neighbor matching algorithm with a caliper width of 0.2 standard deviations of the logit of the propensity score. This approach ensured a balanced comparison between the DLSD and control groups. Sensitivity analyses were performed to verify the robustness of the matching process, confirming consistency across different caliper widths.
After matching, independent t-tests were used to compare CPET-derived continuous variables between groups. Prior to applying parametric tests, the normality of continuous variables was evaluated using the Shapiro-Wilk test, and the homogeneity of variances was assessed with Levene's test. For variables showing mild deviations from normality, additional sensitivity analyses were performed using the nonparametric Mann-Whitney U test. Logistic regression analyses (both univariable and multivariable) were conducted to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for variables associated with DLSD. The assumptions of logistic regression, including independence of observations, adequate sample size, absence of multicollinearity among predictors, and linearity of continuous variables in the logit, were considered and satisfied. A two-tailed P-value of <0.05 was considered statistically significant.
3. Results
3.1 Baseline characteristics of patients with and without DLSD
In this study, a total of 530 patients who underwent CPET were initially screened. After excluding individuals younger than 50 years of age (n = 70) and those with incomplete data (n = 65), 395 patients with HF were included in the final analysis. Among them, 160 patients diagnosed with DLSD were classified into the experimental group, while the remaining 235 patients without DLSD comprised the control group. Baseline demographic comparisons revealed significant differences between the two groups, most notably a higher mean age in the DLSD group (70.98 vs. 64.80 years). To improve comparability and reduce potential confounding, 1:1 propensity score matching was performed based on age, sex, and BMI, resulting in 143 matched patients per group. Post-matching statistical analysis confirmed that there were no significant differences in age, sex, BMI, or comorbidity profiles between the two groups (Table 1).
3.2 Comparison of cardiopulmonary parameters between DLSD and control groups
All enrolled patients were classified as NYHA functional class II or III. The exercise capacity parameters from CPET and 6MWT for patients in the DLSD and control groups are summarized in Table 2.
The DLSD group demonstrated significantly poorer cardiopulmonary performance than the control group, with lower OUES (1.05 ± 0.44 vs. 1.17 ± 0.45; p = 0.017) and shorter 6MWT distance (244.9 ± 130.36 vs. 283.36 ± 132.22 meter; p = 0.014). Although not reaching statistical significance, several other CPET indices, including anaerobic threshold VO₂ (AT VO₂), peak VO₂, and VO₂/work rate (WR) slope, showed a trend toward reduced values in the DLSD group, suggesting a trend toward generally lower cardiopulmonary performance. No significant differences were observed between groups in resting systolic or diastolic blood pressure, heart rate responses, VE/VCO₂ slope, RER, or end-tidal CO₂ tension (PETCO₂) at various time points during the test. These findings suggest that DLSD is associated with impaired exercise tolerance or may be more prevalent among patients with more advanced HF symptoms.
Baseline characteristics of study subjects (before and after propensity score matching)
| Before matching | After matching a | ||||||
|---|---|---|---|---|---|---|---|
| DLSD cohort (n=160) | Control cohort (n=235) | P value | DLSD cohort (n=143) | Control cohort (n=143) | P value | ||
| Age at index(year) | |||||||
| Mean±SD | 70.98±11.253 | 64.8±10.29 | <0.01 | 68.99±10.04 | 68.73±10.07 | 0.833 | |
| Sex | |||||||
| Male | 173(73.6%) | 115(71.9%) | 0.7 | 106(74.1%) | 104(72.7%) | 0.789 | |
| Female | 62(26.4%) | 45(28.1%) | 37(25.9%) | 39(27.3%) | |||
| Height (cm) | 161.71±9.03 | 163.64±10.46 | 0.058 | 162.43±8.50 | 162.59±10.03 | 0.885 | |
| Weight (kg) | 63.49±14.84 | 65.49±14.85 | 0.189 | 63.99±12.05 | 64.49±14.56 | 0.751 | |
| BMI(kg/m2) | 24.07±3.57 | 24.16±4.05 | 0.826 | 24.08±4.08 | 24.12±4.04 | 0.955 | |
| Comorbidities | |||||||
| Hypertension | 115(71.9%) | 155(66.0%) | 0.214 | 99(69.2%) | 95(66.4%) | 0.61 | |
| Diabetes mellitus | 84(52.5%) | 101(43.0%) | 0.063 | 77(53.8%) | 67(46.9%) | 0.237 | |
| Hyperlipidemia | 80(50.0%) | 116(49.4%) | 0.901 | 70(49.0%) | 70(49.0%) | 1 | |
| COPD | 10(6.3%) | 15(6.4%) | 0.958 | 9(6.3%) | 14(9.8%) | 0.277 | |
| ESRD | 8(5.0%) | 14(6.0%) | 0.684 | 8(5.6%) | 12(8.4%) | 0.354 | |
| Liver cirrhosis | 5(3.1%) | 3(1.3%) | 0.2 | 5(3.5%) | 1(0.7%) | 0.099 | |
| Pulmonary hypertension | 1(0.6%) | 11(4.7%) | 0.021 | 0(0.0%) | 7(4.9%) | 0.007* | |
| PAOD | 9(5.6%) | 9(3.8%) | 0.401 | 8(5.6%) | 8(5.6%) | 1 | |
| a Propensity score matching was performed on age at index, sex, body mass index | |||||||
BMI: body mass index; COPD: chronic obstructive pulmonary disease; DLSD: degenerative lumbar spine disease; ESRD: end-stage renal disease; PAOD: peripheral arterial occlusion disease
* p < 0.05
Comparison of cardiopulmonary exercise testing parameters between heart failure patients with and without degenerative lumbar spine disease
| DLSD cohort(n=143) | Control cohort(n=143) | P value | 95%CI | |
|---|---|---|---|---|
| SBP when rest | 118.76±20.84 | 119.74±23.07 | 0.707 | (-4.139 - 6.097) |
| DBP when rest | 69.22±11.45 | 68.87±10.75 | 0.79 | (-2.934 - 2.235) |
| HR when rest | 73.69±13.38 | 75±13.77 | 0.414 | (-1.846 - 4.475) |
| AT VO2(ml/min/kg) | 8.08±2.25 | 8.57±2.4 | 0.076 | (-0.0515 - 1.030) |
| AT Heart rate | 92.92±16.29 | 93.59±18.2 | 0.745 | (-3.356 - 4.685) |
| WATT max | 45.64±24.44 | 48.03±22.41 | 0.389 | (-3.066 - 7.849) |
| PeakVO2(ml/min/kg) | 11.44±3.47 | 12.13±3.54 | 0.098 | (-0.128 - 1.502) |
| Peak HR | 106.08±21.29 | 107.76±23.82 | 0.53 | (-3.580 - 6.936) |
| Peak VE | 33.11±12.08 | 32.54±11.28 | 0.681 | (-3.289 - 2.152) |
| Peak RER | 1.09±0.09 | 1.08±0.1 | 0.319 | (-0.034 - 0.011) |
| 6MWT | 244.9±130.36 | 283.36±132.22 | 0.014* | (7.894 - 69.020) |
| Peak SBP | 145.85±28.02 | 145.35±28.41 | 0.882 | (-7.065 - 6.072) |
| Peak DBP | 72.2±13.34 | 73.12±14.49 | 0.579 | (-2.326 - 4.158) |
| HRR | 9.38±7.66 | 10.04±7.67 | 0.469 | (-1.127 - 2.442) |
| VE/VCO2 slope | 39.66±12.25 | 37.16±14.74 | 0.12 | (-5.654 - 0.656) |
| OUES | 1.05±0.44 | 1.17±0.45 | 0.017* | (0.023 - 0.231) |
| VO2/WR slope | 6.99±2.93 | 7.67±3.15 | 0.057 | (-0.022 - 1.394) |
| PETCO2 when rest | 29.59±5.16 | 30.17±5.24 | 0.34 | (-0.623 - 1.798) |
| PRTCO2 when AT | 33.23±6.55 | 33.15±6.27 | 0.93 | (-1.864 - 1.703) |
| PETCO2 when max | 34.83±6.13 | 35.45±5.66 | 0.368 | (-0.744 - 2.003) |
AT: anaerobic threshold; DBP: diastolic blood pressure; DLSD: degenerative lumbar spine disease; HR: heart rate; HRR: heart rate reserve; OUES: oxygen uptake efficiency slope; PET CO₂: end-tidal carbon dioxide tension; RER: respiratory exchange ratio; SBP: systolic blood pressure; VCO₂: volume of exhaled carbon dioxide; VE: minute ventilation; VO₂: oxygen uptake; WR: work rate; 6MWT: six-minute walk test
* p < 0.05
Factors associated with degenerative lumbar spine disease
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | 1.003 | (0.980 - 1.026) | 0.832 | |||
| Sex | ||||||
| Male | Ref. | |||||
| Female | 0.931 | (0.511 - 1.573) | 0.789 | |||
| BMI | 0.99 | (0.945 - 1.037) | 0.662 | |||
| Comorbidities | ||||||
| Hypertension | 0.88 | (0.535 - 1.445) | 0.613 | |||
| Diabetes mellitus | 0.756 | (0.475 - 1.203) | 0.237 | |||
| Hyperlipidemia | 1 | (0.629 - 1.590) | 1 | |||
| COPD | 1.616 | (0.676 - 3.863) | 0.281 | |||
| ESRD | 1.546 | (0.612 - 3.903) | 0.357 | |||
| Liver cirrhosis | 0.194 | (0.022 - 1.685) | 0.137 | |||
| PAOD | 1 | (0.365 - 2.742) | 1 | |||
| CPET | ||||||
| OUES | 0.527 | (0.31 - 0.896) | 0.018* | 0.477 | (0.259 - 0.879) | 0.018* |
| 6WMT | 0.998 | (0.996 - 1.000) | 0.015* | 0.997 | (0.995 - 0.999) | 0.008* |
The effects of age, sex, BMI and Comorbidities (hypertension, diabetes mellitus, hyperlipidemia, COPD, ESRD, liver cirrhosis, and PAOD) were adjusted in the multivariable regression analysis
COPD: chronic obstructive pulmonary disease; ESRD: end-stage renal disease; OR: odds ratio; OUES: oxygen uptake efficiency slope; PAOD: peripheral arterial occlusion disease; 6MWT: six-minute walk test
* p < 0.05
3.3 Logistic regression analysis of factors associated with DLSD
To further investigate the relationship between exercise capacity and the presence of DLSD in patients with HF, both univariate and multivariate logistic regression analyses were conducted (Table 3). In the univariate analysis, OUES and 6MWT were significantly associated with DLSD. Specifically, lower OUES was associated with increased odds of DLSD (OR = 0.527; 95% CI: 0.31-0.896; p = 0.018), and shorter 6MWT distances also correlated with higher DLSD risk (OR = 0.998; 95% CI: 0.996-1.000; p = 0.015).
To adjust for potential confounders, multivariate logistic regression analysis was performed, controlling for age, sex, BMI, and relevant comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), liver cirrhosis, and peripheral arterial occlusive disease (PAOD). After adjustment, both OUES (adjusted OR = 0.477; 95% CI: 0.259-0.879; p = 0.018) and 6MWT (adjusted OR = 0.997; 95% CI: 0.995-0.999; p = 0.008) remained independently and significantly associated with lower DLSD risk. These findings underscore the clinical relevance of submaximal and peak exercise performance measures in identifying HF patients at greater risk for coexisting spinal degenerative changes.
4. Discussion
In this study, we identified a significant association between DLSD and impaired cardiopulmonary fitness in patients with HF. Patients with DLSD exhibited poorer exercise capacity compared to those without DLSD, as reflected by lower OUES and shorter 6MWT distances. Furthermore, both OUES and 6MWT were independently associated with the presence of DLSD after adjusting for age, sex, BMI, and comorbidities. These findings underscore a potential bidirectional relationship between DLSD and HF, in which musculoskeletal degeneration may contribute to reduced physical performance, while impaired cardiovascular function may in turn accelerate spinal deterioration. Collectively, these results may prompt clinicians to incorporate spinal health as an integral component of cardiopulmonary assessment in patients with HF.
Exercise intolerance in patients with HF arises from multiple contributing factors, including impaired cardiac and pulmonary reserve as well as reduced skeletal muscle perfusion and function[6]. This condition has a profound impact on both quality of life and mortality[43]. CPET serves as a key prognostic tool and is widely utilized to evaluate exercise tolerance in individuals with HF[44]. Several CPET-derived parameters, such as a peak VO₂ ≤ 50% of the predicted value[45], a ventilatory threshold <11 mL/kg/min[13], and a VE/VCO2 slope >34[13] have been strongly associated with reduced cardiac output, more severe HF symptoms, and elevated mortality risk. Furthermore, the OUES has emerged as a valuable predictor of outcomes, particularly in patients with end-stage HF or those unable to complete maximal exercise testing[46, 47]. Prior research has demonstrated a survival benefit in patients with OUES ≥ 1.6[46], while individuals with an OUES <1.25 faced a 5.421-fold increased risk of major adverse cardiovascular events within one year[47]. In our study, the OUES was significantly lower in HF patients with coexisting DLSD compared to those without DLSD (1.05 ± 0.44 vs. 1.17 ± 0.45; p = 0.017), suggesting that this subgroup may be at heightened risk for adverse cardiovascular outcomes. Notably, that both groups exhibited mean OUES values below the 1.25 threshold, indicating an elevated risk of major adverse cardiovascular events across the entire cohort. This may be attributed to the fact that the CPET data analyzed in this study were obtained following acute decompensated HF episodes, specifically using the first post-discharge CPET, which may reflect a period of ongoing physiological recovery and functional limitation.
The 6MWT, valued for its simplicity, low cost, and high patient tolerability, is widely regarded as a practical alternative for individuals unable to undergo maximal exercise testing. In a retrospective study of patients with New York Heart Association (NYHA) class III or IV heart failure symptoms, the 6MWT was significantly associated with both mortality and hospitalization risk[48]. From a prognostic perspective, patients with chronic HF who walked less than 300 meters were found to have poorer outcomes[49], while those walking less than 200 meters faced a markedly increased risk of death[50]. In our study, HF patients with coexisting DLSD demonstrated significantly shorter 6MWT distances compared to controls, suggesting that this subgroup may be at heightened risk for adverse cardiovascular outcomes.
DLSD, encompassing conditions such as spondylitis, osteoporotic vertebral fractures, FOA, intervertebral disk degeneration, and lumbar spinal canal stenosis due to ligamentous changes, represents a major contributor to LBP[22, 24, 51]. Emerging evidence has highlighted a strong association between degenerative joint diseases and cardiovascular pathology. For instance, an observational study involving 153 symptomatic patients with DLSD reported that 66% were diagnosed with metabolic syndrome, thereby substantially elevating their CVD risk[52]. Another population-based study found that individuals aged 50 years or older with lumbar OA exhibited a significantly higher incidence of myocardial infarction or angina compared to age-matched controls[53]. Peripheral joint degeneration appears to exert similar cardiovascular implications. A large pooled analysis of 32,278,744 individuals revealed that the prevalence of combined CVD among patients with OA was approximately 38.4% (95% CI, 37.2%-39.6%). Notably, these patients had a 2~3-fold increased risk of developing HF [Relative Risk (RR), 2.80; 95% CI, 2.25-3.49] and ischemic heart disease (RR, 1.78; 95% CI, 1.18-2.69) compared to controls[54]. These findings align with prior studies showing a consistent link between joint degeneration and cardiovascular outcomes[33, 55]. Moreover, beyond lower limb joints, symptomatic hand OA has also been associated with increased coronary heart disease risk, with a hazard ratio of 2.26 (95% CI, 1.22-4.18)[56].
From another perspective, CVD has also been implicated as a contributing factor to the development of OA. Even after adjusting for various epidemiologic and cardiovascular risk factors associated with spinal degeneration, abdominal aortic calcification has been linked to a twofold increased risk of FOA[28] and lumbar disc degeneration[28, 57]. Beyond aortic atherosclerosis, systematic reviews have emphasized a strong association between stenosis of the lumbar spine's feeding arteries and both disc degeneration and LBP[58]. Supporting this, studies from China have shown that individuals with a history of heart disease have a 1.4-fold increased risk of developing symptomatic knee OA (OR, 1.40; 95% CI, 1.07-1.82)[59, 60].
In summary, beyond well-established biomechanical contributors, several potentially modifiable risk factors for DLSD have been identified. Evidence increasingly supports a bidirectional relationship between CVD and systemic degenerative joint disease. One proposed mechanism involves impaired nutrient delivery to the spine due to arterial stenosis, which may accelerate intervertebral disc degeneration[61]. Moreover, systemic inflammation, a known contributor to both vascular disease and OA may act as a shared pathophysiological link[62, 63]. Local inflammatory mediators such as cyclooxygenase-2 have been implicated in the progression of arterial calcification, atherosclerosis, and OA. In turn, OA may exacerbate systemic inflammation, potentially promoting further vascular damage[64]. These conditions also share common risk factors, including advanced age and obesity. Joint degeneration contributes to reduced mobility, physical deconditioning, and disability, which in turn elevate cardiovascular risk[65]. In severe OA, for example, patients often exhibit markedly reduced peak oxygen consumption[66, 67]. Functional impairment caused by arthritis-induced inactivity may ultimately lead to cardiovascular dysfunction. Our findings highlight the importance of early intervention and structured exercise programs for patients with DLSD to help preserve cardiovascular health and prevent further deterioration.
This study has several limitations. First, the homogeneity of the study population, compromising predominantly male veterans, limits the generalizability of the findings, particularly to females and non-veterans. Future studies should recruit more diverse and representative cohorts and include sex-stratified analyses to clarify potential sex-specific differences. Second, the sample size was relatively modest. Although 395 patients with heart failure were initially enrolled, the cohort decreased to 286 after propensity score matching, which may have reduced statistical power. Moreover, although the cohort included patients with HFrEF, HFmrEF, and HFpEF, subgroup analyses stratified by heart failure phenotype were not feasible. As cardiopulmonary performance and musculoskeletal interactions may vary across subtypes, larger studies are warranted to explore phenotype-specific associations. Third, it should be emphasized that, due to the retrospective observational design, the present study cannot establish a causal relationship between DLSD and impaired cardiopulmonary fitness. Nonetheless, the study identified a significant association between the two conditions. The observed associations should therefore be interpreted as correlations and prospective or interventional studies are needed to clarify causality. Finally, other musculoskeletal disorders common in patients with heart failure, such as knee or hip OA and sarcopenia, were not evaluated or excluded. These conditions may influence exercise capacity and functional performance, potentially confounding the relationship between DLSD and cardiopulmonary fitness. Future research should incorporate comprehensive musculoskeletal assessments to better delineate their impact on CPET and 6MWT outcomes.
5. Conclusion
This study demonstrates a significant association between DLSD and impaired cardiopulmonary fitness in patients with HF. Specifically, HF patients with DLSD exhibited lower OUES and shorter 6MWT distances compared to matched controls, suggesting poorer exercise tolerance and potentially heightened cardiovascular risk. Both OUES and 6MWT remained independently associated with DLSD after adjusting for demographic and clinical confounders. Our results support the hypothesis of a bidirectional relationship between musculoskeletal and cardiovascular health, underscoring the importance of spinal health assessment in HF management. Given the shared pathophysiological pathways, such as systemic inflammation, vascular dysfunction, and physical deconditioning, early recognition and targeted interventions for DLSD in HF patients may help preserve functional capacity and reduce long-term cardiovascular risk. Collectively, these insights may prompt clinicians to consider spinal health as an integral component of cardiopulmonary assessment in patients with HF.
Abbreviations
HF: heart failure; DLSD: degenerative lumbar spine disease; CPET: cardiopulmonary exercise testing; 6MWT: six-minute walk test; NYHA: New York Heart Association; HFpEF: heart failure with preserved ejection fraction; HFmrEF: heart failure with mildly reduced ejection fraction; AT: anaerobic threshold; VE/VCO₂ slope: ventilatory efficiency; OUES: oxygen uptake efficiency slope; LBP: low back pain; CVD: cardiovascular disease; MET: metabolic equivalent; RER: respiratory exchange ratio; VO₂: oxygen consumption; VCO₂: carbon dioxide production; VE: minute ventilation; ECG: electrocardiogram; BMI: body mass index; ICD-10: International Classification of Diseases, 10th Revision; FOA: facet joint osteoarthritis; CT: computed tomography; COPD: chronic obstructive pulmonary disease; ESRD: end-stage renal disease; PAOD: peripheral arterial occlusive disease; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; HRR: heart rate reserve; PETCO₂: end-tidal carbon dioxide tension; OR: odds ratio; CI: confidence interval; OA: osteoarthritis; RR: relative risk.
Acknowledgements
We are grateful to Dr. Meng-Yuan Tsai, a radiology specialist, for his expert assistance in reviewing imaging studies and confirming the presence or absence of DLSD in the study participants.
Author contributions
All authors were involved in manuscript drafting or revision of the article, and all authors approved the final version to be published.
Study design and conception: Sheng-Hui Tuan, Ko-Long Lin, Wen-Hwa Wang, Shu-Fen Sun, and I-Hsiu Liou.
Acquisition of data: Zong-Han Lin, Wan-Yun Huang, Ruei-Sian Ding.
Analysis and interpretation of data: Zong-Han Lin, Ruei-Sian Ding.
Writing (original draft preparation): Zong-Han Lin, Sheng-Hui Tuan, Wen-Hwa Wang, and Ruei-Sian Ding.
Writing (review and editing): Ko-Long Lin, Wan-Yun Huang, Shu-Fen Sun, and I-Hsiu Liou.
Data availability statement
Deidentified individual participant data underlying the findings of this study may be made available upon reasonable request. Interested researchers should submit their proposals to isliuvghks@gmail.com for consideration.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M. et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38-360
2. Global regional, national incidence prevalence, years lived with disability for 354 diseases injuries for 195 countries, territories 1990-2017. a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-858
3. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE. et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539-50
4. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79-96
5. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-9
6. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL. et al. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2209-25
7. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444-50
8. Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009;39:495-501
9. Fuentes-Abolafio IJ, Stubbs B, Pérez-Belmonte LM, Bernal-López MR, Gómez-Huelgas R, Cuesta-Vargas AI. Physical functional performance and prognosis in patients with heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2020;20:512
10. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016;4:607-16
11. Juarez M, Castillo-Rodriguez C, Soliman D, Del Rio-Pertuz G, Nugent K. Cardiopulmonary Exercise Testing in Heart Failure. J Cardiovasc Dev Dis. 2024 11
12. Sato T, Yoshihisa A, Kanno Y, Suzuki S, Yamaki T, Sugimoto K. et al. Cardiopulmonary exercise testing as prognostic indicators: Comparisons among heart failure patients with reduced, mid-range and preserved ejection fraction. Eur J Prev Cardiol. 2017;24:1979-87
13. Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M. et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079-84
14. Tuan SH, Huang IC, Huang WC, Chen GB, Sun SF, Lin KL. Minute Ventilation/Carbon Dioxide Production Slope Could Predict Short- and Long-Term Prognosis of Patients After Acute Decompensated Heart Failure. Life (Basel). 2024 14
15. Gordon J, Michelis KC, Pandey A, Ayers C, Thibodeau JT, Grodin JL. et al. Oxygen Uptake Efficiency Slope and Prognosis in Heart Failure With Reduced Ejection Fraction. Am J Cardiol. 2023;201:273-80
16. Cassidy JD, Carroll LJ, Côté P. The Saskatchewan health and back pain survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976). 1998;23:1860-6 discussion 7
17. Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976). 1987;12:264-8
18. Kalichman L, Li L, Kim DH, Guermazi A, Berkin V, O'Donnell CJ. et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila Pa 1976). 2008;33:2560-5
19. Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15:305
20. Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37:69-80
21. Hassett G, Hart DJ, Manek NJ, Doyle DV, Spector TD. Risk factors for progression of lumbar spine disc degeneration: the Chingford Study. Arthritis Rheum. 2003;48:3112-7
22. Parenteau CS, Lau EC, Campbell IC, Courtney A. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci Rep. 2021;11:5389
23. Abbas J, Peled N, Hershkovitz I, Hamoud K. Facet Tropism and Orientation: Risk Factors for Degenerative Lumbar Spinal Stenosis. Biomed Res Int. 2020;2020:2453503
24. Morimoto T, Kobayashi T, Kakiuchi T, Esaki M, Tsukamoto M, Yoshihara T. et al. Gut-spine axis: a possible correlation between gut microbiota and spinal degenerative diseases. Front Microbiol. 2023;14:1290858
25. Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut-disc axis: A cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31:917-25
26. Hoffeld K, Lenz M, Egenolf P, Weber M, Heck V, Eysel P. et al. Patient-related risk factors and lifestyle factors for lumbar degenerative disc disease: a systematic review. Neurochirurgie. 2023;69:101482
27. Findlay DM. Vascular pathology and osteoarthritis. Rheumatology (Oxford). 2007;46:1763-8
28. Suri P, Katz JN, Rainville J, Kalichman L, Guermazi A, Hunter DJ. Vascular disease is associated with facet joint osteoarthritis. Osteoarthritis Cartilage. 2010;18:1127-32
29. Schönnagel L, Muellner M, Suwalski P, Zhu J, Guven AE, Caffard T. et al. Abdominal aortic calcification is independently associated with lumbar endplate degeneration. Eur Spine J. 2023;32:3387-93
30. Ain DL, Slovut DP, Kamath R, Jaff MR. The association between peripheral artery and lumbar spine disease: a single-center study. Am J Med. 2012;125:411-5
31. Chen J, Zhang Y, Simonsick E, Starkweather A, Chen MH, McCauley P. et al. Back pain and heart failure in community-dwelling older adults: Findings from the Health ABC study. Geriatr Nurs. 2021;42:643-9
32. McBeth J, Symmons DP, Silman AJ, Allison T, Webb R, Brammah T. et al. Musculoskeletal pain is associated with a long-term increased risk of cancer and cardiovascular-related mortality. Rheumatology (Oxford). 2009;48:74-7
33. Veronese N, Trevisan C, De Rui M, Bolzetta F, Maggi S, Zambon S. et al. Association of Osteoarthritis With Increased Risk of Cardiovascular Diseases in the Elderly: Findings From the Progetto Veneto Anziano Study Cohort. Arthritis Rheumatol. 2016;68:1136-44
34. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM. et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. JACC. 2022;79:e263-e421
35. Ferguson B. ACSM's Guidelines for Exercise Testing and Prescription 9th Ed. 2014: J Can Chiropr Assoc. 2014 Sep;58(3):328
36. Che L, Wang LM, Jiang JF, Xu WJ, Zhang QP. [Effects of early submaximal cardiopulmonary exercise test and cardiac rehabilitation for patients with acute myocardial infarction after percutaneous coronary intervention: a comparative study]. Zhonghua Yi Xue Za Zhi. 2008;88:1820-3
37. Poole DC, Rossiter HB, Brooks GA, Gladden LB. The anaerobic threshold: 50+ years of controversy. J Physiol. 2021;599:737-67
38. Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol. 2000;36:194-201
39. Chaumont M, Forton K, Gillet A, Tcheutchoua Nzokou D, Lamotte M. How Does the Method Used to Measure the VE/VCO(2) Slope Affect Its Value? A Cross-Sectional and Retrospective Cohort Study. Healthcare (Basel). 2023 11
40. ATS statement. guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-7
41. Varlotta GP, Lefkowitz TR, Schweitzer M, Errico TJ, Spivak J, Bendo JA. et al. The lumbar facet joint: a review of current knowledge: part 1: anatomy, biomechanics, and grading. Skeletal Radiol. 2011;40:13-23
42. Chamoro M, de Luca K, Ozbulut O, Oei EHG, Vleggeert-Lankamp CLA, Koes BW. et al. Association between clinical findings and the presence of lumbar spine osteoarthritis imaging features: A systematic review. Osteoarthritis Cartilage. 2023;31:1158-75
43. Alba AC, Adamson MW, MacIsaac J, Lalonde SD, Chan WS, Delgado DH. et al. The Added Value of Exercise Variables in Heart Failure Prognosis. J Card Fail. 2016;22:492-7
44. Paolillo S, Veglia F, Salvioni E, Corrà U, Piepoli M, Lagioia R. et al. Heart failure prognosis over time: how the prognostic role of oxygen consumption and ventilatory efficiency during exercise has changed in the last 20 years. Eur J Heart Fail. 2019;21:208-17
45. de Groote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004;43:1584-9
46. Lin YS, Huang HY, Lin WH, Wei J, Chen JC, Kuo LY. et al. Oxygen Uptake Efficiency Slope Predicts Major Cardiac Events in Patients With End-Stage Heart Failure. Transplant Proc. 2016;48:956-8
47. Huang IC, Chen YJ, Chen CH, Huang WC, Lin KL. The Pre-Discharge Oxygen Uptake Efficiency Slope Predicts One-Year Cardiovascular Events in Acute Decompensated Heart Failure Patients. Life (Basel). 2022 12
48. Shah MR, Hasselblad V, Gheorghiade M, Adams KF Jr, Swedberg K, Califf RM. et al. Prognostic usefulness of the six-minute walk in patients with advanced congestive heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2001;88:987-93
49. Giannitsi S, Bougiakli M, Bechlioulis A, Kotsia A, Michalis LK, Naka KK. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. 2019;13:1753944719870084
50. Fan Y, Gu X, Zhang H. Prognostic value of six-minute walk distance in patients with heart failure: A meta-analysis. Eur J Prev Cardiol. 2019;26:664-7
51. Imajo Y, Taguchi T, Yone K, Okawa A, Otani K, Ogata T. et al. Japanese 2011 nationwide survey on complications from spine surgery. J Orthop Sci. 2015;20:38-54
52. Yang JH, Lee BH, Eum KS, Suk KS, Park JO, Kim HS. et al. Prevalence of Gastrointestinal and Cardiovascular Risk in Patients with Degenerative Lumbar Spinal Disease. Clin Orthop Surg. 2020;12:343-52
53. Kim SK, Choe JY. Comorbidities and Health-Related Quality of Life in Subjects with Spine Osteoarthritis at 50 Years of Age or Older: Data from the Korea National Health and Nutrition Examination Survey. Medicina (Kaunas). 2022 58
54. Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:938-46
55. Swain S, Coupland C, Mallen C, Kuo CF, Sarmanova A, Bierma-Zeinstra SMA. et al. Temporal relationship between osteoarthritis and comorbidities: a combined case control and cohort study in the UK primary care setting. Rheumatology (Oxford). 2021;60:4327-39
56. Haugen IK, Ramachandran VS, Misra D, Neogi T, Niu J, Yang T. et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis. 2015;74:74-81
57. Kauppila LI, McAlindon T, Evans S, Wilson PW, Kiel D, Felson DT. Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976). 1997;22:1642-7 discussion 8-9
58. Kauppila LI. Atherosclerosis and disc degeneration/low-back pain-a systematic review. Eur J Vasc Endovasc Surg. 2009;37:661-70
59. Zhang J, Song L, Liu G, Zhang A, Dong H, Liu Z. et al. Risk factors for and prevalence of knee osteoarthritis in the rural areas of Shanxi Province, North China: a COPCORD study. Rheumatol Int. 2013;33:2783-8
60. Ren Y, Hu J, Tan J, Tang X, Li Q, Yang H. et al. Incidence and risk factors of symptomatic knee osteoarthritis among the Chinese population: analysis from a nationwide longitudinal study. BMC Public Health. 2020;20:1491
61. Karasik D, Kiel DP, Kiely DK, Cupples LA, Wilson PW, O'Donnell CJ. et al. Abdominal aortic calcification and exostoses at the hand and lumbar spine: the Framingham Study. Calcif Tissue Int. 2006;78:1-8
62. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M. et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511
63. Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67(Suppl 3):iii75-82
64. Hoeven TA, Kavousi M, Ikram MA, van Meurs JB, Bindels PJ, Hofman A. et al. Markers of atherosclerosis in relation to presence and progression of knee osteoarthritis: a population-based cohort study. Rheumatology (Oxford). 2015;54:1692-8
65. Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S. et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol. 2015;65:976-83
66. Philbin EF, Groff GD, Ries MD, Miller TE. Cardiovascular fitness and health in patients with end-stage osteoarthritis. Arthritis Rheum. 1995;38:799-805
67. Ries MD, Philbin EF, Groff GD. Relationship between severity of gonarthrosis and cardiovascular fitness. Clin Orthop Relat Res. 1995:169-76
Author contact
![]() Corresponding author: Dr. I-Hsiu Liou, Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital. No.386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City 813, Taiwan (R.O.C.), Phone number: +886-7-3422121 ext 4211, Fax number: +886-7-3468205, e-mail: isliuvghkscom.
Corresponding author: Dr. I-Hsiu Liou, Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital. No.386, Dazhong 1st Rd., Zuoying Dist., Kaohsiung City 813, Taiwan (R.O.C.), Phone number: +886-7-3422121 ext 4211, Fax number: +886-7-3468205, e-mail: isliuvghkscom.

 Global reach, higher impact
Global reach, higher impact