Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(15):4049-4062. doi:10.7150/ijms.118252 This issue Cite
Research Paper
Tetrahydrocurcumin Protects Microglial Cells Against Pseudomonas aeruginosa Lipopolysaccharide-Induced Reactive Oxygen Species Production and Cathepsin B to Activate NLRP3 Inflammasome-Mediated Pyroptosis Via the HO-1 and p38/JNK Pathway
1. Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
2. Department of Medical Research, Chung Shan Medical University Hospital, 40201, Taichung, Taiwan.
3. Rehabilitation Sciences & Technology, University of Wisconsin-Milwaukee, Milwaukee, WI, 53211, USA.
4. Department of Microbiology and Immunology, School of Medicine, Chung-Shan Medical University, and Clinical Laboratory, Chung Shan Medical University Hospital, Taichung 40201, Taiwan.
5. Department of Optometry, Asia University, Taichung 413, Taiwan.
6. Department of Nursing, Chung Shan Medical University, Taichung, 40201, Taiwan.
7. Emergency department, St. Martin De Porres Hospital, Chiayi, 60069, Taiwan.
Received 2025-5-26; Accepted 2025-8-19; Published 2025-9-21
Abstract
Tetrahydrocurcumin (THC), a major curcuminoid metabolite known for its antioxidant and anti-inflammatory properties, has potential for reducing brain inflammation. This study investigated THC's anti-inflammatory responses and molecular mechanisms against Pseudomonas aeruginosa LPS (P.a. LPS)-induced NLRP3 inflammasome and ROS-producing cell pyroptosis. In the current study, we showed that THC significantly attenuated P.a. LPS-induced inflammasome factor IL-18 production through lysosomal dysfunction and leakage of cathepsin B. Importantly, THC reduced the levels of NLRP3 inflammasome and pyroptosis-related proteins, including NLRP3, active caspase-1, ASC, N-GSDMD, and IL-18. We also demonstrate that the inhibition of NLRP3 inflammasome activation by THC is ROS-dependent, via inhibition of H2O2 production, SOD activity, and enhancement of GSH activity. Subsequently, we demonstrated that THC treatment significantly reduced LPS-induced phosphorylation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and increased heme oxygenase-1 (HO-1) expression in BV-2 cells. Furthermore, we used MAPKs (SB203580, SP600125, and U0126) and HO-1 (SnPP) inhibitors to demonstrate that THC modulated inflammasome-mediated pyroptosis may be related to p38 and JNK MAPK and HO-1-dependent inflammatory signaling. Overall, THC can inhibit ROS-triggered NLRP3 inflammasome-mediated pyroptosis by promoting GSH activity and HO-1 expression via modulating the p38 and JNK signaling pathways in P.a. LPS-treated BV-2 cells.
Keywords: Tetrahydrocurcumin (THC), Pseudomonas aeruginosa LPS (P.a. LPS), NLRP3, inflammasome, pyroptosis, BV-2 cells
1. Introduction
The prevalence of neurodegenerative diseases, particularly Parkinson's and Alzheimer's disease, has markedly increased in recent decades [1, 2]. While the precise mechanism remains elusive, excessive microglial activation has emerged as a contributing factor to the pathological progression of these conditions and the cascade of inflammatory responses in the brain [3]. Activated microglia can release molecules that activate the inflammasome leading to induced pyroptosis in nearby cells, thereby promoting the accumulation of amyloid beta (Aβ) and neurofibrillary tangles in the brain [4]. Conversely, attenuating microglia activation has shown promise in restoring synaptic integrity and improving functional outcomes in neurodegenerative diseases. This underscores the pivotal role of microglial immune responses in the exacerbation of neuroinflammation and neurodegenerative diseases [5].
Oxidative stress is also induced during inflammation and can modulate microglial activity. In the context of exogenous encephalitis infection, characterized by the presence of bacterial lipopolysaccharide (LPS), a study conducted by Park and colleagues revealed that LPS is proficient in elevating the levels of mitochondrial reactive oxygen species (ROS) in microglia [6]. This increase subsequently triggers the production of tumor necrosis factor-α (TNF-α), interleukin (IL-1β, IL-6), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) through mitogen‑activated protein kinase (MAPK signaling pathways extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK) pathway regulation [6]. In another study, Han et al. Using quercetin inhibits LPS-induced mitochondrial ROS-mediated NLRP3 inflammasome activation in microglia by promoting mitochondrial autophagy [7]. Pyroptosis and oxidative stress can perpetuate each other; for example, pyroptosis can lead to the release of cellular contents, including reactive oxygen species, which can exacerbate oxidative stress [6]. A notable mechanism through which this threat manifests is the activation of the NOD-like receptor protein 3 (NLRP3) inflammasome during the occurrence of pyroptosis [8]. Pyroptosis is a programmed cell death pathway that is often associated with inflammation, characterized by the activation of proinflammatory factors, such as IL-1β, IL-18, and NLRP3, the formation of pores in the cell membrane, and the release of cytoplasmic contents, which can trigger an immune response. However, cytokine maturation and release are dependent on Gasdermin D (GSDMD) in pyroptotic cells [9]. Conversely, oxidative stress can promote the activation of inflammasomes and the induction of pyroptosis, leading to a cascade of inflammatory events and potential harm to the central nervous system [4, 9, 10]. Therefore, understanding these interactions is crucial for developing therapeutic strategies to mitigate the progression of those diseases.
Tetrahydrocurcumin (THC), the major active metabolite of the natural antioxidant curcumin, is highly bioavailable [11, 12] and bioactive and has antioxidant, anti-inflammatory [13], and neuroprotective effects [14]. We previously used a nitric oxide assay to demonstrate the protective effects of THC against Pseudomonas aeruginosa LPS (P.a. LPS)-induced hyperactivation of microglia and discovered that THC inhibited the STAT/JAK signaling pathway-mediated activation of inflammatory factors [15]. Studies have also found that THC exhibits protective potential against septic cardiomyopathy by reducing oxidative stress and inflammation through modulation of JNK/ERK signaling [16]. Although previous studies have illustrated the benefits of THC in antioxidant and anti-inflammatory related diseases, its antioxidant and anti-inflammatory effects on LPS-induced microglia have not been fully studied. Therefore, we aimed to investigate whether the role of THC in P.a. LPS-induced reactive oxygen species production to activate NLRP3 inflammasome-mediated pyroptosis is regulated via the HO-1 and MAPK pathway in BV-2 cells (a mouse microglial cell line).
2. Materials and Methods
2.1. Chemicals
SB203580 (p38 inhibitor, Cat. S1076), U0126 (ERK-1/2 inhibitor, S1102), and SP600125 (JNK inhibitor, S1460) were purchased from Selleck Chemicals (Houston, TX, USA). Tin protoporphyrin IX (SnPP; HO-1 inhibitor, Cat. HY101194) was purchased from MedChemExpress (NJ, USA).
2.2. Microglial cell culture
The murine microglial BV-2 cell line was obtained from Dr. Dah-Yu Lu (China Medical University, Taichung, Taiwan), who purchased the cell line from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI 1640 medium (Gibco, NY, USA) containing 10% fetal bovine serum (No.10437, Gibco) and 100 U/mL penicillin-100 U/mL streptomycin (Anti-Anti; Gibco). All cells were cultured at 37°C in an atmosphere of 5% CO2 in air. BV-2 cells were seeded in 12-well plates overnight at 2.5 × 105 cells/well before treatment to ensure attachment. Cells were pretreated with different concentrations of THC (0, 10, 20, and 40 μM) for 1.5 h and then treated with P. aeruginosa LPS (0.1 μg/mL) for subsequent analysis.
2.3. Cell viability assay
After the designed reaction, 10 μL of CCK-8 reagent (Dojindo Molecular Technologies, Japan) was added to each group of BV-2 cells, which were then dissolved in 500 μL of RPMI medium and incubated at 37°C for 30 min. Next, 200 μL of the supernatant was transferred to a 96-well plate, and absorbance at 450-595 nm was quantified using a Multiskan SkyHigh microplate spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). All percentages were calculated as absorbance values compared with the control.
2.4. Enzyme-linked immunosorbent assay
After the designed reaction, the cultured medium of each group was centrifuged at 12,000 × g for 5 min. Extracellular IL-18 levels were measured by using a sandwich ELISA kit (DY122-05, Lot 340606; R&D Systems, MN, USA) according to the manufacturer's instructions.
2.5. Measurement of intracellular ROS levels
After the designed reaction, the cell culture medium of each group was replaced with 10% RPMI containing 2 μM 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA; No. 15240; AAT Bioquest, Pleasanton, CA, USA) and incubated at 37°C for 30 min. BV-2 cells were separated by 0.25% trypsin-EDTA (No. 25200056, Thermo Fisher Scientific), and their absorbance at 485 nm was measured using a NovoCyte flow cytometer (Agilent, CA, USA). All percentages were calculated as absorbance values compared with the control.
2.6. Measurement of antioxidant enzyme activity and hydrogen peroxide levels
After the designed reaction, the cell lysates of each group were collected, and the activities of SOD (Cat. 706002), catalase (Cat. 707002), and glutathione (Cat. 703002) were measured using detection kits purchased from Cayman (Ann Arbor, Michigan, USA). The cultured medium was centrifuged at 12,000 × g for 5 min, and hydrogen peroxide (H2O2) levels were measured using an assay kit (K265200, Biovision Research, Milpitas, CA, USA). All percentages were calculated as values compared with the control.
2.7. Acridine orange staining
After the designed reaction, the cell culture medium of each group was replaced with 10% RPMI containing 5 μM acridine orange (ab270791, Abcam, UK) and incubated at 37°C for 30 min. BV-2 cells were separated using 0.25% trypsin-EDTA (No. 25200056, Thermo Fisher Scientific), and absorbance at 615 nm (red fluorescence) was measured using a NovoCyte flow cytometer (Agilent). All percentages were calculated as absorbance values compared with the control.
2.8. Annexin V and propidium iodide staining
After the designed reaction, BV-2 cells were separated using 0.25% trypsin-EDTA and treated with 0.25 µg/mL annexin V-fluorescein isothiocyanate and 1 µg/mL propidium iodide (PI) for 1 h in the dark. Absorbance was measured using a flow cytometer. Annexin V-fluorescein isothiocyanate was detected at 485 nm, and PI was detected at 615 nm.
2.9. Western blotting
After the designed reaction, cell lysates were diluted in RIPA buffer and boiled at 95°C for 5 min. Proteins from each group were separated using SDS-PAGE (8%-12%) and transferred to polyvinylidene fluoride membranes (Pall Life Sciences, NY, USA). The membranes were soaked in 5% nonfat milk (in 1x PBST) for 2 h and then incubated with primary antibodies GSDMD (Lot.5500013333, ABclonal, MA, USA), NLRP3 (NBP2-12446, Novus Biologicals, CO, USA), AIM2 (B8, Santa Cruz), ASC (F-9, Santa Cruz, Tx, USA), cleaved-caspase-1 (Asp 927, Santa Cruz), IL-1β (ab9722, Abcam), IL-18 (ab191152, Abcam), and GAPDH (6C5, Santa Cruz) overnight at 4 °C. The membranes were then washed and incubated with a secondary antibody (anti-rabbit/mouse IgG) conjugated with horseradish peroxidase (Thermo Fisher Scientific) for 1 h. Proteins were detected using an enhanced chemiluminescence primer (Cytiva, MA, USA). Protein intensities were quantified using AlphaImager 2200 (Alpha Innotech, San Leandro, CA, USA), and folds were compared with a control group.
2.10. Statistical analysis
The groups and enrollees in this study were randomly assigned. Calculations were performed in Microsoft Excel. Groups were compared using one-way analysis of variance (ANOVA) and post hoc tests of least significant difference. Statistical significance was indicated by p < 0.5.
3. Results
3.1. THC decreased NLRP3 inflammasome-mediated pyroptosis in the P.a. LPS-induced BV2 cells
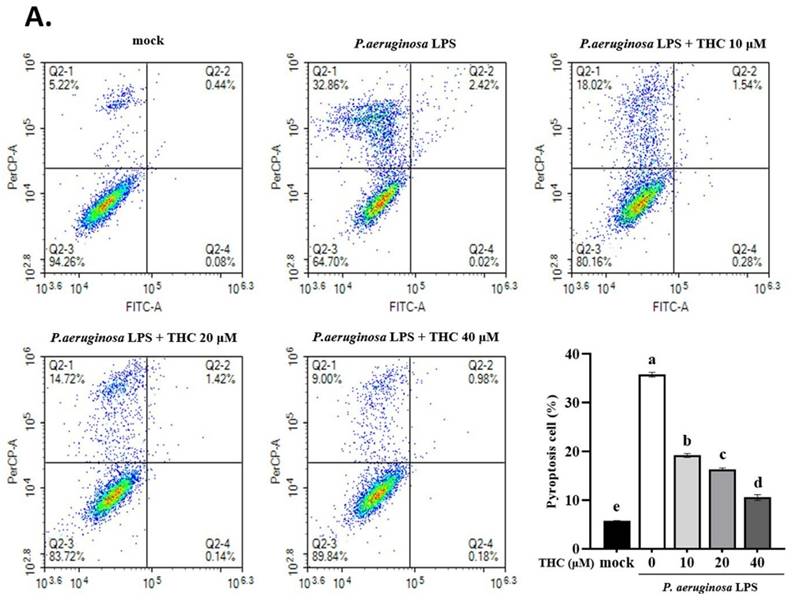
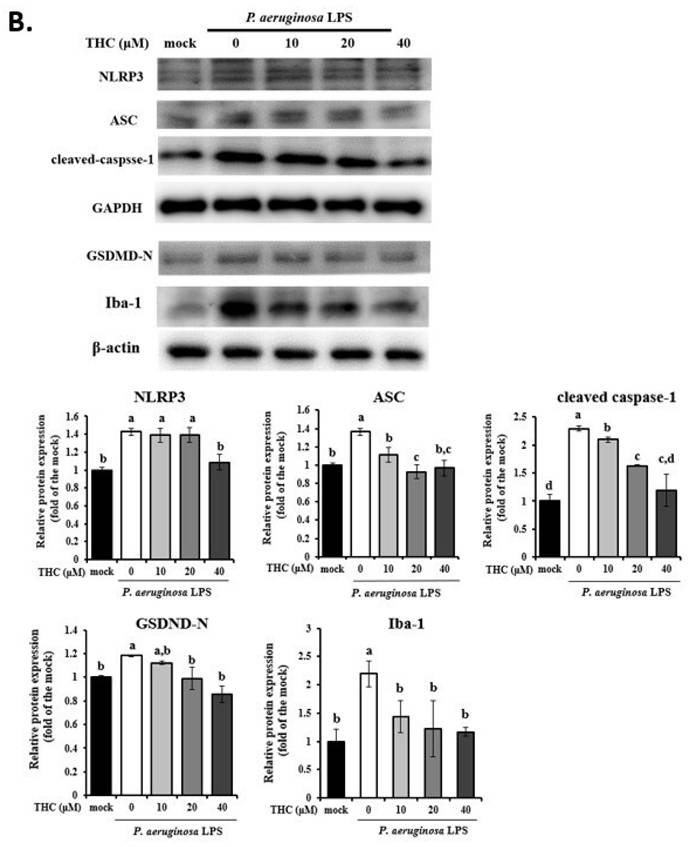
THC is known to exert anti-inflammatory effects by inhibiting the expression of pro-inflammatory cytokines, but its mechanism of action is still unclear, whether it is through blocking NLRP3 inflammasome activation and cell pyroptosis [17]. As demonstrated in Fig. 1A, the rate of pyroptosis (annexin V-/PI+) in the P.a. LPS-treated BV-2 cells was significantly higher than the mock group; however, cells pretreated with 40 µM THC were significantly lower than the P.a. LPS-treated group. We also collected BV-2 cell lysates to analyze protein expression by western blotting. We found that THC protected P.a. LPS-induced BV-2 cells notably downregulated the NLRP3, decreased cleavage-caspase-1, and ASC protein expression (Fig. 1B). Previous studies have shown that inflammatory caspase-induced gasdermin D (GSDMD) activation leads to increased expression of N-terminal GSDMD (GSDMD-N) protein, which induces cell pyroptosis [18]. To further confirm whether THC can affect the pathogenesis of P.a. LPS-induced pyroptosis, we performed western blotting to study its GSDMD-N manifestations. The data showed that the P.a. LPS-induced BV-2 cells have higher GSDMD-N expression than the mock groups, but THC pretreatment led to a significant reduction of its expression. Moreover, THC was found to reduce the Iba-1 in P.a. LPS-induced BV-2 cells (Fig. 1B). These results suggest that THC can regulate the activation of BV-2 cells by inhibiting P.a. LPS-induced NLRP3 inflammasome leading to cell pyroptosis.
3.2. THC suppresses cathepsin B (CTSB) protein to activate inflammasomes-derived IL-18 secretion in P.a. LPS-induced BV-2 cells
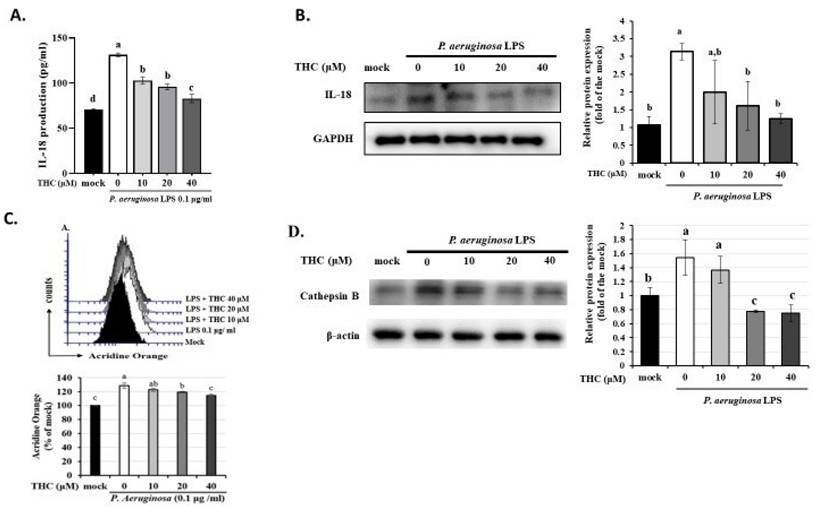
We then used ELISA and western blotting to investigate whether THC can inhibit the formation of the inflammasome and thereby protect microglia by inhibiting the expression of IL-18 in P.a. LPS-treated BV-2 cells. According to Fig. 2A, P.a. LPS markedly increased extracellular IL-18 levels compared to the control group. However, as shown in Fig. 2B, pretreatment with THC significantly reduced IL-18 levels in a dose-dependent manner, with the most pronounced effect observed at the highest concentration (40 µM) of THC. These findings confirm that THC effectively suppresses microglial inflammatory responses.
Effects of THC on pyroptosis production in P.a. LPS-treated BV-2 cells. The BV-2 cells were pretreated with THC (0, 10, 20, or 40 µM) for 1.5 h, and then treated with 0.1 µg/mL P.a. LPS for 24 h. (A.) The BV-2 cells were stained with annexin V and propidium iodide (PI) to isolate pyroptotic cells. The cells were divided into four regions {Q2-1 = annexin V (-), PI (+); Q2-2 = annexin (+), PI (+); Q2-3 = annexin V (-), PI (-); Q2-4 = annexin V (+), PI (-)}, and pyroptotic cells were counted (Q2-1). The pyroptotic cells were expressed as a percentage compared with the mock. (B.) The cell lysates were analyzed to evaluate proteins associated with NLRP3 inflammasome via western blotting. Relative expression of proteins was expressed as a fold compared with the control group (mock). All data are presented as mean ± standard deviation (n = 3). a-e with the same symbol means no statistical difference was observed between groups (p < 0.05).
Lysosomal dysfunction plays an essential role in NLRP3 inflammasome activation [19]. Acridine Orange emits red fluorescence upon accumulation in intact lysosomes, but shifts to green fluorescence when released from ruptured lysosomes and dispersed into the cytosol and nuclei [20]. To evaluate the effect of THC on the lysosomal membrane stability of P.a. LPS-treated BV-2 cells were analyzed by AO staining. As demonstrated in fig. 2C, P.a. LPS increased the acridine orange red fluorescence in BV2 cells; however, pretreatment with 40 µM THC decreased the fluorescence. Recent studies have shown that the integrity of the lysosomal membrane is disrupted, which may lead to the release of the lysosomal protease cathepsin B, thereby inducing NLRP3 activation [21]. The results suggest that THC also significantly lowered the expression of cathepsin B in P.a. LPS-treated BV-2 cells (Fig. 2D). These findings indicate that THC might inhibit NLRP3 inflammasome activation by maintaining the integrity of lysosomes.
3.3. THC inhibited P.a. LPS-induced ROS production
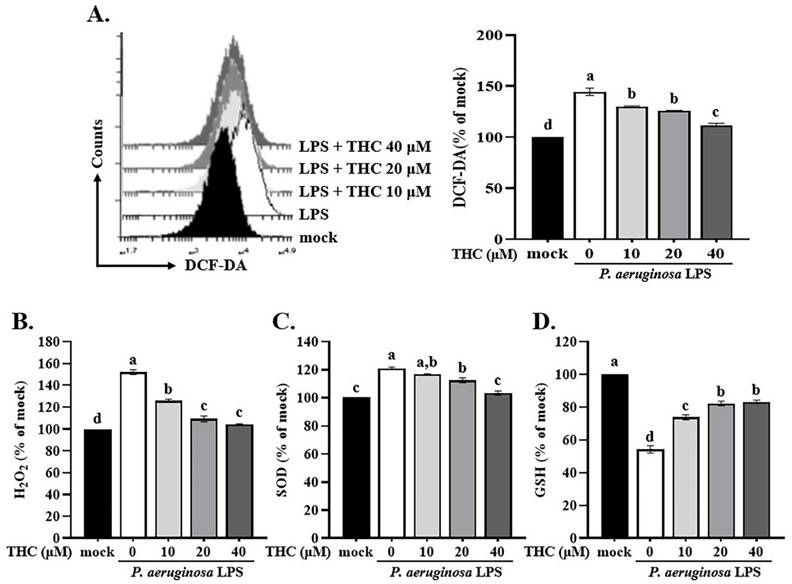
ROS are an important factor in immune regulation and a potential risk factor for an excessive inflammatory response [22]. Previous studies have shown that NF-κB or ROS inhibitors attenuate the LPS-induced upregulation of NLRP3 mRNA, highlighting the critical involvement of NF-κB and ROS in the regulation of NLRP3 gene expression [23, 24]. However, whether THC regulates ROS production or interferes with activation of the NLRP3 inflammasome is unclear. P.a. LPS increased ROS in BV-2 cells, as shown by stronger H2DCF-DA fluorescence. However, in cells pretreated with 40 μM THC, the fluorescence was reduced, indicating lower ROS levels (Fig. 3A).
H2O2 is a ROS that in high concentrations, can cause oxidative damage to cells. An imbalance between superoxide dismutase (SOD) and glutathione can lead to H2O2 levels increase and oxidative stress [25], which has been implicated in various diseases, including cancer, neurodegenerative disorders, and cardiovascular disease [24]. To explore THC's regulation of P.a. LPS-induced ROS production in BV-2 cells, we examined the activity levels of antioxidant enzymes and intracellular H2O2 levels. BV-2 cells treated with P.a. LPS showed higher levels of H₂O₂ compared to the mock group (Fig. 3B). In addition, P.a. LPS increased the activity of the antioxidant enzyme SOD while decreasing GSH activity (Fig. 3C and D), which is consistent with the findings of Park and Chun, who reported that P.a. LPS contributes to microglial inflammatory response [26]. However, pretreatment with 40 µM THC reversed these effects, reducing SOD activity and restoring GSH levels, suggesting a protective role of THC against oxidative stress in BV-2 cells.
Effects of THC on the regulation of cathepsin B (CTSB) protein to activate inflammasomes-derived IL-18 secretion in P.a. LPS-treated BV-2 cells. The BV-2 cells were pretreated with THC (0, 10, 20, or 40 µM) for 1.5 h, and then treated with 0.1 µg/mL P.a. LPS for 24 h. (A.) ELISA was used to detect the production of IL-18 in the cell culture supernatants. (B.) The cell lysates were analyzed to evaluate IL-18 via western blotting. Fluorescence intensity was quantified as a percentage compared with the control group (mock). Relative expression of proteins was expressed as a fold compared with the control group (mock). (C.) Acridine Orange staining for inflammasomes study by flow cytometry. (D.) The cell lysates were analyzed to evaluate cathepsin B expression via western blotting. a-d with the same symbol means no statistical difference was observed between groups (p < 0.05).
Effects of THC on ROS levels and antioxidant enzyme activity in P.a. LPS-treated BV-2 cells. After pretreatment with THC (0, 10, 20 or 40 µM) for 1.5 h and treatment with 0.1 µg/mL P.a. LPS for 24 h, (A) BV-2 cells in each group were stained with H2DCF-DA, intracellular ROS levels were examined through flow cytometry, and the activity levels of (B) H2O2, (C) SOD and (D) GSH were measured. a-d with the same symbol means no statistical difference was observed between groups (p < 0.05).
3.4. THC reduced p38/JNK signaling pathway in P. a. LPS-induced BV-2 cells
The MAPKs/NFκB signaling pathway is a typical regulator of inflammatory responses. This pathway signals the activation of microglial inflammasomes under various stresses, according to various in vivo and in vitro models involving LPS-induced ischemia-reperfusion injury [19, 27]. To assess whether THC regulated the activation of MAPK signaling pathways by P.a. LPS-induced BV2 cells, we used western blot and ELISA to examine the signal-related protein and inflammatory cytokines.
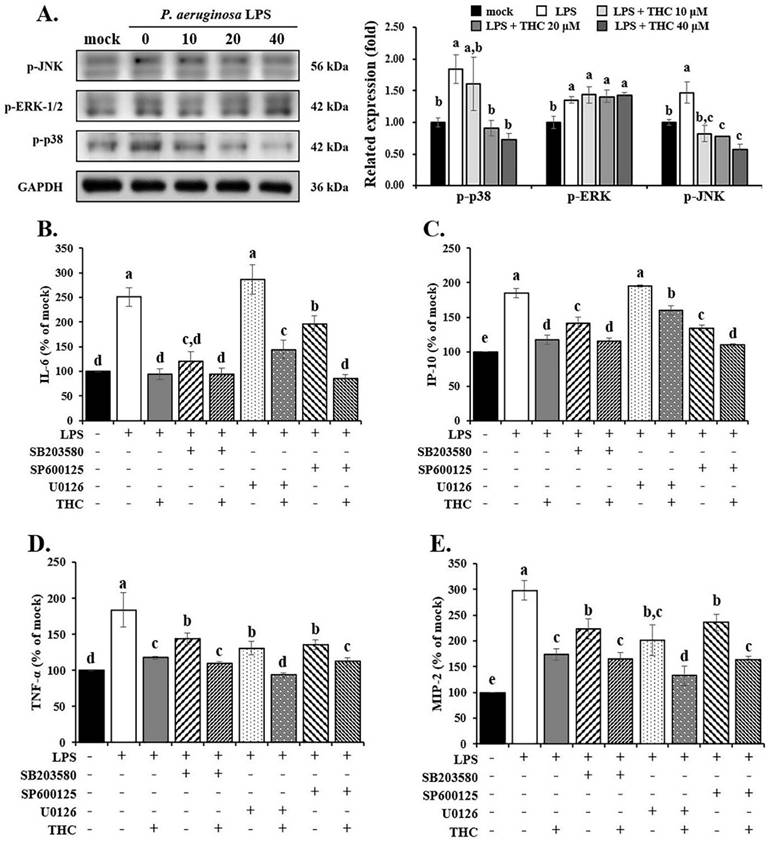
As shown in Fig. 4A, the protein expression of p-ERK-1/2, p-p38, and p-JNK was raised in P.a. LPS-treated BV-2 cells. However, in the THC experimental group, p-p38 and p-JNK expression were significantly reduced, especially in the 40 µM THC group, but no inhibition of p-ERK-1/2 was observed.
In addition, we also determined the effect of THC on the inflammatory factors by LPS-induced BV2 cells using ELISA. The results showed that using SB203580 (a p38 inhibitor, 10 µM) or SP600125 (a JNK inhibitor, 20 µM) [28, 29] on their own lowered the levels of IL-6, IP-10, TNF-α, and MIP-2 (Fig. 4B-E). In contrast, U0126 (an ERK inhibitor, 10 µM) [28] had little to no effect on IL-6 and IP-10 (Fig. 4B and C). However, when THC was added together with these inhibitors in LPS-treated BV-2 cells, it further reduced the levels of all four inflammatory markers more than the inhibitors alone.
3.5. THC prevented P. a. LPS-induced pyroptosis via the p38/JNK signaling pathway
To investigate whether THC affects the mechanisms of LPS-induced inflammation and pyroptosis through the MAPK pathway. We pretreated BV-2 cells with THC and MAPK inhibitors, including SB203580, U0126, and SP600125 for 1.5 h and then treated the BV-2 cells with 0.1 µg/mL P.a. LPS for 24 h. The cells were stained with annexin V/PI to check for pyroptosis using a flow cytometer. In addition, the culture medium was collected to measure the amount of IL-18 released outside the cells using an ELISA test.
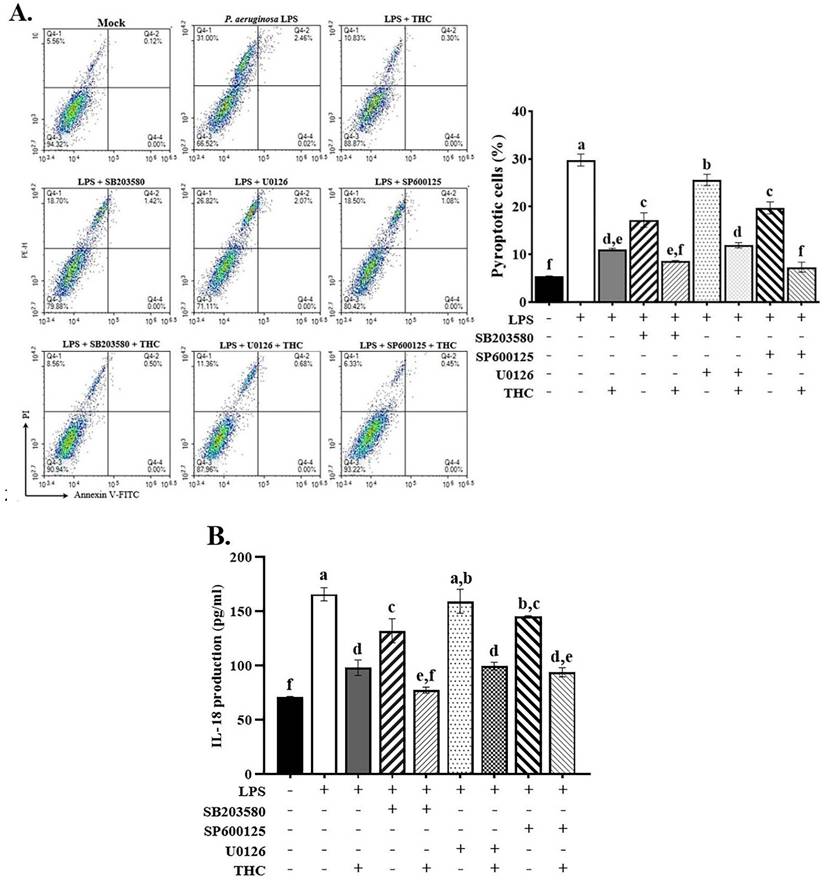
Treatment with THC or MAPK inhibitors markedly reduced the number of pyroptotic cells, with ERK inhibitors showing the least pronounced effect. The combined administration of THC and these inhibitors led to an even greater suppression of P.a. LPS-induced pyroptosis (Fig. 5A). The ELISA data demonstrated that p38 and JNK inhibitors significantly reduced extracellular IL-18 levels, whereas the ERK inhibitor did not produce a significant change. Likewise, co-treatment with THC and the inhibitors led to a further reduction in IL-18 levels (Fig. 5B). These data indicate that THC reduces p38 and JNK expression in LPS-treated BV-2 cells, implying that the regulation of NLRP3 inflammasome-mediated pyroptosis by THC may be related to p38 and JNK MAPK-dependent inflammatory signaling.
Effects of THC on P.a. LPS-mediated MAPK signaling pathways. (A) After pretreatment with THC (0-40 µM) for 1.5 h and treatment with 0.1 µg/mL P.a. LPS for 24 h, cell lysates in each group were collected to analyze the proteins expression of p-JNK, p-ERK and p-p38 through western blotting. After pretreatment with THC (0 or 40 µM), SB 203580 (0 or 10 µM), U0126 (0 or 10 µM), or SP 600125 (0 or 20 µM) for 1.5 h and treatment with 0.1 µg/mL P.a. LPS for 24 h, the levels of (B) IL-6, (C) IP-10, (D) TNF-α and (E) MIP-2 were measured using ELISA kits. The relative expression of proteins was expressed as a percentage compared with the control group (mock). a-e with the same symbol means no statistical difference was observed between groups (p < 0.05).
Effects of THC on P.a. LPS-induced pyroptosis in BV-2 cells by MAPK inhibitors. After pretreatment with THC (0 or 40 µM) and SB 203580 (0 or 10 µM), U0126 (0 or 10 µM), or SP 600125 (0 or 20 µM) for 1.5 h and treatment with 0.1 µg/mL P.a. LPS for 24 h, (A) BV-2 cells were stained with annexin V and propidium iodide (PI) to isolate pyroptotic cells, and (B) extracellular IL-18 levels were measured in the supernatant using ELISA kits. a-f with the same symbol means no statistical difference was observed between groups (p < 0.05).
3.6. THC alleviated ROS-mediated pyroptosis in P. aeruginosa LPS-induced BV2 cells
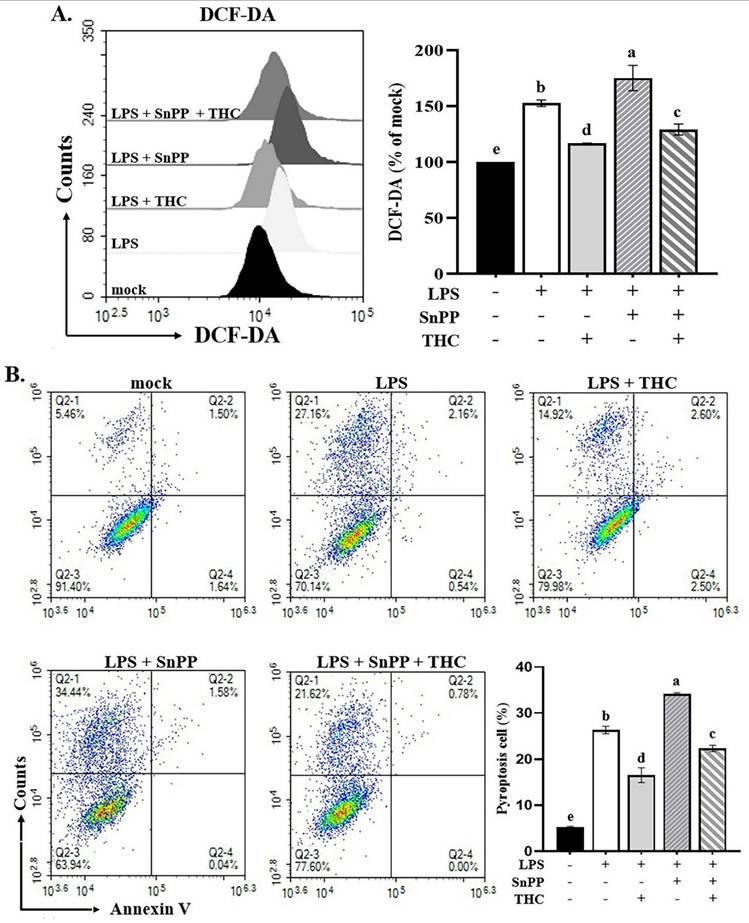
In our previous studies, we have shown that THC moderates LPS-induced inflammatory responses through the Nrf-2/HO-1 signaling pathway [15]. Park et al. demonstrated that the Nrf-2/HO-1 signaling pathway was involved in decreasing LPS-induced ROS production in BV-2 cells [30]. It is not clear whether THC can protect BV-2 cells from ROS-mediated inflammasome activation induced by P.a. LPS through the Nrf-2/HO-1 signaling pathway. We further examined the effects of THC and the HO-1 inhibitor SnPP (20 μM) [15] on ROS production and pyroptosis. As shown in Fig. 6A, pretreatment with THC significantly reduced ROS levels in P.a. LPS-LPS-stimulated BV-2 cells, whereas SnPP increased ROS levels. Interestingly, co-treatment with THC and SnPP attenuated this ROS elevation. Consistently, Fig. 6B demonstrates that SnPP markedly enhanced pyroptosis, while co-administration of THC suppressed this effect. These findings indicate that THC inhibits ROS-mediated NLRP3 inflammasome activation through the p38/JNK and Nrf-2/HO-1 signaling pathways.
Effects of THC on ROS production and pyroptosis in BV-2 cells with or without HO-1 inhibitor. After pretreatment with THC (0 or 40 µM) and with HO-1 inhibitor SnPP (0 or 20 µM) for 1.5 h and treatment with 0.1 µg/mL P.a. LPS for 24 h, BV-2 cells in each group were (A) stained with H2DCF-DA to measure intracellular ROS levels or (B) stained with annexin V and propidium iodide (PI) to isolate pyroptotic cells. DHICA fluorescence intensity was quantified as a percentage compared with the control group (mock). a-e with the same symbol means no statistical difference was observed between groups (p < 0.05).
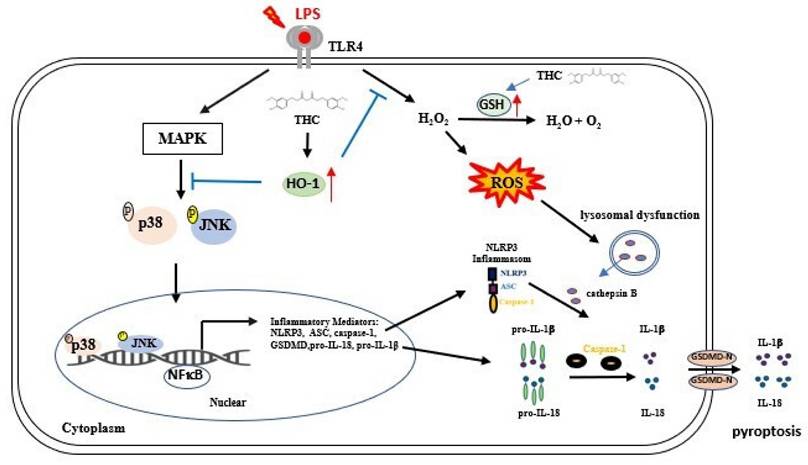
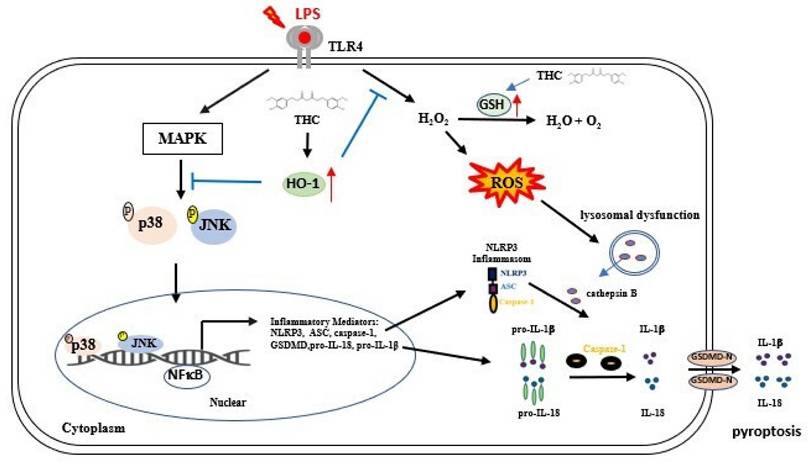
Schematic diagram of THC regulation in P.a. LPS-induced NLRP3 inflammasome led to pyroptosis in BV-2 cells. THC regulates the NLRP3 inflammasome signaling pathway at different levels. P.a. LPS induction NLRP3 inflammasomes-associated proteins through the MAPK/NFκB signaling pathway and inhibits GSH activity, thus raising intracellular H2O2 levels causing ROS accumulation. Additionally, the induction of lysosomal dysfunction release of cathepsin B triggers the activation of the NLRP3 inflammasome leading to pyroptosis, resulting in the release of IL-1β, and IL-18. However, THC inhibited NLRP3 inflammasome activation leading to pyroptosis by inhibiting the p38/JNK/NFκB signaling pathway and inducting GSH activation alleviated ROS production within cells through the signaling pathway Nrf-2/HO-1, thus inhibiting pyroptosis.
4. Discussion
As the global population ages, the prevalence of neurodegenerative diseases is expected to continue increasing. Neurodegeneration refers to the complex process of progressive degeneration or abnormal death of neurons, resulting in a series of incurable and debilitating diseases [31]. From a pathological perspective, neuronal loss associated with gliomas, protein misfolding and deposition, resulting in abnormal filamentous deposits is the main symptom of NDs [32]. There has been evidence that neuronal degeneration is related to pyroptosis, which is mediated by the NLRP3 inflammasome [33]. Excessive pyroptosis can lead to tissue damage and plays a crucial role in infectious illnesses [20]. LPS-treated BV-2 cells have been used to mimic brain microglial inflammation patterns for many years [34]. Curcumin has been shown to protect against apoptosis and to have antioxidant and anti-inflammatory effects in in vitro models of various diseases; however, curcumin is not highly bioavailable in vivo [35, 36]. Aggarwal et al. and Zhang et al. have revealed that THC, a natural derivative of curcumin, retains the biological activities of curcumin and has the advantages of permeability and residence time in vivo [11, 12]. We previously demonstrated the protective effect of THC on P.a. LPS-induced inflammation by inhibiting the JAK/STAT and Nrf2/HO-1 pathway in microglia [15]. However, whether THC can inhibit P.a. LPS-induced NLRP3 inflammasome leading to pyroptosis in microglia remains unclear, so we designed this study to explore these effects.
Pyroptosis is an inflammatory form of cell death characterized by the activation of the NLRP3 inflammasome, which transforms procaspase-1 into cleaved caspase-1 and promotes the GSDMD to generate GSDMD N-termini (GSDMD-N) then the release of proinflammatory cytokines such as IL-1β and IL-18 [37]. In this study, we found that P.a. LPS induced pyroptosis in BV-2 cells, while THC pretreatment significantly suppressed this effect (Fig. 1A). Furthermore, THC also reduced the expression of key NLRP3 inflammasome-related proteins (including NLRP3, ASC, cleaved caspase-1, and GSDMD-N) in BV-2 cells stimulated with P.a. LPS (Fig. 1B). In our study, IL-18 levels were also assessed, and the results confirmed that THC reduced IL-18 concentrations in P.a. LPS-stimulated BV-2 cells, further supporting its inhibitory effect on inflammasome activation. (Figure 2A and 2B). These findings confirm that THC can inhibit LPS-induced NLRP3 inflammasome-mediated pyroptosis.
Cells were stained with acridine orange to study the integrity of lysosomes, which accumulate in acidic compartments and emit red fluorescence. Our results showed that P.a. LPS increased red fluorescence, whereas THC pretreatment reduced this effect (Fig. 2C), suggesting that THC could prevent the formation of lysozyme. Previous studies have shown that nicotine, free fatty acids, and Lactobacillus casei wall components can trigger NLRP3 activation through lysosomal damage and the release of cathepsin B [38; 39]. In our results, we also found that THC could inhibit the expression of cathepsin B (Fig. 2D). Therefore, we speculated that THC might inhibit NLRP3 activation by reducing the release of cathepsin B through reducing lysozyme production.
ROS are recognized as key triggers of NLRP3 inflammasome activation [40]. In this study, we observed that P.a. LPS stimulation increased ROS levels in BV-2 cells, an effect that was reversed by THC pretreatment (Fig. 3A). The H₂O₂ a type of ROS, plays an important role in various cellular functions. Under physiological conditions, it is efficiently broken down by cellular enzymes to prevent oxidative damage. However, in response to cellular injury, H₂O₂ levels can rise sharply, leading to oxidative stress and potential cellular dysfunction [41, 42]. The SOD converts superoxide anions (O₂•-) into H₂O₂ and oxygen, while catalase and glutathione further break down H₂O₂ into water and oxygen [43]. Our results show that P.a. LPS increases ROS production by elevating H₂O₂ levels and inhibiting GSH activity in BV-2 cells. THC pretreatment counteracts this effect by enhancing GSH activity and reducing H₂O₂ accumulation (Fig. 3B-D). These findings suggest that THC reduces ROS production by inhibiting SOD and enhancing GSH, thereby suppressing NLRP3 inflammasome activation and ultimately preventing pyroptosis.
Excessive ROS production activates MAPK pathways and triggers inflammatory signaling in microglia [44]. Studies have shown that ROS-dependent MAPK signaling plays a key role in regulating NFκB transcription and inflammasome formation in LPS-stimulated BV-2 cells [45, 46]. In our study, THC promoted the phosphorylation of p38 and JNK, but had minimal effect on ERK in BV-2 cells stimulated with P. a. LPS (Fig. 4). As shown in Fig. 5A, treatment with the p38 inhibitor SB203580 and the JNK inhibitor SP600125 significantly reduced IL-18 levels, whereas the ERK inhibitor U0126 did not show a notable effect (Fig. 5B). We also found that all three inhibitors reduced pyroptosis, but U0126 was the least effective (Fig. 5A). These findings suggest that THC regulates NLRP3 inflammasome-mediated pyroptosis primarily through p38- and JNK-dependent MAPK signaling in P. a. LPS-stimulated BV-2 cells.
ROS can activate the NLRP3 inflammasome and aggravate the subsequent inflammatory cascade; however, this phenomenon can be inhibited by activating Nrf-2 [47]. Nrf-2 modulates inflammation by upregulating HO-1, which has anti-inflammatory effects, and inhibiting NLRP3 inflammasome activation through ROS reduction. Previous studies have shown that THC can upregulate the Nrf-2/HO-1 signaling pathway in P.a. LPS-treated BV-2 cells to counteract the production of inflammatory factors IL-6, TNF-α, MIP-2, and IP-10 [15]; however, whether THC can inhibit NLRP3 inflammation and pyroptosis by activating HO-1 has not been demonstrated. Chen et al. suggested that the inhibition of Nrf-2/HO-1 accelerates the activation of inflammasomes stimulated by ROS [47]. This effect was shown to be achieved through the MAPK-mediated NFκB signaling pathway [48]. Therefore, we wished to determine whether THC-regulated Nrf-2/HO-1 confers protection against pyroptosis. BV-2 cells were pretreated with the HO-1 inhibitor SnPP alone or combined with THC before P.a. LPS treatment to assess the impact of HO-1 inhibition on pyroptosis. Our results showed that SnPP inhibited HO-1-induced ROS production and pyroptosis, which were inhibited by pretreatment with THC (Figure 6). This indicates that HO-1 activation plays a role in THC's protection against P.a. LPS-induced NLRP3 inflammasome-mediated pyroptosis in BV-2 cells.
In conclusion, this study provides the first evidence that THC attenuates NLRP3 inflammasome-mediated pyroptosis in P.a. LPS-stimulated BV-2 microglial cells through the inhibition of the p38 and JNK MAPK signaling pathways. Furthermore, THC suppresses ROS-induced pyroptosis by upregulating the expression of antioxidant molecules GSH and HO-1 (Fig. 7). Collectively, these findings highlight the anti-inflammatory properties of THC in microglial cells and suggest its therapeutic potential for the treatment of neuroinflammatory and neurodegenerative disorders.
Acknowledgements
We express our gratitude to the Instrument Resource Center of Chung Shan Medical University for their technical support.
Funding
This study was supported by the Ministry of Science and Technology, Taiwan (project numbers: MOST-109-2320-B-468-004-MY3 and MOST 111-2320-B-040-006-MY3).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
H-W Lin and C-J Chuang planned work and designed experiments. I Wang, L Sun, and Y-Y Chang wrote the manuscript. T-C Chen, J-H Yeh, S-C Tsou, and C-J Chuang collected data, performed experiments, and conducted statistical analysis. All authors analyzed and discussed the results and commented on the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cabreira V, Massano J. Doença de Parkinson: Revisão clínica e atualização. Acta Médica Portuguesa. 2019 32
2. Breijyeh Z, Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. 2020;25:5789
3. Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T. et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Frontiers in cellular neuroscience. 2018;12:488
4. Wang C, Zong S, Cui X, Wang X, Wu S, Wang L. et al. The effects of microglia-associated neuroinflammation on Alzheimer's disease. Frontiers in immunology. 2023;14:1117172
5. Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. Journal of neuroinflammation. 2021;18:258
6. Park J, Min J-S, Kim B, Chae U-B, Yun JW, Choi M-S. et al. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neuroscience letters. 2015;584:191-6
7. Han X, Xu T, Fang Q, Zhang H, Yue L, Hu G. et al. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox biology. 2021;44:102010
8. Coll RC, Schroder K, Pelegrín P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends in pharmacological sciences. 2022;43:653-68
9. Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C. et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310
10. Tufekci KU, Ercan I, Isci KB, Olcum M, Tastan B, Gonul CP. et al. Sulforaphane inhibits NLRP3 inflammasome activation in microglia through Nrf2-mediated miRNA alteration. Immunology letters. 2021;233:20-30
11. Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2014;20:185-205
12. Zhang Z-B, Luo D-D, Xie J-H, Xian Y-F, Lai Z-Q, Liu Y-H. et al. Curcumin's metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effects in vivo through suppression of TAK1-NF-κB pathway. Frontiers in pharmacology. 2018;9:1181
13. Nakmareong S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Kongyingyoes B, Donpunha W. et al. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertension research. 2012;35:418-25
14. Park C-H, Song JH, Kim S-N, Lee JH, Lee H-J, Kang KS. et al. Neuroprotective effects of tetrahydrocurcumin against glutamate-induced oxidative stress in hippocampal HT22 cells. Molecules. 2019;25:144
15. Lin H-W, Chen T-C, Yeh J-H, Tsou S-C, Wang I, Shen T-J. et al. Suppressive effect of Tetrahydrocurcumin on Pseudomonas aeruginosa lipopolysaccharide-induced inflammation by suppressing JAK/STAT and Nrf2/HO-1 pathways in microglial cells. Oxidative Medicine and Cellular Longevity. 2022;2022:4978556
16. Zhu H, Zhang L, Jia H, Xu L, Cao Y, Zhai M. et al. Tetrahydrocurcumin improves lipopolysaccharide-induced myocardial dysfunction by inhibiting oxidative stress and inflammation via JNK/ERK signaling pathway regulation. Phytomedicine. 2022;104:154283
17. Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X. et al. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circulation research. 2016;118:1525-39
18. Li T, Sun H, Li Y, Su L, Jiang J, Liu Y. et al. Downregulation of macrophage migration inhibitory factor attenuates NLRP3 inflammasome mediated pyroptosis in sepsis-induced AKI. Cell death discovery. 2022;8:61
19. Chen X, Wang Y, Yao N, Lin Z. Immunoproteasome modulates NLRP3 inflammasome-mediated neuroinflammation under cerebral ischaemia and reperfusion conditions. Journal of cellular and molecular medicine. 2022;26:462-74
20. Aziz M, Jacob A, Wang P. Revisiting caspases in sepsis. Cell death & disease. 2014;5:e1526-e
21. Eriksson I, Vainikka L, Persson HL, Öllinger K. Real-time monitoring of lysosomal membrane permeabilization using acridine orange. Methods and Protocols. 2023;6:72
22. Zhao W, Ma L, Cai C, Gong X. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. International journal of biological sciences. 2019;15:1571
23. Cunha LD, Silva AL, Ribeiro JM, Mascarenhas DP, Quirino GF, Santos LL. et al. AIM2 engages active but unprocessed caspase-1 to induce noncanonical activation of the NLRP3 inflammasome. Cell reports. 2017;20:794-805
24. Zhao LR, Xing RL, Wang PM, Zhang NS, Yin SJ, Li XC. et al. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Molecular medicine reports. 2018;17:5463-9
25. Liochev SI, Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radical Biology and Medicine. 2007;42:1465-9
26. Park E, Chun HS. Melatonin attenuates manganese and lipopolysaccharide-induced inflammatory activation of BV2 microglia. Neurochemical research. 2017;42:656-66
27. Lo J, Liu C-C, Li Y-S, Lee P-Y, Liu P-L, Wu P-C. et al. Punicalagin attenuates LPS-induced inflammation and ROS production in microglia by inhibiting the MAPK/NF-κB signaling pathway and NLRP3 inflammasome activation. Journal of Inflammation Research. 2022:5347-59
28. Qin S, Yang C, Huang W, Du S, Mai H, Xiao J. et al. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacological Research. 2018;133:218-35
29. Liu P, Gao Q, Guan L, Sheng W, Hu Y, Gao T. et al. Atorvastatin attenuates isoflurane-induced activation of ROS-p38MAPK/ATF2 pathway, neuronal degeneration, and cognitive impairment of the aged mice. Frontiers in aging neuroscience. 2021;12:620946
30. Park YJ, Yang HJ, Li W, Oh Y-C, Go Y. Menthae herba attenuates neuroinflammation by regulating CREB/Nrf2/HO-1 pathway in BV2 microglial cells. Antioxidants. 2022;11:649
31. Ajoolabady A, Pratico D, Tang D, Zhou S, Franceschi C, Ren J. Immunosenescence and inflammaging: Mechanisms and role in diseases. Ageing Research Reviews. 2024;101:102540
32. Ikram M, Park TJ, Ali T, Kim MO. Antioxidant and neuroprotective effects of caffeine against Alzheimer's and Parkinson's disease: Insight into the role of Nrf-2 and A2AR signaling. Antioxidants. 2020;9:902
33. Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death & Differentiation. 2021;28:2029-44
34. Skrzypczak-Wiercioch A, Sałat K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules. 2022;27:5481
35. Yin H, Guo Q, Li X, Tang T, Li C, Wang H. et al. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. The Journal of Immunology. 2018;200:2835-46
36. Liang W-F, Gong Y-X, Li H-F, Sun F-L, Li W-L, Chen D-q. et al. Curcumin activates ROS signaling to promote pyroptosis in hepatocellular carcinoma HepG2 cells. in vivo. 2021;35:249-57
37. Liu Z, Yao X, Sun B, Jiang W, Liao C, Dai X. et al. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radical Biology and Medicine. 2021;168:142-54
38. Zhang Y, Chen Y, Zhang Y, Li P-L, Li X. Contribution of cathepsin B-dependent Nlrp3 inflammasome activation to nicotine-induced endothelial barrier dysfunction. European journal of pharmacology. 2019;865:172795
39. Hong F, Zhao M, Xue L-L, Ma X, Liu L, Cai X-Y. et al. The ethanolic extract of Artemisia anomala exerts anti-inflammatory effects via inhibition of NLRP3 inflammasome. Phytomedicine. 2022;102:154163
40. Sho T, Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnology and applied biochemistry. 2019;66:4-13
41. Dong M-J, Li W, Xiang Q, Tan Y, Xing X, Wu C. et al. Engineering Metal-Organic Framework Hybrid AIEgens with Tumor-Activated Accumulation and Emission for the Image-Guided GSH Depletion ROS Therapy. ACS Applied Materials & Interfaces. 2022;14:29599-612
42. Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nature reviews Molecular cell biology. 2022;23:499-515
43. Lei P, Li Z, Hua Q, Song P, Gao L, Zhou L. et al. Ursolic acid alleviates neuroinflammation after intracerebral hemorrhage by mediating microglial pyroptosis via the NF-κB/NLRP3/GSDMD pathway. International Journal of Molecular Sciences. 2023;24:14771
44. Liu Z, Yao X, Jiang W, Li W, Zhu S, Liao C. et al. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Journal of neuroinflammation. 2020;17:90
45. Kang C-H, Jayasooriya RGPT, Dilshara MG, Choi YH, Jeong Y-K, Kim ND. et al. Caffeine suppresses lipopolysaccharide-stimulated BV2 microglial cells by suppressing Akt-mediated NF-κB activation and ERK phosphorylation. Food and chemical toxicology. 2012;50:4270-6
46. Huang M-Y, Tu C-E, Wang S-C, Hung Y-L, Su C-C, Fang S-H. et al. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC complementary and alternative medicine. 2018;18:221
47. Zou Y, Luo X, Feng Y, Fang S, Tian J, Yu B. et al. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chemico-biological interactions. 2021;345:109573
48. Chen Z, Zhong H, Wei J, Lin S, Zong Z, Gong F. et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis research & therapy. 2019;21:300
Author contact
![]() Corresponding authors: Chen-Ju Chuang: ilovespurs168com; and Yuan-Yen Chang: cyy0709edu.tw.
Corresponding authors: Chen-Ju Chuang: ilovespurs168com; and Yuan-Yen Chang: cyy0709edu.tw.

 Global reach, higher impact
Global reach, higher impact