Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(15):3994-4002. doi:10.7150/ijms.111747 This issue Cite
Research Paper
Cerebrospinal fluid profile in coronavirus disease 2019, a prospective case series study
1. Department of Neurology, Fujian Medical University Union Hospital, Fuzhou, China.
2. Clinical Research Center for Precision Diagnosis and Treatment of Neurological Diseases of Fujian Province, Fuzhou, China.
3. Department of Laboratory Medicine, Fujian Medical University Union Hospital, Fuzhou, China.
4. Department of Neurology, Longyan First Hospital, Longyan, China.
5. Department of Rehabilitation Medicine, Fujian Medical University Union Hospital, Fuzhou, China.
6. Institute of Clinical Neurology, Fujian Medical University, China.
*These authors contributed equally and shared co-first author.
Received 2025-2-8; Accepted 2025-7-10; Published 2025-9-8
Abstract
Background: Cerebrospinal fluid (CSF) analysis in patients with Coronavirus disease 2019 (COVID-19) and co-existing acute neurological involvement remains poorly understood.
Objective: To investigate the CSF profile in patients with COVID-19 and co-existing acute neurological involvement.
Methods: This prospective case series study included patients with confirmed severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection and co-existing acute neurological involvement who underwent lumbar puncture in two teaching hospitals between November 2022 and April 2023. Demographics, clinical characteristics, and CSF profile, including leukocyte count, total protein, and glucose levels, oligoclonal band (OCB) patterns, blood-CSF barrier function, SARS-CoV-2 mRNA, and SARS-CoV-2 antibodies were described.
Results: A total of 26 participants were analyzed. The median age was 51 (interquartile range [IQR] 39-76) years, and 18 (69.2%) were male. The median open CSF pressure was 140mm (IQR 110-183) water column, and the median CSF total protein was slightly elevated (485 [IQR 350-611] mg/L). The most frequent pathological finding was elevated CSF total protein (12 [46.2%] samples) and blood-CSF barrier dysfunction (12 [46.2%] samples). SARS-CoV-2 was undetectable in all CSF samples using the reverse-transcriptase-polymerase-chain-reaction detection. SARS-CoV-2-IgG-antibody was positive in five CSF samples, while SARS-Cov-2 IgM antibodies were not detected in all participants.
Conclusions: This study showed that some patients with COVID-19 and co-existing acute neurological involvement presented non-specific inflammatory CSF abnormalities despite no SARS-CoV-2 being detected in the CSF. Our findings suggest that neurological injury is related to disordered immune responses associated with systemic inflammation rather than direct virus invasion.
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus type 2, nervous system disease, cerebrospinal fluid, neurological involvement
Introduction
The global outbreak of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has killed millions of people worldwide [1,2]. There is accumulating evidence of central neurological symptoms and conditions seen in the context of coronavirus disease 2019 (COVID-19), including headache, encephalopathy, seizures, stroke, Guillain-Barré syndrome, and acute disseminated encephalomyelitis [3-6]. Cytokine storm or neurological verification in cerebrospinal fluid have been reported, but it is unknown whether it is caused by direct viral infection or immune response [6].
The COVID-19 pandemic highlighted the existence of neurological symptoms associated with SARS-CoV-2 infection and raised the question of the neuropathogenicity of coronaviruses [7]. Few case reports have identified SARS-CoV-2 in the CSF by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) or sequencing techniques [8]. However, a comprehensive study of 150 lumbar punctures in 127 patients with COVID-19-associated neurological disease showed that although inflammatory changes were common, direct detection of SARS-CoV-2 in CSF was rare [5]. CSF biomarkers can characterize the central nervous syndrome response to infection by directly detecting invading pathogens and host inflammatory responses [9]. To date, comprehensive data on the cerebrospinal fluid (CSF) profile in patients with COVID-19 and co-existing acute neurological involvement remains not well understood. In this prospective observational study, we aimed to describe the CSF profile in patients with acute neurological diseases associated with COVID-19.
Methods
Participants
This prospective case series study included consecutive adult patients (i.e., 18 years or older) with confirmed SARS-CoV-2 infection who underwent lumbar puncture for CSF testing due to co-existing acute neurological involvement in two teaching hospitals during the Omicron epidemic in mainland China, between November 2022 and April 2023. Patients with pre-existing inflammatory or cerebrovascular conditions were excluded since these conditions may cause CSF changes and are considered less likely related to COVID-19. Confirmed SARS-CoV-2 infection was defined by the detection of SARS-CoV-2 mRNA in nasopharyngeal swabs using qRT-PCR detection, with a cycle threshold value of 37 or lower [10].
Data collection
Demographics, clinical characteristics, indications for lumber puncture, and CSF parameters were collected through the electronic medical database.
CSF assessment
CSF samples were immediately processed to determine cell counts, total protein, and glucose levels. SARS-CoV-2 mRNA in CSF samples was detected using the RT-qPCR kit (Zhengzhou Autobio, China). Detection of oligoclonal bands (OCB) in the CSF was performed by isoelectric focusing on comparing paired CSF and serum samples. Oligoclonal IgG bands were assessed by isoelectric focusing and evaluated according to an international consensus. Immunoglobulins and albumin were measured immunonephelometrically [11]. Oligoclonal bands (OCB) patterns were classified as type 1, OCB detected in neither CSF nor serum; type 2, oligoclonal IgG bands detected in only CSF; type 3, OCB in CSF and serum with additional bands in CSF; type4, identical OCB in CSF and serum; and type 5, monoclonal bands in CSF and serum. Types 2 and 3 indicate intrathecal IgG synthesis, type 4 indicates a systemic, ongoing inflammatory process, and type 5 indicates systemic paraproteinemia [3].
Evaluation of blood-CSF barrier function
Blood-CSF barrier function was assessed using the CSF/serum albumin quotient: QAlb = AlbCSF [mg/L]/Albserum [g/L]. As the upper reference limit of QAlb is age-dependent, Qlim (Alb) was calculated as (4+[a/15])×10-3, with a representing the patient's age. Dysfunction of the blood-CSF barrier was defined as QAlb > Qlim (Alb) [12].
Statistical analyses
Continuous variables were summarized as mean with standard variations if normally distributed or median with interquartile range (IQR) if not normally distributed. Categorical variables were expressed as frequencies with percentages. Spearman's rho test was used to assess correlations. P values < 0.05 were considered statistically significant. All analyses were conducted using SPSS Statistics Version 25.0 (IBM, Chicago, IL, USA).
Results
We included 26 consecutive patients with confirmed SARS-CoV-2 infection who received lumbar puncture due to co-existing acute neurological involvement in the final analysis. The median age was 51 (IQR 39-76), and 18 (69.2%) were male. Table 1 summarizes the demographics and clinical characteristics. The most commonly reported neurological symptoms were limb weakness or numbness (12 cases [46.2%]), followed by dizziness (10 cases [38.5%]), headache (8 cases [30.8%]), and impaired consciousness (8 cases [30.8%]). The clinical diagnosis of neurological involvement included Herpes Simplex encephalitis (2 cases [7.7%]), Streptococcus pneumoniae encephalitis (1 case [3.8%]), encephalitis with unknown etiology (4 cases [15.4%]), acute myelitis (2 cases [7.7%]), and acute cerebellitis (2 cases [7.7%]), cranial neuritis (2 cases [7.7%]), stroke (7 cases, [26.9%]), Guillain-Barre syndrome (3 cases [11.5%]), demyelinating encephalopathy (1 case [3.8%]), epilepsy with unknown etiology (1 case [3.8%]), extrapyramidal disorder with unknown etiology (1 case [3.8%]).
Table 2 lists the significant findings of the CSF profile. The median open CSF pressure was 140mm [IQR 110-183] water column, the median leucocyte count was 2×106/L, and the median CSF total protein level was 485 mg/L (IQR 350-611). Six (23.1%) cases had elevated open CSF pressure (ranging from 195 to 350 mmH2O), 6 (23.1%) had elevated leucocyte counts (ranging from 19 to 225×106/L), and 12 (46.2%) of our participants had increased CSF total protein (ranging from 491 to 803 mg/L).
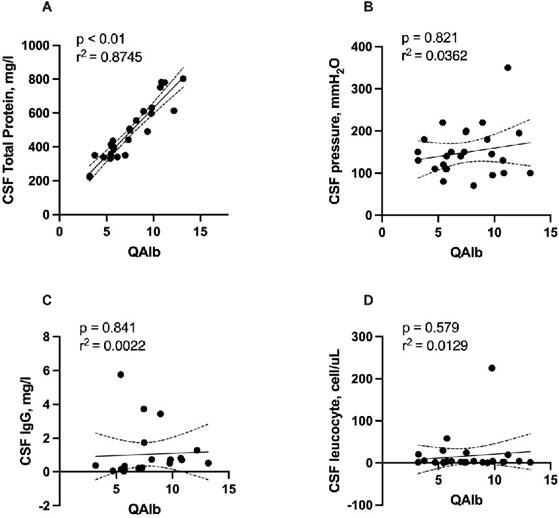
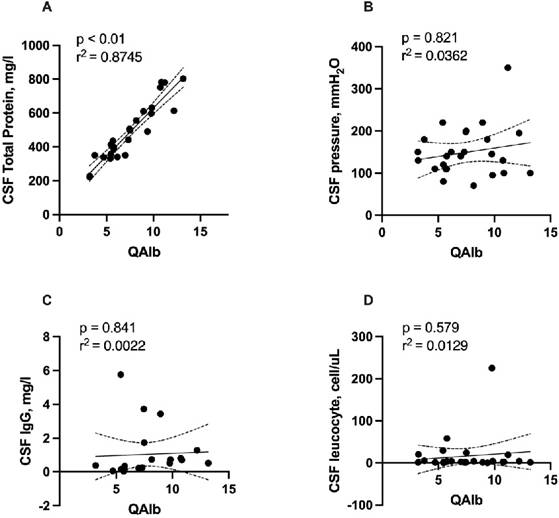
Elevation of QAlb, a sign of disruption of the blood-CSF barrier, was found in 12 (46.2%) of patients. Patients with blood-CSF barrier dysfunction had a median QAlb of 7.45 (IQR 5.52-9.67; range: 3.19-13.19). Total protein concentration was significantly correlated with QAlb (r = 0.926, r2 = 0.8745, p < 0.001, Figure 1A). No significant correlation was detected of QAlb with opening CSF pressure, CSF IgG, and leucocyte counts. (Figure 1B, 1C, 1D). We detected no significant association between CSF albumin concentration and serum albumin levels using Spearman's rho test (r=0.0117, p = 0.599, Figure 2).
SARS-Cov-2 IgM antibody was undetectable in all CSF samples, while SARS-CoV-2 IgG antibodies were positive in 5 (19.2%) patients (Cases No. 5, 13, 16, 17, 20). Two of these five patients with positive SARS-CoV-2 IgG antibody presented blood-CSF barrier dysfunction.
Demographics and clinical characteristics of 26 participants.
| Cases | Sex | Age | COVID-19 vaccine doses | Day from COVID-19 to lumber puncture | Hyper-tension | Diabetes | Cardio-cerebrova-scular disease | Chronic kidney disease | Limb numbness | Dizziness | Headache | Impaired consciousness | Clinical diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 51 | 3 | 8 | No | No | No | No | Yes | No | No | No | Guillain-Barre syndrome |
| 2 | Male | 24 | 3 | 18 | No | No | No | No | Yes | Yes | No | No | Acute myelitis |
| 3 | Male | 35 | 3 | 34 | No | No | No | No | Yes | No | No | No | Cerebral infarction |
| 4 | Male | 76 | 2 | 24 | Yes | No | No | No | No | Yes | Yes | No | Encephalitis with unknown etiology |
| 5 | Male | 85 | 1 | 28 | No | No | No | No | Yes | No | No | No | Cerebral infarction |
| 6 | Female | 52 | 2 | 43 | No | No | No | No | Yes | No | No | No | Acute myelitis |
| 7 | Male | 75 | 3 | 32 | No | No | No | No | Yes | Yes | No | No | Guillain-Barre syndrome |
| 8 | Male | 80 | 2 | 7 | No | No | No | No | No | No | No | No | Extrapyramidal disorder |
| 9 | Male | 64 | 3 | 11 | No | No | No | No | No | No | No | No | Acute cerebellitis |
| 10 | Male | 16 | 3 | 24 | No | No | No | No | No | Yes | Yes | Yes | HSV-7 virus encephalitis |
| 11 | Female | 60 | 2 | 23 | No | No | No | No | No | No | Yes | No | Cranial neuritis |
| 12 | Male | 40 | 3 | 37 | No | No | No | No | No | No | Yes | Yes | Encephalitis with unknown etiology |
| 13 | Male | 51 | 2 | 10 | No | No | No | No | No | Yes | Yes | No | Streptococcus pneumoniae encelphaltis |
| 14 | Male | 51 | 3 | 13 | Yes | No | No | No | No | No | No | No | Brainstem infarction |
| 15 | Female | 79 | 3 | 88 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Cerebral infarction |
| 16 | Male | 76 | 1 | 97 | Yes | No | No | No | No | Yes | Yes | No | Acute cerebellitis |
| 17 | Male | 47 | 2 | NA | Yes | No | No | No | No | No | No | No | Cranial neuritis |
| 18 | Female | 19 | 3 | 108 | No | No | No | No | No | Yes | Yes | Yes | Encephalitis with unknown etiology |
| 19 | Female | 56 | 3 | NA | Yes | Yes | No | No | Yes | No | No | No | Cerebral infarction |
| 20 | Female | 83 | 2 | 3 | Yes | No | No | No | Yes | Yes | No | No | Cerebral infarction |
| 21 | Female | 45 | 3 | 6 | Yes | No | No | No | No | Yes | No | No | Demyelinating encephalopathy |
| 22 | Male | 71 | 3 | 9 | No | No | No | No | No | No | No | Yes | Epilepsy |
| 23 | Female | 78 | 3 | 6 | Yes | No | Yes | No | Yes | No | No | Yes | Intracranial venous sinus thrombosis |
| 24 | Male | 45 | 3 | 26 | Yes | No | No | No | No | No | No | Yes | Encephalitis with unknown etiology |
| 25 | Male | 35 | 3 | 3 | No | No | No | No | Yes | No | No | Yes | HSV-2 virus encephalitis |
| 26 | Male | 34 | 3 | 1 | No | No | No | No | Yes | No | No | No | Guillain-Barre syndrome |
Abbreviations: COVID-19 = Coronavirus disease 2019; HSV = Herpes Simplex
Correlation of QAlb with total protein concentration, opening CSF pressure. CSF IgG, and leucocyte counts. Abbreviations: CSF = cerebrospinal fluid; QAlb = AlbCSF[mg/L]/Albserum[g/L]
Correlation of Serum Albumin with CSF total protein concentration. Abbreviations: CSF = cerebrospinal fluid; TP = total protein.
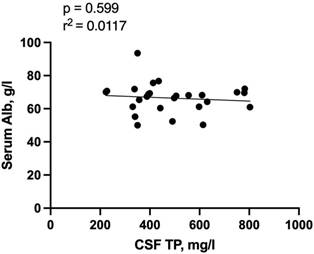
Table 3 shows the percentage of abnormal CSF parameters in different central nervous system disorders. Patients were categorized into four groups of clinical syndromes to overcome the small number of cases: (1) inflammatory neurological diseases, which included encephalitis, myelitis, cerebellitis, and cranial neuritis; (2) stroke; (3) Guillain-Barré Syndrome; and (4) other diseases including extrapyramidal disorder, demyelinating encephalopathy and epilepsy. Among 13 patients with inflammatory neurological disease, 5 (38.5%) presented with increased CSF leucocyte counts, 6 (46.2%) had elevated CSF total protein concentrations and blood-CSF barrier dysfunction, and 4 (30.8%) patients presented elevated CSF pressure. In seven patients with stroke, 3 (42.9%) indicated elevated CSF total protein levels, 2 (28.6%) had increased CSF pressure and blood-CSF barrier dysfunction, and 1 (14.3%) showed elevated CSF leukocyte counts. Among the three patients with Guillain-Barré Syndrome, all exhibited elevated CSF total protein levels and blood-CSF barrier dysfunction. Among three cases with other syndromes, only one (33.3%) exhibited increased CSF total protein concentrations and blood-CSF barrier dysfunction (Table 3).
CSF profile of included participants.
| Cases | CSF pressure (mmHg) | Leukocyte count (cells/μL) | Total protein (mg/L) | Glucose (mmol/L) | IgG (mg/L) | QAlb | Qlim | Blood-CSF barrier dysfunction | SARS-CoV-2 mRNA | SARS-CoV-2 IgG (S/C.O) | SARS-CoV-2 IgG | SARS-CoV-2 IgM | Combined with other virus infections |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 1 | 803 | 3.95 | 0.52 | 13.18 | 7.4 | yes | 0 | 0.52 | negative | negative | No |
| 2 | 150 | 1 | 223 | 4.13 | 0.38 | 3.18 | 5.6 | no | 0 | 0.38 | negative | negative | No |
| 3 | 110 | 4 | 388 | 3.33 | 0.15 | 5.76 | 6.3 | no | 0 | 0.15 | negative | negative | No |
| 4 | 145 | 225 | 598 | 2.86 | 0.51 | 9.77 | 9.1 | yes | 0 | 0.51 | negative | negative | Yes |
| 5 | 195 | 4 | 614 | 3.44 | 1.28 | 12.20 | 9.7 | yes | 0 | 1.28 | positive | negative | No |
| 6 | 140 | 58 | 399 | 4.26 | 0.34 | 5.76 | 7.5 | no | 0 | 0.34 | negative | negative | No |
| 7 | 95 | 4 | 631 | 4.00 | 0.73 | 9.83 | 9 | yes | 0 | 0.73 | negative | negative | No |
| 8 | 110 | 3 | 394 | 3.77 | 0.08 | 5.73 | 9.3 | no | 0 | 0.08 | negative | negative | No |
| 9 | 80 | 0 | 413 | 3.62 | 0.16 | 5.46 | 8.3 | no | 0 | 0.16 | negative | negative | Yes |
| 10 | 70 | 4 | 556 | 3.35 | 0.74 | 8.16 | 5.1 | yes | 0 | 0.74 | negative | negative | Yes |
| 11 | 100 | 2 | 782 | 3.87 | 0.7 | 10.85 | 8 | yes | 0 | 0.70 | negative | negative | No |
| 12 | 110 | 1 | 338 | 3.62 | 0.06 | 4.70 | 6.7 | no | 0 | 0.06 | negative | negative | Yes |
| 13 | 220 | 29 | 331 | 2.70 | 5.76 | 5.41 | 7.4 | no | 0 | 5.76 | positive | negative | No |
| 14 | 130 | 2 | 751 | 2.70 | 0.8 | 10.74 | 7.4 | yes | 0 | 0.80 | negative | negative | No |
| 15 | 150 | 1 | 442 | 5.56 | 0.25 | 7.32 | 9.3 | no | 0 | 0.25 | negative | negative | No |
| 16 | 198 | 1 | 506 | 3.30 | 3.73 | 7.46 | 9.1 | no | 0 | 3.73 | positive | negative | Yes |
| 17 | 220 | 1 | 610 | 3.44 | 3.44 | 8.94 | 7.1 | yes | 0 | 3.44 | positive | negative | No |
| 18 | 110 | 2 | 435 | 3.57 | 0.03 | 5.67 | 5.3 | yes | 0 | 0.03 | negative | negative | Yes |
| 19 | 140 | 2 | 350 | 4.10 | 0.22 | 7.00 | 7.7 | no | 0 | 0.22 | negative | negative | No |
| 20 | 200 | 24 | 498 | 4.47 | 1.73 | 7.49 | 9.5 | no | 0 | 1.73 | positive | negative | No |
| 21 | 120 | 2 | 357 | 3.13 | 0.16 | 5.46 | 7 | no | 0 | 0.16 | negative | negative | No |
| 22 | 180 | 0 | 491 | NA | NA | 9.37 | 8.7 | yes | 0 | 0.11 | negative | negative | No |
| 23 | 150 | 4 | 340 | NA | NA | 6.16 | 9.2 | no | 0 | 1.56 | negative | negative | No |
| 24 | 130 | 20 | 227 | NA | NA | 3.21 | 7 | no | 0 | 2.01 | negative | negative | Yes |
| 25 | 350 | 19 | 780 | NA | NA | 11.20 | 6.3 | yes | 0 | 0.22 | negative | negative | Yes |
| 26 | 180 | 5 | 350 | NA | NA | 3.74 | 6.3 | yes | 0 | 0.31 | negative | negative | No |
Abbreviations: CSF = cerebrospinal fluid; QAlb = AlbCSF[mg/L]/Albserum[g/L]; Qlim = (4+[age/15])×10-3; SARS-CoV-2 = severe acute respiratory syndrome coronavirus type 2
Abnormal CSF parameters in different neurological syndromes.
| Clinical syndrome | Open CSF pressure >180mmH2O | Leucocyte > 5cells/μL | Total protein > 450 mg/L | Abnormal glucosec (mmol/L) | blood-CSF barrier dysfunction | Positive SARS-CoV-2 IgG | Positive SARS-CoV-2 IgM |
|---|---|---|---|---|---|---|---|
| Inflammatory syndromea (n = 13) | 4 (30.8%) | 5 (38.5%) | 6 (46.2%) | 0 | 6 (46.2%) | 3 (23.1%) | 0 |
| Stroke (n = 7) | 2 (28.6%) | 1 (14.3%) | 3 (42.9%) | 1 (14.3%) | 2 (28.6%) | 2 (28.6%) | 0 |
| GBS (n = 3) | 0 | 1 (33.3%) | 3 (100%) | 0 | 3 (100%) | 0 | 0 |
| Other diseasesb (n = 3) | 0 | 0 | 1 (33.3%) | 0 | 1 (33.3%) | 0 | 0 |
aInflammatory neurological diseases included encephalitis (n = 7), myelitis (n = 2), cerebellitis (n = 2) and cranial cranial neuritis (n = 2)
bOther diseases included extrapyramidal diseases (n = 1), demyelinating encephalopathy (n=1) and epilepsy (n=1)
c Abnormal glucose refers to > 4.5 or < 3.6mmol/L
d Blood-CSF barrier dysfunction was defined as QAlb > Qlim (Alb)
Abbreviations: CSF = cerebrospinal fluid; GBS = Guillain-Barré Syndrome; SARS-CoV-2 = severe acute respiratory syndrome coronavirus type 2;
QAlb = AlbCSF[mg/L]/Albserum[g/L]; Qlim = (4+[age/15])×10-3
Data regarding the OCB patterns in the CSF and serum samples were available in 17 out of the 26 participants. Of these 17 CSF samples, only two (11.8%) were classified as type 2, indicating intrathecal synthesis (Table 4). Among these two patients who showed intrathecal synthesis, only one was detected positive for SARS-CoV-2 IgG.
Table 5 lists the peripheral blood profiles of our patients. Serum IgG antibodies against SARS-CoV-2 were detected in 23 out of the 26 participants, and co-existent SARS-CoV-2 IgM were detected in seven participants. Among these 23 patients with serum SARS-CoV-2 IgG, the median albumin level was 68.0 [IQR 61.2-70.1] g/L, the median leucocyte count was 7.3×106/L, and the median glucose level was 5.2 [IQR 4.5-6.0] mmol/L. A total of 21 (91.3%) patients had elevated albumin (ranging from 55.2 to 93.6 g/L), seven (30.4%) had elevated leucocyte counts (ranging from 10.1 to 14.6×109/L), and six (26.1%) patients had increased glucose (ranging from 6.6 to 9.8 mmol/L).
SARS-CoV-2 IgG in the CSF was detected in 23 (88.5%) patients, including 15 patients with SARS-CoV-2 infection only and 8 patients with other viral co-infections. As shown in Table 6, patients with SARS-CoV-2 infection only had similar proportion of elevated CSF pressure (5 [23.3%] vs. 2 [25.0%], p = 0.679), elevated total protein level (7 [46.7%] vs.4 [50.0%], p = 0.879) and blood-brain barrier disruption (8 [53.3%] vs. 4 [50.0%], p = 0.879) compared to those with co-infections. Elevated leukocyte count (2 [13.3%] vs. 3 [37.5%], p = 0.181) and IgG detection (3 [20.0%] vs. 1 [12.5%], p = 0.651) were more frequently seen in patients with SARS-CoV-2 infection only compared to those with other infections, but the differences did not reach statistical significance.
Discussion
The present study yielded several important findings. First, some COVID-19 patients with co-existing neurological disorders may present abnormal CSF profiles despite SARS-CoV-2 mRNA were not detected in the CSF. The most frequent pathological finding was elevated CSF total protein and blood-CSF barrier dysfunction. Moreover, some of the patients tested positive for SARS-CoV-2 IgG antibodies in their CSF, while none tested positive for SARS-CoV-2 IgM antibody. Our findings suggest that CSF analysis is useful in the clinical evaluation of these patients and may contribute to a better understanding of the neurological involvement associated with COVID-19.
In agreement with our findings, another small cohort showed that although SARS-CoV-2 was not detected in CSF samples by qRT-PCR, many patients presented abnormal CSF profiles [8]. Our findings that six (23.1%) cases had elevated open CSF pressure were supported by several previous case series studies that showed open CSF pressure ranged between normal and slightly elevated values in most cases [13]. Moreover, a retrospective study showed that one-third of patients had high open CSF pressure, although this characteristic was not associated with a specific neurological picture [1]. Our data showed that some COVID-19 patients with co-existing neurological disorders presented pleocytosis. In line with our findings, pooled data showed that among 409 patients with central nervous system disorders who had available data of CSF white blood cell count, 29 (7%) had a CSF leukocyte count of 21-100 cells/μL, and 8 (2%) had a CSF leukocyte count of 100 cells/μL or higher [14]. Our data that nearly half of our participants had elevated CSF total protein concentration (>450 mg/L) was supported by pooled observational data showing that among 397 patients who had available data on CSF total protein, 160 (40%) of whom had increased CSF total protein levels [11]. The elevation of CSF total protein levels in our patients can be partly explained by the blood-CSF barrier dysfunction since we detected a significant correlation between CSF total protein concentration with QAlb.
Oligoclonal bands in serum and CSF.
| Cases | Oligoclonal bands in serum | Oligoclonal bands in CSF | Oligoclonal patterns | SARS-CoV-2 IgG |
|---|---|---|---|---|
| 1 | 0 | 0 | Type 1 | negative |
| 2 | 0 | 0 | Type 1 | negative |
| 3 | /* | / | / | negative |
| 4 | 0 | 0 | Type 1 | negative |
| 5 | 0 | 1 | Type 2 | positive |
| 6 | / | / | / | negative |
| 7 | 0 | 0 | Type 1 | negative |
| 8 | 0 | 1 | Type 2 | negative |
| 9 | 0 | 0 | Type 1 | negative |
| 10 | 0 | 0 | Type 1 | negative |
| 11 | 0 | 0 | Type 1 | negative |
| 12 | 0 | 0 | Type 1 | negative |
| 13 | 0 | 0 | Type 1 | positive |
| 14 | 0 | 0 | Type 1 | negative |
| 15 | 0 | 0 | Type 1 | negative |
| 16 | / | / | / | positive |
| 17 | / | / | / | positive |
| 18 | 0 | 0 | Type 1 | negative |
| 19 | 0 | 0 | Type 1 | negative |
| 20 | 1 | 1 | Type 4 | positive |
| 21 | 0 | 0 | Type 1 | negative |
| 22 | / | / | / | negative |
| 23 | / | / | / | negative |
| 24 | / | / | / | negative |
| 25 | / | / | / | negative |
| 26 | / | / | / | negative |
* / = data unavailable
Abbreviations: CSF = cerebrospinal fluid; SARS-CoV-2 = severe acute respiratory syndrome coronavirus type 2
Mechanisms implicated in the above-mentioned abnormal CSF findings remain uncertain but might include neuronal injury associated with direct virus infection, a para- or post-infectious inflammatory disease, or a secondary process due to severe effects of a systemic disorder [15,16]. Our study showed that SARS-Cov-2 mRNA was undetectable using the qRT-PCR technology in all CSF samples. Similar to our findings, data from Sweden showed undetectable SARS-CoV-2 mRNA in all 31 CSF samples [17]. Moreover, a case series study showed that patients with COVID-19 and neurological disorders had undetectable or extremely low levels of SARS-CoV-2 mRNA in the CSF [1]. A prospective observational study of pregnancy women indicated no evidence of SARS-CoV-2 in the CSF of COVID-19 patients with early neurological symptoms.
Peripheral blood profile of included participants.
| Cases | Leukocyte count (cells/µL) | Hemoglobin (g/L) | Platelet count (cells/µL) | Fibrinogen (g/L) | Glucose (mmol/L) | Albumin (g/L) | SARS-CoV-2 IgG | SARS-CoV-2 IgM |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.18 | 141 | 238 | 2.75 | 4.48 | 60.93 | positive | positive |
| 2 | 6.21 | 144 | 300 | 3.01 | 3.34 | 70.13 | positive | negative |
| 3 | 7.02 | 140 | 325 | 2.41 | 4.4 | 67.36 | positive | negative |
| 4 | 5.93 | 136 | 226 | / | 5.74 | 61.21 | positive | negative |
| 5 | 11.22 | 115 | 198 | 3.83 | 5.02 | 50.33 | positive | positive |
| 6 | 8.23 | 115 | 292 | 3.98 | 6.64 | 69.27 | positive | negative |
| 7 | 8.8 | 136 | 224 | 2.71 | 4.73 | 64.19 | positive | negative |
| 8 | 6.06 | 141 | 366 | 4.65 | 5.42 | 68.76 | positive | positive |
| 9 | 5.71 | 138 | 181 | 2.48 | 9.81 | 75.64 | positive | negative |
| 10 | 8.74 | 140 | 193 | 2.44 | 4.38 | 68.14 | positive | negative |
| 11 | 6.38 | 147 | 270 | / | 5.68 | 72.07 | positive | negative |
| 12 | 11.11 | 158 | 179 | / | 4.32 | 71.91 | positive | positive |
| 13 | 13.14 | 144 | 71 | 5.42 | 5.99 | 61.18 | positive | positive |
| 14 | 5.38 | 136 | 204 | 2.65 | 4.63 | 69.93 | positive | negative |
| 15 | 10.85 | 83 | 262 | 4.34 | 6.04 | 60.38 | negative | negative |
| 16 | 5.53 | 120 | 150 | 2.68 | 4.99 | 67.83 | positive | positive |
| 17 | 6.59 | 119 | 201 | 4.01 | 3.21 | 68.23 | positive | positive |
| 18 | 7.00 | 123 | 407 | 3.25 | 4.68 | 76.72 | positive | negative |
| 19 | 7.64 | 142 | 332 | 3.12 | 4.09 | 50.00 | negative | negative |
| 20 | 8.91 | 133 | 2237 | 4.2 | 7.54 | 66.49 | positive | negative |
| 21 | 6.61 | 128 | 270 | 2.17 | 4.71 | 65.38 | negative | negative |
| 22 | 11.15 | 120 | 160 | 4.26 | 7.97 | 52.40 | positive | negative |
| 23 | 3.85 | 109 | 334 | 3.91 | 5.88 | 55.19 | positive | negative |
| 24 | 14.58 | 135 | 214 | 5.98 | 9.04 | 70.72 | positive | negative |
| 25 | 10.71 | 161 | 152 | 4.01 | 7.17 | 69.64 | positive | negative |
| 26 | 10.14 | 158 | 204 | 4.52 | 5.7 | 93.58 | positive | negative |
* / = data unavailable;
Abbreviations: SARS-CoV-2 = severe acute respiratory syndrome coronavirus type 2
CSF characteristics of patients with COVID-19 infection only and co-existing other infections.
| Variable | COVID-19 infection only (n = 15) | Co-existing other infections (n =8) | P value |
|---|---|---|---|
| Elevated CSF pressure, n (%) | 5 (33.3) | 2 (25.0) | 0.679 |
| Elevated leukocyte count, n (%) | 2 (13.3) | 3 (37.5) | 0.181 |
| Elevated total protein, n (%) | 7 (46.7) | 4 (50.0) | 0.879 |
| Positive CSF-IgG, n (%) | 3 (20.0) | 1 (12.5) | 0.651 |
| Blood-CSF barrier dysfunctiona, n (%) | 8 (53.3) | 4 (50.0) | 0.879 |
aBlood-CSF barrier dysfunction was defined as QAlb > Qlim (Alb)
Abbreviations: CSF = cerebrospinal fluid
Therefore, the neurological symptoms may not be caused by the direct damage of the virus to the central nervous system [18]. The findings of the above-mentioned studies may suggest a low likelihood of direct viral involvement in neurological symptoms. Of note, the methodology used for SARS-CoV-2 detection in the CSF may not have been sensitive enough to get low viral loads, leading to potential underestimation of viral involvement. Given that COVID-19 patients with neurologic involvement may present a wide range of clinical manifestations, the neuroinvasion potential of SARS-CoV-2, neuroinflammation as a possible driver, and the connection with blood-brain barrier disruption regardless of intrathecal inflammation should be more investigated in future studies [5,19].
Our data showed that the SARS-CoV-2 IgM antibody was undetectable in all CSF samples, while the SARS-CoV-2 IgG antibody was detected in 5 (19.2%) patients. Two of these five patients with SARS-CoV-2 IgG antibody presented blood-CSF barrier dysfunction. The findings aforementioned may prevent direct conclusions over the active destruction of SARS-CoV-2 infection in the CSF. The detectable SARS-CoV-2 IgG antibodies in some cases may be associated with previous virus exposure or vaccination [20]. Moreover, high levels of circulating inflammatory cytokines after SARS-CoV-2 infection may possibly disrupt the blood-CSF barrier allowing for antibodies and other inflammatory mediators to enter the brain parenchyma [21]. Thus, SARS-CoV-2 antibodies in the CSF of COVID-19 patients possibly reflect blood-CSF barrier disruption associated with systemic inflammation. Although we did not have data available to assess antibody-index, a previous study with antibody-index calculations did not support a direct SARS-CoV-2 brain infection [22]. Whether the SARS-CoV-2 antibodies detection may have diagnostic and prognostic implications needs to be investigated in future studies.
Our data showed that two patients (11.8%) had the type 2 OCB pattern consistent with a possible intrathecal IgG synthesis. Similar to our findings, data from Germany showed that 5/83 (6%) patients with post-COVID-19 syndrome presented CSF-restricted OCB (pattern 2 in 4/83 [5%], pattern 3 in 1/83 [1%]) [23]. Moreover, a case series study showed that CSF-restricted OCB was detected in 11.1% (5/45) of patients with neuroimmune complications of COVID-19 [24]. Further studies are required to validate these findings, contributing to better elucidating the pathophysiological mechanisms.
Comparative analysis of CSF samples from patients with COVID-19 infection only and those with co-existing other infections showed no statistically significant differences in CSF profiles (e.g., leukocyte count, total protein, and open CSF pressures). This finding suggests that the neurological changes observed in the CSF may not be solely attributed to COVID-19 and could potentially be influenced by other underlying infections. This issue needs to be investigated in future studies.
Our finding should be interpreted with caution due to its small size, which may not enough represent the broader population of patients with COVID-19 and neurological complications. Although comparing CSF characteristics of general COVID-19 infected patients to neurological involvement COVID-19 patients may better demonstrate our conclusions, it is not feasible to collect CSF of general COVID-19 patients without neurological involvement in clinical practice. Moreover, comparing the peripheral blood characteristics of COVID-19 individuals with and without neurological involvement may better observe the changes caused by neurological injury or a possible connection with the blood-brain barrier. However, only including COVID-19 patients with neurological involvement in this study does not allow us to make these comparisons, which needs to be investigated in future studies. Notably, the timing of lumbar puncture and the time of COVID-19 infection were uneven among our patients, which may affect our findings. Beyond routine hematological parameters, these comparisons could include biomarkers associated with inflammatory responses and blood-brain barrier function, such as cytokines, chemokines, and blood-brain barrier-specific proteins. Appropriate statistical methods will be employed to identify differentially expressed markers in patients with neurological involvement and to analyze the relationship between these alterations and neural injury or blood-brain barrier dysfunction. This approach aims to further elucidate the patho-physiological mechanisms underlying neurological involvement in COVID-19 patients. Moreover, some CSF parameters, such as concentration of total Tau, neurofilament light chain proteins [25], and inflammatory index, including CD4/CD8, cytokines [26], the cellularity of B cells, T-subsets of lymphocytes, the activation of lymphocytes, and other cells (e.q.,neutrophils) were not routinely measured in our patients due to Medicare policy. A multicenter study of 150 lumbar punctures in 127 COVID-19 patients with neurological symptoms showed that cytokine levels were frequently elevated in the CSF (often associated with blood-brain barrier dysfunction), partly remaining positive at high levels for weeks to months [5]. Also, we only analyzed Chinese participants; whether our findings may be generalizable to other populations needs to be validated in future studies. Lastly, the lack of longitudinal data does not allow us to determine these CSF changes over time.
Conclusion
This study showed that some patients with COVID-19 and co-existing neurological involvement may present non-specific inflammatory CSF abnormalities despite no SARS-CoV-2 being detected in the CSF. Our findings suggest that neurological injury is related to misdirected immune responses associated with systemic inflammation rather than direct virus invasion.
Abbreviations
COVID-19: coronavirus disease 2019; CSF: cerebrospinal fluid; IQR: interquatile range; qRT-PCR: quantitative reverse transcriptase polymerase chain reaction; OCB: oligoclonal band; SARS-CoV-2: severe acute respiratory syndrome coronavirus type 2.
Acknowledgements
The authors are thankful to all participants in this study.
Funding
This study was supported by the Fujian Provincial Key Clinical Specialty of Neurology (05029001) and Clinical Research Center for Precision Diagnosis and Treatment of Neurological Diseases of Fujian Province (2022Y2005). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Ethics approval and consent to participate
The study was approved by the ethics committee of Fujian Medical University Union Hospital (2023KY120). All clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki. All patients or their legally accepted representatives provided written informed consent before study entry for the present analysis.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but can be obtained from the corresponding author upon reasonable request.
Author contributions
Drs H Du contributed to study concept and design. Drs H Lin, X Zhang, H Li, and H Du contributed to acquisition, analysis, or interpretation of data. Drs H Lin, X Zhang and H Du contributed to drafting of the manuscript. Drs H Li, S Fang, H Huang, N Liu and H Du contributed to critical revision of the manuscript for important intellectual content. H Lin, X Zhang and S Fang contributed to Tables 1-6. H Lin and H Li contributed to Figures 1-2. Dr. H Du had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782-793
2. Menni C, Valdes AM, Polidori L. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618-1624
3. Espíndola OM, Brandão CO, Gomes YCP. et al. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int J Infect Dis. 2021;102:155-162
4. Ellul MA, Benjamin L, Singh B. et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767-783
5. Jarius S, Pache F, Körtvelyessy P. et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation. 2022;19(1):19
6. Garcia MA, Barreras PV, Lewis A. et al. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. Preprint. medRxiv. 2021. 2021 01.10.20249014
7. Maury A, Lyoubi A, Peiffer-Smadja N, de Broucker T, Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev Neurol (Paris). 2021;177(1-2):51-64
8. Miller EH, Namale VS, Kim C. et al. Cerebrospinal Analysis in Patients With COVID-19. Open Forum Infect Dis. 2020 7(11)
9. Edén A, Kanberg N, Gostner J. et al. CSF Biomarkers in Patients With COVID-19 and Neurologic Symptoms: A Case Series. Neurology. 2021;96(2):e294-e300
10. Technical guidelines for laboratory testing of pneumonia for COVID-19 infection. In: COVID-19 Infection Prevention and Control Plan (9th). Chinese center for disease control and prevention.2022. Online: https://www.chinacdc.cn/jkyj/crb2/yl/xxgzbdgr/jsfa_xg/202409/W020240906512407062778.pdf. Accessed 27 Jun. 2022
11. Andersson M, Alvarez-Cermeño J, Bernardi G. et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry. 1994;57(8):897-902
12. Reiber H. Flow rate of cerebrospinal fluid (CSF)-a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122(2):189-203
13. Domingues RB, Leite FBVM, Senne C. Cerebrospinal fluid analysis in patients with COVID-19-associated central nervous system manifestations: a systematic review. Arq Neuropsiquiatr. 2022;80(3):296-305
14. Lewis A, Frontera J, Placantonakis DG. et al. Cerebrospinal fluid in COVID-19: A systematic review of the literature. J Neurol Sci. 2021;421:117316
15. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020;77(8):1018-1027
16. Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological Involvement in COVID-19 and Potential Mechanisms: A Review. Neurocrit Care. 2021;34(3):1062-1071
17. Kanberg N, Grahn A, Stentoft E. et al. COVID-19 Recovery: Consistent Absence of Cerebrospinal Fluid Biomarker Abnormalities in Patients With Neurocognitive Post-COVID Complications. J Infect Dis. 2024;229(2):493-501
18. Sasaki LP, Fernandes GM, Silva APD. et al. Cerebrospinal fluid analysis of pregnant women at early stages of COVID-19. Taiwan J Obstet Gynecol. 2022;61(4):672-674 doi:10.1016/j.tjog.2022.03.043
19. Cardoso CO, Rodrigues Sandoval ES, de Oliveira Chagas LBM. et al. Neurologic manifestations of COVID-19 and viral test in cerebrospinal fluid. PLoS One. 2025;20(3):e0312621
20. Selma-Royo M, Bäuerl C, Mena-Tudela D. et al. Anti-SARS-CoV-2 IgA and IgG in human milk after vaccination is dependent on vaccine type and previous SARS-CoV-2 exposure: a longitudinal study. Genome Med. 2022;14(1):42
21. Bodro M, Compta Y, Llansó L. et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e821
22. Nersesjan V, Boldingh MI, Paulsen EQ. et al. Antibodies against SARS-CoV-2 spike protein in the cerebrospinal fluid of COVID-19 patients and vaccinated controls: a multicentre study. J Neurol. 2024;272(1):60
23. Alexopoulos H, Magira E, Bitzogli K. et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e893
24. Gong S, Deng B, Yu H, Zhang X, Yang W, Chen X. Clinical and immunological features in patients with neuroimmune complications of COVID-19 during Omicron wave in China: a case series. Front Immunol. 2024;15:1499082
25. Domingues KZA, Cobre AF, Lazo REL. et al. Systematic review and evidence gap mapping of biomarkers associated with neurological manifestations in patients with COVID-19. J Neurol. 2024;271(1):1-23
26. Michael BD, Dunai C, Needham EJ. et al. Para-infectious brain injury in COVID-19 persists at follow-up despite attenuated cytokine and autoantibody responses. Nat Commun. 2023;14(1):8487
Author contact
![]() Corresponding author: Dr. Houwei Du, Department of Neurology, Fujian Medical University Union Hospital, 29 Xinquan Road, Gulou District, 350001, Fuzhou, China. Tel.: 0086 591 83346181; FAX: 0086 591 83346181, Email: houweiduedu.cn ORCID: 0000-0002-5978-9734.
Corresponding author: Dr. Houwei Du, Department of Neurology, Fujian Medical University Union Hospital, 29 Xinquan Road, Gulou District, 350001, Fuzhou, China. Tel.: 0086 591 83346181; FAX: 0086 591 83346181, Email: houweiduedu.cn ORCID: 0000-0002-5978-9734.

 Global reach, higher impact
Global reach, higher impact