Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(15):3939-3945. doi:10.7150/ijms.109707 This issue Cite
Research Paper
Drug Interaction of Dasatinib with Thymoquinone: A Pharmacokinetic Study in Rats
1. Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia.
2. Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia.
3. Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia.
4. Quality Assurance Unit, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia.
Received 2024-12-31; Accepted 2025-4-28; Published 2025-9-3
Abstract
Dasatinib (DAS), a multi-kinase inhibitor targeting Src and BCR-ABL families, is approved for Ph+ acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CML). Its metabolism by CYP3A4 and transported by efflux pump P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP) render it susceptible to drug, food, and herbal interactions. This study investigated the potential impact of thymoquinone (TQ) co-administration on dasatinib pharmacokinetics (PK) and the subsequent risk of altered efficacy or toxicity. Wistar rats were pretreated with a daily oral dose of TQ (40 mg/kg) for one week before receiving a single oral dose of DAS (25 mg/kg). Blood samples were collected at different time points, and plasma concentrations of DAS were measured using UPLC-MS/MS. A non-compartmental analysis was applied to calculate the PK parameters of DAS. Additionally, the impact of TQ treatment on hepatic and intestinal protein expressions of CYP3A4, Pgp and BCRP were investigated using Western blot analysis. TQ pretreatment significantly altered the disposition of DAS in animals as compared to untreated animals. More specifically, a substantial increase in DAS Cmax (213.26%), AUC0-t (166.53%), AUMC0-∞ (0.34%), Kel (93.85%) and Tmax (83.33%) were observed (p<0.05). In addition, a significant reduction in DAS Vd (67.70%) and clearance CL (36.35%) of were evident in the TQ treatment group (p<0.05). Furthermore, TQ pretreatment inhibited CYP3A4 and Pgp and BCRP1 in the liver and lumen tissues. TQ pretreatment significantly alters the disposition of DAS in rats. This is likely due to an increase in DAS bioavailability via a modulation in the protein expressions of CYP3A4 and Pgp and BCRP1 in the liver and lumen tissues. Thus, the concurrent intake of TQ-containing products with DAS can lead to a serious interaction. Further clinical studies are warranted to evaluate the clinical impact of such observations.
Keywords: Dasatinib, thymoquinone, pharmacokinetic, interaction, CYP3A2, Pgp, BCPR.
Introduction
The tyrosine kinase (TK), a pivotal regulator within the cell cycle signaling pathways, has been linked to controlling proliferation, apoptosis, cell division, and metabolism [1]. Thus, TK pathway dysfunction (overexpression or somatic mutation) causes an array of human diseases, especially cancers [2]. In recent times, tyrosine kinase inhibitors (TKIs) have become among the most renowned pathway-directed chemotherapy medications for treating several types of cancer and exhibit fewer adverse reactions than conventional chemotherapy regimens [3, 4]. Dasatinib (DAS, Figure 1A) is a potent orally available TKI, for PDGFR-β5, BCR-ABL, SRC family kinases, and c-KIT. DAS has been utilized in the treatment of both acute lymphoblastic leukemia (CML) and acute lymphocytic leukemia (ALL) [5-7]. DAS is broadly absorbed from the intestine and is primarily metabolized in the liver [8-11]. Unambiguously, DAS undergoes CYP3A4 facilitated metabolism and the key metabolites are M4, M5, M6, M20 and M24 [7, 12, 13]. M4 is formed due to N-dealkylation by CYP3A4, while M20 and M24 are formed by hydroxylation [9, 10]. Not only DAS serves as a substrate for CYP3A4, but it also a target for efflux transporters such as P-glycoprotein (Pgp) and Breast Cancer Resistance Protein (BCRP) [14-19]. Treatment with DAS has been linked to some serious toxicities, including heart failure, peripheral arterial occlusive disease (PAOD), QT prolongation, hypertension, reactivation of Hepatitis B virus (HBV), thrombosis, hyperglycemia, myelosuppression, hypocalcemia, cytopenia, hyperlipidemia, pulmonary hypertension, pneumonitis, and pleural effusion [20, 21].
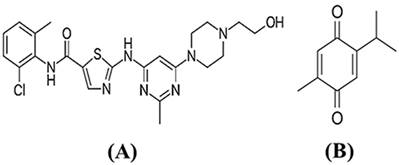
(A) Chemical structure of dasatinib 2-amino-1,3-thiazole-5-carboxylic acid and at position 6 by a 4-(2-hydroxyethyl)piperazin-1-yl. (B) TQ chemical structure (2-Methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione).
Thymoquinone (TQ, Figure 1) is a bioactive polyphenol present in Nigella sativa (black seed) [22, 23]. Because of its many pharmacological properties, TQ is commonly used in food and nutraceutical products. It has demonstrated antineoplastic, antioxidant, anti-inflammatory, and antidiabetic, activities [23, 24]. Drug-drug interactions (DDIs) are increasingly being recognized as significant clinical events which can be detrimental [25-27]. Several studies have suggested that TQ functions as an inhibitor of CYP3A4 [23, 28-30] and Pgp and BCRP [31]. Therefore, possible interactions with TQ are more likely to occur with drugs that undergo a high first pass metabolism. Both the liver and lumen mucosa contain the CYP3A4 enzyme. After absorption by the mucosa, vulnerable drugs may be pumped back into the intestine lumen by Pgp and BCRP, and metabolized by CYP3A4. Consequently, it's crucial to carefully assess the safety implications of using TQ products concurrently with DAS. Concerns about such interactions have been raised due to the consumption of products containing TQ alongside with prescribed medications, which could potentially alter the pharmacokinetics (PK) and the pharmacodynamics (PD) of interacting drugs [31, 32]. Therefore, the aim of this study is to investigate the potential PK interaction between DAS and TQ; also, to explore the possible underlying mechanism of such consequence, if any.
Materials and Methods
Chemicals
Ammonium acetate, Ibrutinib (IRB), TQ, DAS were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, acetonitrile, and formic acid (HPLC grade) were procured from BDH Chemicals (Poole, UK). Anti- Pgp (sc-55510), CYP3A2 (sc-271033), BCPR (sc-58222) and β-actin (sc-47778) antibodies were obtained Santa Cruz Biotechology (Dallas, TX, USA).
Animals and pharmacokinetic studies
The adult male Wistar rats (204-226 g) were procured from the College of Pharmacy Animal Care and Use Facility, King Saud University, Riyadh, Saudi Arabia. The animals were hold in polycarbonate rat cages, with 12h light/dark cycle at 25 ± 2 ºC. All groups were allowed ad libitum before the study. This study was conducted following the ethical guidelines approved by the facility and the study was approved by the King Saud University College of Pharmacy Research Ethics Committee (KSU-SE-21-58). Overnight fasted animals (n=24) were divided into four groups (n=6 per group). Group I (normal controls) and Group II were administered normal saline by oral gavage for seven consecutive days. On the seventh day, Group II received a single dose of DAS (25 mg/kg, p.o.) via oral gavage. Group III received TQ (40 mg/kg, p.o.) via oral gavage for seven days, and two hours after the final TQ dose, DAS (25 mg/kg, p.o.) was administered by oral gavage. Group IV received TQ (40 mg/kg/day) by oral gavage for seven consecutive days. For the PK study, blood samples were collected via a tail vein in heparinized vacutainers at various time intervals (i.e., 0, 0.5, 1, 2, 4, 6, 8, 10, 12 h post DAS dosing). The collected blood was centrifuged at 3000 ×g for 10 minutes to separate the plasma, which was then collected and stored in Eppendorf tubes for UHPLC-MS/MS analysis. After completion of the study, the animals were euthanized, and liver and lumen tissues were collected for Western blot analysis. DAS concentrations in plasma samples were determined using an UHPLC-MS/MS method according to a previously described method [17]. Briefly, DAS and ibrutinib (IBR;(internal standard, IS) were separated on an Acquity BEH C18 column. Prior to sample analysis, the method underwent validation for precision and accuracy [33]. A non-compartmental analysis was empoyed to calculate the PK parameters of DAS using the PK Solver software (version 1.01).
Western blotting
The liver and intestine tissues were homogenized in RIPA lysis buffer and incubated for 30 minutes, followed by centrifugation at 10,000 rpm for 20 minutes at 4 °C. The supernatants were then collected and stored at -20 °C. Total protein content was determined using the bicinchoninic acid (BCA) assay. Protein expression of CYP3A2, Pgp, and BCRP proteins were analyzed according to previously published methods [15, 17, 34, 35].
Statistical analysis
All data are expressed as the mean ± SEM. The pharmacokinetic and protein expression data were compared using one-way analysis of variance followed by Dunnett's test and a p value of ≤0.05 was used for statistical significance.
Results
Table 1 provides the PK parameters of DAS following an oral administration in rats. TQ pretreatment resulted in a substantial increase in DAS Cmax (213.26%), AUC0-t (166.53%), AUMC0-∞ (0.34%), Kel constant (93.85%) and Tmax (83.33%), while the MRT (49.12%), T1/2 (11.56%), Vd (67.70%) and CL (36.35%) were significantly decreased as compared to the control group (p<0.05). Figure 2 demonstrated the plasma concentration profiles of DAS alone and with TQ pretreatment.
The non-compartmental pharmacokinetic parameters for oral dasatinib (DAS, 25 mg/kg) with and without the co-administration of TQ, 40 mg/kg p.o. in rats (n= per group).
| Pk Parameters | DAS | DAS+TQ | % Change |
|---|---|---|---|
| Mean ±SEM | Mean ± SEM | ||
| Kel (1/h) | 0.09 ±0.01 | 0.18 ±0.01 | 93.85* |
| T1/2 (h) | 7.77 ±0.65 | 3.95 ±0.21 | -49.19* |
| Tmax (h) | 1.00 ±0.00 | 1.83 ±0.17 | 83.33* |
| Cmax (ng/ml) | 246.08 ±3.34 | 770.88 ±232.55 | 213.27* |
| AUC0-t (ng/ml*h) | 1614.93 ±28.86 | 4304.28 ±1289.08 | 166.53* |
| AUC0-∞ (ng/ml*h) | 2501.79 ±112.63 | 4931.04 ±1507.76 | 97.10* |
| AUMC0-∞ (ng/ml*h2) | 29259.52 ±3397.55 | 29359.54 ±9463.06 | 0.34* |
| MRT (h) | 11.53 ±0.81 | 5.86 ±0.15 | -49.13* |
| Vd (mg/kg)/(ng/ml) | 0.11 ±0.00 | 0.04 ±0.004 | -67.71* |
| CL (mg/kg)/(ng/ml)/h | 0.01 ±0.00 | 0.01 ±0.001 | -36.35 |
Data are presented as mean ±SEM. *indicates p<0.05.
As illustrated in Figure 3 (A, B), the protein expressions of CYP3A2 in the liver and lumen tissues was significantly elevated by 91.84% and 93.47%, respectively, following DAS administration to rats (p<0.05). However, pretreatment with TQ for seven days significantly suppressed this CYP3A2 upregulation by 53.98% in the liver and by 40.30% in the lumen, of rats which administered DAS. TQ alone also did not significantly altered CYP3A2 expressions as compared to normal controls.
Dasatinib (DAS) plasma concentrations with and without TQ co-administration in rats. Data represent mean ± SEM. *p<0.05 vs. controls; #p<0.05 vs. the DAS group (n= 6 per group).
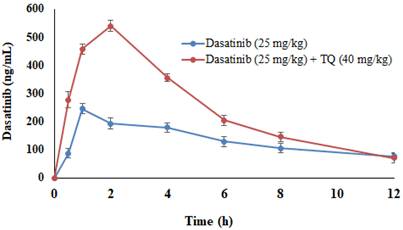
Figure illustrates the impact of TQ pretreatment on the expressions of CYP3A2, in the liver (A) and the intestinal lumen (B) of rats following dasatinib (DAS) administration. All data are presented as the mean ± SEM. * indicates p<0.05 vs. normal controls; # indicates p<0.05 vs. the DAS group (n= 6 per group).
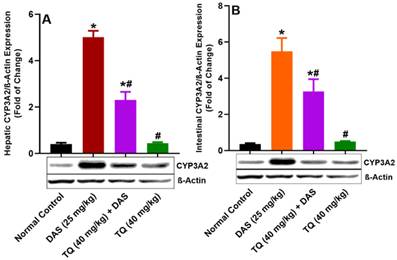
Figure illustrates the impact of TQ pretreatment on the expressions of Pgp, in the liver (A) and the intestinal lumen (B) of rats following dasatinib (DAS) administration. All data are presented as the mean ± SEM. * indicates p<0.05 vs. normal controls; # indicates p<0.05 vs. the DAS group (n= 6 per group).
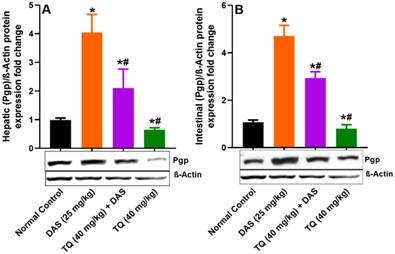
Figure 4 (A, B) demonstrates the inhibitory effect of TQ on Pgp protein expression. Liver and lumen Pgp expressions were significantly increased by 75.62% and 77.19%, respectively (p<0.05), in rats treated with DAS compared to untreated animals. However, pretreatment with TQ significant suppressed the Pgp expressions in the liver and lumen tissues of rats treated with DAS by 47.97% and 37.72%, respectively (p<0.05). Similar to CYP3A2, TQ pretreatment alone did not affect the liver and lumen Pgp protein expressions compared to untreated animals.
Figure illustrates the impact of TQ pretreatment on the expressions of BCRP in the liver (A) and the intestinal lumen (B) of rats following dasatinib (DAS) administration. All data are presented as the mean ± SEM. * indicates p<0.05 vs. normal controls; # indicates p<0.05 vs. the DAS group (n= 6 per group).
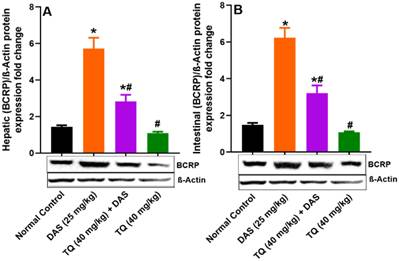
Figure 5 (A, B) presents the investigation into the potential inhibitory effect of TQ on BCRP protein expression, revealing no significant changes in the liver and lumen BCPR protein levels following TQ treatment compared to normal controls. Contrarily, oral administration of DAS resulted in a significant induction of BCRP protein expression in the liver and the lumen by 74.82% and 76.05%, respectively (p<0.05) as compared to untreated animals. Finally, TQ pretreatment in the DAS treatment group resulted in a significant inhibition of BCRP protein expression in the Liver by 50.48% and in the lumen tissues by 48.46% (p<0.05).
Discussion
TKIs can be beneficial for the specific targeted treatment of an array of malignancies. The first medication to be employed in clinical oncology of this class of drugs was imatinib. Others such as inhibitorssunitinib, gefitinib, sorafenib, erlotinib, and dasatinib have emerged later [4]. TKIs can induce toxicity to several organs, including the lungs, the kidneys, the heart, the liver, and the thyroid gland., In addition, blood coagulation, skin and nervous system reactions, and gastrointestinal tract adverse effects have been reported, irrespective of how well TKIs work as inhibitors targeting and translating this into therapeutic applications [36, 37]. Cancer patients often use alternative therapies such as herbal and nutraceutical products and supplements, to enhance their general well-being and reduce the negative effects of chemotherapy [38, 39]. The benefits of using such products are questionable due to the possibility of herb-drug interaction (HDI). The main mode that HDI work is by inhibiting or enhancing the expression of drug transporters and/or drug-metabolizing enzymes [40, 41]. The benefits of using herbal products are questionable due to the possibility of herb-drug interaction (HDI). The main way that HDI interactions work is by inhibiting or enhancing the expression of drug transporters and/or drug-metabolizing enzymes (DME) [40, 41]. DAS has been approved by FDA for use in patients with CML and Ph+ ALL who do not improve with first-line treatment. BCR-ABL and Src family kinases are its several targets that it inhibits [42]. The CYP3A primarily metabolised DAS in liver, intestine and kidney. M4, M5, M6, M20, and M24 are the primary metabolites of DAS [9-11]. Food substances have a wide range of interactions with proteins that are implicated in drug PK/PD profiles. These interactions can occur at any point from the absorption phase to potential changes in clinical efficacy, and they may also be related to pharmacological refractoriness, therapeutic side effects, or unanticipated adverse events. Considering that DAS is a substrate for CYP3A4, PGP/MDR1, and BCPR1 and has a narrow therapeutic spectrum [15, 17, 19, 35, 43-45]. The concominant use of TQ along with prescribed drug DAS a potential pharmacokinetic interaction may occur the current investigation was proposed to verify whether or not TQ has a significant pharmacokinetic interaction with DAS, and also what possible mechanism(s) could be implicated. DAS has a dose-proportional pharmacokinetic profile and a linear elimination between 15 mg/day (0.15 times the lowest approved recommended dose) and 240 mg/day (1.7 times the highest approved recommended dose).The dose selected for DAS based on highest recommended dose human clinical dose that is 240 mg per day for adult (average weight 65 kg) is converted to rat dose which is 22.73 and acute toxicity studies demonstrated that 30 mg/kg DAS is well tolerated by rats therefore 25 mg/kg DAS selected for pharmacokinetic studies and it is within the limit.
Previous studies demonstrated administration of TQ to wistar rats of both sexes, the maximum tolerated doses (MTDs) for i.p. injection were 22.5 and 15 mg/kg in male and female rats, while MTD was 250 mg/kg in both male and female rats that received oral TQ. The dose selection was based on the basis of previous studies [46, 47]. The previous studies reported therapeutic dose [48-51] hence selection of doses for DAS and TQ pharmacokinetic study are within the clinical limits. The pharmacokinetic study reveals that several fold increased in systemic exposure of DAS in TQ preteated animals as compared to DAS alone animals. TQ pretreatment or parallel administration of DAS (25 mg/kg) resulted in a substantial increase in the Cmax (ng/ml), AUC0-t (ng/(ml h), AUMC0-∞ (ng/(ml h), Kel (h-1) constant and Tmax and MRT, volumes of distribution Vd (mg/kg)/(ng/ml) and rate of clearance CL (mg/kg)/(ng/ml) of DAS (p<0.05) as compared to the normal control (DAS 25 mg/kg). These findings indicate that both systemic bioavailability and DAS exposures have risen. Most likely TQ, a strong inhibitor of drug-metabolizing enzymes (such as CYP3A4) and drug transporters, such as Pgp/MDR1 and BCRP1, may cause pharmacokinetic interactions. The literature shows that TQ inhibitors of CYP3A4 a drug metabolizing enzyme [28, 52-54] and drug transporters Pgp/MDR1 and BCRP1[55-58]. The pharmacokinetic parameters indicated an increase in the bioavailability of DAS as demonstrated by a rise in Cmax and AUC, and a reduction in its elimination as reflected by a decreased in T ½ (h) and CL. The increased in bioavailability as revealed by PK parameters may be due to suppression of CYP3A2, Pgp/MDR1, and BCPR/ABCG2-mediated dasatinib metabolism in the liver and intestine as demonstrated in the current study. The inhibition of ABCG2 and BCPR increased the absorption of drug and prevent elimination as evident by decreased T ½ (h) and CL. These results are in line with previous reports [45, 59-62]. The prior studies conducted in our labs demonstrated that polyphenols (apigenin, sinapic acid and naringenin) have increased the systemic bioavailability of DAS [15, 17, 18, 35]. HDI between TQ and DAS is likely due to TQ, a strong inhibitor of the CYPP3A2 and the drug transporters Pgp and BCRP [28, 52-54]. Involvement of efflux transporters are evident as there is significant reduction in PK parameters MRT, T1/2, Vd and CL led to PK interaction that may cause accumulation of drug and inhibition of drug metabolism [14, 44, 45, 55].
The data of immunoblot experiments carried on the liver and lumen tissues demonstrated that TQ significantly reduced the upregulated CYP3A4, Pgp and BCRP protein expressions as compared to the DAS treatment group. This indicates that TQ has ability to modulate the expression of CYP3A2/Pgp/BCRP in the liver and lumen. This in accordance with increased DAS systemic bioavailability as evident by increased in its Cmax, Tmax, and AUC and decrease in Vd, MRT, CL of were evident in the TQ treatment group. TQ inhibits CYP3A2/Pgp/BCRP [28], Therefore, a thorough clinical investigation on the potential interaction between DAS and TQ-containing products is warranted for an appropriate regulation of the safety and effectiveness of drug therapy. Meanwhile, it is prudent to avoid the cocurrent use of TQ-containing products with DAS.
Conclusion
The current study demonstrated that TQ pretreatment can significantly alters the disposition of DAS in an animal model. The increased in systemic bioavailability and is likely due to modulation of protein expression of CYP3A4 and Pgp and BCRP in the liver and lumen tissues. Thus, consuming TQ-containing food with DAS can lead to serious interactions and may pose a risk to patients. Furthermore, a clinical evaluation is needed to confirm such observations in humans.
Acknowledgements
The authors would like to extend their sincere appreciation to the Ongoing Research Funding Program, (ORF-2025-957), King Saud University, Riyadh, Saudi Arabia.
Funding
Ongoing Research Funding Program, (ORF-2025-957), King Saud University, Riyadh, Saudi Arabia.
Data availability statement
The Data generated from the study has been clearly presented in the results and discussion sections
Ethics approval
The study was conducted as per the animal guidelines and was approved by the King Saud University College of Pharmacy Research Ethics Committee (KSU-SE-21-58).
Author contributions
Conceptualization and Methodology: Ajaz Ahmad, Mohammad Raish; Experimental work: Yousef A Bin Jardan, Abdul Ahad, Mohd Abul Kalam Muzaffer Iqbal, Ibrahim A. Abdelrahman, Naushad Ali, Ali Akhtar; Data Analysis and writing of original draft: Ajaz Ahmad and Mohammad Raish: Writing, Reiewing and Editing: Khalid M. Alkharfy; Fahad I Al-Jenoobi, Ajaz Ahmad and Mohammad Raish. All authors agreed for the publication of this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Paul MK, Mukhopadhyay AK. Tyrosine kinase - Role and significance in Cancer. Int J Med Sci. 2004;1:101-15
2. Teo YL, Ho HK, Chan A. Metabolism-related pharmacokinetic drug-drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. Br J Clin Pharmacol. 2015;79:241-53
3. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-42
4. Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10:470-81
5. Bonvin A, Mesnil A, Nicolini FE, Cotte L, Michallet M, Descotes J. et al. Dasatinib-induced acute hepatitis. Leuk Lymphoma. 2008;49:1630-2
6. Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K. et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino) thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. Journal of medicinal chemistry. 2004;47:6658-61
7. Kamath AV, Wang J, Lee FY, Marathe PH. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol. 2008;61:365-76
8. Rochat B, Fayet A, Widmer N, Lahrichi SL, Pesse B, Decosterd LA. et al. Imatinib metabolite profiling in parallel to imatinib quantification in plasma of treated patients using liquid chromatography-mass spectrometry. J Mass Spectrom. 2008;43:736-52
9. Di Gion P, Kanefendt F, Lindauer A, Scheffler M, Doroshyenko O, Fuhr U. et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on pyrimidines, pyridines and pyrroles. Clin Pharmacokinet. 2011;50:551-603
10. Wang L, Christopher LJ, Cui D, Li W, Iyer R, Humphreys WG. et al. Identification of the human enzymes involved in the oxidative metabolism of dasatinib: an effective approach for determining metabolite formation kinetics. Drug Metab Dispos. 2008;36:1828-39
11. Christopher LJ, Cui D, Wu C, Luo R, Manning JA, Bonacorsi SJ. et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008;36:1357-64
12. Keam S. Dasatinib. Bio-Drugs. 2008;22:59-69
13. Squibb B-MJP, NJ, Bristol-Myers Squibb. Sprycel (dasatinib). 2017.
14. Deng J, Shao J, Markowitz JS, An G. ABC transporters in multi-drug resistance and ADME-Tox of small molecule tyrosine kinase inhibitors. Pharm Res. 2014;31:2237-55
15. Shahid M, Ahmad A, Raish M, Bin Jardan YA, Alkharfy KM, Ahad A. et al. Herb-drug interaction: Effect of sinapic acid on the pharmacokinetics of dasatinib in rats. Saudi Pharm J. 2023;31:101819
16. Alanazi AZ, Alhazzani K, Alrewily SQ, Aljerian K, Algahtani MM, Alqahtani QH. et al. The Potential Protective Role of Naringenin against Dasatinib-Induced Hepatotoxicity. Pharmaceuticals (Basel). 2023 16
17. Raish M, Ahmad A, Shahid M, Jardan YAB, Ahad A, Kalam MA. et al. Effects of Apigenin on Pharmacokinetics of Dasatinib and Probable Interaction Mechanism. Molecules. 2023 28
18. Abdelgalil AA, Alam MA, Raish M, Mohammed IE, Hassan Mohammed AE, Ansari MA. et al. Dasatinib significantly reduced in vivo exposure to cyclosporine in a rat model: The possible involvement of CYP3A induction. Pharmacol Rep. 2019;71:201-5
19. Fleisher B, Unum J, Shao J, An G. Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci. 2015;104:266-75
20. Steegmann JL, Baccarani M, Breccia M, Casado LF, Garcia-Gutierrez V, Hochhaus A. et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648-71
21. Herviou P, Thivat E, Richard D, Roche L, Dohou J, Pouget M. et al. Therapeutic drug monitoring and tyrosine kinase inhibitors. Oncol Lett. 2016;12:1223-32
22. Mohtashami A, Entezari MH. Effects of Nigella sativa supplementation on blood parameters and anthropometric indices in adults: A systematic review on clinical trials. J Res Med Sci. 2016;21:3
23. Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients. 2021; 13
24. Awad AS, Kamel R, Sherief MA. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N-1-acetyl-transferase gene expression induced by renal ischaemia-reperfusion in rats. J Pharm Pharmacol. 2011;63:1037-42
25. Alzoman NZ, Maher HM, Shehata SM, Abanmy NO. UPLC-MS/MS study of the effect of dandelion root extract on the plasma levels of the selected irreversible tyrosine kinase inhibitors dasatinib, imatinib and nilotinib in rats: Potential risk of pharmacokinetic interactions. Biomed Chromatogr. 2019;33:e4674
26. Zeng W, Jin L, Zhang F, Zhang C, Liang W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol Res. 2018;135:122-6
27. Zhou Y, Wang S, Ding T, Chen M, Wang L, Wu M. et al. Evaluation of the effect of apatinib (YN968D1) on cytochrome P450 enzymes with cocktail probe drugs in rats by UPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;973C:68-75
28. Albassam AA, Ahad A, Alsultan A, Al-Jenoobi FI. Inhibition of cytochrome P450 enzymes by thymoquinone in human liver microsomes. Saudi Pharm J. 2018;26:673-7
29. Manthalkar L, Ajazuddin, Bhattacharya S. Evidence-based capacity of natural cytochrome enzyme inhibitors to increase the effectivity of antineoplastic drugs. Discov Oncol. 2022;13:142
30. Zuo HL, Huang HY, Lin YC, Cai XX, Kong XJ, Luo DL. et al. Enzyme Activity of Natural Products on Cytochrome P450. Molecules. 2022 27
31. Nabekura T, Kawasaki T, Furuta M, Kaneko T, Uwai Y. Effects of Natural Polyphenols on the Expression of Drug Efflux Transporter P-Glycoprotein in Human Intestinal Cells. ACS Omega. 2018;3:1621-6
32. Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397-421
33. Meesters R, Voswinkel S. Bioanalytical Method Development and Validation: from the USFDA 2001 to the USFDA 2018 Guidance for Industry. Journal of Applied Bioanalysis. 2018;4:67-73
34. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350-4
35. Raish M, Ahmad A, Karim BA, Jardan YAB, Ahad A, Iqbal M. et al. Pharmacokinetics of Dasatinib in Rats: a Potential Food-Drug Interaction with Naringenin. Eur J Drug Metab Pharmacokinet. 2024;49:239-47
36. Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36:413-26
37. Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther. 2023;8:262
38. Krejbich P, Birringer M. The Self-Administered Use of Complementary and Alternative Medicine (CAM) Supplements and Antioxidants in Cancer Therapy and the Critical Role of Nrf-2-A Systematic Review. Antioxidants (Basel). 2022 11
39. Fouladbakhsh JM, Balneaves L, Jenuwine E. Understanding CAM Natural Health Products: Implications of Use Among Cancer Patients and Survivors. J Adv Pract Oncol. 2013;4:289-306
40. Fasinu PS, Rapp GK. Herbal Interaction With Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front Oncol. 2019;9:1356
41. Bazrafshani MS, Pardakhty A, Kalantari Khandani B, Tajadini H, Ghazanfari Pour S, Hashemi S. et al. The prevalence and predictors of herb-drug interactions among Iranian cancer patients during chemotherapy courses. BMC Complement Med Ther. 2023;23:41
42. Chen R, Chen B. The role of dasatinib in the management of chronic myeloid leukemia. Drug Des Devel Ther. 2015;9:773-9
43. Yang S, Zhang X, Wang Y, Wen C, Wang C, Zhou Z. et al. Development of UPLC-MS/MS Method for Studying the Pharmacokinetic Interaction Between Dasatinib and Posaconazole in Rats. Drug Des Devel Ther. 2021;15:2171-8
44. Mittapalli RK, Chung AH, Parrish KE, Crabtree D, Halvorson KG, Hu G. et al. ABCG2 and ABCB1 Limit the Efficacy of Dasatinib in a PDGF-B-Driven Brainstem Glioma Model. Mol Cancer Ther. 2016;15:819-29
45. Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956-63
46. Abukhader MM. The effect of route of administration in thymoquinone toxicity in male and female rats. Indian J Pharm Sci. 2012;74:195-200
47. Ahmad A, Alqahtani S, Jan BL, Raish M, Rabba AK, Alkharfy KM. Gender effect on the pharmacokinetics of thymoquinone: Preclinical investigation and in silico modeling in male and female rats. Saudi Pharm J. 2020;28:403-8
48. Butt MS, Imran M, Imran A, Arshad MS, Saeed F, Gondal TA. et al. Therapeutic perspective of thymoquinone: A mechanistic treatise. Food Sci Nutr. 2021;9:1792-809
49. Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin-nicotinamide induced diabetic rats. Life sciences. 2009;85:830-4
50. Bouhlel A, Ben Mosbah I, Hadj Abdallah N, Ribault C, Viel R, Mannai S. et al. Thymoquinone prevents endoplasmic reticulum stress and mitochondria-induced apoptosis in a rat model of partial hepatic warm ischemia reperfusion. Biomed Pharmacother. 2017;94:964-73
51. Jaswal A, Sinha N, Bhadauria M, Shrivastava S, Shukla S. Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage. Environmental Toxicology and Pharmacology. 2013;36:779-86
52. Adiwidjaja J, Sasongko L. Effect of Nigella sativa oil on pharmacokinetics and pharmacodynamics of gliclazide in rats. Biopharm Drug Dispos. 2021;42:359-71
53. Elbarbry F, Ung A, Abdelkawy K. Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities. Pharmacogn Mag. 2018;13:S895-S9
54. Elbarbry F, Ragheb A, Marfleet T, Shoker A. Modulation of hepatic drug metabolizing enzymes by dietary doses of thymoquinone in female New Zealand White rabbits. Phytother Res. 2012;26:1726-30
55. Keyvani V, Nasserifar Z, Saberi MR, Mohajeri SA, Arabzadeh S, Shahriari Ahmadi F. et al. Evaluation the interaction of ABC multidrug transporter MDR1 with thymoquinone: substrate or inhibitor? Iran J Basic Med Sci. 2020;23:1360-6
56. Thabet NA, El-Khouly D, Sayed-Ahmed MM, Omran MM. Thymoquinone chemosensitizes human colorectal cancer cells to imatinib via uptake/efflux genes modulation. Clin Exp Pharmacol Physiol. 2021;48:911-20
57. Alkhatib MH, Bawadud RS, Gashlan HM. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci Rep. 2020;10:18124
58. Ma J, Hu X, Li J, Wu D, Lan Q, Wang Q. et al. Enhancing conventional chemotherapy drug cisplatin-induced anti-tumor effects on human gastric cancer cells both in vitro and in vivo by Thymoquinone targeting PTEN gene. Oncotarget. 2017;8:85926-39
59. Hegedus C, Ozvegy-Laczka C, Apati A, Magocsi M, Nemet K, Orfi L. et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153-64
60. Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A. et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485-95
61. Kakumoto M, Sakaeda T, Takara K, Nakamura T, Kita T, Yagami T. et al. Effects of carvedilol on MDR1-mediated multidrug resistance: comparison with verapamil. Cancer Sci. 2003;94:81-6
62. Martinez C, Albet C, Agundez JA, Herrero E, Carrillo JA, Marquez M. et al. Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin Pharmacol Ther. 1999;65:369-76
Author contact
![]() Corresponding author: Prof. Mohammad Raish, Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; Email: mraishedu.sa.
Corresponding author: Prof. Mohammad Raish, Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; Email: mraishedu.sa.

 Global reach, higher impact
Global reach, higher impact