Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(15):3878-3894. doi:10.7150/ijms.116512 This issue Cite
Review
Association between Disorders of Lipid Metabolism and Oculopathy: An Overview
1. Shandong University of Traditional Chinese Medicine, Jinan 250002, China.
2. Shandong Provincial Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Therapy of Ocular Diseases; Shandong Academy of Eye Disease Prevention and Therapy, Jinan 250002, China.
3. Medical College of Optometry and Ophthalmology, Shandong University of Traditional Chinese Medicine, Jinan 250002, China.
4. Affiliated Eye Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250002, China.
Received 2025-4-27; Accepted 2025-8-4; Published 2025-8-22
Abstract
Lipid metabolism disorders, which lead to lipid deposition or changes in blood lipid composition, play a significant role in inducing or exacerbating the pathogenesis of diseases such as hypercholesterolemia and diabetes. Moreover, these disorders are closely associated with the development and progression of ocular diseases, including corneal degeneration, cataracts, glaucoma, age-related macular degeneration, and diabetic retinopathy. Studies have shown that lipid metabolism disorders critically impact the structure and function of ocular tissues through mechanisms such as lipid deposition, disrupted cholesterol synthesis, abnormal lipid concentrations, or impaired lipid transport. These disorders can damage cellular structures, induce oxidative stress, disrupt signal transduction, and lead to apoptosis of ocular tissue cells, mitochondrial dysfunction, and osmotic imbalance, ultimately impairing normal physiological functions and contributing to the onset of various eye diseases. This article reviews the association between lipid metabolism disorders and the development of various ocular diseases and explores the mechanisms underlying the interaction between lipid metabolism abnormalities and eye diseases, as well as the preventive role of lipid metabolism regulation in ocular diseases.
Keywords: metabolic disorder, eye diseases, cholesterol, lipoprotein, lipid deposition.
1. Introduction
Lipid metabolism is a complex process involving the synthesis, breakdown, transformation, and transport of lipid substances in organisms, essential for maintaining normal physiological functions and life activities. Lipids mainly comprise triglyceride (TG), cholesterol, and lipoproteins, which serve essential functions such as energy storage, cell membrane integrity, bioactive molecule synthesis/signaling, and lipid transport. Lipid metabolism disorders refer to pathological states characterized by abnormalities in the synthesis, degradation, or transport of lipids, resulting in dysregulated serum lipid levels (e.g., high cholesterol, high TG, low high-density lipoprotein cholesterol (HDL-C)) or metabolic dysfunction. These are collectively termed dyslipidemia, including abnormal lipid levels such as high/low cholesterol, hypertriglyceridemia, or imbalanced lipoprotein profiles (e.g., LDL/HDL). Throughout this review, unless otherwise specified, we use "dyslipidemia" to describe lipid metabolic disorders (e.g., hyperlipidemia, hypolipidemia.).
In recent years, with changing lifestyles and an aging population, the prevalence of lipid metabolism disorders and related diseases has been steadily increasing. Metabolic disorders such as hypercholesterolemia have become a major global health challenge [1]. Analysis of national data from South Korea revealed that the prevalence of hypercholesterolemia rose from 9.0% in 2007 to 20.7% in 2018. In 2018, the overall prevalence of dyslipidemia was 45.6% in men and 31.3% in women, showing an age-dependent increase. The number of dyslipidemia cases surged nearly 8-fold, from 1.5 million in 2002 to 11.6 million in 2018 [2]. Similarly, dyslipidemia represents a significant public health burden in China. A study of Chinese adults found that 42.1% had dyslipidemia, low HDL-C as the most common subtype, followed by high TG (15.4%), high total cholesterol (TC) (8.3%), and high low-density lipoprotein cholesterol (LDL-C) (7.1%). Dyslipidemia was more common in men (47.3%) than women (38.8%) [3]. Given these trends, exploring the association between lipid metabolism disorders and disease progression is crucial for improving global health outcomes.
Lipid metabolism plays a pivotal role in maintaining normal physiological functions. Cholesterol, serving as a precursor for steroid hormones, participates in the metabolism, stress response and reproductive functions. Abnormal cholesterol metabolism not only disrupts hormone secretion [4], but also interacts with glucose metabolism disorders to form "glucolipotoxicity", leading to mitochondrial dysfunction and reactive oxygen species production [5], thereby impairing normal physiological functions. Furthermore, lipoproteins transport lipids through the bloodstream to fulfill energy supply and tissue repair. Elevated lipoprotein levels can trigger inflammation, vascular stenosis/occlusion, consequently inducing atherosclerosis, cardiovascular diseases and diabetes [6, 7]. This explains the growing body of research investigating the association between dyslipidemia and these related diseases. For instance: Atherosclerotic plaques are present in over 50% of hypercholesterolemia patients [8]; the U.S. reported 613,969 deaths linked to coronary artery disease and dyslipidemia between 1999-2020 [9]; Approximately 96.6% of diabetic patients exhibit various patterns of dyslipidemia [10].
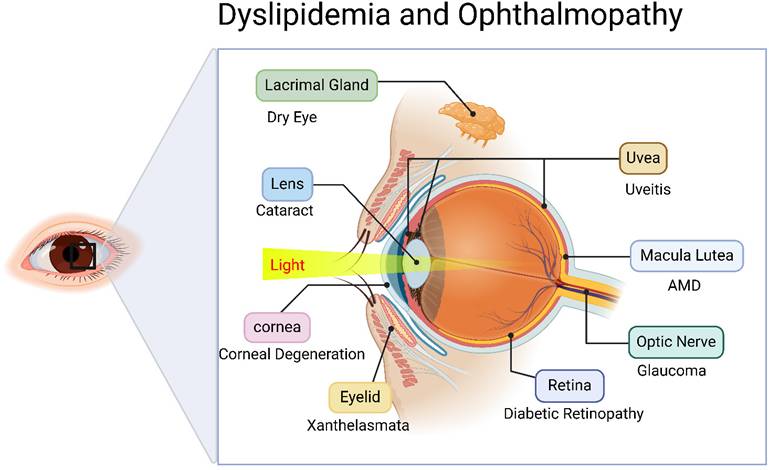
However, lipid metabolism disorders leading to lipid deposition or functional impairment can adversely affect ocular health, potentially inducing sight-threatening ocular diseases impairing visual function. Consequently, it is critical to elucidate these associations and mechanisms. However, current research on dyslipidemia-associated major eye diseases (cataract, glaucoma, AMD, and DR) remains fragmented, lacking comprehensive systematic reviews. This study aims to critically review the mechanistic roles and research advances of lipid metabolism disorders in various ophthalmic pathologies, while exploring lipid modulation as a potential therapeutic strategy for ocular disease intervention (Figure 1).
Relationship between disorders of lipid metabolism and oculopathy. Created in BioRender. 1, 1. (2025) https://BioRender.com/avh63pc.
2. Association Between Lipid Metabolism Disorders and Major Ophthalmic Diseases
2.1 Corneal diseases
2.1.1 Corneal degeneration
Corneal degeneration refers to non-inflammatory, progressive structural deterioration and functional deterioration of corneal tissue caused by metabolic disorders, aging, diseases, or environmental factors. Among these conditions, corneal arcus (also known as arcus senilis) represents a characteristic lipid deposition disorder at the corneal periphery, with significantly higher prevalence in patients with familial hypercholesterolemia (FH) and hypertriglyceridemia. A study of 1,031 FH patients found corneal arcus in 36% of cases, with prevalence rising to ~45% by age 40 and increasing further with age [11]. The Blue Mountains Eye Study, a cross-sectional investigation of 3,654 participants (82.4% aged ≥ 49 years), further established that corneal arcus was significantly associated with progressively elevated TC (> 5 mmol/L), hypercholesterolemia, and hypertriglyceridemia in elderly populations [12]. The mechanistic links of corneal arcus in hypercholesterolemic patients may be mechanistically linked to: Single-base deletion (211delG) in the LDL receptor (LDLR) gene [13]; Biallelic pathogenic variants (c.1069G > A and c.2034C > A) in LDLR [14]; FH-related genetic mutations [15].
The first reported case analysis of dense deposit disease with corneal opacity and juvenile corneal arcus demonstrated a significant association between corneal manifestations and dyslipidemia, characterized by a markedly abnormal lipid profile including elevated serum TC (285 mg/dL; normal range: 112-232 mg/dL), elevated VLDL (66 mg/dL; normal range: 8.1-30.2 mg/dL), elevated low-density lipoprotein (LDL) (204 mg/dL; normal range: < 120 mg/dL), hypertriglyceridemia (334 mg/dL; normal range: < 150 mg/dL), and low high-density lipoprotein (HDL) (14 mg/dL; normal range: 27-77 mg/dL), with ocular dense deposits potentially linked to systemic lipid metabolism dysfunction [16]. Studies of middle-aged Taiwanese populations further revealed that elevated TC, LDL-C, non-HDL-C, and TC/HDL-C ratios were significantly associated with increased corneal arcus risk [17].
Deficiency of lecithin-cholesterol acyltransferase (LCAT) or reduction in its activity or dysfunction may also lead to corneal arcus formation [18]. This condition results from an LCAT defect caused by a single nucleotide substitution at codon 123 of the LCAT gene, which alters plasma lipoprotein composition and impairs cholesterol efflux capacity, particularly affecting HDL levels [19]. Histopathological examination reveals extracellular vacuolization and amyloid deposition throughout stromal layers, with notable accumulation at Descemet's membrane [20]. The underlying mechanism likely involves impaired cholesterol esterification, leading to deposition of unesterified cholesterol in the corneal stroma [21].
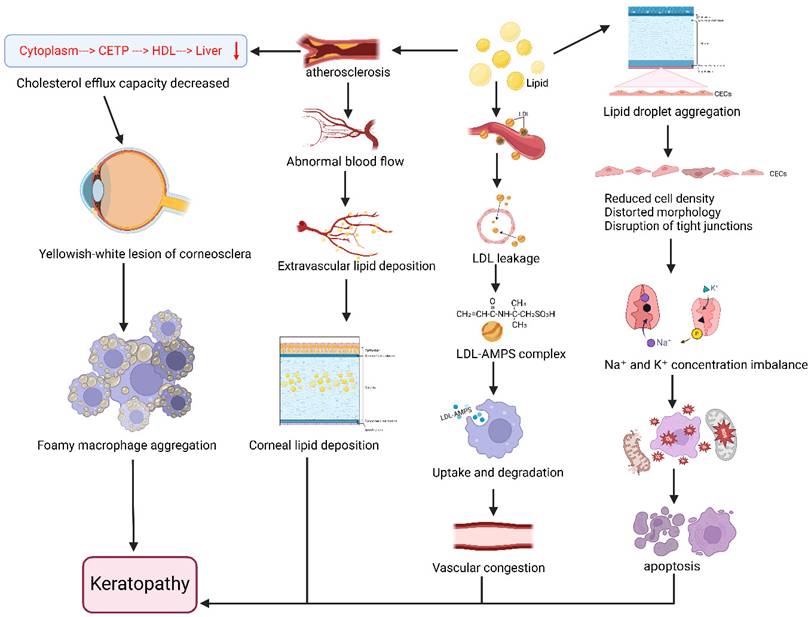
Animal studies have further confirmed that high-cholesterol diets can induce corneal lipid deposition [22], inducing intracellular lipid droplet accumulation in corneal endothelial cells (CECs). These dietary interventions cause disruption of tight junctions and adherens junctions in CECs, reduction of surface microvilli, downregulation of Na⁺-K⁺-ATPase expression and function, oxidative stress activation, mitochondrial ultrastructural alterations, and increased apoptosis. The pathological changes in corneal endothelium were found to correlate with dose-dependent cytotoxicity in CECs, morphological changes, decreased pump function, and oxidative stress induction [23]. In rabbit models of hyper-ß-lipoproteinemia, ocular lipid deposition was observed primarily in the cornea, iris, and uveal tract. Lipids initially existed as LDL distributed perivascularly, later infiltrating tissues through insudation processes and becoming selectively entrapped in various ocular locations through interactions with acid mucopolysaccharides (AMPS). Phagocytic cells subsequently uptake and degrade LDL-AMPS complexes, potentially releasing cholesterol and other lipids into tissues and triggering inflammatory hyperemia, followed by sclerotic reactions. The study also revealed associations between hyperlipoproteinemia and increased vascular permeability, where additional congestion and enhanced permeability accelerated the rate and intensity of ocular lipid deposition [24]. Furthermore, foam cell aggregation in the cornea may contribute to lipid accumulation [25]. Muramatsu M et al. demonstrated that combined deficiency of apolipoprotein E (ApoE) and Down syndrome critical region-1 (Dscr-1) in Dscr-1-/- and ApoE-/- mice led to dramatic increases in serum cholesterol levels along with severe corneal opacity and complete penetrance [26]. These symptoms were alleviated following treatment with atorvastatin (20 mg/d) and a low-cholesterol diet [27], demonstrating that lipid-lowering therapy can partially mitigate or prevent corneal degeneration (Figure 2).
Relationship between disorders of lipid metabolism and the progression of keratoconus. Lipid accumulation in the body affects corneal health in a number of ways. Lipid deposition leads to the aggregation of lipid droplets within corneal endothelial cells (CECs), which results in decreased density of CECs, distorted cell morphology, disruption of intercellular tight junctions and adhesion junctions, reduction in the number of surface microvilli, reduction in the expression and function of intracellular Na+/K+-ATPase, imbalance in the concentration of sodium and potassium ions, activation of oxidative stress and mitochondrial dysfunction, and ultimately, corneal endothelial cytotoxicity and apoptosis. Meanwhile, lipids are initially distributed as low-density lipoproteins (LDL) around blood vessels and leak into tissues through an insulating process, after which LDL interacts with 2-acrylamide-2-methylpropanesulfonic acid (AMPS) to form LDL-AMPS complexes, which are selectively entrapped to different parts of the eye, and then phagocytosis uptakes and degrades the LDL-AMPS complexes and then releases the Cholesterol and other lipids are released into the ocular tissues, causing inflammatory congestion and vascular obstruction near the cornea. In addition to this, high lipid can lead to atherosclerosis, which on the one hand increases vascular permeability and abnormal blood flow, causing extravascular lipid deposition in ocular tissues, and ultimately lipid deposition occurs in the stromal layer of the cornea, forming corneal rings; on the other hand, the cycle of cytoplasm→cholesterol transfer protein (CETP)→high-density lipoprotein (HDL)→liver decreases, and the ability to exocytosis of cholesterol decreases, resulting in the occurrence of a corneo-scleral at yellowish-white lesions, foamy macrophage aggregates at the cornea, and corneal clouding. Together, these pathways contribute to the development and progression of corneal lesions. Created in BioRender. 1, 1. (2025) https://BioRender.com/rtkqkrf.
Corneal degeneration arises from metabolic, age-related, and environmental factors, with dyslipidemia being a significant contributor to the development of corneal arcus. Notably, the juvenile corneal arcus may serve as an important clinical warning sign of underlying lipid metabolism disorders [28, 29]. Lipid metabolism abnormalities thus play a pivotal role in the pathogenesis of corneal arcus, where genetic or protein-level disruptions in lipid pathways can induce structural changes in corneal tissue leading to arcus formation. Elucidating the mechanistic role of lipid metabolism in this pathological process and its associated tissue changes provides novel insights for developing targeted therapeutic strategies.
2.1.2 Corneal dystrophy
Corneal dystrophies represent a group of progressive corneal disorders with familial inheritance patterns, among which lipid metabolism disorders play a critical role in the pathogenesis of Schnyder corneal dystrophy (SCD) [30] and Bietti crystalline dystrophy (BCD).
SCD is characterized by structural abnormalities of the anterior corneal stroma and Bowman's layer, featuring abnormal cholesterol and cholesterol esters deposits [31]. Corneal crystalline deposits mimicking SCD's clinical phenotype [32] have been observed in hyperlipoproteinemia patients (particularly type IIa) [33], suggesting plasma lipoprotein abnormalities may induce localized metabolic disturbances in corneal tissue [34].
Studies of a French family [35] and a large Nova Scotian pedigree [36] revealed that this dystrophy originates from mutations in the UBIAD1 gene, which encodes prenyltransferase 1 - an enzyme involved in vitamin K1-to-K2 conversion and cholesterol biosynthesis. UBIAD1 mutations lead to dysfunctional prenyltransferase 1, resulting in systemic cholesterol crystal accumulation, corneal thickening, central corneal deposits, and potentially blindness in severe cases. Additional studies indicate SCD correlates with foam cell deficiency, lipid accumulation in corneal fibroblasts, and localized fibroblast apoptosis [37]. BCD, caused by CYP4V2 gene mutations, results from defective lipid metabolism due to the encoded cytochrome P450 enzyme. Cyp4v3(-/-) mouse models demonstrate that CYP4V2 mutations induce systemic lipid metabolism disorders with subsequent lipid crystal deposition in both cornea and retina [38].
Therapeutic evidence suggests that low-cholesterol diets and intake of unsaturated fatty acids may improve corneal opacity and reduce cardiovascular risks [39]. Studies of corneal stromal dystrophy (CSD) in Boxer dogs further support that low-fat diets improve lipoproteins and corneal lesions [40]. These findings highlight the therapeutic potential of lipid metabolism regulation in corneal dystrophy, with lipid-lowering therapy a promising therapeutic strategy. Importantly, dietary modification and habit adjustment may prevent lipid metabolism disorder-induced corneal pathology.
2.2 Diseases of the ocular surface
2.2.1 Dry eye
Dry eye disease (DED; keratoconjunctivitis sicca) is a chronic ocular surface disorder caused by abnormalities in tear quality/quantity or impaired tear film dynamics. Population studies in regions like Saudi Arabia revealed a 55.9% prevalence of dyslipidemia (95% CI 40.1-71.7) among DED patients, with significantly greater severity than the general population [41]. A nationwide Korean survey of 17,364 adults (≥20 years) demonstrated that DED patients had higher systemic comorbidity rates, showing significant association between dyslipidemia (adjusted odds ratio: 1.63) and DED prevalence [42]. Similar correlations were observed between dyslipidemia (elevated serum cholesterol and triglycerides) and DED in studies of 5,627 Korean women [43] and 15,294 general Korean adults [44]. Notably, research on DED patients aged 25-70 years identified particular susceptibility in female populations [45]. Retrospective analysis of 306 DED patients (18-87 years) further confirmed significantly increased DED incidence among women >40 years and dyslipidemic individuals [46]. Systematic reviews and meta-analyses established that elevated TC, LDL-C, and HDL-C levels correlated with higher DED risk [47], especially in women [48]. Cross-sectional studies also linked abnormal TC, HDL, LDL, and TG levels to DED progression, potentially through impaired tear film stability and meibomian gland dysfunction (MGD) [49].
Animal models provide mechanistic insights, showing that ACAT-1 deficiency with ApoE or LDLR knockout exacerbates dry eye symptoms by impairing lacrimal function and tear film stability [50]. The underlying mechanism involves dysregulation of multiple oxidative stress pathways, characterized by significantly elevated key oxidative markers (4-HNE and MDA) and hyperactivation of oxidases (MPO, NOS3, and XOR). These alterations trigger inflammatory cascades through the TLR4/NF-κB pathway, while abnormally elevated systemic inflammation indices (NLR, PLR) reflect chronic inflammatory status. Notably, triglyceride accumulation in meibum directly activates these inflammatory pathways, establishing a vicious cycle between oxidative stress and inflammation [51, 52].
In conclusion, lipid metabolism may influence DED pathogenesis through lipoprotein-mediated pathways. Early DED screening for dyslipidemic patients could enable timely intervention to mitigate ocular health impacts. However, current evidence remains limited regarding precise mechanistic links between dyslipidemia and DED etiology, warranting further rigorous investigation into lipid-related pathophysiological roles. Furthermore, research on the application of lipid-lowering medications (particularly statins) in dry eye disease remains relatively scarce. Whether lipid-modulating therapies can prevent or alleviate dry eye symptoms requires further clinical investigation and validation.
2.2.2 Meibomian gland dysfunction
The meibomian glands (MGs) primarily function to synthesize and secrete lipids that maintain tear film stability, prevent evaporation, and reduce friction. MGD is pathologically characterized by terminal duct obstruction and/or abnormal quality/quantity of meibum secretion. Dyslipidemia has been identified as a contributing factor to MGD development. A study of 116 patients aged 18-65 years demonstrated that MGD patients generally exhibited elevated serum lipid levels compared to healthy individuals [53], with particularly significant correlations observed between dyslipidemia [54] and MGD severity. Clinical observations of 66 moderate-to-severe MGD cases revealed a higher incidence of dyslipidemia compared to the general population, specifically showing elevated TC (> 200 mg/dL) in 67.4% vs. 45.1% of controls (P = 0.0012), while low HDL (HDL < 40 mg/dL) was observed in 6.5% vs. 15.7% of controls (P = 0.045) [55]. Systematic reviews and meta-analyses [56] confirmed significant associations between dyslipidemia (particularly elevated TC and triglycerides) and MGD prevalence: among MGD patients, 20.0-77.6% had TC ≥200 mg/dL and 8.3-89.7% had TG ≥150 mg/dL, compared to 6.1-45.1% and 1.1-47.8% in age-matched controls.
Dyslipidemia may influence MG acinar cell differentiation, maturation, and lipid synthesis through both direct and indirect mechanisms [57]. Excessive cholesterol accumulation alters meibum composition, disrupting the tear film lipid layer and aqueous retention, thereby exacerbating MGD pathology [58]. Lipidomics studies and dyslipidemic mouse models have provided initial insights into the association between dyslipidemia and altered meibum composition [59]. Research using obese mouse models demonstrated that high-fat diet-induced dyslipidemia resulted in meibomian gland hypertrophy, altered meibum composition, and increased saturated fatty acyl lipids in both plasma and meibum [60]. Transcriptomic analysis revealed that dyslipidemia disrupts lipid metabolic homeostasis by disrupting rhythmic expression of core circadian clock genes (Clock, Per2, Per3) in meibomian glands, ultimately leading to gland dysfunction. These findings demonstrate a direct correlation between circadian rhythm disruption and morphological abnormalities of the glands, providing novel mechanistic insights into metabolism-associated meibomian gland dysfunction [61].
Notably, MG atrophy and deteriorated meibum quality may persist in dyslipidemic patients even during statin therapy [62]. Therefore, the therapeutic efficacy of lipid-lowering therapy for meibomian gland dysfunction remains to be clinically validated.
In summary, dyslipidemia can contribute to MGD pathogenesis by altering meibum composition and impairing MG cellular function. However, current understanding of lipid metabolism's role in MGD pathogenesis remains limited. Future studies should further elucidate the specific role of dyslipidemia in MGD development to inform novel preventive and therapeutic strategies.
2.3 Eyelid diseases
2.3.1 Xanthelasma
Xanthelasma is a lipid deposition disorder primarily caused by cholesterol accumulation in the eyelid region [63], representing a classic manifestation of lipid and lipoprotein metabolism disorders. Epidemiological investigations in Taiwan revealed that approximately 50% of xanthelasma patients have dyslipidemia [64], with hypercholesterolemia being particularly prevalent in this population [65]. A study of 896 xanthelasma cases demonstrated uniform prevalence of hypercholesterolemia comorbidity across all age groups above 30 years [66]. Cross-sectional studies further found an 80.00% dyslipidemia prevalence (95% CI 74-86) among xanthelasma patients [67]. Clinical analyses indicate xanthelasma primarily affects middle-aged women, most commonly located on the upper eyelids [68]. Nair PA's research validated this demographic trend, showing peak incidence in women aged 50-60 years, with associations to both dyslipidemia and potential early coronary artery disease [69].
The Lipid Research Clinics Population Study found highest prevalence xanthelasma prevalence in type II phenotype individuals, increasing with age. The condition is associated with elevated plasma cholesterol and LDL-C levels, particularly among young males [70]. Notably, xanthelasma may develop even in normolipidemic patients, possibly due to atherogenic lipoprotein components [71]. Chang HC's study demonstrated significantly increased apolipoprotein B levels (SMD, 1.036; 95% CI, 0.361 to 1.711; P = 0.003) and decreased atheroprotective apolipoprotein A1 (SMD, -0.328; 95% CI, -0.704 to 0.048; P = 0.087) in xanthelasma patients, potentially contributing to increased carotid intima-media thickness and elevated anti-atherogenic risk [72].
Therapeutic evidence shows cholesterol reduction reduces xanthelasma severity or induces regression xanthelasma. Probucol treatment in FH patients not only significantly reduced serum cholesterol but also induced xanthelasma regression [73], though non-macrophage lipid deposits may persist, suggesting probucol may redistribute cholesterol from macrophages to other cells. Furthermore, extremely low LDL cholesterol concentrations may lead to regression of cutaneous lipid disorders such as xanthelasma [74]. Lipid-lowering therapy has also proven effective in treating familial hypercholesterolemia, thereby reducing xanthomatous manifestations including xanthelasma [75, 76]. However, some patients may develop recurrence of lesions due to persistent lipid metabolism abnormalities despite treatment [77].
While existing studies confirm associations between dyslipidemia and xanthelasma, the precise mechanisms remain incompletely understood. Further research is needed to clarify the role of lipid metabolism in xanthelasma pathogenesis and develop targeted therapeutic strategies.
2.3.2 Upper eyelid ptosis
Ptosis, as a common ocular condition, can impair visual acuity and function. Emerging evidence indicates associations between dyslipidemia and ptosis development. A study of 251 Japanese patients (aged ≥ 60 years) with age-related degenerative ptosis identified non-HDL cholesterol (p = 0.003) and HDL cholesterol (p = 0.044) as independent correlates, suggesting dyslipidemia as a potential risk factor for senile ptosis, though causal relationships require further investigation [78]. Patients with mitochondrial DNA deletion-associated chronic progressive external ophthalmoplegia (CPEO) complicated by FH frequently exhibit bilateral ptosis alongside elevated serum cholesterol levels [79].
Certain genetic disorders demonstrate close links between impaired cholesterol biosynthesis and ptosis pathogenesis [80]. For instance, Smith-Lemli-Opitz syndrome (SLOS), caused by DHCR7 gene mutations impairing cholesterol synthesis, leads to accumulated 7-dehydrocholesterol (7-DHC) disrupting normal cholesterol function in tissues, ultimately causing ptosis [81]. Dhcr7(-/-) mouse models demonstrate that 7-DHC supports Ret signaling, but its deficiency impairs cholesterol synthesis, potentially compromising sympathetic nervous system function through disrupted Ret signaling pathways and inducing ptosis [82].
Lipid metabolism abnormalities may contribute to ptosis development by affecting neural transmission, tissue structure, and cellular signaling pathways. However, direct evidence linking dyslipidemia to ptosis remains limited, and Lipid-targeted therapies (e.g., cholesterol-lowering agents) are not yet established as primary interventions for blepharoptosis. Further in-depth investigations are required to elucidate the underlying pathological mechanisms and explore potential therapeutic approaches.
2.4 Cataract
Cataract, characterized by lens opacity due to reduced transparency or altered coloration of the crystalline lens, represents a degenerative change compromising optical quality. Substantial evidence establishes significant associations between dyslipidemia and cataract development. Analysis of 715,554 adults ≥ 40 years from the 2008-2012 Korean Community Health Survey demonstrated dyslipidemia as a significant risk factor for cataract [83]. Park YH's study using 2008-2010 Korea National Health and Nutrition Examination Survey data demonstrated metabolic syndrome (MetS) and its components specifically correlated with age-related cataracts, particularly nuclear cataracts, in Korean women [84]. Furthermore, Rim TH's research revealed a 40.1% cataract prevalence among adults > 40 years, with MetS and hypercholesterolemia identified as independent risk factors, suggesting cholesterol control may help reduce cataract incidence in Korea's general population [85]. Cholesterol synthesis disorders and elevated cholesterol levels can induce lens fiber breakage, vacuolation [86], and retinal cell dysfunction [87], ultimately contributing to lens opacification.
Hypercholesterolemic rat models exhibit increased extracellular signal-regulated kinase (ERK) and malondialdehyde (MDA) alongside reduced glutathione (GSH), indicating enhanced oxidative stress [88]. This oxidative imbalance further disrupts mitochondrial dynamics [89], leading to reactive oxygen species promote crystallin protein denaturation and aggregation—key processes in cataractogenesis. Cholesterol-induced mitochondrial oxidative stress also promotes intracellular lipoprotein accumulation [90]. Spontaneously diabetic Torii fatty rats developed cortical and posterior subcapsular cataracts more rapidly than Sprague-Dawley controls, suggesting a synergistic effect by hypercholesterolemia and diabetes [91]. High-fat diet mouse models demonstrated lipid peroxidation impairs Na+/K+-ATPase (NKA) function through NKAα1 subunit imbalance, disrupting oxidation-autophagy equilibrium and causing cellular damage [92]. NKA dysfunction also alters ionic gradients, resulting in lens osmotic imbalance and cortical liquefaction.
Genetic disorders of cholesterol metabolism like SLOS and cerebrotendinous xanthomatosis (CTX) also are associated with cataracts [93]. SLOS involves post-squalene pathway defects leading to elevated specific sterol intermediates that induce cataracts [94], while CTX - caused by CYP27A1 mutations impairing mitochondrial sterol 27-hydroxylase - leads to cholesterol accumulation resulting in cataracts and tendon xanthomas [95]. A Chinese case report of cerebrotendinous xanthomatosis patients showed that lipid-lowering therapy normalized serum free fatty acid levels and alleviated disease-related symptoms [96] (Figure 3).
Collective evidence indicates lipid metabolism dysregulation as a significant cataract risk factor, particularly through cholesterol-related pathways. Maintaining lipid homeostasis may help prevent or delay cataract onset, though targeted interventions based on lipid abnormalities warrant further exploration.
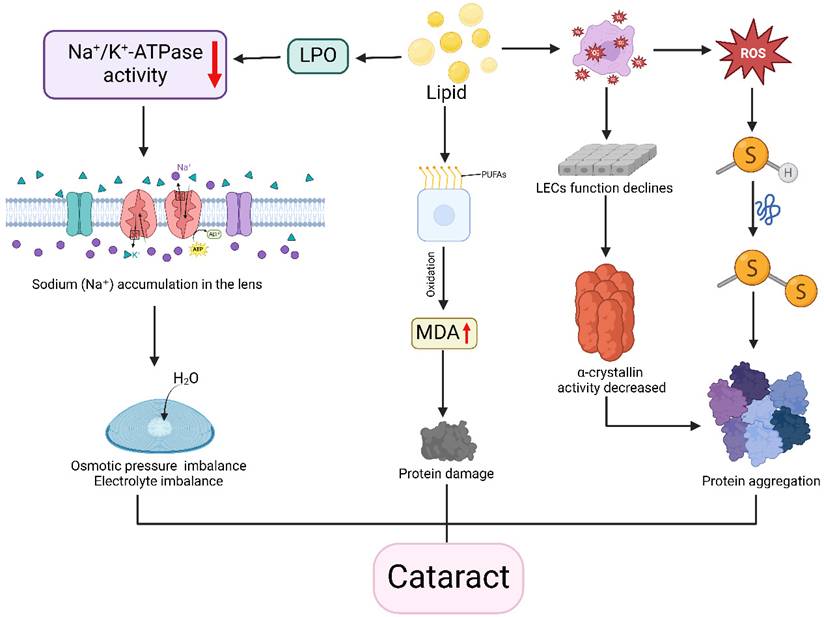
Association between disorders of lipid metabolism and cataracts. Disorders of lipid metabolism and imbalances in lipid peroxide (LPO) levels reduce the activity of the Na+/K+-ATPase enzyme, leading to sodium ion accumulation in the lens, osmotic pressure and electrolyte imbalances, and water influx into the lens, resulting in lens edema and cortical liquefaction. Also, metabolic disorders cause oxidation of polyunsaturated fatty acids PUFAs in cell membranes to produce malondialdehyde (MDA), resulting in protein damage. In addition, lipid imbalance also triggers oxidative stress. On the one hand, the generated reactive oxygen species attack protein sulfhydryl groups (-SH), denaturing proteins and forming disulfide bonds (-S-S-), which leads to protein aggregation; on the other hand, oxidative stress decreases the function of lens epithelial cells and reduces the function of α-lens proteins, which can likewise lead to denaturation and aggregation of proteins. The combined effect of these mechanisms contributes to the development of cataract. Created in BioRender. 1, 1. (2025) https://BioRender.com/rtb0ufa.
2.5 Glaucoma
Glaucoma, a disease characterized by optic nerve damage due to elevated intraocular pressure (IOP), has been increasingly linked to dyslipidemia in its pathogenesis [97]. A study of 18,161 Italian glaucoma patients identified hypertension (60.2%) as the most prevalent comorbidity, followed by dyslipidemia (29.7%) [98]. Baseline data from the China Health and Retirement Longitudinal Study demonstrated significant association between glaucoma and dyslipidemia (OR 1.757; 95% CI 1.157-3.650), with notable regional prevalence variations [99]. Analysis of 2015-2021 Korea National Health and Nutrition Examination Survey data confirmed dyslipidemia (OR 1.529) as a glaucoma risk factor [100]. Case-control studies revealed abnormal TG levels increased primary open-angle glaucoma (POAG) risk by 16.9-fold compared to normolipidemic individuals [101]. Younger-onset POAG showed stronger dyslipidemia association (OR 1.49; 95% CI 1.07-2.08) versus elderly patients [102]. Mendelian randomization supports dyslipidemia as an independent glaucoma predictor [103], where HDL-C inversely correlates with risk [104] while LDL-C [105] and TG show positive associations [106]. Dysregulated LDL metabolism alters trabecular meshwork cholesterol homeostasis, modulating mechanical signaling and aqueous humor outflow [107]. Lipid deposits in the trabecular meshwork induce chronic inflammation, fibrosis, and extracellular matrix remodeling, ultimately elevating IOP. GPR18 lipid receptor signaling activation similarly modulates murine IOP.
Hypercholesterolemia may exacerbate glaucoma through NOS-2-mediated oxidative damage to retinal ganglion cells [108] or vascular dysfunction impairing optic nerve perfusion [109, 110]. Comparative studies demonstrate elevated oxidative status, stress indices, and TC in normal-tension and pseudoexfoliation glaucoma patients versus controls, with altered PON1 enzyme activity [111], suggesting oxidative stress and dyslipidemia contribute across glaucoma subtypes (Figure 4).
Statins demonstrate protective effects against glaucoma progression, particularly in patients exhibiting intraocular pressure (IOP) reduction but persistent visual field loss. These agents may reduce glaucoma risk and serve as a novel therapeutic strategy [112, 113]. Talwar N et al. further demonstrated that compared to non-users, patients receiving continuous statin therapy for 2 years exhibited a 21% reduction in glaucoma risk (adjusted HR, 0.79; 95% CI, 0.66-0.96; P = 0.02) [114]. In JAMA Ophthalmology, Kang et al. [115] analyzed three large longitudinal cohorts and established an inverse association between prolonged statin use (≥5 years) and primary open-angle glaucoma (POAG) incidence. In addition, LDL-C lowering medications demonstrate efficacy in reducing glaucoma risk, providing additional therapeutic rationale for dyslipidemic glaucoma patients [116].
Collectively, dyslipidemia is significantly associated with glaucoma pathogenesis. Emerging evidence suggests anti-glaucoma medications may exert therapeutic effects through systemic lipid profile modulation, potentially regulating intraocular pressure via pathogenic lipid reduction.
2.6 Retinopathy
The retina, a critical component of the human visual system, is essential for receiving and transmitting visual information. Dyslipidemia has been strongly associated with the pathogenesis and progression of various retinal diseases, with studies showing that abnormal lipid profiles increase the risk of DR [117, 118], retinal vein/artery occlusion (RVO/RAO) [119], age-related macular degeneration (AMD), and retinal microvascular abnormalities [120], among others.
Link between disorders of lipid metabolism and the pathogenesis of glaucoma. Lipid metabolism disorders, with increased saturated fatty acids in the body, trigger endoplasmic reticulum stress (ERS), exacerbate mitochondrial dysfunction and reactive oxygen species production, activate the JNK/p38 MAPK pathway, and an imbalance in the Bax/Bcl-2 ratio, causing apoptosis of the retinal ganglion cells (RGC). Lipids also promote atherosclerosis and vascular endothelial cell damage, resulting in microcirculatory impairment of blood flow, reduced blood supply, and decreased blood supply to the optic nerve from the short posterior ciliary artery, leading to optic nerve atrophy. In addition, lipid disorders affect SREPB-1 expression, resulting in lipid deposition in the trabecular meshwork, causing lectin-like oxidized LDL receptor-1 (Lox-1) uptake of oxidized LDL, activation of the NF-kB signaling pathway, and elevated expression of IL-6 and TNF-α, resulting in inflammatory damage to the optic nerve. Lipid deposition in the trabecular meshwork also causes oxidative stress and remodeling of the trabecular meshwork extracellular matrix (ECM), resulting in trabecular meshwork cellular damage, increased resistance to outflow of aqueous humor, and impaired aqueous drainage, which ultimately leads to elevated intraocular pressure. The mechanisms of action, such as changes in intraocular pressure and optic nerve atrophy, are coordinated to promote glaucoma in the figure. Created in BioRender. 1, 1. (2025) https://BioRender.com/qwzyxcw.
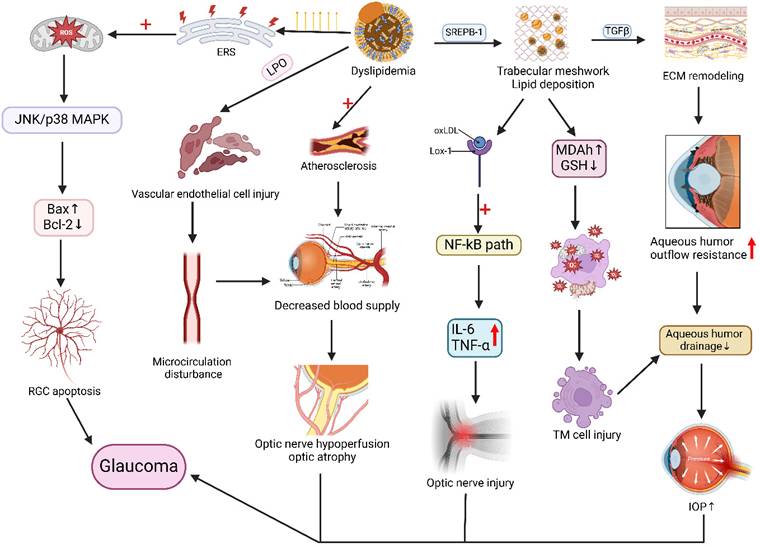
2.6.1 Diabetic retinopathy
In DR, Shi R et al. found that dyslipidemia significantly correlates with reduced retinal blood flow in patients, potentially leading to thinning of the retinal nerve fiber and ganglion cell layers [121]. Abnormal lipid metabolism is strongly associated with both the onset and severity of DR [122], with apolipoproteins such as apoB and apoC-II/apoC-III are implicated in DR pathogenesis while apoA-I/A-II may serve protective roles [123]. Strict control of serum apolipoprotein B (apoB) levels may serve as an effective therapeutic strategy for DR.
During DR progression, increased extravasation of modified LDL is observed, with highly oxidized glycated LDL (HOG-LDL) exacerbating DR progression by inducing caspase activation, mitochondrial dysfunction, and pericyte apoptosis in human retinal capillary pericytes [124]. Additionally, lipotoxicity can induce accumulation of mononuclear phagocytes expressing the lipid-load marker Perilipin 2 in retinal regions, where pro-inflammatory macrophages further promote capillary degenerative changes [125]. Dyslipidemia may also disrupt the blood-retinal barrier, leading to extravasation and local modification of lipoproteins that ausing significant tissue damage [126]. Studies in Zucker diabetic fatty (ZDF) rats, a type 2 diabetes model, demonstrated that dyslipidemia induces staged retinal alterations: elevated cytoplasmic reactive oxygen species (ROS) at early stages (6 weeks), mitochondrial dysfunction at intermediate phases (12 weeks), and mitochondrial DNA damage in advanced disease (≥20 weeks), indicating lipid metabolism disorders accelerate retinopathy through oxidative stress and mitochondrial impairment by disrupting energy metabolism and induce apoptosis [127]. In type 1 diabetic mouse models, extravasated modified plasma lipoproteins drive DR progression, with intravitreal injection of human HOG-LDL causing severe progressive retinal damage characterized by morphological abnormalities, ERG changes, vascular leakage, VEGF overexpression, gliosis, endoplasmic reticulum stress, and apoptosis [128] (Figure 5).
Mechanism of action between disorders of lipid metabolism and the development of diabetic retinal degeneration. Disorders of lipid metabolism cause aggregation of the monocyte macrophage system (MPS), which activates PPARγ signaling and affects Perilipin2 protein expression, resulting in capillary degeneration. Lipid deposition also causes changes in the expression of intercellular tight junction proteins (JAM, Occludin, and Claudin), which increases the permeability of the blood-retinal barrier and the infiltration of plasma components into the retina, causing changes in retinal structure. Abnormal lipid metabolism also affects the expression of various factors (LOX-1, HIF-1α, VEGF-A, NOX-1, NOX-2), activating oxidative stress and causing endothelial dysfunction (ETDF). In addition, lipid abnormalities lead to endoplasmic reticulum stress and mitochondrial dysfunction, reactive oxygen species (ROS) generation, and activation of STING signaling through the IRE1α-XBP1 axis in human retinal endothelial cells (HRVECs), triggering vascular endothelial (VE) inflammatory responses. Meanwhile, mitochondrial DNA from retinal endothelial cells leaks into the cytosol, and glycolipotoxicity damages mtDNA and affects cytochrome B (CYTB) transcription, ultimately leading to disruption of mitochondrial homeostasis. Created in BioRender. 1, 1. (2025) https://BioRender.com/ncg8p6q.
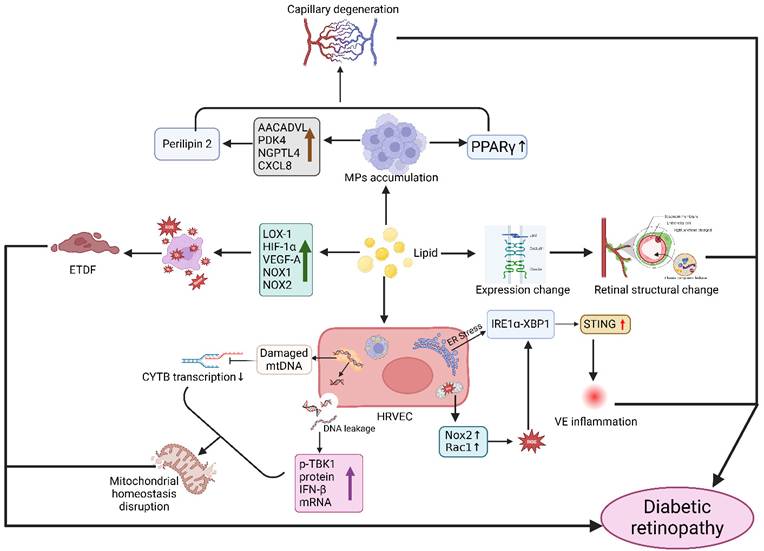
For diabetes-related complications, lipid-lowering therapy offers a potential therapeutic approach. The 2024 AMD Annals Program revealed that 72.0% of patients showed improvement with lipid-modifying medications [129]. This therapeutic feasibility was further corroborated by Italian diabetic patients attaining LDL target levels through lipid-lowering regimens [130].
2.6.2 Age-related macular degeneration
Age-related macular degeneration (AMD), a chronic progressive degenerative disease of the macular region, primarily involves pathological changes in the retinal pigment epithelium (RPE)-Bruch's membrane-choriocapillaris complex. The Blue Mountains Eye Study in Australia demonstrated that metabolic syndrome components including obesity, hyperglycemia, and hypertriglyceridemia correlate with increased AMD risk during 10-year follow-up of participants aged ≥49 years [131, 132].
Dyslipidemia may impair retinal function by disrupting intracellular organelle homeostasis. Peroxisomes, which are critical for lipid metabolism (including fatty acid oxidation, phospholipid synthesis, and ROS regulation), become dysregulated under lipid overload. This leads to oxidative stress, mitochondrial lipid deposition, and inflammatory damage, contributing to drusen formation, macular injury, and RPE/photoreceptor apoptosis [133]. Abnormal apolipoprotein E metabolism also triggers retinal inflammation and drusen generation [134]. While lipid accumulation damages RPE cells, it concurrently injures Bruch's membrane [135, 136], ultimately causing photoreceptor degeneration and retinal dystrophy [137]. Animal models of Cyp27a1(-/-)Cyp46a1(-/-) mice confirm that cholesteryl ester accumulation may accelerate photoreceptor damage and macular degeneration [138] (Figure 6).
Illustration of the association between disorders of lipid metabolism and age-related macular degeneration (AMD). Lipid metabolism disorders affect peroxidase expression levels, and the interaction between the two is such that peroxidase accumulates very long chain fatty acids (VLCFA), which are deposited on mitochondrial membranes affecting membrane fluidity, destabilizing the electron transport chain (ETC) complex, and leading to reduced ATP production and cell death. Peroxidase also blocks glucose oxidation by inhibiting pyruvate dehydrogenase (PDH) activity, leading to retinal photoreceptor cell (PRC), muller cell, and ganglion damage, macular edema, and an inflammatory response that results in an imbalance of apolipoproteins (APOEs) and complement, leading to the development of vitreous warts. In addition peroxidase induces oxidative stress and changes in the NAD(+)/NADH redox state, leading to the release of cytochrome C from the mitochondria, dysregulation of the expression of Sirtuins and HIF-1α, and ultimately retinal pigment epithelial (RPE) cell death. Together, these mechanisms contribute to the development of AMD. Created in BioRender. 1, 1. (2025) https://BioRender.com/exw8si8.
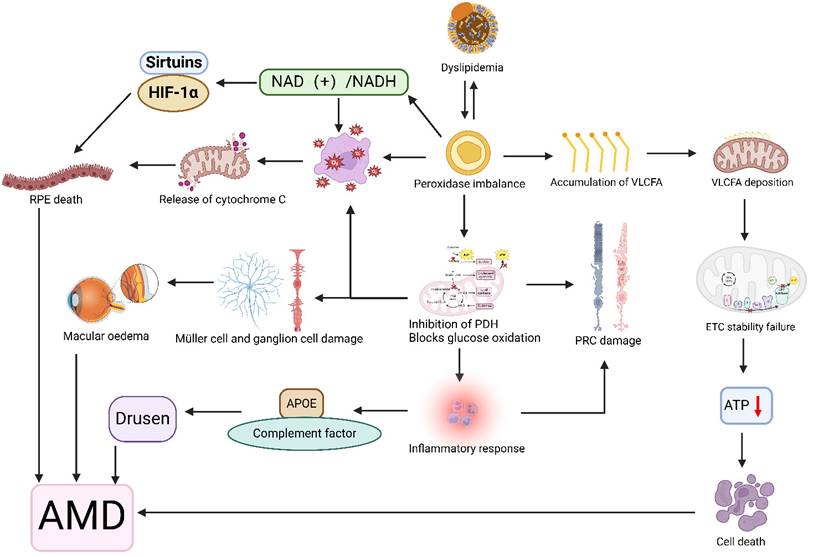
Furthermore, multiple studies have demonstrated that lipid-lowering medication use exerts potentially beneficial effects on AMD prevalence, reducing AMD risk by 35-50% [139, 140]. These findings provide additional therapeutic potential for managing age-related macular degeneration.
2.6.3 Retinal occlusive diseases
In retinal occlusive diseases, studies have identified dyslipidemia as a potential key factor, with 5 out of 6 patients (83.3%) with concurrent branch retinal artery and vein occlusion showing abnormal lipid profiles. This suggests lipid metabolism disorders may contribute to these rare vascular events through atherosclerosis, increased blood viscosity, and endothelial dysfunction [141]. A large multicenter Italian case-control study identified dyslipidemia (OR: 2.255 [1.352-3.762], p=0.002) as an independent risk factor for central retinal vein occlusion (CRVO) [142]. Multiple studies demonstrate hypercholesterolemia, hypertriglyceridemia, and low HDL-C are independently associated with retinal vein/artery occlusion (RVO/RAO) [143-145], possibly through mechanisms such as increased plasma viscosity, endothelial dysfunction, or thrombogenesis [146, 147]. Porcine models of hypercholesterolemia show retinal ultrastructural changes mediated by increased oxidative stress, NO metabolites, and superoxide anion release [148].
2.6.4 Other retinopathies
Lipid abnormalities are implicated in other retinopathies like lipemia retinalis, where severe hypertriglyceridemia induces milky retinal vasculature which resolves with dietary intervention [149]. Murine models indicate dyslipidemia modifies circRNA expression profiles, regulating focal adhesion signaling and endothelial function during retinal vascular dysfunction [150]. Additionally, dyslipidemia induces retinal microvascular damage [151], manifesting as arteriolar narrowing, reduced vessel density, and nerve fiber layer thinning, mediated by endothelial dysfunction and inflammation [152].
In summary, the key pathological mechanisms include: oxidative stress and inflammation via Nox2-derived ROS activation of STING/IRE1α-XBP1 pathways [153, 154]; vascular dysfunction driven by cholesterol-induced endothelial activation and impaired microcirculation [155]; and metabolic-autophagy imbalance where lipid accumulation disrupts energy homeostasis and exacerbates neurodegeneration [156, 157]. Statins and fenofibrate may mitigate DR progression through combined lipid-lowering, anti-inflammatory, and antioxidant effects to reduce macular edema and vascular leakage [158, 159]. Optimized dyslipidemia management (e.g., statins, fibrates) improves retinopathy outcomes [160], highlighting its therapeutic relevance. Lipid metabolism disturbances impact retinal structure/function through multifaceted mechanisms. Future research should elucidate molecular links between lipid profiles and retinal microstructural changes to inform novel treatment strategies.
2.7 Myopia
Myopia, a common refractive error, occurs when parallel light rays focus anterior to the retina when the eye is in a relaxed state, resulting in blurred distance vision while maintaining relatively clear near vision. Emerging evidence links myopia development to lipid metabolism. Che et al. identified five differential lipids in myopic patients, with three—BMP (20:3/22:3), PS (14:1/22:4), and TG (55:3)_FA18:1—showing significant correlations with spherical equivalent refraction. BMP and PS additionally associated with axial length [161]. SWATH-based quantitative proteomics revealed marked lipid metabolic alterations in early-stage myopic guinea pig retinas [162]. Mendelian randomization demonstrated causal relationships between myopia and intermediate-density lipoprotein components: TC (OR 0.90, 95% CI 0.86-0.95, P=0.00021) and cholesteryl esters (OR 0.85, 95% CI 0.79-0.92, P=0.0006) [163].
Myopic patients exhibit significant retinal lipid peroxidation [164], which may further induce damage to both retinal tissue and ganglion cells [165]. In vitro studies suggest human scleral fibroblasts' bioactivity promotes scleral elongation through sterol regulatory element-binding protein-mediated regulation of fatty acid, TG, and cholesterol synthesis - a proposed key mechanism for axial elongation [166]. Murine myopia models show elevated LILRB2/Pirb protein expression promotes fatty acid synthesis and lipid accumulation, disrupting choroidal function and accelerating pathological myopia progression [167]. High-fat diet-fed mice demonstrate excessive retinal lipid droplet accumulation, elevated oxidative stress and inflammatory signaling, accompanied by RPE degeneration, Bruch's membrane thickening, and photoreceptor dysfunction [168]. Studies in dragon-eye goldfish reveal PPAR signaling pathway upregulation concurrent with increased lipid accumulation may trigger ocular morphological changes resembling myopic defects [169].
While myopia pathogenesis involves multifactorial mechanisms, lipid metabolism abnormalities contribute through disrupted lipid synthesis, peroxidation, and retinal structural alterations. However, current research has not yet identified lipid-lowering therapy as an effective treatment for myopia, and whether reducing relevant lipid levels can prevent or slow myopia progression remains unverified. Further research should elucidate precise roles of lipids and metabolites in myopia development to inform preventive strategies against pathological myopia and its complications.
3. Conclusion
In summary, lipid metabolism is closely associated with ocular health, maintaining visual function, retinal homeostasis, and cellular membrane stability. Lipids serve not only as essential structural components of ocular tissues including the retina, cornea, optic nerve, and aqueous humor but also participate in critical biological processes such as signal transduction, energy metabolism, and immune regulation. Dyslipidemia, beyond its systemic metabolic effects, is implicated in various ocular pathologies including xanthelasma, glaucoma, corneal disorders, and DR. These diseases likely arise from imbalances in ocular lipid composition, deficiency of key enzymes, or genetic mutations, which induce pathological changes such as lipid deposition, inflammatory responses, oxidative stress, osmotic imbalance, and cellular apoptosis, ultimately leading to structural and functional impairments of ocular tissues.
Despite established associations between dyslipidemia and ocular diseases, several challenges remain. The precise dynamics of lipid metabolic cycles within ocular tissues and their underlying molecular mechanisms require further elucidation. Although lipid abnormalities can be mitigated through dietary modifications, pharmacological interventions, and lifestyle adjustments, the pathogenesis of ophthalmic diseases involves complex interactions among multiple factors. Future research should investigate the interplay between lipid metabolism and other contributing elements (e.g., genetic predisposition, inflammation, vascular abnormalities) to develop more effective prevention and treatment strategies.
Abbreviations
AMD: age-related macular degeneration; LDL: low-density lipoprotein; HDL: high-density lipoprotein; FH: familial hypercholesterolemia; LDLR: low-density lipoprotein receptor; LDL-C: low-density lipoprotein cholesterol; LCAT: lecithin-cholesterol acyltransferase; SCD: Schnyder corneal dystrophy; BCD: Bietti crystalline dystrophy; DED: Dry eye disease; SLOS: Smith-Lemli-Opitz syndrome; TC: total cholesterol; TG: triglyceride; MGD: Meibomian gland dysfunction; 7-DHC: 7-dehydrocholestero; HDL-C: high-density lipoprotein cholesterol; IOP: intraocular pressure; DR: diabetic retinopathy; RPE: retinal pigment epithelium.
Acknowledgements
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the natural science foundation of Shandong province (ZR2024MH057) and the National Key R&D Program of China [grant numbers 2021YFC2702103].
Author contributions
Conceptualization & Supervision: Dadong Guo; Writing Original Draft&Image Drafts: Yuanting Yang; Visualization: Miao Zhang; Writing Review & Editing: Dadong Guo, Ruixue Zhang, Zhaohui Yang, Zhongyu Ma; Investigation: Yinqiao Zhang, Mengke Wu.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhang H, Zhou XD, Shapiro MD, Lip GYH, Tilg H, Valenti L. et al. Global burden of metabolic diseases, 1990-2021. Metabolism. 2024;160:155999
2. Cho SMJ, Lee H, Lee HH, Baek J, Heo JE, Joo HJ. et al. Dyslipidemia Fact Sheets in Korea 2020: an Analysis of Nationwide Population-based Data. J Lipid Atheroscler. 2021;10:202-9
3. Xia Q, Chen Y, Yu Z, Huang Z, Yang Y, Mao A. et al. Prevalence, awareness, treatment, and control of dyslipidemia in Chinese adults: a systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:1186330
4. Fan J, Papadopoulos V. Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS One. 2013;8:e76701
5. El-Assaad W, Joly E, Barbeau A, Sladek R, Buteau J, Maestre I. et al. Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 ss-cells. Endocrinology. 2010;151:3061-73
6. Martini T, Gobet C, Salati A, Blanc J, Mookhoek A, Reinehr M. et al. A sexually dimorphic hepatic cycle of periportal VLDL generation and subsequent pericentral VLDLR-mediated re-uptake. Nat Commun. 2024;15:8422
7. Skoumas I, Andrikou I, Simantiris S, Grigoriou K, Dima I, Terentes-Printzios D. et al. Lipoprotein(a) in familial dyslipidemias: The effect on cardiovascular prognosis in patients with familial hypercholesterolemia or familial combined hyperlipidemia. Nutr Metab Cardiovasc Dis. 2025: 103867.
8. Giral P, Pithois-Merli I, Filitti V, Levenson J, Plainfosse MC, Mainardi C. et al. Risk factors and early extracoronary atherosclerotic plaques detected by three-site ultrasound imaging in hypercholesterolemic men. Prévention Cardio-vasculaire en Médecine du Travail METRA Group. Arch Intern Med. 1991;151:950-6
9. Cheema MR, Ahmed F, Ali F, Baloch ZQ, Minhas AM, Khosa F. et al. Trends in coronary artery disease and dyslipidemia-related mortality in the USA from 1999-2020. Minerva Cardiol Angiol. 2025
10. Muhammad AA, Afzaal M, Khan MJ, Baig AM, Aasim M. Frequency and pattern of dyslipidemia and its association with other risk factors among Type-2 Diabetics. Pak J Med Sci. 2025;41:472-7
11. Firth JC, Marais AD. Familial hypercholesterolaemia: the Cape Town experience. S Afr Med J. 2008;98:99-104
12. Chua BE, Mitchell P, Wang JJ, Rochtchina E. Corneal arcus and hyperlipidemia: findings from an older population. Am J Ophthalmol. 2004;137:363-5
13. Ward AJ, O'Kane M, Nicholls DP, Young IS, Nevin NC, Graham CA. A novel single base deletion in the LDLR gene (211delG): Effect on serum lipid profiles and the influence of other genetic polymorphisms in the ACE, APOE and APOB genes. Atherosclerosis. 1996;120:83-91
14. Lock JH, Ross CA, Flaherty M. Corneal arcus as the presenting sign of familial hypercholesterolemia in a young child. J aapos. 2018;22:467-8
15. Silva PRS, Jannes CE, Oliveira TGM, Miname MH, Rocha VZ, Chacra AP. et al. Evaluation of clinical and laboratory parameters used in the identification of index cases for genetic screening of familial hypercholesterolemia in Brazil. Atherosclerosis. 2017;263:257-62
16. Sahay P, Pandya I, Maharana PK, Titiyal JS. Cloudy Cornea with Arcus Juvenilis in a Case of Dense Deposit Disease. BMJ Case Rep. 2018. 2018
17. Chen HT, Chen HC, Hsiao CH, Ma DH, Chen YT, Lin KK. Corneal arcus and cardiovascular risk factors in middle-aged subjects in Taiwan. Am J Med Sci. 2009;338:268-72
18. Naghashpour M, Cualing H. Splenomegaly with sea-blue histiocytosis, dyslipidemia, and nephropathy in a patient with lecithin-cholesterol acyltransferase deficiency: a clinicopathologic correlation. Metabolism. 2009;58:1459-64
19. Clerc M, Pouliquen Y. [Arcus juvenilis and lecithin:cholesterol acyltransferase functions. Report of a case of familial fish-eye-disease]. Bull Acad Natl Med. 1993;177:807-20 discussion 20-2
20. Viestenz A, Schlötzer-Schrehardt U, Hofmann-Rummelt C, Seitz B, Küchle M. Histopathology of corneal changes in lecithin-cholesterol acyltransferase deficiency. Cornea. 2002;21:834-7
21. Gjone E. Familial lecithin: cholesterol acyltransferase (LCAT) deficiency. An updated review Spring 1988. Ophthalmic Paediatr Genet. 1988;9:167-9
22. Zauberman H, Livni N. Experimental vascular occlusion in hypercholesterolemic rabbits. Invest Ophthalmol Vis Sci. 1981;21:248-55
23. Bu J, Yu J, Wu Y, Cai X, Li K, Tang L. et al. Hyperlipidemia Affects Tight Junctions and Pump Function in the Corneal Endothelium. Am J Pathol. 2020;190:563-76
24. Walton KW, Dunkerley DJ. Studies on the pathogenesis of corneal arcus formation II. Immunofluorescent studies on lipid deposition in the eye of the lipid-fed rabbit. J Pathol. 1974;114:217-29
25. Kouchi M, Ueda Y, Horie H, Tanaka K. Ocular lesions in Watanabe heritable hyperlipidemic rabbits. Vet Ophthalmol. 2006;9:145-8
26. Muramatsu M, Nakagawa S, Osawa T, Toyono T, Uemura A, Kidoya H. et al. Loss of Down Syndrome Critical Region-1 Mediated-Hypercholesterolemia Accelerates Corneal Opacity Via Pathological Neovessel Formation. Arterioscler Thromb Vasc Biol. 2020;40:2425-39
27. Widmer C, Hättenschwiler A, Gross B, Schulthess G. ["Gout tophi" and heart disease in the family]. Praxis (Bern 1994). 2003;92:1912-5
28. Sharma VK, Khurana S, Kaur S, Ram J. Arcus lipoides juvenilis: a presenting sign of dyslipidaemia. Qjm. 2021;114:333-4
29. Macchiaiolo M, Buonuomo PS, Valente P, Rana I, Lepri FR, Gonfiantini MV. et al. Corneal arcus as first sign of familial hypercholesterolemia. J Pediatr. 2014;164:670
30. Kurtul BE, Elbeyli A, Ozcan DO, Ozcan SC, Karaaslan A. Schnyder Corneal Dystrophy: A Rare Case Report. Nepal J Ophthalmol. 2020;12:110-3
31. Weiss JS. Schnyder corneal dystrophy. Curr Opin Ophthalmol. 2009;20:292-8
32. Thiel HJ, Voigt GJ, Parwaresch MR. [Crystalline corneal dystrophy (Schnyder) in the presence of familial type IIa hyperlipoproteinaemia (author's transl)]. Klin Monbl Augenheilkd. 1977;171:678-84
33. Brownstein S, Jackson WB, Onerheim RM. Schnyder's crystalline corneal dystrophy in association with hyperlipoproteinemia: histopathological and ultrastructural findings. Can J Ophthalmol. 1991;26:273-9
34. Bron AJ, Williams HP, Carruthers ME. Hereditary crystalline stromal dystrophy of Schnyder. I. Clinical features of a family with hyperlipoproteinaemia. Br J Ophthalmol. 1972;56:383-99
35. Gonzalvez M, Ho Wang Yin G, Gascon P, Denis D, Hoffart L. Clinical and para-clinical description of a novel mutation for Schnyder dystrophy in a French family. J Fr Ophtalmol. 2018;41:920-5
36. Orr A, Dubé MP, Marcadier J, Jiang H, Federico A, George S. et al. Mutations in the UBIAD1 gene, encoding a potential prenyltransferase, are causal for Schnyder crystalline corneal dystrophy. PLoS One. 2007;2:e685
37. Crispin S. Ocular lipid deposition and hyperlipoproteinaemia. Prog Retin Eye Res. 2002;21:169-224
38. Lockhart CM, Nakano M, Rettie AE, Kelly EJ. Generation and characterization of a murine model of Bietti crystalline dystrophy. Invest Ophthalmol Vis Sci. 2014;55:5572-81
39. Miettinen M, Turpeinen O, Karvonen MJ, Elosuo R, Paavilainen E. Effect of cholesterol-lowering diet on mortality from coronary heart-disease and other causes. A twelve-year clinical trial in men and women. Lancet. 1972;2:835-8
40. Barsotti G, Pasquini A, Busillo L, Senese M, Cardini G, Guidi G. Corneal crystalline stromal dystrophy and lipidic metabolism in the dog. Vet Res Commun. 2008;32(Suppl 1):S227-9
41. Al Houssien AO, Al Houssien RO, Al-Hawass A. Magnitude of diabetes and hypertension among patients with Dry Eye Syndrome at a tertiary hospital of Riyadh, Saudi Arabia - A case series. Saudi J Ophthalmol. 2017;31:91-4
42. Roh HC, Lee JK, Kim M, Oh JH, Chang MW, Chuck RS. et al. Systemic Comorbidities of Dry Eye Syndrome: The Korean National Health and Nutrition Examination Survey V, 2010 to 2012. Cornea. 2016;35:187-92
43. Chun YH, Kim HR, Han K, Park YG, Song HJ, Na KS. Total cholesterol and lipoprotein composition are associated with dry eye disease in Korean women. Lipids Health Dis. 2013;12:84
44. Park HW, Park JW. The Association between Symptoms of Dry Eye Syndrome and Metabolic Outcome in a General Population in Korea. J Korean Med Sci. 2016;31:1121-6
45. Rathnakumar K, Ramachandran K, Baba D, Ramesh V, Anebaracy V, Vidhya R. et al. Prevalence of dry eye disease and its association with dyslipidemia. J Basic Clin Physiol Pharmacol. 2018;29:195-9
46. Posa A, Sel S, Dietz R, Sander R, Paulsen F, Bräuer L. et al. Historical Profiling of Dry Eye Patients - Potential Trigger Factors and Comorbidities. Klin Monbl Augenheilkd. 2024;241:110-8
47. Li Y, Xie L, Song W, Chen S, Cheng Y, Gao Y. et al. Association between dyslipidaemia and dry eye disease: a systematic review and meta-analysis. BMJ Open. 2023;13:e069283
48. Wang TH, Tsai YJ, Wang YH, Wu CL, Lin IC. Relationship between Dry Eye Disease and Dyslipidemia: A Systematic Review. J Clin Med. 2023 12
49. Serrano-Morales JM, Álvarez-Santaliestra N, Sánchez-González MC, Ballesteros-Sánchez A, Sánchez-González JM. Impact of Dyslipidemia on Tear Film and Meibomian Gland Dysfunction: A Cross-Sectional Study of the Interplay between Serum Lipid Profile and Ocular Surface Health. J Ophthalmol. 2024;2024:7345270
50. Yagyu H, Kitamine T, Osuga J, Tozawa R, Chen Z, Kaji Y. et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J Biol Chem. 2000;275:21324-30
51. Pieńczykowska K, Bryl A, Mrugacz M. Link Between Metabolic Syndrome, Inflammation, and Eye Diseases. Int J Mol Sci. 2025 26
52. Chang K, Luo P, Guo Z, Yang L, Pu J, Han F. et al. Lipid Metabolism: An Emerging Player in Sjögren's Syndrome. Clin Rev Allergy Immunol. 2025;68:15
53. Irfan KSA, Agrawal A, Singh A, Mittal SK, Samanta R, Shrinkhal. Association of Lipid Profile with Severity of Meibomian Gland Dysfunction. Nepal J Ophthalmol. 2020;12:216-35
54. Pinna A, Blasetti F, Zinellu A, Carru C, Solinas G. Meibomian gland dysfunction and hypercholesterolemia. Ophthalmology. 2013;120:2385-9
55. Dao AH, Spindle JD, Harp BA, Jacob A, Chuang AZ, Yee RW. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am J Ophthalmol. 2010;150:371-5.e1
56. Tomioka Y, Kitazawa K, Yamashita Y, Numa K, Inomata T, Hughes JB. et al. Dyslipidemia Exacerbates Meibomian Gland Dysfunction: A Systematic Review and Meta-Analysis. J Clin Med. 2023 12
57. Yoo YS, Park SK, Hwang HS, Kim HS, Arita R, Na KS. Association of Serum Lipid Level with Meibum Biosynthesis and Meibomian Gland Dysfunction: A Review. J Clin Med. 2022 11
58. Ooi KG, Watson SL. Rosacea Meibomian Gland Dysfunction Posterior Blepharitis May Be a Marker for Earlier Associated Dyslipidaemia and Inflammation Detection and Treatment with Statins. Metabolites. 2023 13
59. Osae EA, Steven P, Redfern R, Hanlon S, Smith CW, Rumbaut RE. et al. Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model. Int J Mol Sci. 2019 20
60. Osae EA, Bullock T, Chintapalati M, Brodesser S, Hanlon S, Redfern R. et al. Obese Mice with Dyslipidemia Exhibit Meibomian Gland Hypertrophy and Alterations in Meibum Composition and Aqueous Tear Production. Int J Mol Sci. 2020 21
61. Zhang Q, Su J, Chen J, Wu S, Qi X, Chu M. et al. Diurnal rhythm-modulated transcriptome analysis of meibomian gland in hyperlipidemic mice using RNA sequencing. Int Ophthalmol. 2025;45:57
62. Wu KI, Chen CY, Jou TS, Jimmy Juang JM, Lu JY, Wang IJ. Effect of 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase Inhibitors on the Meibomian Gland Morphology in Patients with Dyslipidemia. Am J Ophthalmol. 2020;219:240-52
63. Elghazi T, Hafidi Z. [Xanthelasma]. Pan Afr Med J. 2016;25:41
64. Wang KY, Hsu KC, Liu WC, Yang KC, Chen LW. Relationship Between Xanthelasma Palpebrarum and Hyperlipidemia. Ann Plast Surg. 2018;80:S84-s6
65. Rubinstein TJ, Mehta MP, Schoenfield L, Perry JD. Orbital xanthogranuloma in an adult patient with xanthelasma palpebrarum and hypercholesterolemia. Ophthalmic Plast Reconstr Surg. 2014;30:e6-8
66. Pedace FJ, Winkelmann RK. XANTHELASMA PALPEBRARUM. Jama. 1965;193:893-4
67. Rai A, Karki S, Prasad Sah S, Narayan Kamat L, Pradhan M. Dyslipidemia in Patients with Xanthelasma Palpebrarum Visiting the Department of Dermatology of a Tertiary Care Centre: A Descriptive Cross-sectional Study. JNMA J Nepal Med Assoc. 2022;60:529-32
68. Kavoussi H, Ebrahimi A, Rezaei M, Ramezani M, Najafi B, Kavoussi R. Serum lipid profile and clinical characteristics of patients with xanthelasma palpebrarum. An Bras Dermatol. 2016;91:468-71
69. Nair PA, Patel CR, Ganjiwale JD, Diwan NG, Jivani NB. Xanthelasma Palpebrarum with Arcus Cornea: A Clinical and Biochemical Study. Indian J Dermatol. 2016;61:295-300
70. Segal P, Insull W Jr, Chambless LE, Stinnett S, LaRosa JC, Weissfeld L. et al. The association of dyslipoproteinemia with corneal arcus and xanthelasma. The Lipid Research Clinics Program Prevalence Study. Circulation. 1986;73:I108-18
71. Douste-Blazy P, Marcel YL, Cohen L, Giroux JM, Davignon J. Increased Frequency of Apo E-ND phenotype and hyperapobeta-lipoproteinemia in normolipidemic subjects with xanthelasmas of the eyelids. Ann Intern Med. 1982;96:164-9
72. Chang HC, Sung CW, Lin MH. Serum lipids and risk of atherosclerosis in xanthelasma palpebrarum: A systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:596-605
73. Nakamura T, Ueyama Y, Funahashi T, Yamashita S, Takemura KK, Kubo M. et al. Non-macrophage-related accumulation of cholesterol during probucol treatment in familial hypercholesterolemia: report of two cases. Atherosclerosis. 1992;92:193-202
74. Civeira F, Perez-Calahorra S, Mateo-Gallego R. Rapid resolution of xanthelasmas after treatment with alirocumab. J Clin Lipidol. 2016;10:1259-61
75. Fularski P, Hajdys J, Majchrowicz G, Stabrawa M, Młynarska E, Rysz J. et al. Unveiling Familial Hypercholesterolemia-Review, Cardiovascular Complications, Lipid-Lowering Treatment and Its Efficacy. Int J Mol Sci. 2024 25
76. Zha S, Yu X, Wang X, Gu Y, Tan Y, Lu Y. et al. Topical Simvastatin Improves Lesions of Diffuse Normolipemic Plane Xanthoma by Inhibiting Foam Cell Pyroptosis. Front Immunol. 2022;13:865704
77. Al Aboud AM, Shah SS, Blair K, Al Aboud DM. Xanthelasma Palpebrarum. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC. 2025
78. Shirado M. Dyslipidaemia and age-related involutional blepharoptosis. J Plast Reconstr Aesthet Surg. 2012;65:e146-50
79. Orimo S, Arai M, Hiyamuta E, Goto Y. [A case of chronic progressive external ophthalmoplegia associated with familial hypercholesterolemia]. Rinsho Shinkeigaku. 1992;32:37-41
80. Atchaneeyasakul LO, Linck LM, Connor WE, Weleber RG, Steiner RD. Eye findings in 8 children and a spontaneously aborted fetus with RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 1998;80:501-5
81. Porter FD. RSH/Smith-Lemli-Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol Genet Metab. 2000;71:163-74
82. Gou-Fàbregas M, Macià A, Anerillas C, Vaquero M, Jové M, Jain S. et al. 7-dehydrocholesterol efficiently supports Ret signaling in a mouse model of Smith-Opitz-Lemli syndrome. Sci Rep. 2016;6:28534
83. Rim TH, Kim DW, Kim SE, Kim SS. Factors Associated with Cataract in Korea: A Community Health Survey 2008-2012. Yonsei Med J. 2015;56:1663-70
84. Park YH, Shin JA, Han K, Yim HW, Lee WC, Park YM. Gender difference in the association of metabolic syndrome and its components with age-related cataract: the Korea National Health and Nutrition Examination Survey 2008-2010. PLoS One. 2014;9:e85068
85. Rim TH, Kim MH, Kim WC, Kim TI, Kim EK. Cataract subtype risk factors identified from the Korea National Health and Nutrition Examination survey 2008-2010. BMC Ophthalmol. 2014;14:4
86. Goodwin H, Brooks BP, Porter FD. Acute postnatal cataract formation in Smith-Lemli-Opitz syndrome. Am J Med Genet A. 2008;146a:208-11
87. El-Sayyad HI, Elmansi AA, Bakr EH. Hypercholesterolemia-induced ocular disorder: Ameliorating role of phytotherapy. Nutrition. 2015;31:1307-16
88. Abd-Al-Ameer DR, Albazi W, Muhammed HA. Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Vet J. 2024;14:1836-42
89. Chen S, Li Q, Shi H, Li F, Duan Y, Guo Q. New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases. Biomed Pharmacother. 2024;178:117084
90. Fima R, Dussaud S, Benbida C, Blanchet M, Lanthiez F, Poupel L. et al. Loss of embryonically-derived Kupffer cells during hypercholesterolemia accelerates atherosclerosis development. Nat Commun. 2024;15:8341
91. Kikuchi K, Murata M, Noda K, Kase S, Tagawa Y, Kageyama Y. et al. Diabetic Cataract in Spontaneously Diabetic Torii Fatty Rats. J Diabetes Res. 2020;2020:3058547
92. Zhu XX, Su JB, Wang FM, Chai XY, Chen G, Xu AJ. et al. Sodium pump subunit NKAα1 protects against diabetic endothelial dysfunction by inhibiting ferroptosis through the autophagy-lysosome degradation of ACSL4. Clin Transl Med. 2025;15:e70221
93. Schaefer EJ, Tint GS, Duell PB, Steiner RD. Cerebrotendinous xanthomatosis, sitosterolemia, Smith-Lemli-Opitz syndrome and the seminal contributions of Gerald Salen, MD (1935-2020). J Clin Lipidol. 2021;15:540-4
94. Jira P. Cholesterol metabolism deficiency. Handb Clin Neurol. 2013;113:1845-50
95. Di Taranto MD, Gelzo M, Giacobbe C, Gentile M, Marotta G, Savastano S. et al. Cerebrotendinous xanthomatosis, a metabolic disease with different neurological signs: two case reports. Metab Brain Dis. 2016;31:1185-8
96. Cao LX, Yang M, Liu Y, Long WY, Zhao GH. Chinese patient with cerebrotendinous xanthomatosis confirmed by genetic testing: A case report and literature review. World J Clin Cases. 2020;8:5446-56
97. Jung Y, Han K, Park HYL, Lee SH, Park CK. Metabolic Health, Obesity, and the Risk of Developing Open-Angle Glaucoma: Metabolically Healthy Obese Patients versus Metabolically Unhealthy but Normal Weight Patients. Diabetes Metab J. 2020;44:414-25
98. Perrone V, Formica D, Piergentili B, Rossetti L, Degli Esposti L. Real-World Analysis on the Characteristics, Therapeutic Paths and Economic Burden for Patients Treated for Glaucoma in Italy. Healthcare (Basel). 2023 11
99. Sun J, Li T, Zhao X, Lu B, Chen J, Liu W. et al. Prevalence and Risk Factors of Glaucoma Among Chinese People from the China Health and Retirement Longitudinal Study. J Glaucoma. 2022;31:789-95
100. Kim HJ, Ryu YK, Shin YJ. Impact of COVID-19 pandemic on ocular disease: KNHANES 2015-2021. Sci Rep. 2024;14:20706
101. Mohammad Hossien D, Toba K, Azame R. A Survey of the Relationship Between Serum Cholesterol and Triglyceride to Glaucoma: A Case Control Study. Journal of Basic & Applied Sciences. 2021;10:39-43
102. Lee SH, Kim GA, Lee W, Bae HW, Seong GJ, Kim CY. Vascular and metabolic comorbidities in open-angle glaucoma with low- and high-teen intraocular pressure: a cross-sectional study from South Korea. Acta Ophthalmol. 2017;95:e564-e74
103. Kang T, Zhou Y, Fan C, Zhang Y, Yang Y, Jiang J. Genetic association of lipid traits and lipid-related drug targets with normal tension glaucoma: a Mendelian randomization study for predictive preventive and personalized medicine. Epma j. 2024;15:511-24
104. Huang G, Wang J, Li L, Gao Y, Yan Y. Meta-Analysis of Dyslipidemia and Blood Lipid Parameters on the Risk of Primary Open-Angle Glaucoma. Comput Math Methods Med. 2022;2022:1122994
105. Joshi RS, Adatiya VH. Study of the relationship between serum lipid levels and primary open-angle glaucoma. Indian J Ophthalmol. 2023;71:1948-52
106. Zhang Y, Zhang Q, Thomas R, Li SZ, Wang NL. Association of Hypertriglyceridemia and Incident Glaucoma in a Rural Chinese Population: The Handan Eye Study. Transl Vis Sci Technol. 2021;10:25
107. Lakk M, Hoffmann GF, Gorusupudi A, Enyong E, Lin A, Bernstein PS. et al. Membrane cholesterol regulates TRPV4 function, cytoskeletal expression, and the cellular response to tension. J Lipid Res. 2021;62:100145
108. Yücel I, Akar Y, Yücel G, Ciftçioğlu MA, Keleş N, Aslan M. Effect of hypercholesterolemia on inducible nitric oxide synthase expression in a rat model of elevated intraocular pressure. Vision Res. 2005;45:1107-14
109. Khamar MB, Sthapak AP, Vijayevarshcini D, Patel PM. Association between hypertriglyceridemia and open angle glaucoma: A case report. Indian J Ophthalmol. 2019;67:1202-4
110. Jünemann AG, Huchzermeyer C, Rejdak R, Hohberger B. [Dyslipidaemia and glaucoma]. Klin Monbl Augenheilkd. 2014;231:1203-14
111. Yilmaz N, Coban DT, Bayindir A, Erol MK, Ellidag HY, Giray O. et al. Higher serum lipids and oxidative stress in patients with normal tension glaucoma, but not pseudoexfoliative glaucoma. Bosn J Basic Med Sci. 2016;16:21-7
112. Xiao Z, Gong X. Use of Statins and Risk of Reducing Glaucoma: Is There a Link? Med Princ Pract. 2017;26:296
113. Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119:2074-81
114. Talwar N, Musch DC, Stein JD. Association of Daily Dosage and Type of Statin Agent with Risk of Open-Angle Glaucoma. JAMA Ophthalmol. 2017;135:263-7
115. Kang JH, Boumenna T, Stein JD, Khawaja A, Rosner BA, Wiggs JL. et al. Notice of Retraction and Replacement. Kang et al. Association of statin use and high serum cholesterol levels with risk of primary open-angle glaucoma. JAMA Ophthalmol. 2019;137(7):756-765. JAMA Ophthalmol. 2020;138:588-9
116. Liu Y, Li T. Causal Association Between Lipid-Lowering Drugs and Glaucoma: A Drug-Targeted Mendelian Randomization Study. Invest Ophthalmol Vis Sci. 2024;65:23
117. Tomić M, Vrabec R, Vidas Pauk S, Bulum T, Ljubić S. Systemic inflammation and dyslipidemia are associated with retinopathy in type 2 but not in type 1 diabetes. Scand J Clin Lab Invest. 2020;80:484-90
118. Chadalavada SH, Shaia JK, Russell MW, Talcott KE, Singh RP. Impact of Dyslipidemia Medications on the Prevalence of Diabetic Retinopathy Among a Large US Cohort. Ophthalmic Surg Lasers Imaging Retina. 2023;54:626-33
119. Chapin J, Carlson K, Christos PJ, DeSancho MT. Risk Factors and Treatment Strategies in Patients with Retinal Vascular Occlusions. Clin Appl Thromb Hemost. 2015;21:672-7
120. Wong TY, Duncan BB, Golden SH, Klein R, Couper DJ, Klein BE. et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949-54
121. Shi R, Lu Y, Liu D, Guo Z. Association of serum apolipoprotein B with retinal neurovascular structural alterations in patients with type 2 diabetes: an optical coherence tomography angiography study. Acta Diabetol. 2021;58:1673-81
122. Shukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC. 2025
123. Zhang X, Nie Y, Gong Z, Zhu M, Qiu B, Wang Q. Plasma Apolipoproteins Predicting the Occurrence and Severity of Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:915575
124. Wu M, Chen Y, Wilson K, Chirindel A, Ihnat MA, Yu Y. et al. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2679-85
125. Blot G, Karadayi R, Przegralek L, Sartoris TM, Charles-Messance H, Augustin S. et al. Perilipin 2-positive mononuclear phagocytes accumulate in the diabetic retina and promote PPARγ-dependent vasodegeneration. J Clin Invest. 2023 133
126. Modjtahedi BS, Bose N, Papakostas TD, Morse L, Vavvas DG, Kishan AU. Lipids and Diabetic Retinopathy. Semin Ophthalmol. 2016;31:10-8
127. Kowluru RA, Mishra M, Kowluru A, Kumar B. Hyperlipidemia and the development of diabetic retinopathy: Comparison between type 1 and type 2 animal models. Metabolism. 2016;65:1570-81
128. Yu JY, Du M, Elliott MH, Wu M, Fu D, Yang S. et al. Extravascular modified lipoproteins: a role in the propagation of diabetic retinopathy in a mouse model of type 1 diabetes. Diabetologia. 2016;59:2026-35
129. Russo GT, De Cosmo S, Nicolucci A, Manicardi V, Rocca A, Lucisano G. et al. Type 2 diabetes specialist care in Italy in the AMD Annals initiative 2024: The path is traced. Diabetes Res Clin Pract. 2025;225:112273
130. Rossi A, Masi D, Zilich R, Baccetti F, Baronti W, Falcetta P. et al. Lipid-lowering therapy and LDL target attainment in type 2 diabetes: trends from the Italian Associations of Medical Diabetologists database. Cardiovasc Diabetol. 2025;24:94
131. Ghaem Maralani H, Tai BC, Wong TY, Tai ES, Li J, Wang JJ. et al. Metabolic syndrome and risk of age-related macular degeneration. Retina. 2015;35:459-66
132. Lin JB, Halawa OA, Husain D, Miller JW, Vavvas DG. Dyslipidemia in age-related macular degeneration. Eye (Lond). 2022;36:312-8
133. Chen CT, Shao Z, Fu Z. Dysfunctional peroxisomal lipid metabolisms and their ocular manifestations. Front Cell Dev Biol. 2022;10:982564
134. Hu ML, Quinn J, Xue K. Interactions between Apolipoprotein E Metabolism and Retinal Inflammation in Age-Related Macular Degeneration. Life (Basel). 2021 11
135. van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A. et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res. 2018;67:56-86
136. Miceli MV, Newsome DA, Tate DJ Jr, Sarphie TG. Pathologic changes in the retinal pigment epithelium and Bruch's membrane of fat-fed atherogenic mice. Curr Eye Res. 2000;20:8-16
137. Barathi VA, Yeo SW, Guymer RH, Wong TY, Luu CD. Effects of simvastatin on retinal structure and function of a high-fat atherogenic mouse model of thickened Bruch's membrane. Invest Ophthalmol Vis Sci. 2014;55:460-8
138. Saadane A, Mast N, Dao T, Ahmad B, Pikuleva IA. Retinal Hypercholesterolemia Triggers Cholesterol Accumulation and Esterification in Photoreceptor Cells. J Biol Chem. 2016;291:20427-39
139. Mauschitz MM, Verzijden T, Schuster AK, Elbaz H, Pfeiffer N, Khawaja A. et al. Association of lipid-lowering drugs and antidiabetic drugs with age-related macular degeneration: a meta-analysis in Europeans. Br J Ophthalmol. 2023;107:1880-6
140. Xue CC, Teo KYC, Tham YC, Li H, Thakur S, Sabanayagam C. et al. Lipid-lowering drug and complement factor H genotyping-personalized treatment strategy for age-related macular degeneration. iScience. 2024;27:111344
141. Sengupta S, Pan U. Combined branch retinal vein and branch retinal artery occlusion - clinical features, systemic associations, and outcomes. Indian J Ophthalmol. 2017;65:238-41
142. Trovato Battagliola E, Pacella F, Malvasi M, Scalinci SZ, Turchetti P, Pacella E. et al. Risk factors in central retinal vein occlusion: A multi-center case-control study conducted on the Italian population: Demographic, environmental, systemic, and ocular factors that increase the risk for major thrombotic events in the retinal venous system. Eur J Ophthalmol. 2022;32:2801-9
143. Marcucci R, Sodi A, Giambene B, Liotta AA, Poli D, Mannini L. et al. Cardiovascular and thrombophilic risk factors in patients with retinal artery occlusion. Blood Coagul Fibrinolysis. 2007;18:321-6
144. Pan M, Zhou P, Guo J, An G, Liu Z, Du L. et al. Elevated Neutrophil Counts, Triglycerides, Monocyte/High-Density Lipoprotein Ratios, and Lower High-Density Lipoprotein in Patients with Retinal Vein Occlusion. Ophthalmic Res. 2023;66:265-71
145. Zheng C, Lin Y, Jiang B, Zhu X, Lin Q, Luo W. et al. Plasma lipid levels and risk of retinal vascular occlusion: A genetic study using Mendelian randomization. Front Endocrinol (Lausanne). 2022;13:954453
146. Garnavou-Xirou C, Bontzos G, Smoustopoulos G, Velissaris S, Papadopoulos A, Georgopoulos E. et al. Systemic Risk Factors in Branch Retinal Vein Occlusion: a Comprehensive Review. Maedica (Bucur). 2024;19:380-7
147. Ponto KA, Scharrer I, Binder H, Korb C, Rosner AK, Ehlers TO. et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg Retinal Vein Occlusion Study. J Hypertens. 2019;37:1372-83
148. Fernandez-Robredo P, Moya D, Rodriguez JA, Garcia-Layana A. Vitamins C and e reduce retinal oxidative stress and nitric oxide metabolites and prevent ultrastructural alterations in porcine hypercholesterolemia. Invest Ophthalmol Vis Sci. 2005;46:1140-6
149. Bouraoui R, El Matri K, Derouiche K, Falfoul Y, Amrouche C, Hachicha I. et al. Case Report: Swept-source Optical Coherence Tomography and Angiography Findings in Lipemia Retinalis. Optom Vis Sci. 2022;99:76-81
150. Sun YN, Liu B, Wang JJ, Li XM, Zhu JY, Liu C. et al. Identification of aberrantly expressed circular RNAs in hyperlipidemia-induced retinal vascular dysfunction in mice. Genomics. 2021;113:593-600
151. Lee DH, Yi HC, Bae SH, Cho JH, Choi SW, Kim H. Risk factors for retinal microvascular impairment in type 2 diabetic patients without diabetic retinopathy. PLoS One. 2018;13:e0202103
152. Nguyen TT, Wong TY. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol Metab. 2006;17:262-8
153. Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:2985-92
154. Wen Z, He X, Wang J, Wang H, Li T, Wen S. et al. Hyperlipidemia induces proinflammatory responses by activating STING pathway through IRE1α-XBP1 in retinal endothelial cells. J Nutr Biochem. 2023;112:109213
155. Acharya NK, Qi X, Goldwaser EL, Godsey GA, Wu H, Kosciuk MC. et al. Retinal pathology is associated with increased blood-retina barrier permeability in a diabetic and hypercholesterolaemic pig model: Beneficial effects of the LpPLA(2) inhibitor Darapladib. Diab Vasc Dis Res. 2017;14:200-13
156. Fu Z, Chen CT, Cagnone G, Heckel E, Sun Y, Cakir B. et al. Dyslipidemia in retinal metabolic disorders. EMBO Mol Med. 2019;11:e10473
157. Heckel E, Cagnone G, Agnihotri T, Cakir B, Das A, Kim JS. et al. Triglyceride-derived fatty acids reduce autophagy in a model of retinal angiomatous proliferation. JCI Insight. 2022 7
158. Stewart S, Lois N. Fenofibrate for Diabetic Retinopathy. Asia Pac J Ophthalmol (Phila). 2018;7:422-6
159. Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675-82
160. Kang EY, Chen TH, Garg SJ, Sun CC, Kang JH, Wu WC. et al. Association of Statin Therapy with Prevention of Vision-Threatening Diabetic Retinopathy. JAMA Ophthalmol. 2019;137:363-71
161. Che D, Lv L, Cao Y, Zhang Y, Yu Q, Li F. et al. Lipid profile in the aqueous humor of patients with myopia. Exp Eye Res. 2024;247:110023
162. Bian J, Sze YH, Tse DY, To CH, McFadden SA, Lam CS. et al. SWATH Based Quantitative Proteomics Reveals Significant Lipid Metabolism in Early Myopic Guinea Pig Retina. Int J Mol Sci. 2021 22
163. Hui J, Tang K, Zhou Y, Cui X, Han Q. The causal impact of gut microbiota and metabolites on myopia and pathological myopia: a mediation Mendelian randomization study. Sci Rep. 2025;15:12928
164. Liu S, Fang W, Pei Y, Chen T, Li B, Liang Y. et al. Rhodopsin Induces Myopia via Lipid Peroxidation in Zebrafish Reared in a Dark Environment. Faseb j. 2025;39:e70612
165. Ye H, Feng Y, Xiang W, Lin Z, Li Y, Hu W. et al. Ferroptosis Contributes to Retinal Ganglion Cell Loss in GLAST Knockout Mouse Model of Normal Tension Glaucoma. Invest Ophthalmol Vis Sci. 2025;66:26
166. Ohguro H, Umetsu A, Sato T, Furuhashi M, Watanabe M. Lipid Metabolism Regulators Are the Possible Determinant for Characteristics of Myopic Human Scleral Stroma Fibroblasts (HSSFs). Int J Mol Sci. 2023 25
167. Jiang L, Huang L, Dai C, Zheng R, Miyake M, Mori Y. et al. Genome-Wide Association Analysis Identifies LILRB2 Gene for Pathological Myopia. Adv Sci (Weinh). 2024;11:e2308968
168. Zhang Y, Huang J, Liang Y, Huang J, Fu Y, Chen N. et al. Clearance of lipid droplets by chimeric autophagy-tethering compound ameliorates the age-related macular degeneration phenotype in mice lacking APOE. Autophagy. 2023;19:2668-81
169. Yu P, Wang Y, Yang WT, Li Z, Zhang XJ, Zhou L. et al. Upregulation of the PPAR signaling pathway and accumulation of lipids are related to the morphological and structural transformation of the dragon-eye goldfish eye. Sci China Life Sci. 2021;64:1031-49
Author contact
![]() Corresponding author: Hongsheng Bi, No. 48#, Yingxiongshan Road, Jinan 250002, P. R. China; Email: azuresky1999com; Dadong Guo, No. 48#, Yingxiongshan Road, Jinan 250002, P. R. China; Email: dadonggenecom.
Corresponding author: Hongsheng Bi, No. 48#, Yingxiongshan Road, Jinan 250002, P. R. China; Email: azuresky1999com; Dadong Guo, No. 48#, Yingxiongshan Road, Jinan 250002, P. R. China; Email: dadonggenecom.

 Global reach, higher impact
Global reach, higher impact