Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(14):3692-3708. doi:10.7150/ijms.115650 This issue Cite
Review
Mesenchymal Stem Cells Senescence: Mechanism and Rejuvenation Interventions
1. Department of Clinical Laboratory Medicine, The Eco-city Hospital of Tianjin Fifth Central Hospital, Tianjin, China.
2. Clinical Laboratory, Wanxin Street Community Healthcare Center, Tianjin, China.
3. Unicell Life Science Development Co., Ltd, Tianjin, China.
4. The Second Hospital of Tianjin Medical University, Tianjin Institute of Urology, Tianjin, China.
5. Tianjin Children's Hospital, Tianjin, China.
6. Children's Hospital, Tianjin University, Tianjin, China.
* These authors contributed equally to this work.
Received 2025-4-13; Accepted 2025-7-21; Published 2025-7-28
Abstract
Aging has become one of the most significant challenges and burdens on public health and healthcare systems worldwide. However, it is possible to slow down the aging process through various interventions. Mesenchymal stromal/stem cells (MSCs) have emerged as one of the most promising therapeutic agents for combating aging and treating various age-related chronic medical conditions. This is primarily due to their well-known cellular plasticity and potent multipotency, which enable them to promote tissue repair and regeneration, as well as address inflammatory conditions. Remarkably, the high quality and functional activity of MSCs are negatively affected by cellular senescence, particularly in both healthy-aging MSCs and replicative senescent MSCs. This is a critical issue when considering the provision of "personalized" or "universal" clinical-grade products. Therefore, this review aims to summarize the biological properties, immunomodulatory dysfunction, and underlying mechanisms of senescent MSCs. Additionally, it discusses the current promising techniques published for rejuvenating senescent MSCs and optimizing their therapeutic potential.
Keywords: mesenchymal stromal/stem cells, ageing, cellular senescence, priming interventions
1. Introduction
Mesenchymal stromal/stem cells (MSCs), as multipotent mesoderm-derived progenitor cells, can promote tissue repair and regeneration, and alleviate organ dysfunction [1]. Human MSCs are at the forefront of medical research and are being evaluated as therapeutic agents in multiple clinical trials. There are currently almost 1,500 MSC-based clinical trials registered at clinicaltrials.gov database [2], ranging from Phase I to III, with many either completed or currently ongoing. Accumulating clinical evidence highlights the efficacy and safety of MSCs in treating severe COVID-19 and acute respiratory distress syndrome [3]. Based on these findings, we propose the term of “MSCs +” (a rhetorical device). This term leverages the pluripotent and paracrine factors of MSCs (e.g., secretome, microvesicles, or exosomes) to achieve immunomodulation, anti-inflammatory, antiviral, anti-apoptotic, anti-fibrotic, and regenerative effects, offering potent therapeutic potential for various clinical conditions [4,5].
Ageing, a natural, multifactorial, and degenerative process, influences socioeconomic and public policies over time, representing a significant challenge [6]. According to the National Bureau of Statistics of China, the population aged 65 and above exceeded 200 million, accounting for 14.9% of the total population by the end of 2022. This figure is expected to surpass 400 million, representing more than 30% of the population by 2050, marking the onset of severe aging in China [7]. Additionally, World Health Organization predicts that one in six people worldwide will be over 65 by 2050 [8]. This indicates that a globally aging society will pose a significant challenge to the healthcare system and humanity, raising a foreseeable concern. MSCs are increasingly recognized as viable and ideal therapeutic agents for combating the effects of aging and treating various age-related diseases in the elderly, such as malignancies, cardiovascular conditions, skeletal disorders, immune/inflammatory diseases, and cognitive impairments [9]. However, despite successful clinical applications, we cannot overlook the senescence-related changes in MSCs, which are natural responses to aging and accumulated stress from excessive cell proliferation. These changes reduce regenerative potential and undermine the therapeutic efficacy of MSCs in clinical trials. On one hand, although autologous MSCs are favored over allogeneic ones, their function declines with donor age, leading to senescence and reduced therapeutic efficacy [10]. On the other hand, MSCs isolated from young donors require extensive monolayer culture and in vitro expansion to achieve sufficient quantities for clinical use. However, this process induces replicative senescence (in vitro aging), with each generation leading to a loss of stemness. This includes changes in intrinsic MSC characteristics, such as pluripotency, immunomodulatory properties, and paracrine function—inevitable aspects of cell manufacturing [11].
The acquisition of high-quality MSCs is currently considered one of the biggest technical challenges, particularly in preventing their premature senescence in vitro or in vivo during translation to clinical practice [12]. To address these challenges, it is crucial to understand the features of senescence and develop specific strategies to rejuvenate senescent MSCs, aiming to generate a large number of youthful and viable MSCs for cell-based therapies. This review summarizes the biological properties, immunomodulatory dysfunctions, and driving mechanisms of senescent MSCs, while discussing promising techniques for rejuvenating these cells and optimizing their therapeutic potential.
2. Identification of MSC Senescence
As MSCs undergo senescence, their characteristics change significantly, including alterations in cellular morphology, phenotype, cell cycle, intracellular senescence-associated β-galactosidase (SA-β-gal) activity, senescence-related genes, the senescence-associated secretory phenotype, and intracellular adenosine triphosphate levels [13].
2.1 Morphological changes
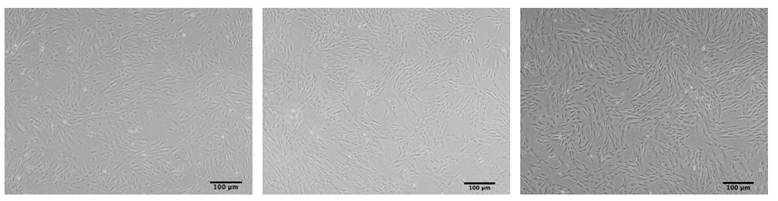
MSCs typically exhibit an elongated fibroblast like morphology when cultured under standard conditions. However, MSCs derived from late passages in vitro or from elderly donors, particularly those over 70 years old, often exhibit a noticeable morphological change, becoming flattened and enlarged, resembling a "fried egg" with constricted nuclei and granular cytoplasm [14-16]. This alteration in cell size and morphology serves as a hallmark for evaluating cellular senescence, as exhibited in Figure 1 [17]. Furthermore, nuclear morphometric analysis (NMA), based on quantitative parameters of geometric features such as cells, nuclei, or nucleoli, provides a valuable tool for monitoring MSC senescence [18]. He et al. recently developed a Cascade region-based convolutional neural network (Cascade R-CNN) system to detect both replicative and drug-induced senescent MSCs by identifying single cells of various sizes and shapes in multicellular images, providing a real-time platform for morphological image analysis [19].
The morphology of ADSCs. ADSCs at passage 3 (left), ADSCs at passage 10 (middle) and ADSCs at passage 12 (“fried egg” shape) (right) (scale bars: 100 μm).
2.2 Cell phenotypic changes
2.2.1 Specific surface markers
According to the guidelines of the International Society for Cellular Therapy (ISCT), cultured MSCs are identified by the positive expression of cell surface markers CD73, CD90, and CD105, and the absence of endothelial and hematopoietic markers such as CD45 and CD34. Evidence indicates that the expression levels of these positive surface markers are downregulated with donor age or prolonged culture. For instance, Truong et al. [20] observed that the expression of CD105 dropped from 92.43% in early-passaged (p5) to 48.87% in late-passaged (p15) MSCs during in vitro culture. In another study, Martini et al. [21] suggested that the decrease in CD90 expression could serve as a distinct marker for the aging of cardiac-derived MSCs (cMSCs).
2.2.2 Other surface markers
Additionally, evidence suggests that other surface marker expression profiles play an important role in identifying senescent MSCs. For instance, CD106 (vascular cell adhesion molecule-1) and CD228 (melanotransferrin) were significantly downregulated in late-passaged (p14) and (p16-18) MSCs, respectively, and were associated with reduced cell adhesion, migration, and multi-differentiation capabilities [22,23]. It has been reported that a decrease in CD146 expression accelerates cellular senescence by enhancing the expression of p53, p16, and p21, while inhibiting Bmi-1 expression in senescent MSCs. Additionally, several studies have observed an increase in senescence-related surface markers, such as CD264 and CD26, in MSCs, suggesting that these markers could serve as indicators of MSC quality. For example, MSCs showed a gradual increase in CD264 expression, accompanied by co-expression of p53 and p21 during subculture expansion, which strongly negatively correlated with their proliferation and multi-differentiation capabilities [24]. Several studies have shown that CD26 expression is upregulated during the MSC senescence process both in vitro and in vivo, along with an increase in the expression of cell cycle arrest genes such as p16 and p21. This suggests that CD26 could serve as a senescence marker for human MSCs. Furthermore, CD26high MSCs were less effective at suppressing T cell proliferation compared to CD26low MSCs [25,26]. Notably, inhibiting CD26 could reverse senescence-associated gene expression changes (p53 and p21), increase MSC surface adhesion, and enhance therapeutic effects in mouse lung emphysema [26]. The surface phenotype mentioned above are listed in Table 1.
The surface phenotype associated with MSCs senescence
| Species | Tissue source | Age (passage number) | Altered phenotype | Refs |
|---|---|---|---|---|
| Human | Adipose tissue | From early (p0) to late (p15) | CD105 ↓ | [20] |
| - | From early (p5) to late (p14) | CD106 ↓ | [22] | |
| Bone marrow | From early (p4-6) to late | CD228 ↓ | [23] | |
| Bone marrow | From young (20-40 years) to aged donors (45-60 years); From early (p3) to late (p14) | CD264 ↑ | [24] | |
| Adipose tissue | From early (p5) to late (p15) | CD26 ↑ | [25] | |
| Umbilical cord blood | From early (p4) to late (p10) | CD26 ↑ | [26] | |
| Bone marrow | From young (<35 years) to aged donors (≥70 years) | CD146 ↓ | [27] | |
| Bone marrow | From early (p2) to late (p10) | CD105 ↓, CD106 ↓ | [28] | |
| Mice | Myocardium | From young (4 months) to aged mice (20 months) | CD90 ↓ | [21] |
↑ increased; ↓ decreased.
2.3 Intracellular SA-β-gal and senescence-related genes
2.3.1 SA-β-gal and senescence-related genes
A well-established method for evaluating senescent cells and the senescence process is the detection of elevated intracellular senescence-associated β-galactosidase (SA-β-gal) activity [29]. In addition to SA-β-gal, increased expression of p16, p21, and p53 are also recognized markers of cellular senescence. Previous research has shown increased SA-β-gal activity, upregulated p16 and p21 gene expression, and cell cycle arrest in late-passaged (p9~p14) human bone marrow-derived MSCs (BM-MSCs), (p18) umbilical cord blood-derived MSCs (UCB-MSCs), and (p10) adipose-derived MSCs (ADSCs) [30-32]. It is worth noting that SA-β-gal activity is expressed by GLB1, the gene encoding lysosomal beta-D-galactosidase. When MSCs were genetically deficient in the GLB1 gene or completely lacked lysosomal β-galactosidase, aged MSCs did not express SA-β-gal [33].
2.3.2 α-fuc
Recently, researchers identified α-l-fucosidase (α-fuc) as a biomarker for cellular senescence and developed an α-fuc aggregation-induced emission (AIE) probe, QM-NHαfuc. This probe enabled real-time tracking of senescent cells that lack β-galactosidase expression, both in vitro and in vivo, overcoming the limitations of traditional markers like SA-β-gal [34].
The composition of SASP released in senescent MSCs
| Species | Tissue source | Age (passage number) | Variable factors | Refs |
|---|---|---|---|---|
| Human | Umbilical cord blood | From early (p4) to late (p10) | IL-6 ↑, IL-8 ↑, MCP-1 ↑, and CXCL5 ↑ | [26] |
| Human | Umbilical cord blood | From early (p4-5) to late (more than p11) | MCP-1 ↑ | [42] |
| Human | Umbilical cord blood | From early (p5) to late (p20) | IL-6 ↑, IGFBP-4 ↑, IGFBP-7 ↑ and MCP-1 ↑ | [43] |
| Human | Bone marrow | From young (<35years) to aged donors (≥70 years) | IL6 ↑, MCP-1 ↑, IL8 ↑, IL-1α ↑, CXCL2 ↑ | [27] |
| human | Bone marrow | From early (p2-5) to late (p9-14) | IL-1α ↑, and IL-8 ↑ | [30] |
| Mouse | Bone marrow | From young (8 weeks) to aged (78 weeks); From early (p3) to late(p10) | IL-6 ↑, IL-10 ↓ | [35] |
| Mouse | Bone marrow | From young (2 months) to aged (18 months) | IL-1α ↑, IL-5 ↑, IL-6 ↑, MCP-5 ↑ | [44] |
MCP-1, monocyte chemoattractant protein-1; CXCL5, C-X-C motif chemokine ligand 5; IGFBP, insulin-like growth factor binding protein; CXCL2, C-X-C motif chemokine ligand 2. ↑ increased; ↓ decreased.
2.4 Senescence-associated secretory phenotype
2.4.1 SASP production
When senescence occurs, MSCs also exhibit the senescence-associated secretory phenotype (SASP), which helps maintain cellular metabolism. This phenotype involves a variety of secretory proteins, including proinflammatory cytokines, chemokines, growth regulators, angiogenic factors, and matrix metalloproteinases (MMPs). These proteins are synthesized non-autonomously and secreted by senescent MSCs into the surrounding microenvironment through both autocrine and paracrine mechanisms. This process induces cells to exit the cell cycle and enter a state of cellular senescence via a regulatory network of cascades and feedback loops.
2.4.2 The effect of SASP
The biologically active components of the SASP, such as interleukin (IL)-6, IL-8/CXCL8, and monocyte chemoattractant protein-1/CCL2 (MCP-1/CCL2), are released by MSCs from elderly donors into the microenvironment. These components play a role in evaluating the typical characteristics of senescent cells and can lead to negative health consequences. These include activating a cellular senescence program in early-passaged MSCs, triggering premature senescence in young MSCs, increasing inflammation and pathological changes in the bone marrow microenvironment, and contributing to a higher incidence of age-related osteoporosis [35]. Alessio et al. observed certain components of insulin-like growth factor binding proteins (IGFBPs) in the SASP of senescent MSCs, and these IGFBPs could induce senescence in neighboring cells. Inactivating IGFBPs in the SASP abolished their pro-senescent effect [36]. We compiled and compared the composition of the SASP released by different types of senescent MSCs (Table 2).
2.4.3 Related signaling pathways
Notably, the composition of the SASP released by senescent MSCs may contribute to the activation of a cellular senescence program through various signaling pathways, each with distinct functions. These include: p38MAPK/MAPKAPK-2-mediated growth arrest by increasing NF-κB transcriptional activity [18]; p53/p21/Rb- and p16/Rb-mediated cell growth arrest by inhibiting cyclin-dependent kinase (CDK), thus blocking E2F-mediated transcription of genes involved in cell proliferation [37]; phosphatidylinositide 3-kinases (PI3K)/protein kinase B (PKB)/mammalian target of rapamycin (mTOR)-mediated autophagy, which synthesizes SASP factors through mTOR complex 1 bound to lysosomes [38]; and ROS-prostaglandin signaling, which is mediated by internalization via caveolae and interaction with RARα [36]. Currently, inhibitors or shRNA transfection targeting these relevant pathways are considered effective in suppressing SASP production, reducing inflammatory responses, and treating age-related diseases such as UVA-induced skin photoaging [39], degenerative disc disease [40], and osteoarthritis (OA) [41].
2.5 ATP and eccDNA
2.5.1 ATP
Mitochondria are well-known cellular organelles responsible for maintaining cellular energy balance and metabolism through the production of adenosine triphosphate (ATP), a high-energy phosphate compound [45]. However, aging is associated with cellular dysfunction and a decline in the energy required for cellular physiological functions, which directly impacts ATP availability [46]. In a 2021 study by Herzig et al. [47], a reliable assessment of the immunosuppressive properties of MSCs on PBMCs was conducted using a luminescent ATP assay, which streamlined the analysis for comparing the potency of different MSC preparations. Therefore, we suggest that intracellular ATP could serve as an effective indicator in analyzing aging MSCs from donors or those undergoing replicative senescence.
2.5.2 eccDNA
Furthermore, extrachromosomal circular DNA (eccDNA) is a form of double-stranded, closed, non-structured circular DNA that exists outside the chromosomes. Yang et al. [48] discovered that senescent BM-MSCs exhibited a distinct eccDNA landscape and expression pattern, and that a specific eccDNA could serve as a biomarker for BM-MSCs senescence.
3. Biological properties
Proliferative, migratory, anti-oxidative, and multilineage differentiation potentials are key biological properties of MSCs. However, MSCs gradually lose these properties as their proliferative and migratory capacities decline, oxidative stress resistance decreases, and differentiation becomes imbalanced, ultimately leading to senescence.
3.1 Proliferation, migration and anti-oxidation
MSCs senescence limits their clinical efficacy, primarily due to reduced proliferative and migratory capacities, as well as decreased tolerance to oxidative stress.
3.1.1 Proliferation
Senescent MSCs are characterized by decreased levels of fibroblast colony-forming units (CFU-F) and increased population doubling time (PDT). More importantly, they exhibit a significant reduction in the number of cells in the S phase, along with a decline in their self-renewal capacity [15,31,49,50]. After prolonged cell division during long-term in vitro culture, MSCs enter permanent G0 phase arrest, and mitosis is inhibited, although they remain viable. Several studies have shown that this process impairs the G1/G2-to-S phase and G2/M transitions of the cell cycle, upregulates cell cycle arrest-related genes such as p16, p21, and p27, and is primarily modulated through activation of the p53/p21WAF1/CIP1, Cyclin D1/CDK4/E2F1, and p16INK4A/Rb signaling pathways, resulting in cellular senescence [51,52].
3.1.2 Migration
Given that migration and homing ability are key for MSCs in tissue repair and immunomodulation, previous studies have shown that with donor aging and cellular replicative senescence, the migration ability of MSCs is impaired, correlating with decreased levels of migration-related proteins such as chemokine receptors (CXCR4 and CXCR7) and MMP family members (MMP3, MMP9, MMP11, MMP13, and MMP14) [49,53,54]. Similarly, our study demonstrated a significant inhibition of proliferation and migration potential in senescent MSCs, as detected by xCELLigence RTCA [17]. Moreover, Amini-Nik et al. reported that senescence could affect MSCs migration to the wound bed, contributing to impaired regenerative capacities of MSCs in wound healing [55].
3.1.3 Anti-oxidation
In addition to weakened proliferative and migratory capacities, the antioxidant ability of MSCs from elderly individuals was significantly reduced, as evidenced by lower activity of the antioxidant enzyme SOD and decreased resistance to hydrogen peroxide (H2O2) compared to MSCs from adults [56]. More specifically, the expression levels of SOD1 to SOD3 genes in MSCs from elderly individuals were significantly downregulated [57]. Compared to early-passage MSCs, late-passage MSCs were more sensitive to oxidative stress, exhibiting excessive ROS production and downregulating the expression of antioxidant proteins such as Cu/Zn-SOD, glutathione peroxidase (GPX), and catalase (CAT). This imbalance could impair cellular function and induce apoptosis, ultimately compromising MSCs quality for therapeutic use [58].
3.2 Tri-lineage differentiation
3.2.1 MSCs differentiation
According to the standard definition, MSCs possess differentiation potential toward multiple cell types under specific culture conditions, including adipocytes, osteoblasts, chondroblasts, islet-like cells, hepatocyte-like cells, neural-like cells, and skeletal myocytes. This differentiation capacity confers significant therapeutic efficacy for regenerative medicine, as MSCs can migrate to tissue injury sites and differentiate into organ-specific cell types.
3.2.2 Tri-lineage differentiation fates
Notably, senescent MSCs can affect their capacity for tri-lineage differentiation and differentiation direction, as confirmed by both common histological staining techniques and gene expression analysis. For example, using widely applied techniques, osteogenic differentiation declines, as indicated by decreased mineral deposition in Alizarin red-S staining and, particularly, downregulation of osteoblast markers such as alkaline phosphatase (ALP) and osteocalcin (OCN) [15,17,20,28,35,50,53,56,59,60].
Impaired chondrogenic lineage is evidenced by decreased sulfated proteoglycans in the cartilage matrix, stained with Alcian blue, and reduced gene expression levels of collagen type II (Col-II) [15,20,56,57,59,61].
While adipogenic differentiation potential is retained or even enhanced with aging [15,17,53,56,60], prolonged culture leads to a decline in adipogenic differentiation in MSCs undergoing in vitro senescence [28,35,50]. This decline is primarily evidenced by lipid droplet accumulation, revealed through Oil Red O staining, and downregulation of adipogenic markers, including peroxisome proliferator-activated receptor gamma (PPARγ) and lipoprotein lipase (LPL) gene expression in senescent MSCs.
However, some evidence suggests that age has no significant effect on the tri-lineage differentiation of MSCs [62,63]. One key reason for these contradictory findings is that MSCs are a heterogeneous population, meaning that MSCs from different donors or subgroups may yield different results [64]. Table 3 summarizes the changes in tri-lineage differentiation direction of MSCs during in vivo and in vitro senescence.
4. Immunomodulatory Functions
4.1 Immune cells polarization status
It is well established that restoring the normal immunomodulatory function of immune cells is crucial for improving treatment outcomes in various age-related inflammatory diseases [65]. As promising modulators of the immune system and immune responses, MSCs regulate both the innate and adaptive immune responses through direct cell contact with immune cells and paracrine effects. These include mediating the polarization of pro-inflammatory M1-like macrophages (M1) to anti-inflammatory M2-like macrophages (M2) and T helper 1 (Th1) and Th17 cells to regulatory T cells (Tregs), promoting the resolution of inflammation, cell and tissue regeneration, and damage repair [66-68].
Changes in tri-lineage differentiation direction of MSCs in vivo and in vitro senescence
| Species | Tissue source | Age (passage number) | Osteogenic differentiation | Chondrogenic differentiation | Adipogenic differentiation | Changes in specific transcription factor | Refs |
|---|---|---|---|---|---|---|---|
| In vivo | |||||||
| Human | Adipose tissue | From young (>20 years) to aged (>70 years) | ↓ | ↓ | ↑ | Osteogenic: BMP-2 ↓, OPN ↓, OCN ↓; Chondrogenic: Col-II ↓; Adipogenic: PPAR-γ ↑ | [15] |
| Mice | Bone marrow | From young (5 weeks) to aged (50 weeks) | ↓ | - | ↑ | Osteogenic: ALP ↓, OCN ↓; Adipogenic: PPAR-γ ↑, LPL ↑ | [53] |
| Human | Adipose tissue | From young (2-6 years) to aged ( > 50 years) | ↓ | - | No differences | Osteogenic: OPN ↓ | [17] |
| Human | Adipose tissue | From young ( <30 years) to aged (>60 years) | ↓ | ↓ | No differences | Osteogenic: OCN ↓, ALP ↓; Chondrogenic: ACAN ↓, Col-II ↓ | [56] |
| Human | Adipose tissue | Infant Adult | ↑ | ↓ | ↓ | Osteogenic: OCN ↑, RUNX2 ↑; Chondrogenic: SOX9 ↓, Col-II ↓, COL10 ↓; Adipogenic:PPARγ ↓, LPL ↓ | [57] |
| Horses | Bone marrow | From young (0 days) to aged (≥ 22 years) | ↓ | ↓ | - | Osteogenic: RUNX2 ↓; Chondrogenic: COMP↓ , ACAN ↓, MIA ↓, COL11A1 ↓ | [59] |
| Rat | Bone marrow | From young (1-month) to aged (21-month) | ↓ | - | ↑ | Osteogenic: RUNX2 ↓, OSX ↓, ALP ↓, BSP ↓, OPN ↓, OCN ↓; Adipogenic: PPAR-γ ↑, aP2 ↑, RTN ↑ | [60] |
| Human | Bone marrow | From young (18-49 years) to aged (≥50 years) | No differences | No differences | No differences | - | [62] |
| Human | Adipose tissue | From young (≤ 35 years) to aged (≥ 55 years) | No differences | - | No differences | - | [63] |
| In vitro | |||||||
| Human | Adipose tissue | P0-P15 | ↓ | ↓ | No differences | - | [20] |
| Human | Bone marrow | From early (p2) to late(p10) | ↓ | - | ↓ | Osteogenic: RUNX2↓; Adipogenic: PPAR-γ↓ | [28] |
| Rat | Bone marrow | From early (p3) to late (p10) | ↓ | - | ↓ | Osteogenic: OCN ↓, ALP ↓, RUNX2 ↓, OSX ↓; Adipogenic: PPAR-γ ↓, Fabp4 ↓, adiponectin ↓, perilipin A ↓ | [35] |
| Human | Bone marrow | From early (p4) to late (p12) | ↓ | - | ↓ | - | [50] |
| Human | Tonsil | From early (p3) to late (p15) | p3-p10 ↑; p10-p15 ↓ | ↓ | ↓ | Osteogenic: OCN ↓ (after P10) | [61] |
BMP-2, bone morphogenetic protein 2; OPN, osteopontin; OCN, osteocalcin; Col-II, collagen types II; PPAR-γ, peroxisome proliferator-activated receptor γ; ALP, alkaline phosphatase; LPL, lipoprotein lipase; ACAN, aggrecan core protein; RUNX, runt-related transcription factor 1; SOX9, sex determining region Y box protein 9; COL10, recombinant collagen type X; COMP, cartilage oligomeric matrix protein; MIA, cartilage-derived retinoic acid-sensitive protein; COL11A1, recombinant collagen type XI alpha 1 (COL11a1); OSX, osterix; BSP, bone sialoprotein; aP2, adipocyte protein 2; RTN, resistin; Fabp4, fatty acid binding protein 4. ↑ increased or upregulated; ↓ decreased or down-regulated.
Molecular mechanisms associated with MSC senescence.
Indeed, our recent study adds to the growing body of evidence that ADSCs contribute to the immunosuppressive effects in colorectal cancer patients with severe COVID-19, suggesting that interventions involving ADSCs could be a beneficial approach for patients who fail to respond to conventional therapies [69].
4.2 MSC2 to MSC1
With aging, MSCs derived from elderly donors undergo a shift from the anti-inflammatory MSC2 phenotype to the pro-inflammatory MSC1 phenotype, which triggers early immune and inflammatory responses in tissues. This shift promotes the activation and proliferation of effector T cells, favors the expansion and differentiation of Th1 and Th17 cells, and inhibits Tregs. This process is driven by the downregulation of intracellular proteins, including indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and programmed cell death 1 ligand 1 (PD-L1), as well as a decrease in factors such as transforming growth factor β (TGF-β) and IL-10 [70]. The downregulation of PD-L1 was observed during doxorubicin- or H2O2-induced UCB-MSCs senescence and in late-passaged UCB-MSCs. After the addition of an anti-PD-L1 blocker, the ability to inhibit T cell activation was significantly reduced, suggesting that the reduction in PD-L1 expression may be a key factor in the decline of their immunomodulatory capacity [71]. Monitoring PD-L1 expression levels could provide a rapid assay for MSC-mediated immune suppression, which would align with the cell manufacturing process. Other studies of replicative-induced senescent MSCs have reported similar findings [31], indicating that the transformation of MSCs to the MSC1 phenotype is crucial in the development of senescence-associated immunomodulatory dysfunction and pro-aging activities.
4.3 Cellular microenvironment
Furthermore, senescent MSCs can alter the cellular microenvironment by secreting SASP factors such as chemokine ligand 2 (CCL2), tumor necrosis factor-α (TNF-α), interferon-inducible protein 10 (IP10), IL-6, and interferon-gamma (IFN-γ) in a paracrine manner. This contributes to the differentiation of macrophages into the M1-like polarization state, enhancing the inflammatory phase [72].
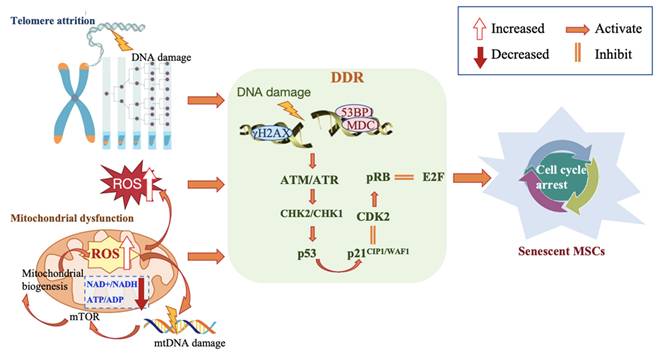
5. Mechanisms of MSC Senescence
Driving MSCs senescence involves various interrelated molecular mechanisms, triggered by DNA damage, telomere attrition, reactive oxygen species (ROS), and mitochondrial dysfunction. These lead to progressive impairment or loss of self-renewal capacity and functional exhaustion, ultimately accelerating cell senescence (Figure 2).
5.1 DNA Damage
DNA damage contributes to persistent replicative senescence and the natural aging of MSCs. Continuous DNA damage accumulation results in reduced expression of stemness-related genes, disrupts transcriptional processes, and activates the DNA damage response (DDR) signaling pathway. Activation of DDR halts the cell cycle, inducing cellular senescence and progressive functional deterioration [73,74].
5.1.1 DDR associated molecules
p16, phosphorylated-p53, and p21Cip1/Waf1 are DDR-associated molecules found to be upregulated in late-passaged MSCs [74]. The p53 binding protein 1 (53BP1), phosphorylated histone H2AX (γH2AX), and the ATM pathway are key components of the DNA damage response and have been used to assess DNA damage [12]. Gnani et al. reported a significant increase in the percentage of DDR marker 53BP1 foci-positive cells in MSCs derived from aged donors, along with activation of the phosphorylated form of the upstream DDR kinase ATM and the double-strand breaks (DSBs) marker γH2AX in MSCs [27]. Similarly, cardiac MSCs from aged mice (20 months ± 2.55) also exhibited accumulated DNA damage, as evidenced by an increased percentage of γH2AX-positive cells [21]. Evidence suggests that MSCs' ability to recognize endogenous and radiation-induced DNA double-strand breaks (DSBs) declines during long-term culture, characterized by a decrease in the number of γH2AX/53BP1 DSB repair foci and a gradual loss of ATM pathway activity [75].
5.1.2 DNA repair mechanisms
Additionally, DNA repair pathways can recognize DNA damage and restore DNA integrity in MSCs through various mechanisms, including base excision repair (BER), nucleotide excision repair (NER), and DNA mismatch repair (MMR) [76]. DNA repair deficiency increases the accumulation of DNA damage, leading to suppression of MSCs growth and self-renewal capacity, ultimately driving cell fate toward DNA damage-related senescence both in vitro and in vivo.
5.2 Telomere attrition
5.2.1 Telomere attrition
MSCs senescence is closely associated with the loss of telomere length maintenance. Progressive or replicative telomere shortening is a major contributor to DNA damage, which exacerbates cell cycle arrest, apoptosis, differentiation bias or loss, and impaired replicative capacity of MSCs [77]. Telomere attrition triggers the formation of telomere-associated DDR foci (TAFs) or telomere-induced DNA damage foci (TIFs), leading to overexpression of p53, p16, and p21, and attenuates mitochondrial metabolic activity through the peroxisome proliferator-activated receptor gamma co-activators 1α/β (PGC-1α/β). This, in turn, forcibly inhibits cell proliferation, sustains a persistent DNA damage signal, and increases MSCs susceptibility to senescence, even driving apoptosis or autophagy [78,79].
5.2.2 POT1a
Protection of telomeres 1a (POT1a), a telomere-binding protein, has been found to maintain telomere length and genomic integrity. Recently, Nakashima et al. reported that POT1a-deficient MSCs led to the intracellular accumulation of excessive ROS and DNA damage, favoring adipogenesis over osteogenesis, which resulted in skeletal retardation in MSC-specific POT1a-deficient mice [80].
5.2.3 TERT
Notably, telomerase reverse transcriptase (TERT) is the key determinant of telomerase activity, which is essential for maintaining telomere length. Telomerase deficiency accelerates telomere shortening, significantly reducing migratory and multi-differentiation capabilities, even in early-passaged MSCs [81,82]. One study showed that overexpression of TERT enabled ADSCs to resist DNA damage accumulation during long-term culture and increased their tolerance to oxidative stress, thereby activating telomerase activity and preserving MSC biological functions [83].
5.3 ROS and mitochondrial dysfunction
Mitochondrial dysfunction is a hallmark and therapeutic target of the MSCs senescence process, driven by a series of pathological changes, including an increase in mitochondrial DNA (mtDNA) mutations, endogenous ROS accumulation, and ATP depletion. These factors contribute to the acceleration of senescence-associated DNA damage, ultimately impairing cellular functions [84].
5.3.1 ROS dysfunction
The study reported that normal physiological ROS production was essential and beneficial for MSCs self-renewal. However, when unregulated intracellular ROS levels, particularly from mitochondria, increase dramatically and antioxidant defenses are significantly impaired, it is considered one of the mechanisms underlying replicative senescence in MSCs. This weakens self-renewal, proliferation, and osteogenic differentiation capabilities, induces cellular dysfunction and apoptosis, and promotes and exacerbates inflammation, leading to a stagnation in repopulating capacity [85,86]. One example of its impact is that excessive production of intracellular ROS during aging can trigger DDR, which activates complex I of the mammalian target of rapamycin (mTORC1), leading to mitochondrial biogenesis and further increasing the generation of harmful ROS [87].
5.3.2 Mitochondrial dysfunction
Researchers reported that senescent MSCs exhibited downregulation in the expression of mitochondrial biogenesis genes, such as peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), SIRT, mammalian target of rapamycin (mTOR), and adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK). They also displayed lower mitochondrial membrane potential, reduced NAD+/NADH and ATP/ADP ratios [88], as well as altered mitochondrial morphology and bioenergetics [89], all of which were associated with MSCs senescence [90]. Therefore, regulating mitochondrial function could rejuvenate senescent MSCs by photobiomodulation, altering their energy metabolism-related factors and pathways [91,92].
5.3.3 Mtophagy and MT
Additionally, dysfunctional mitochondria are typically degraded through mitophagy, which protects cells from various cytotoxic stimuli and slows senescence in MSCs, thereby maintaining homeostasis and stemness. An overabundance of autophagy or genetic damage to the autophagy process can lead to the accumulation of dysfunctional organelles, promoting aging [93]. Recent evidence has shown that harnessing the beneficial effects of intercellular mitochondrial transfer (MT) from MSCs can restore the bioenergetic profile and cell viability, increase mtDNA content, and reduce oxidative stress and inflammation [94], thereby delaying MSCs senescence or rejuvenating biological functions lost during this process.
6. Anti-Senescence Interventions
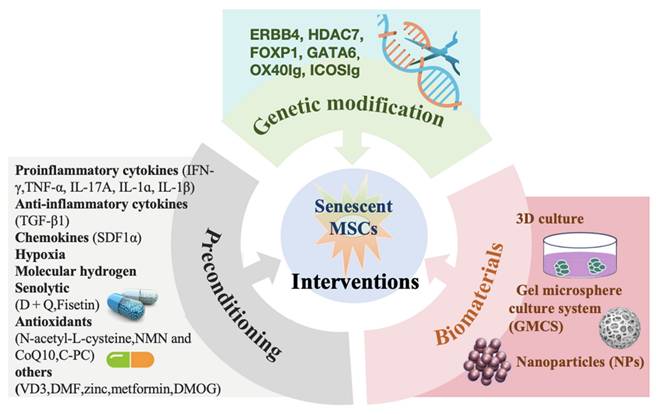
Given the challenges outlined above, various specific priming interventions have been proposed to enhance the functionality and therapeutic potential of MSCs through preconditioning, targeted genetic modification, and biomaterials. These interventions aim to repair self-renewal capacity and reshape the immunoregulatory and regenerative abilities of MSCs, ensuring optimal survival and function (Figure 3).
6.1 Preconditioning
6.1.1 Inflammatory factor preconditioning
Preconditioning MSCs with specific or conditioned media containing proinflammatory cytokines before application is a widely used strategy to enhance the therapeutic effect of MSCs. This approach restores self-renewal capacity, increases in vivo cell survival, augments paracrine activity, and enhances tri-lineage differentiation capabilities of MSCs.
Rejuvenation interventions of senescent MSCs.
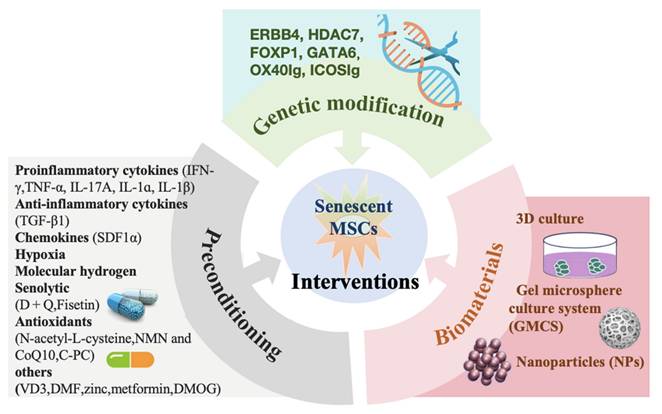
Specifically, preconditioning with IFN-γ has been reported to enhance the immunosuppressive phenotype, characterized by high IDO activity, and counteract the induction of paracrine senescence in cultured MSCs, alleviating the symptoms of graft-versus-host disease (GVHD) [95-97]. Researchers designed an injectable synthetic hydrogel containing a biologically active form of IFN-γ, which induced strong immunomodulatory and anti-inflammatory capabilities in MSCs. This inhibited both activated T cell proliferation and monocyte differentiation into dendritic cells, accelerating the healing of mucosal wounds in a mouse colonic wound surgery model, and eliminating the need for in vitro manipulation [98]. Additionally, TNF-α-preconditioned MSCs exhibited anti-inflammatory properties by reducing the capacity of peripheral immune cells to produce pro-inflammatory cytokines. This was accompanied by a decrease in low-density lipoprotein (LDL) levels and an elevation in high-density lipoprotein (HDL) levels, thereby suppressing systemic inflammation and reducing the size of atherosclerotic plaques [99]. A recent study found that preconditioning ADSCs with IL-17A significantly enhanced their immunosuppressive responses on activated T cells and induced Treg expansion compared to naive MSCs, despite the similarity in their immunophenotype [100].
Notably, the use of cocktail treatments (IFN-γ synergized with TNF-α) to precondition MSCs can stimulate MSCs to synthesize abundant growth factors associated with wound repair and promote dermal fibroblast migration, proliferation, and activation, thus improving wound healing [101]. Moreover, preconditioning with chemokines [106] and anti-inflammatory cytokines [107,108] can also prolong the effectiveness of therapy.
6.1.2 Hypoxic preconditioning
Hypoxic preconditioning is another approach to reversing MSCs senescence, effectively enhancing MSCs biological functions, such as survival, proliferation, migration, and multilineage differentiation. This is achieved through activation of the PI3K/AKT-HIF-1α-CXCR4/CXCR7 pathway, upregulation of key cell cycle regulators (lncRNA SNHG16), and activation of the HIF-1α/Apelin/APJ axis [109,110]. Furthermore, hypoxic preconditioning has been shown to significantly enhance the paracrine activity of MSCs, including increased expression of vascular endothelial growth factors, angiopoietin-1, erythropoietin, monocyte chemoattractant protein 1 (MCP-1), hepatocyte growth factor (HGF), and hypoxia-inducible factor-1α (HIF-1α), thereby improving the in vitro expansion of MSCs [109-111]. Hypoxic preconditioning has also been shown to increase survival capacity, delay olfactory mucosa MSC (OM-MSC) senescence by upregulating miR-326/PTBP1/PI3K-mediated autophagy, and enhance neuroprotection, thereby augmenting therapeutic efficacy in intracerebral hemorrhage (ICH) [112].
In addition to hypoxic preconditioning, priming MSCs with molecular hydrogen has emerged as a promising approach to overcome cellular senescence. Molecular hydrogen preconditioning induces antioxidant, anti-inflammatory, and anti-apoptotic effects, primarily mediated by the ROS/p53/p21 signaling pathway, thereby improving therapeutic outcomes in a wide range of diseases [113].
6.1.3 Senolytic drugs preconditioning
Given that senolytic drugs can effectively eliminate senescent cells, they have become a common approach to intervene in MSC senescence in recent years. For example, preconditioning MSCs with a senolytic mixture such as dasatinib (D) and quercetin (Q) has been shown to reduce the expression of senescence-related markers like p21 and p16, enhance mobilization and proliferation, and promote osteogenic differentiation of senescent BM-MSCs, resulting in bone organoids with restored bone remodeling [115]. A recent study reported for the first time that the senolytic agent fisetin attenuated reactive oxygen species, SA-β-gal, and senescence-associated heterochromatin foci produced during the culture expansion of ADSCs, while maintaining the multilineage differentiation potential of amplified ADSCs [116].
Currently, the use of antioxidants offers a feasible strategy for MSCs rejuvenation. N-acetyl-L-cysteine [117], β-nicotinamide mononucleotide (NMN), and Coenzyme Q10 (CoQ10) [114] have been reported to maintain genome stability, telomerase activity, and telomere length, reduce TNF and IL-17 inflammatory signaling pathways, and enhance DNA repair even after prolonged expansion, thereby inhibiting MSCs senescence. A recent study by Liu et al. reported for the first time that C-phycocyanin (C-PC) could ameliorate MSCs senescence, specifically by restoring the proliferative activity of senescent MSCs to some extent, reducing harmful ROS production and aging-related phenotypes, and promoting adipogenic and osteogenic differentiation [119].
The development of additional drugs holds potential for effectively reversing MSC aging, enhancing therapeutic efficacy, and preventing age-related diseases. Examples include Vitamin D3 (VD3) [120], dimethyl fumarate (DMF) [121], zinc [122], metformin [123,124], and dimethyloxalylglycine (DMOG) [125]. These drugs could induce ultrastructural and metabolic changes in MSCs, along with modifications in their secretome profiles, thereby delaying MSCs senescence. However, challenges remain in determining the optimal dosages, exposure duration, and managing potential therapeutic side effects. We summarize most priming preconditioning, as shown in Table 4.
6.2 Genetic modification
Over the past decade, various techniques for genetically modifying MSCs have been rapidly developed to enhance the secretion and expression of beneficial gene products, thereby imparting new characteristics to the cells and improving their therapeutic efficacy [126]. For instance, MSCs genetically engineered to overexpress Erb-B2 receptor tyrosine kinase 4 (ERBB4) have been shown to reduce oxidative stress, decrease the senescent phenotype in vitro, and improve heart function by enhancing blood vessel density and reducing cardiac remodeling [127]. Similarly, Li et al. demonstrated that MSCs overexpressing forkhead box P1 (FOXP1) played a crucial role in cell cycle regulation, enhancing MSCs self-renewal by suppressing p16INK4A activation [128]. Further studies have shown that MSCs genetically engineered to overexpress both FOXP1 and histone deacetylase 7 (HDAC7) cooperate to promote MSCs replicative capacity, reduce cellular senescence, and enhance osteogenic potential in vitro, compared to MSCs overexpressing FOXP1 alone [129]. Recently, MSCs transduced with either OX40Ig or ICOSIg have been reported to regulate MSCs function without altering their intrinsic characteristics and to alleviate various inflammatory diseases by blocking the OX40/OX40L or ICOS/ICOSL co-stimulatory pathways [130-132]. In summary, gene overexpression imparts new characteristics to MSCs, leading to more potent targeted therapeutic effects.
In addition to gene overexpression, gene knockdown has also been shown to reverse MSCs senescence. Jiao et al. demonstrated that knockdown of the age-related pro-senescence factor GATA binding protein 6 (GATA6) significantly reduced the expression of p53 and p21CIP1, while downregulating the pro-inflammatory cytokines IL-6 and IL-1β, which are known to induce cellular senescence, in late-passaged MSCs, thereby mitigating MSCs senescence [133]. Lin et al. further emphasized the significance of the GATA6/SOCS3/PDL1 pathway in regulating aging-associated changes in the immunomodulatory activity of MSCs [134].
Notably, Ruetz et al. [135] developed high-throughput in vitro and in vivo CRISPR-Cas9 screening platforms to identify genetic interventions that enhance the function of elderly neural stem cells, potentially delaying or reversing aging features and counteracting senescence in MSCs. To date, numerous technical challenges remain, including durability and safety concerns related to genetic engineering that still need to be addressed.
6.3 Biomaterials
Considering the biomimetic environment required for MSCs to maintain their properties and the targeted, controlled release of MSCs, advances in biomaterial engineering play a crucial role in improving the biophysical properties of MSCs, thereby enhancing their therapeutic efficacy. MSCs cultured using three-dimensional (3D) culture techniques or the gel microsphere culture system (GMCS) have been shown to enhance the integrated stress response of MSCs, preserving higher stemness, behaviors, and functionality compared to those cultured on conventional substrates [136,137]. The 3D culturing system enhanced cell migration, proliferation, and multilineage differentiation capabilities [138], demonstrating superior bone regeneration in a bone defect model [139]. Furthermore, this method improved therapeutic efficacy in treating inflammatory pulmonary diseases by enhancing the pulmonary delivery and in vivo survival of MSCs [140].
In recent years, nanoparticles (NPs) have played an increasingly important role as a biomaterial in supporting MSCs proliferation and stimulating multi-differentiation, ultimately enhancing tissue repair. For example, MSCs cultured on dentin disks embedded with silver nanoparticles (AgNPs) showed a significant increase in proliferation, migration, osteogenic differentiation, and improved wound repair and closure [141]. Wang et al. developed an NAD+-dependent SIRT1-activated nanoplatform that dual-delivers resveratrol (RSV) and nicotinamide riboside (NR), which could delay MSCs senescence and promote bone formation [142].
Recently, Yang et al. loaded TNF-α-treated MSCs-derived exosomes onto micro/nano-network titanium (Ti) surfaces, which increased the secretion of anti-inflammatory cytokines by targeting the PI3K/AKT/mTOR pathway, and enhanced angiogenesis and osteogenesis through M2-macrophage polarization, thereby accelerating osseointegration in type 2 diabetes (T2D) conditions [143]. Current research on the effects of applied mechanical forces in reducing MSCs senescence has shown that mechanical conditioning can rejuvenate senescing MSCs from elderly patients through oxidative stress and DNA damage repair mechanisms [144].
Multiple preconditioning regulating MSC senescence
| Preconditioning | Model / tissue source | Effect | Mechanism | Disease | Refs |
|---|---|---|---|---|---|
| Proinflammatory cytokines | |||||
| IFN-γ | Mouse | The symptoms of GVHD in NOD-SCID mice ↓ | Induction of IDO expression in MSCs via the IFN-γ-JAK-STAT1 pathway | GVHD | [97] |
| IFN-γ | Mouse/ Bone marrow | MSCs immunomodulatory function ↑, colonic mucosal wound healing rate ↑ | IDO and PD-L1 expression ↑ | Colonic wound | [98] |
| TNF-α | Mouse/ Bone marrow | Total cholesterol and LDL levels ↓, TNF-α and IFN-γ ↓, the spleens' weights ↓; HDL levels ↑, IL-10 ↑; | Tregs and Th1 ↑ | Atherosclerosis | [99] |
| Bone marrow | Chondrogenic differentiation ↑ | Modulating SOX11 levels and WNT/β-catenin signaling | - | [102] | |
| IL-17A | Human / Adipose tissue | The inhibitory rate of lymphocyte proliferation increased gradually | Tregs ↓ | - | [100] |
| IL-1ɑ | Mouse / Bone marrow | Lesion volume ↓, infarct volume ↓, neurological deficits ↓; CBF in the ipsilateral hemisphere ↑ | - | Ischemic stroke | [103] |
| IL-1β | Human / Umbilical cord blood | Migration ↑ | Matrix metalloproteinase-3 via ERK1/2 pathway ↑ | - | [104] |
| IFN-γ + TNF-α | Umbilical cord blood | proliferation, migration and activation of dermal fibroblasts ↑, type III collagen expression ↑, wound healing ↑ | Expression of PDGF-BB via the PI3K/Akt signaling pathway ↑ | - | [101] |
| IFN-γ + IL-1α, IFN-γ + IL-1β, IFN-γ + TNF-α | Human / Bone marrow | PBMC proliferation ↓ | - | Parkinson's disease | [105] |
| Chemokines | |||||
| SDF1α | Rat / Bone marrow | MSCs survival ↑, cardiac function ↑, the peri-infarct capillary density ↑; reduced scar size ↓ | Lactate dehydrogenase release ↓ | Infarcted Myocardium | [106] |
| Anti-inflammatory cytokines | |||||
| TGF-β1 | Mouse | MSCs ability to suppress T cell proliferation ↑, hepatic homing ↑, anti-inflammatory efficacy ↑ | Surface expression of CXCR3 ↑ | Acute carbon tetrachloride | [107] |
| Rat / Umbilical cord blood | MSC proliferation and survival rate ↑; LPS-induced systemic injury ↓ | RhoA expression ↑, phosphorylation of SMAD3 ↑ | Lipopolysaccharide-induced acute lung injury | [108] | |
| Hypoxia | |||||
| Placenta-derived | proliferation capacity ↑; cell death and senescence ↓ | PI3K/AKT pathway ↑ | - | [109] | |
| Mouse / Bone marrow | New vessel formation during skeletal muscle reconstruction ↑ | the level of VEGF and SDF-1 expression ↑ | Muscle injury | [110] | |
| Mouse / Umbilical cord blood | Bone fracture healing ↑, MSCs proliferation and migration ability ↑, tube formation in umbilical vein endothelial cell ↑ | Production of exosomal miR-126 through the activation of HIF-1α ↑ | Bone fracture | [111] | |
| Mouse / Olfactory mucosa | MSCs senescence ↓; neuroprotection following intracerebral hemorrhage ↑ | MiR-326/PTBP1/PI3K-mediated autophagy ↑ | Intracerebral hemorrhage | [112] | |
| Olfactory mucosa | Angiogenesis-stimulatory activity of HBMECs ↑ | MiR-612-TP53-HIF-1α-VEGF axis↑ | - | [114] | |
| Senolytics | |||||
| Dasatinib+ Quercetin (D + Q) | Mouse / Bone marrow | SA-β-gal+ cells ↓, p21 ↓, p16 ↓, IL6 ↓; proliferation rate↑, in vitro and in vivo osteogenic capacity ↑ | Clearance of senescent cells | Calvarial defect | [114] |
| Fisetin | Human adipose tissue | ROS ↓, SA-β-gal ↓, senescence-associated heterochromatin foci ↓ | - | - | [116] |
| Antioxidants | |||||
| N-acetyl-L-cysteine | Human bone marrow | Proliferation rate and multiple linage differentiation ability ↑; retains BM-MSCs with stable chromosome, DNA, telomere length, and telomerase activity | - | - | [117] |
| NMN and CoQ10 | Human umbilical cord blood | H2O2-induced senescence ↓, TNFα mRNA level ↓; proliferation migration ability ↑ | DNA repair ability↑, cyclin expression ↑; TNF and IL-17 inflammatory signaling pathways ↓ | - | [118] |
| C-PC | Rat / Adipose tissue | Proliferation ↑, adipogenic and osteogenic differentiation ↑, anti-inflammatory factors ↑, SOD activity ↑; ROS or MDA ↓, | ZDHHC5-Mediated Autophagy via PI3K/AKT/mTOR Pathway | D-Gal-induced accelerated aging | [119] |
| Other drugs | |||||
| VD3 | Human bone marrow | Proliferation ↑, osteogenic differentiation ↑; replicative senescence ↓ | SIRT1-FoxO3 signaling pathways ↑ | - | [120] |
| DMF | Rat / Adipose tissue | Survivability ↑, proliferation and antioxidant capability ↑, neuroprotective effect ↑; restore impaired learning and memory | Nrf2 ↑ | Alzheimer's disease | [121] |
| Zinc | Human umbilical cord blood | Cell adhesion, migration and self-renewal potential ↑ | - | - | [122] |
| Metformin | Human bone marrow | Percentage of SA-β-Gal-positive cells ↓, the levels of p53, p21, and p16 ↓. | Autophagy by activating the AMPK pathway ↑ | - | [123] |
| Human dental pulp | Percentage of SA-β-gal-positive cells ↓, the levels of p53, p21, and p16 ↓ | Targeting CAB39 through the AMPK/mTOR signaling pathway | - | [124] | |
| DMOG | Human umbilical cord blood | Migratory and proliferative capacities ↑; altered ultrastructural morphology | - | - | [125] |
GVHD, graft-versus-host disease; IDO, intracellular protein indoleamine 2,3-dioxygenase; JAK-STAT, janus kinase - signal transducer and activator of transcription; PD-L1, rogrammed cell death 1 ligand 1; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SOX11, SRY-box transcription factor 11; CBF, cerebral blood flow; ERK, extracellular regulated protein kinases; PDGF-BB, platelet-derived growth factor-BB; PI3K/Akt, phosphatidylinositol 3-kinase / protein kinase B; PBMC, peripheral blood mononuclear cell; SDF1α, stromal cellderived factor 1α; CXCR3, chemokine C-X-C-motif receptor 3; SMAD3, mothers against DPP homolog 3; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia inducible factor-1α; PTBP1, polypyrimidine tract binding protein 1; ROS, reactive oxygen species; NMN, β-nicotinamide mononucleotide; CoQ10, Coenzyme Q10; C-PC, C-phycocyanin; SOD, superoxide dismutase; MDA, malondialdehyde; VD3, Vitamin D3; SIRT1, Sirtuin 1; FoxO3, forkhead box O3; DMF, dimethyl fumarate; Nrf2, nuclear factor erythroid 2-related factor 2; AMPK, adenosine 5'-monophosphate-activated protein kinase; CAB39, calcium binding protein 39; DMOG, dimethyloxalylglycine. ↑ increased, upregulated or enhanced; ↓ decreased, down-regulated or impaired.
7. Conclusions
Numerous bench-to-bedside studies have shown that MSCs offer significant advantages. However, the potential negative impact of in vivo versus ex vivo senescence of MSCs must be carefully considered. The progressive senescence of MSCs not only leads to a gradual loss of cellular stemness but also causes broad immunomodulatory dysfunction, thereby hindering the therapeutic benefits for age-related diseases driven by senescent MSCs. Current anti-senescent interventions aim to rejuvenate senescing MSCs, restoring them from age-related decline to youthful vigor, preserving self-renewal capabilities, and reshaping their immunoregulatory and regenerative functions, while achieving a favorable safety/efficacy profile and minimizing side effects. Despite extensive research and efforts, each technique still has certain limitations and may vary between individuals. Therefore, from a treatment perspective, there is an urgent need to continue developing and optimizing novel approaches to reverse cellular senescence, thereby enhancing the therapeutic outcomes of off-the-shelf MSCs therapy for specific disorders while minimizing side effects. With advances in modern science, artificial intelligence (AI) could play a crucial role in MSC therapy, offering significant benefits to regenerative medicine in the future. The ultimate goal is to delay aging and combat the development of aging-related diseases, thereby improving health and extending longevity.
Acknowledgements
This work was supported by the Key Project of Tianjin Natural Science Foundation (No. 20JCZDJC00660).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Xu W, Yang Y, Li N, Hua J. Interaction between Mesenchymal Stem Cells and Immune Cells during Bone Injury Repair. Int J Mol Sci. 2023;24(19):14484 doi: 10.3390/ijms241914484
2. National Library of Medicine. ClinicalTrials.gov. https://www.clinicaltrials.gov/
3. Lu W, Yan L, Tang X, Wang X, Du J, Zou Z. et al. Efficacy and safety of mesenchymal stem cells therapy in COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2024;22(1):550 doi: 10.1186/s12967-024-05358-6
4. Chen X, Wang F, Huang Z, Wu Y, Geng J, Wang Y. Clinical applications of mesenchymal stromal cell-based therapies for pulmonary diseases: An Update and Concise Review. Int J Med Sci. 2021;18:2849-2870 doi: 10.7150/ijms.59218
5. Klimczak A. Mesenchymal Stem/Progenitor Cells and Their Derivates in Tissue Regeneration-Part II. Int J Mol Sci. 2024;25(9):4937 doi: 10.3390/ijms25094937
6. Santos CJ, Paciência I, Ribeiro AI. Neighbourhood Socioeconomic Processes and Dynamics and Healthy Ageing: A Scoping Review. Int J Environ Res Public Health. 2022;19(11):6745 doi: 10.3390/ijerph19116745
7. National Bureau of Statistics of China. Wang Pingping: The total population decreased slightly and the level of urbanization continued to improve. 2023. https://www.stats.gov.cn/sj/sjjd/202302/t20230202_1896742.html
8. World Health Organization. Ageing and health. 2022. https://www.who.int/zh/news-room/fact-sheets/detail/ageing-and-health
9. Vasanthan J, Gurusamy N, Rajasingh S, Sigamani V, Kirankumar S, Thomas EL. et al. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells. 2020;10(1):54 doi: 10.3390/cells10010054
10. Chen H, Liu O, Chen S, Zhou Y. Aging and Mesenchymal Stem Cells: Therapeutic Opportunities and Challenges in the Older Group. Gerontology. 2022;68(3):339-352 doi: 10.1159/000516668
11. Olmedo-Moreno L, Aguilera Y, Baliña-Sánchez C, Martín-Montalvo A, Capilla-González V. Heterogeneity of In Vitro Expanded Mesenchymal Stromal Cells and Strategies to Improve Their Therapeutic Actions. Pharmaceutics. 2022;14(5):1112 doi: 10.3390/pharmaceutics14051112
12. Zhou X, Hong Y, Zhang H, Li X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front Cell Dev Biol. 2020;8:364 doi: 10.3389/fcell.2020.00364
13. Weng Z, Wang Y, Ouchi T, Liu H, Qiao X, Wu C. et al. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl Med. 2022;11(4):356-371 doi: 10.1093/stcltm/szac004
14. Oja S, Komulainen P, Penttilä A, Nystedt J, Korhonen M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res Ther. 2018;9(1):6 doi: 10.1186/s13287-017-0740-x
15. Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:2152435 doi: 10.1155/2016/2152435
16. Massaro F, Corrillon F, Stamatopoulos B, Dubois N, Ruer A, Meuleman N. et al. Age-related changes in human bone marrow mesenchymal stromal cells: morphology, gene expression profile, immunomodulatory activity and miRNA expression. Front Immunol. 2023;14:1267550 doi: 10.3389/fimmu
17. Du P, Wang F, Chen XB, Zheng Y, Wu QQ, Liu WJ. et al. Impact of age on the biological properties of human adipose tissue-derived mesenchymal stromal cells. Tianjin Med J. 2022;50(09):912-916 doi: 10.11958/20220808
18. Grun LK, Maurmann RM, Scholl JN, Fogaça ME, Schmitz CRR, Dias CK. et al. Obesity drives adipose-derived stem cells into a senescent and dysfunctional phenotype associated with P38MAPK/NF-KB axis. Immun Ageing. 2023;20(1):51 doi: 10.1186/s12979-023-00378-0
19. He L, Li M, Wang X, Wu X, Yue G, Wang T. et al. Morphology-based deep learning enables accurate detection of senescence in mesenchymal stem cell cultures. BMC Biol. 2024;22(1):1 doi: 10.1186/s12915-023-01780-2
20. Truong NC, Bui KH, Van Pham P. Characterization of Senescence of Human Adipose-Derived Stem Cells After Long-Term Expansion. Adv Exp Med Biol. 2019;1084:109-128 doi: 10.1007/5584_2018_235
21. Martini H, Iacovoni JS, Maggiorani D, Dutaur M, Marsal DJ, Roncalli J. et al. Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90 + subset. Aging Cell. 2019;18(5):e13015 doi: 10.1111/acel.13015
22. Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK, Min JK. et al. Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells. Biochem Biophys Res Commun. 2011;404(1):463-9 doi: 10.1016/j.bbrc.2010.12.003
23. Dubon M, Lee S, Park JH, Lee JY, Kang D. The Role of Melanotransferrin (CD228) in the regulation of the differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells (hBM-MSC). Int J Med Sci. 2021;18(7):1580-1591 doi: 10.7150/ijms.53650
24. Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA, O'Connor KC. Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):201 doi: 10.1186/s13287-017-0649-4
25. Psaroudis RT, Singh U, Lora M, Jeon P, Boursiquot A, Stochaj U. et al. CD26 is a senescence marker associated with reduced immunopotency of human adipose tissue-derived multipotent mesenchymal stromal cells. Stem Cell Res Ther. 2022;13(1):358 doi: 10.1186/s13287-022-03026-4
26. Kim M, Go J, Kwon JH, Jin HJ, Bae YK, Kim EY. et al. CD26 Inhibition Potentiates the Therapeutic Effects of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells by Delaying Cellular Senescence. Front Cell Dev Biol. 2022;9:803645 doi: 10.3389/fcell.2021.803645
27. Gnani D, Crippa S, Della Volpe L, Rossella V, Conti A, Lettera E. et al. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell. 2019;18(3):e12933 doi: 10.1111/acel.12933
28. Moravcikova E, Meyer EM, Corselli M, Donnenberg VS, Donnenberg AD. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytometry A. 2018;93(9):894-904 doi: 10.1002/cyto.a.23574
29. Valieva Y, Ivanova E, Fayzullin A, Kurkov A, Igrunkova A. Senescence-Associated β-Galactosidase Detection in Pathology. Diagnostics (Basel). 2022;12(10):2309 doi: 10.3390/diagnostics12102309
30. Chou LY, Ho CT, Hung SC. Paracrine Senescence of Mesenchymal Stromal Cells Involves Inflammatory Cytokines and the NF-κB Pathway. Cells. 2022;11(20):3324 doi: 10.3390/cells11203324
31. Jie Z, Huan Y, Mengyun W, Yasha L, Huafeng P, Ke Y. Nrf2 modulates immunosuppressive ability and cellular senescence of human umbilical cord mesenchymal stem cells. Biochem Biophys Res Commun. 2020;526(4):1021-1027 doi: 10.1016/j.bbrc.2020.03.175
32. Yang Y, Liu S, He C, Lv T, Zeng L, Zhang F. et al. LncRNA LYPLAL1-AS1 rejuvenates human adipose-derived mesenchymal stem cell senescence via transcriptional MIRLET7B inactivation. Cell Biosci. 2022;12(1):45 doi: 10.1186/s13578-022-00782-x
33. Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC. et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187-95 doi: 10.1111/j.1474-9726.2006.00199.x
34. Koo S, Won M, Li H, Kim WY, Li M, Yan C. et al. Harnessing α-l-fucosidase for in vivo cellular senescence imaging. Chem Sci. 2021;12(29):10054-10062 doi: 10.1039/d1sc02259h
35. Peng X, Zhou X, Yin Y, Luo B, Liu Y, Yang C. Inflammatory Microenvironment Accelerates Bone Marrow Mesenchymal Stem Cell Aging. Front Bioeng Biotechnol. 2022;10:870324 doi: 10.3389/fbioe.2022.870324
36. Alessio N, Aprile D, Peluso G, Mazzone V, Patrone D, Di Bernardo G. et al. IGFBP5 is released by senescent cells and is internalized by healthy cells, promoting their senescence through interaction with retinoic receptors. Cell Commun Signal. 2024;22(1):122 doi: 10.1186/s12964-024-01469-1
37. Lee JY, Yu KR, Lee BC, Kang I, Kim JJ, Jung EJ. et al. GATA4-dependent regulation of the secretory phenotype via MCP-1 underlies lamin A-mediated human mesenchymal stem cell aging. Exp Mol Med. 2018;50(5):1-12 doi: 10.1038/s12276-018-0092-3
38. Carroll B, Nelson G, Rabanal-Ruiz Y, Kucheryavenko O, Dunhill-Turner NA, Chesterman CC. et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J Cell Biol. 2017;216(7):1949-1957 doi: 10.1083/jcb.201610113
39. Chen Q, Zhang H, Yang Y, Zhang S, Wang J, Zhang D. et al. Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. Int J Mol Sci. 2022;23(13):6960 doi: 10.3390/ijms23136960
40. Yurube T, Ito M, Kakiuchi Y, Kuroda R, Kakutani K. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine. 2020;3(1):e1082 doi: 10.1002/jsp2.1082
41. Ye G, Li J, Yu W, Xie Z, Zheng G, Liu W. et al. ALKBH5 facilitates CYP1B1 mRNA degradation via m6A demethylation to alleviate MSC senescence and osteoarthritis progression. Exp Mol Med. 2023;55(8):1743-1756 doi: 10.1038/s12276-023-01059-0
42. Jin HJ, Lee HJ, Heo J, Lim J, Kim M, Kim MK. et al. Senescence-Associated MCP-1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxid Redox Signal. 2016;24(9):471-85 doi: 10.1089/ars.2015.6359
43. Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A. et al. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics. 2017;7(10):2673-2689 doi: 10.7150/thno.18915
44. Zheng X, Wang Q, Xie Z, Li J. The elevated level of IL-1α in the bone marrow of aged mice leads to MSC senescence partly by down-regulating Bmi-1. Exp Gerontol. 2021;148:111313 doi: 10.1016/j.exger.2021.111313
45. Harrington JS, Ryter SW, Plataki M, Price DR, Choi AMK. Mitochondria in health, disease, and aging. Physiol Rev. 2023;103(4):2349-2422 doi: 10.1152/physrev.00058.2021
46. Malekpour K, Hazrati A, Soudi S, Hashemi SM. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Control Release. 2023;354:755-769 doi: 10.1016/j.jconrel.2023.01.059
47. Herzig MC, Delavan CP, Jensen KJ, Cantu C, Montgomery RK, Christy BA. et al. A streamlined proliferation assay using mixed lymphocytes for evaluation of human mesenchymal stem cell immunomodulation activity. J Immunol Methods. 2021;488:112915 doi: 10.1016/j.jim.2020.112915
48. Yang W, Ji W, Liao B, Li Z, Wang J, Lin H. et al. Genome-wide sequencing identified extrachromosomal circular DNA as a transcription factor-binding motif of the senescence genes that govern replicative senescence in human mesenchymal stem cells. Front Cell Neurosci. 2024;18:1421342 doi: 10.3389/fncel.2024.1421342
49. Liu M, Lei H, Dong P, Fu X, Yang Z, Yang Y. et al. Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell Transplant. 2017;26(9):1505-1519 doi: 10.1177/0963689717721221
50. Wang S, Wang Z, Su H, Chen F, Ma M, Yu W. et al. Effects of long-term culture on the biological characteristics and RNA profiles of human bone-marrow-derived mesenchymal stem cells. Mol Ther Nucleic Acids. 2021;26:557-574 doi: 10.1016/j.omtn.2021.08.013
51. Taherian Fard A, Leeson HC, Aguado J, Pietrogrande G, Power D, Gómez-Inclán C. et al. Deconstructing heterogeneity of replicative senescence in human mesenchymal stem cells at single cell resolution. Geroscience. 2024;46(1):999-1015 doi: 10.1007/s11357-023-00829-y
52. Kim HY, Kim HS. Podoplanin depletion in tonsil-derived mesenchymal stem cells induces cellular senescence via regulation of the p16Ink4a/Rb pathway. Cell Commun Signal. 2024;22(1):323 doi: 10.1186/s12964-024-01705-8
53. Aung KT, Akiyama K, Kunitomo M, Mun AY, Tosa I, Nguyen HTT. et al. Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model. Int J Mol Sci. 2020;21(21):8103 doi: 10.3390/ijms21218103
54. Li D, Liu J, Yang C, Tian Y, Yin C, Hu L. et al. Targeting long noncoding RNA PMIF facilitates osteoprogenitor cells migrating to bone formation surface to promote bone formation during aging. Theranostics. 2021;11(11):5585-5604 doi: 10.7150/thno.54477
55. Amini-Nik S, Abdullahi A, Vinaik R, Yao RJR, Yu N, Datu A. et al. Aging Impairs the Cellular Interplay between Myeloid Cells and Mesenchymal Cells during Skin Healing in Mice. Aging Dis. 2022;13(2):540-551 doi: 10.14336/AD.2021.1008
56. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8 doi: 10.1186/1479-5876-12-8
57. Wu SH, Yu JH, Liao YT, Liu KH, Chiang ER, Chang MC. et al. Comparison of the Infant and Adult Adipose-Derived Mesenchymal Stem Cells in Proliferation, Senescence, Anti-oxidative Ability and Differentiation Potential. Tissue Eng Regen Med. 2022;19(3):589-601 doi: 10.1007/s13770-022-00431-x
58. Yu J, Shi J, Zhang Y, Zhang Y, Huang Y, Chen Z. et al. The replicative senescent mesenchymal stem / stromal cells defect in DNA damage response and anti-oxidative capacity. Int J Med Sci. 2018;15(8):771-781 doi: 10.7150/ijms.24635
59. Bagge J, Berg LC, Janes J, MacLeod JN. Donor age effects on in vitro chondrogenic and osteogenic differentiation performance of equine bone marrow- and adipose tissue-derived mesenchymal stromal cells. BMC Vet Res. 2022;18(1):388 doi: 10.1186/s12917-022-03475-2
60. Abuna RP, Stringhetta-Garcia CT, Fiori LP, Dornelles RC, Rosa AL, Beloti MM. Aging impairs osteoblast differentiation of mesenchymal stem cells grown on titanium by favoring adipogenesis. J Appl Oral Sci. 2016;24(4):376-82 doi: 10.1590/1678-775720160037
61. Yu Y, Park YS, Kim HS, Kim HY, Jin YM, Jung SC. et al. Characterization of long-term in vitro culture-related alterations of human tonsil-derived mesenchymal stem cells: role for CCN1 in replicative senescence-associated increase in osteogenic differentiation. J Anat. 2014;225(5):510-8 doi: 10.1111/joa.12229
62. Prall WC, Saller MM, Scheumaier A, Tucholski T, Taha S, Böcker W. et al. Proliferative and osteogenic differentiation capacity of mesenchymal stromal cells: Influence of harvesting site and donor age. Injury. 2018;49(8):1504-1512 doi: 10.1016/j.injury.2018.06.024
63. Horinouchi CD, Barisón MJ, Robert AW, Kuligovski C, Aguiar AM, Dallagiovanna B. Influence of donor age on the differentiation and division capacity of human adipose-derived stem cells. World J Stem Cells. 2020;12(12):1640-1651 doi: 10.4252/wjsc.v12.i12.1640
64. Zhang C, Han X, Liu J, Chen L, Lei Y, Chen K. et al. Single-cell Transcriptomic Analysis Reveals the Cellular Heterogeneity of Mesenchymal Stem Cells. Genomics Proteomics Bioinformatics. 2022;20(1):70-86 doi: 10.1016/j.gpb.2022.01.005
65. Wu R, Sun F, Zhang W, Ren J, Liu GH. Targeting aging and age-related diseases with vaccines. Nat Aging. 2024;4(4):464-482 doi: 10.1038/s43587-024-00597-0
66. Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41(9):653-664 doi: 10.1016/j.tips.2020.06.009
67. Yang G, Fan X, Liu Y, Jie P, Mazhar M, Liu Y. et al. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev Rep. 2023;19(5):1214-1231 doi: 10.1007/s12015-023-10539-9
68. Zhang Z, Yang X, Meng Q, Long Y, Shi X, Wang Y. Adipose tissue-derived mesenchymal stromal cells attenuate acute lung injury induced by trauma and haemorrhagic shock. Immunobiology. 2023;228(6):152765 doi: 10.1016/j.imbio.2023.152765
69. Wang JF, Yang XX, Zhang J, Zheng Y, Zhang FQ, Shi XF. et al. Immunomodulation of adipose-derived mesenchymal stem cells on peripheral blood mononuclear cells in colorectal cancer patients with COVID-19. World J Gastrointest Oncol. 2024;16(5):2113-2122 doi: 10.4251/wjgo.v16.i5.2113
70. Özgül Özdemir RB, Özdemir AT, Kırmaz C, Eker Sarıboyacı A, Karaöz E, Erman G. et al. Age-related changes in the immunomodulatory effects of human dental pulp derived mesenchymal stem cells on the CD4+ T cell subsets. Cytokine. 2021;138:155367 doi: 10.1016/j.cyto.2020.155367
71. Gao Y, Chi Y, Chen Y, Wang W, Li H, Zheng W. et al. Multi-omics analysis of human mesenchymal stem cells shows cell aging that alters immunomodulatory activity through the downregulation of PD-L1. Nat Commun. 2023;14(1):4373 doi: 10.1038/s41467-023-39958-5
72. Zhang J, Akiyama K, Mun AY, Tagashira R, Zou T, Matsunaga N. et al. Age-Related Effects on MSC Immunomodulation, Macrophage Polarization, Apoptosis, and Bone Regeneration Correlate with IL-38 Expression. Int J Mol Sci. 2024;25(6):3252 doi: 10.3390/ijms25063252
73. Kapetanos K, Asimakopoulos D, Christodoulou N, Vogt A, Khan W. Chronological Age Affects MSC Senescence In Vitro-A Systematic Review. Int J Mol Sci. 2021;22(15):7945 doi: 10.3390/ijms22157945
74. Goyal A, Afzal M, Khan NH, Goyal K, Srinivasamurthy SK, Gupta G. et al. Targeting p53-p21 signaling to enhance mesenchymal stem cell regenerative potential. Regen Ther. 2025;29:352-363 doi: 10.1016/j.reth.2025.03.007
75. Hladik D, Höfig I, Oestreicher U, Beckers J, Matjanovski M, Bao X. et al. Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Res Ther. 2019;10(1):218 doi: 10.1186/s13287-019-1334-6
76. Banimohamad-Shotorbani B, Kahroba H, Sadeghzadeh H, Wilson DM 3rd, Maadi H, Samadi N. et al. DNA damage repair response in mesenchymal stromal cells: From cellular senescence and aging to apoptosis and differentiation ability. Ageing Res Rev. 2020;62:101125 doi: 10.1016/j.arr.2020.101125
77. Lin J, Epel E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res Rev. 2022;73:101507 doi: 10.1016/j.arr.2021.101507
78. Rossiello F, Jurk D, Passos JF, d'Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135-147 doi: 10.1038/s41556-022-00842-x
79. Sui B, Hu C, Jin Y. Mitochondrial metabolic failure in telomere attrition-provoked aging of bone marrow mesenchymal stem cells. Biogerontology. 2016;17(2):267-279 doi: 10.1007/s10522-015-9609-5
80. Nakashima K, Kunisaki Y, Hosokawa K, Gotoh K, Yao H, Yuta R. et al. POT1a deficiency in mesenchymal niches perturbs B-lymphopoiesis. Commun Biol. 2023;6(1):996 doi: 10.1038/s42003-023-05374-0
81. He C, Zhang X, Li J, Dai C, Wang S, Dai C. et al. Low-dose telomerase is required for the expansion and migration of placental mesenchymal stem cells. Biochem Biophys Res Commun. 2022;636(Pt 2):40-47 doi: 10.1016/j.bbrc.2022.10.093
82. Liu L, DiGirolamo CM, Navarro PA, Blasco MA, Keefe DL. Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp Cell Res. 2004;294(1):1-8 doi: 10.1016/j.yexcr.2003.10.031
83. Trachana V, Petrakis S, Fotiadis Z, Siska EK, Balis V, Gonos ES. et al. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy. 2017;19(7):808-820 doi: 10.1016/j.jcyt.2017.03.078
84. Khan AH, Gu X, Patel RJ, Chuphal P, Viana MP, Brown AI. et al. Mitochondrial protein heterogeneity stems from the stochastic nature of co-translational protein targeting in cell senescence. Nat Commun. 2024;15(1):8274 doi: 10.1038/s41467-024-52183-y
85. Ye G, Xie Z, Zeng H, Wang P, Li J, Zheng G. et al. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis. 2020;11(9):775 doi: 10.1038/s41419-020-02993-x
86. Denu RA, Hematti P. Optimization of oxidative stress for mesenchymal stromal/stem cell engraftment, function and longevity. Free Radic Biol Med. 2021;167:193-200 doi: 10.1016/j.freeradbiomed.2021.02.042
87. Branco A, Moniz I, Ramalho-Santos J. Mitochondria as biological targets for stem cell and organismal senescence. Eur J Cell Biol. 2023;102(2):151289 doi: 10.1016/j.ejcb.2023.151289
88. Li X, Wang X, Zhang C, Wang J, Wang S, Hu L. Dysfunction of metabolic activity of bone marrow mesenchymal stem cells in aged mice. Cell Prolif. 2022;55(3):e13191 doi: 10.1111/cpr.13191
89. Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y. et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell. 2020;19(4):e13128 doi: 10.1111/acel.13128
90. Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208(1):149-53 doi: 10.1002/jcp.20641
91. Eroglu B, Genova E, Zhang Q, Su Y, Shi X, Isales C. et al. Photobiomodulation has rejuvenating effects on aged bone marrow mesenchymal stem cells. Sci Rep. 2021;11(1):13067 doi: 10.1038/s41598-021-92584-3
92. Yan W, Diao S, Fan Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res Ther. 2021;12(1):140 doi: 10.1186/s13287-021-02194-z
93. Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I. et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1(8):634-650 doi: 10.1038/s43587-021-00098-4
94. Velarde F, Ezquerra S, Delbruyere X, Caicedo A, Hidalgo Y, Khoury M. Mesenchymal stem cell-mediated transfer of mitochondria: mechanisms and functional impact. Cell Mol Life Sci. 2022;79(3):177 doi: 10.1007/s00018-022-04207-3
95. Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C, Marchese C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front Cell Dev Biol. 2020;8:236 doi: 10.3389/fcell.2020.00236
96. Mahmoud M, Juntunen M, Adnan A, Kummola L, Junttila IS, Kelloniemi M. et al. Immunomodulatory Functions of Adipose Mesenchymal Stromal/Stem Cell Derived From Donors With Type 2 Diabetes and Obesity on CD4 T Cells. Stem Cells. 2023;41(5):505-519 doi: 10.1093/stmcls/sxad021
97. Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW. et al. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine. 2018;28:261-273 doi: 10.1016/j.ebiom.2018.01.002
98. García JR, Quirós M, Han WM, O'Leary MN, Cox GN, Nusrat A. et al. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials. 2019;220:119403 doi: 10.1016/j.biomaterials.2019.119403
99. Sekenova A, Li Y, Issabekova A, Saparov A, Ogay V. TNF-α Preconditioning Improves the Therapeutic Efficacy of Mesenchymal Stem Cells in an Experimental Model of Atherosclerosis. Cells. 2023;12(18):2262 doi: 10.3390/cells12182262
100. Long YY, Li CL, Du P, Chen XB, Wang YL. Regulation of lymphocyte function by IL-17A preconditioned adipose-derived mesenchymal Stem Cells. Current Immunology. 2024;44(02):121-125 doi: 1001-2478(2024)02-0121-06
101. Liu C, Wang C, Yang F, Lu Y, Du P, Hu K. et al. The conditioned medium from mesenchymal stromal cells pretreated with proinflammatory cytokines promote fibroblasts migration and activation. PLoS One. 2022;17(4):e0265049 doi: 10.1371/journal.pone.0265049
102. Voskamp C, Koevoet WJLM, Somoza RA, Caplan AI, Lefebvre V, van Osch GJVM. et al. Enhanced Chondrogenic Capacity of Mesenchymal Stem Cells After TNFα Pre-treatment. Front Bioeng Biotechnol. 2020;8:658 doi: 10.3389/fbioe.2020.00658
103. Wong R, Smith CJ, Allan SM, Pinteaux E. Preconditioning with interleukin-1 alpha is required for the neuroprotective properties of mesenchymal stem cells after ischemic stroke in mice. J Cereb Blood Flow Metab. 2023;43(12):2040-2048 doi: 10.1177/0271678X231197109
104. Chang CH, Lin YL, Tyan YS, Chiu YH, Liang YH, Chen CP. et al. Interleukin-1β-induced matrix metalloproteinase-3 via ERK1/2 pathway to promote mesenchymal stem cell migration. PLoS One. 2021;16(5):e0252163 doi: 10.1371/journal.pone.0252163
105. Guan YQ, Zhao CS, Zou HQ, Yan XM, Luo LL, Wu JL. et al. Aging, rather than Parkinson's disease, affects the responsiveness of PBMCs to the immunosuppression of bone marrow mesenchymal stem cells. Mol Med Rep. 2019;19(1):165-176 doi: 10.3892/mmr.2018.9670
106. Esmaeili R, Darbandi-Azar A, Sadeghpour A, Majidzadeh-A K, Eini L, Jafarbeik-Iravani N. et al. Mesenchymal Stem Cells Pretreatment With Stromal-Derived Factor-1 Alpha Augments Cardiac Function and Angiogenesis in Infarcted Myocardium. Am J Med Sci. 2021;361(6):765-775 doi: 10.1016/j.amjms.2021.01.025
107. Garg A, Khan S, Luu N, Nicholas DJ, Day V, King AL. et al. TGFβ1 priming enhances CXCR3-mediated mesenchymal stromal cell engraftment to the liver and enhances anti-inflammatory efficacy. J Cell Mol Med. 2023;27(6):864-878 doi: 10.1111/jcmm.17698
108. Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14(2):1681-1692 doi: 10.3892/mmr.2016.5416
109. Feng XD, Zhou JH, Chen JY, Feng B, Hu RT, Wu J. et al. Long non-coding RNA SNHG16 promotes human placenta-derived mesenchymal stem cell proliferation capacity through the PI3K/AKT pathway under hypoxia. World J Stem Cells. 2022;14(9):714-728 doi: 10.4252/wjsc.v14.i9.714
110. Archacka K, Grabowska I, Mierzejewski B, Graffstein J, Górzyńska A, Krawczyk M. et al. Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res Ther. 2021;12(1):448 doi: 10.1186/s13287-021-02530-3
111. Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z. et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212 doi: 10.1016/j.actbio.2019.12.020
112. Liu J, He J, Ge L, Xiao H, Huang Y, Zeng L. et al. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res Ther. 2021;12(1):413 doi: 10.1186/s13287-021-02480-w
113. Artamonov MY, LeBaron TW, Pyatakovich FA, Minenko IA. Mesenchymal Stem Cell Priming: Potential Benefits of Administration of Molecular Hydrogen. Pharmaceuticals (Basel). 2024;17(4):469 doi: 10.3390/ph17040469
114. Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y. et al. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 2021;19(1):380 doi: 10.1186/s12951-021-01126-6
115. Zhou Y, Xin X, Wang L, Wang B, Chen L, Liu O. et al. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen Med. 2021;6(1):34 doi: 10.1038/s41536-021-00145-z
116. Mullen M, Nelson AL, Goff A, Billings J, Kloser H, Huard C. et al. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem Cells. 2023;41(7):698-710 doi: 10.1093/stmcls/sxad036
117. Yang CD, Chuang SC, Cheng TL, Lee MJ, Chen HT, Lin SY. et al. An Intermediate Concentration of Calcium with Antioxidant Supplement in Culture Medium Enhances Proliferation and Decreases the Aging of Bone Marrow Mesenchymal Stem Cells. Int J Mol Sci. 2021;22(4):2095 doi: 10.3390/ijms22042095
118. Zheng Z, Wang X, Ouyang L, Chen W, Zhang L, Cao Y. Antioxidants Improve the Proliferation and Efficacy of hUC-MSCs against H2O2-Induced Senescence. Antioxidants (Basel). 2023;12(7):1334 doi: 10.3390/antiox12071334
119. Liu G, Li X, Yang F, Qi J, Shang L, Zhang H. et al. C-Phycocyanin Ameliorates the Senescence of Mesenchymal Stem Cells through ZDHHC5-Mediated Autophagy via PI3K/AKT/mTOR Pathway. Aging Dis. 2023;14(4):1425-1440 doi: 10.14336/AD.2023.0121
120. Borojević A, Jauković A, Kukolj T, Mojsilović S, Obradović H, Trivanović D. et al. Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling. Biomolecules. 2022;12(2):323 doi: 10.3390/biom12020323
121. Babaei H, Kheirollah A, Ranjbaran M, Cheraghzadeh M, Sarkaki A, Adelipour M. Preconditioning adipose-derived mesenchymal stem cells with dimethyl fumarate promotes their therapeutic efficacy in the brain tissues of rats with Alzheimer's disease. Biochem Biophys Res Commun. 2023;672:120-127 doi: 10.1016/j.bbrc.2023.06.045
122. Sahibdad I, Khalid S, Chaudhry GR, Salim A, Begum S, Khan I. Zinc enhances the cell adhesion, migration, and self-renewal potential of human umbilical cord derived mesenchymal stem cells. World J Stem Cells. 2023;15(7):751-767 doi: 10.4252/wjsc.v15.i7.751
123. Ye P, Feng L, Zhang D, Li R, Wen Y, Tong X. et al. Metformin Ameliorates D-Galactose-Induced Senescent Human Bone Marrow-Derived Mesenchymal Stem Cells by Enhancing Autophagy. Stem Cells Int. 2023;2023:1429642 doi: 10.1155/2023/1429642
124. Zhang S, Zhang R, Qiao P, Ma X, Lu R, Wang F. et al. Metformin-Induced MicroRNA-34a-3p Downregulation Alleviates Senescence in Human Dental Pulp Stem Cells by Targeting CAB39 through the AMPK/mTOR Signaling Pathway. Stem Cells Int. 2021;2021:6616240 doi: 10.1155/2021/6616240
125. Olcar HN, Isildar B, Ozkan S, Ercin M, Gezginci-Oktayoglu S, Koyuturk M. Investigation of conditioned medium properties obtained from human umbilical cord mesenchymal stem/stromal cells preconditioned with dimethyloxalylglycine in a correlation with ultrastructural changes. Microsc Res Tech. 2024;87(1):159-171 doi: 10.1002/jemt.24420
126. Varkouhi AK, Monteiro APT, Tsoporis JN, Mei SHJ, Stewart DJ, Dos Santos CC. Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness. Stem Cell Rev Rep. 2020;16(5):812-827 doi: 10.1007/s12015-020-10000-1
127. Liang X, Ding Y, Lin F, Zhang Y, Zhou X, Meng Q. et al. Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. FASEB J. 2019;33(3):4559-4570 doi: 10.1096/fj.201801690R
128. Li H, Liu P, Xu S, Li Y, Dekker JD, Li B. et al. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J Clin Invest. 2017;127(4):1241-1253 doi: 10.1172/JCI89511
129. Ling S, Chen T, Wang S, Zhang W, Zhou R, Xia X. et al. Deacetylation of FOXP1 by HDAC7 potentiates self-renewal of mesenchymal stem cells. Stem Cell Res Ther. 2023;14(1):188 doi: 10.1186/s13287-023-03376-7
130. Liu T, Zhang Y, Shen Z, Zou X, Chen X, Chen L. et al. Immunomodulatory effects of OX40Ig gene-modified adipose tissue-derived mesenchymal stem cells on rat kidney transplantation. Int J Mol Med. 2017;39(1):144-152 doi: 10.3892/ijmm.2016.2808
131. Geng J, Liu T, Jiang J, Li G, Huang ZW, Wang YL.The expression ICOSIg gene mediated by eukaryotic expression vector in rat adipose tissue-derived mesenchymal stem cells. Tianjin Med J. 2020;48(08):711-714. doi: 10.11958/20193697.
132. Fan X, Xu ZB, Li CL, Zhang HY, Peng YQ, He BX. et al. Mesenchymal stem cells regulate type 2 innate lymphoid cells via regulatory T cells through ICOS-ICOSL interaction. Stem Cells. 2021;39(7):975-987 doi: 10.1002/stem.3369
133. Jiao H, Walczak BE, Lee MS, Lemieux ME, Li WJ. GATA6 regulates aging of human mesenchymal stem/stromal cells. Stem Cells. 2021;39(1):62-77 doi: 10.1002/stem.3297
134. Lin EC, Davis MP, Lee MS, Ma G, Xu W, Chang YI. et al. Advancing immunomodulatory functions in mesenchymal stem/stromal cells through targeting the GATA6-mediated pathway. Cytotherapy. 2024:S1465-3249(24)00823-5. doi: 10.1016/j. jcyt. 2024 08.001
135. Ruetz TJ, Pogson AN, Kashiwagi CM, Gagnon SD, Morton B, Sun ED. et al. CRISPR-Cas9 screens reveal regulators of ageing in neural stem cells. Nature. 2024;634(8036):1150-1159 doi: 10.1038/s41586-024-07972-2
136. Bijonowski BM, Fu Q, Yuan X, Irianto J, Li Y, Grant SC. et al. Aggregation-induced integrated stress response rejuvenates culture-expanded human mesenchymal stem cells. Biotechnol Bioeng. 2020;117(10):3136-3149 doi: 10.1002/bit.27474
137. He Q, Liao Y, Zhang H, Sun W, Zhou W, Lin J. et al. Gel microspheres enhance the stemness of ADSCs by regulating cell-ECM interaction. Biomaterials. 2024;309:122616 doi: 10.1016/j.biomaterials.2024.122616
138. Krasnova O, Kovaleva A, Saveleva A, Kulakova K, Bystrova O, Martynova M. et al. Mesenchymal stem cells lose the senescent phenotype under 3D cultivation. Stem Cell Res Ther. 2023;14(1):373 doi: 10.1186/s13287-023-03599-8
139. Raik S, Sharma P, Kumar S, Rattan V, Das A, Kumar N. et al. Three-dimensional spheroid culture of dental pulp-derived stromal cells enhance their biological and regenerative properties for potential therapeutic applications. Int J Biochem Cell Biol. 2023;160:106422 doi: 10.1016/j.biocel.2023.106422
140. Shimazawa Y, Kusamori K, Tsujimura M, Shimomura A, Takasaki R, Takayama Y. et al. Intravenous injection of mesenchymal stem cell spheroids improves the pulmonary delivery and prolongs in vivo survival. Biotechnol J. 2022;17(1):e2100137 doi: 10.1002/biot.202100137
141. Algazlan AS, Almuraikhi N, Muthurangan M, Balto H, Alsalleeh F. Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Int J Mol Sci. 2022;24(1):702 doi: 10.3390/ijms24010702
142. Wang Y, Xie F, He Z, Che L, Chen X, Yuan Y. et al. Senescence-Targeted and NAD+-Dependent SIRT1-Activated Nanoplatform to Counteract Stem Cell Senescence for Promoting Aged Bone Regeneration. Small. 2024;20(12):e2304433 doi: 10.1002/smll.202304433
143. Yang Y, Wang J, Lin X, Zhang Z, Zhang M, Tang C. et al. TNF-α-licensed exosome-integrated titaniumaccelerated T2D osseointegration by promoting autophagy-regulated M2 macrophage polarization. Biochem Biophys Res Commun. 2024;727:150316 doi: 10.1016/j.bbrc.2024.150316
144. Massidda MW, Demkov A, Sices A, Lee M, Lee J, Paull TT. et al. Mechanical Rejuvenation of Mesenchymal Stem Cells from Aged Patients. bioRxiv [Preprint]. 2024:2024.06.06.597781. doi: 10.1101/2024.06.06.597781.
Author contact
![]() Corresponding authors: lance1971com (W.L.); wangyuliang123edu.cn or wang_yu_lcom (Y.W.).
Corresponding authors: lance1971com (W.L.); wangyuliang123edu.cn or wang_yu_lcom (Y.W.).

 Global reach, higher impact
Global reach, higher impact