Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(12):3101-3111. doi:10.7150/ijms.115217 This issue Cite
Research Paper
Association between Magnesium Depletion Score and Advanced Cardiovascular-Kidney-Metabolic Syndrome Stages in US Adults
1. Department of Endocrinology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing 100020, China.
2. Department of Urology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing 100020, China.
*These authors contributed equally to this work.
Received 2025-4-7; Accepted 2025-6-9; Published 2025-6-23
Abstract
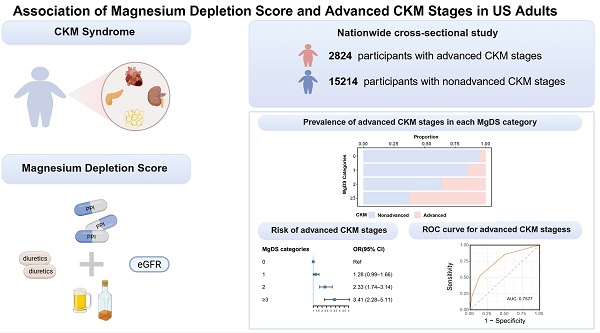
Background: Cardiovascular-kidney-metabolic (CKM) syndrome is highly prevalent. Advanced CKM stages have the potential for multiorgan disease, premature mortality, and excess morbidity. Magnesium deficiency has a prominent impact on the components of CKM syndrome. However, the relationship between magnesium depletion score (MgDS) and the risk of developing advanced stages of CKM syndrome is still unclear.
Methods: We used data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018. To investigate the relationship between MgDS categories and advanced CKM stages, we employed weighted multivariable logistic regression. Stratified and interaction analysis was conducted to find whether some demographics and lifestyle factors modified the association. Restricted cubic spline (RCS) analysis was implemented to investigate the dose-response relationships. Additionally, receiver operating characteristic (ROC) curve was plotted to assess the diagnostic accuracy of MgDS for advanced CKM stages.
Results: The study population comprised 18,038 participants aged 20 to 79 years. The multivariable logistic regression suggested that high MgDS levels (MgDS = 2 and ≥ 3) were associated with higher odds of having advanced CKM stages compared with low MgDS level (MgDS = 0) in all adjusted models (P < 0.05). Stratified and interaction analysis indicated that PIR had significant effects on the association (P for interaction < 0.05). The RCS regression analyses demonstrated a positive linear association between MgDS and the incidence of advanced CKM stages (P for overall = 0.0003, P for nonlinear = 0.1374). The MgDS predicted the area of the ROC curve of 0.7577 (P < 0.05) and the optimal cutoff value was 2.
Conclusions: Magnesium-depleted state as quantified by MgDS (MgDS ≥ 2) is a significant risk factor for advanced CKM stages, which suggests that identifying magnesium deficiency and improving the nutritional status of magnesium might mitigate the risk of advanced CKM stages.
Keywords: cardiovascular-kidney-metabolic syndrome, magnesium, magnesium depletion score, cross-sectional study
Introduction
Cardiovascular-kidney-metabolic (CKM) syndrome was defined as a systemic disorder by the American Heart Association (AHA), which is attributable to the pathophysiological connections among metabolic risk factors, chronic kidney disease (CKD), and cardiovascular disease (CVD) [1]. To delineate the progressive nature of CKM syndrome, it was further categorized into stages: stage 0, absence of CKM risk factors; stage 1, excess and/or dysfunctional adiposity; stage 2, presence of metabolic risk factors, moderate- to high-risk CKD, or both; stage 3, presence of subclinical CVD; and stage 4, presence of clinical CVD [1]. Recent research indicated that there is a high incidence of CKM syndrome in adults, with a higher burden reported among those with insulin resistance, unfavorable social risk profile or social determinants of health [2-4]. Poor CKM health has been demonstrated to have multisystem consequences that extend beyond the simple sum of its components. These consequences may include multiorgan disease, premature mortality, and excess morbidity, which can result in high health care expenditures [1, 5]. There is a critical need to screen for exposures most relevant to CKM health and to establish life-course interventions aimed at averting the progression of CKM syndrome and related outcomes.
Magnesium is essential for the physiological functions of nearly all organs in the human body. Beyond its foundational roles in RNA, DNA, and protein synthesis, magnesium plays a role in various biological processes, including the metabolism of lipids, glucose, bone and calcium (Ca), and the function of the immune system [6, 7]. Magnesium deficiency is commonly seen in cardiovascular, renal, neurological, psychiatric, and metabolic diseases [8-12]. Recent clinical and preclinical studies have suggested potential therapeutic effects of magnesium supplementation in various chronic conditions, including hypertension, stroke, heart failure, diabetes, and CKD [13].
Nonetheless, there is an absence of standardized methods for the precise evaluation of magnesium status. Although serum magnesium is the most commonly used clinical test, it does not always accurately reflect the body's overall magnesium levels, given that less than 1% of magnesium is present in serum [14]. While 24-hour urinary magnesium concentrations can help evaluate magnesium status, they may be unreliable in patients with impaired kidney function and can be affected by diet and certain medications like proton pump inhibitors (PPIs) and diuretics [6]. The magnesium tolerance test (MTT) is a more sensitive assessment [15]. Due to time consuming and labor burden of conducting the MTT, this test is rarely used in clinic. The magnesium depletion score (MgDS) has been demonstrated to be a more accurate and effective predictor of magnesium deficiency status, which takes into account four key factors that influence the absorption and excretion of dietary magnesium, including diuretics and PPI use, deterioration in kidney function, and alcohol consumption [16]. Individuals with a higher MgDS indicate a more severe nutritional state of magnesium shortage.
To our knowledge, there is no study focused on the association between MgDS and advanced CKM stages. It is necessary to identify the contribution of MgDS to advanced stages of CKM syndrome, which will help promote screening for magnesium deficiency and inform future development of strategies to prevent CKM progression. We conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) 1999-2018 to explore the relationship between MgDS and advanced CKM stages.
Materials and Methods
Study population
The data for this cross-sectional study were obtained from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). NHANES is a large-scale nationwide survey with a complex, stratified, multistage probability sampling methodology, which is designed to assess the health and nutritional conditions of the general US population [17]. The research was approved by the Institutional Review Board of the National Center for Health Statistics (NCHS), and written informed consent was obtained from all participants or their guardian prior to data collection.
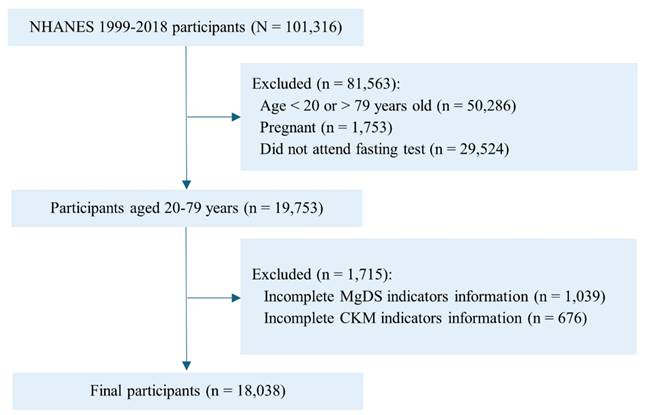
In this study, we selected 101316 participants from 10 cycles of NHANES data (1999-2018). A total of 19753 individuals aged 20 to 79 years were selected after excluding participants who were pregnant and did not attend fasting test in the mobile examination center. We further excluded 1715 participants who did not have sufficient information to determine MgDS (n=1039) and CKM syndrome (n=676). Ultimately, 18038 adults were enrolled in the final analysis (Figure 1).
Magnesium depletion score calculation
The MgDS was calculated by summing points from the following 4 scores: (1) current use of diuretics was scored 1 point; (2) current use of proton pump inhibitor (PPI) was scored 1 point; (3) an estimated glomerular filtration rate (eGFR) more than 90 mL/min/1.73 m2 was scored 0 point, an eGFR between 60 mL/min/1.73 m2 and 90 mL/min/1.73 m2 was scored 1 point, while an eGFR less than 60 mL/min/1.73 m2 was scored 2 points; and (4) heavy drinking (> 1 drink/day for women and > 2 drinks/day for men, according to 2015-2020 Dietary guidelines for Americans) was scored 1 point [16]. The eGFR was calculated using the 2021 race- and ethnicity-free Chronic Kidney Disease Epidemiology Collaboration creatinine equation [18]. According to previous validation studies [19, 20] and the limited sample size in higher MgDS categories (scores 3-5) across CKM stages within our study, MgDS was categorized into 4 groups: MgDS = 0, MgDS = 1, MgDS = 2, and MgDS ≥ 3.
CKM syndrome stages
The definition of CKM syndrome stages is according to the criteria from Li J et al. [3]. Briefly, the stages are delineated as follows:
Stage 0, individuals with normal body mass index (BMI < 25 kg/m2, or < 23 kg/m2 if Asian ancestry), normal waist circumference (waist circumference < 88/102 cm in female/male or if Asian ancestry < 80/90 cm in female/male), without other metabolic disorders (prediabetes, diabetes, hypertension, hypertriglyceridemia [≥ 135 mg/dL], metabolic syndrome), low-risk CKD, predicted 10-year CVD risk < 20%, and no evidence of clinical CVD (history of chronic heart failure, coronary heart disease, heart attack, or stroke).
Stage 1, individuals with overweight/obesity (BMI ≥ 25 kg/m2, or ≥ 23 kg/m2 if Asian ancestry), abdominal obesity (waist circumference ≥ 88/102 cm in female/male or if Asian ancestry ≥ 80/90 cm in female/male), or prediabetes (fasting blood glucose of 100-124 mg/dL or glycosylated hemoglobin A1c of 5.7%-6.4% and without self-reported diagnosis of diabetes, use of insulin, or oral hypoglycemic agents), and meeting all of the following conditions: without other metabolic disorders, low-risk CKD, predicted 10-year CVD risk < 20%, and no evidence of clinical CVD.
Stage 2, individuals with any of the four metabolic disorders (hypertriglyceridemia, hypertension, diabetes, metabolic syndrome), or moderate-to-high-risk CKD, and no evidence of subclinical CVD (very high-risk CKD, predicted 10-year CVD risk ≥ 20%) or clinical CVD.
Stage 3, subclinical CVD with any of the seven metabolic disorders (overweight/obesity, abdominal obesity, prediabetes, diabetes, hypertension, hypertriglyceridemia, metabolic syndrome) or moderate-to-high-risk CKD, and no evidence of clinical CVD.
Stage 4, clinical CVD with any of the seven metabolic disorders (overweight/obesity, abdominal obesity, prediabetes, diabetes, hypertension, hypertriglyceridemia, metabolic syndrome) or CKD.
Participants were also classified into 2 groups: nonadvanced (CKM stages 0, 1, or 2), advanced (CKM stages 3 or 4). Metabolic syndrome (MetS) was diagnosed according to the NCEP-ATP III report [21]. CKD was defined as moderate- to high-risk or high-risk levels of CKD according to Kidney Disease Improving Global Outcomes (KDIGO) 2021 [22]. We calculated the 10-year CVD risk through the AHA PREVENT equations [23].
Flowchart of participant selection from NHANES 1999 to 2018. Abbreviations: NHANES, National Health and Nutrition Examination Survey; CKM, cardiovascular-kidney-metabolic; MgDS, magnesium depletion score.
Recent studies using the NHANES database have applied CKM definitions according to the AHA Presidential Advisory Statement on CKM Syndrome [1]. For example, Zhu R et al. (n=29722) used 10 NHANES cycles with modified criteria from Aggarwal et al., which is similar to our definition but differs in age cut-offs for 10-year CVD risk estimation [4, 24]. Notably, Tu D et al. (n=12245) employed Framingham risk score for 10-year CVD risk prediction [25, 26]. Both Chen Y et al. (n=21609) and Wu S et al. (n=1889) adopted definitions aligned with that used in our study [27, 28]. Collectively, while there are slight variations in the evaluation of CKM stages across these studies, our research maintains methodological consistency with established definitions, providing a robust framework for understanding CKM syndrome.
Covariate assessment
Demographic covariates in this study included age, sex (male, female), race and ethnicity (Mexican American, non-Hispanic Black, non-Hispanic White, and other [other Hispanic or other race, including multiracial]), education level (> High school, High school, < High school), and poverty income ratio (PIR) (< 1.3, 1.3-3.5, > 3.5). Examination data included BMI, waist circumference. Laboratory data included triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), eGFR, HOMA-IR [HOMA-IR = Fasting Glucose (mmol/L) × Fasting Insulin (uU/mL) / 22.5], glycated hemoglobin A1c (HbA1c), serum calcium (Ca). Dietary data included magnesium intake. Lifestyle data included current smoker (yes, no), current drinker (yes, no) and physical activity (active, refers to at least 150 minutes per week of moderate-intensity activity or 75 minutes per week of vigorous-intensity activity; inactive indicates less than these levels).
Statistical analysis
The data were processed according to NHANES analytical guidelines. All analyses used appropriate sample weights and strata because of the complex, multistage, sampling design of NHANES [29]. For continuous variables, statistics were described using the weighted mean ± standard error (SE), and differences between groups were investigated using analysis of variance (ANOVA) or Student's t-test. Categorical variables were described using frequency with weighted percentages, and intergroup differences were assessed using the chi-square test. The relationship between MgDS and advanced CKM Stages was analyzed using weighted multivariable logistic regression. Model 1 was adjusted for age, sex, race, education and PIR. Model 2 was adjusted for the variables in model 1 plus BMI, TG, HDL-c, LDL-c, HOMA-IR, HbA1c, serum calcium (Ca), magnesium intake. Model 3 was adjusted for the variables in model 2 plus current smoker, current drinker, physical activity. The missing data for the participants in this study were shown in Table S1. Two sensitivity analyses were performed to address missing data. First, multiple imputation was conducted on the data using the “jomo” package [30]. In addition, we excluded individuals with any missing values to verify the robustness of the results. The test for multicollinearity (Table S2) showed that the variance inflation factor (VIF) for each covariate was less than 5, indicating the absence of substantial multicollinearity among the covariates [31].
Given that demographics and lifestyle factors were different in MgDS and advanced CKM Stages, stratified analyses were also performed. Restricted cubic spline (RCS) analysis was conducted to investigate the potential nonlinear relationship between MgDS and advanced CKM Stages. To determine the optimal number of knots, we compared models with 3-6 knots using both Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). Given the large-scale nature of our dataset, we prioritize the lowest BIC value for knots selection to reduce the risk of overfitting and improve the interpretability of the model. The results of AIC and BIC calculations and the final selected number of knots are detailed in Table S7. Receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to evaluate the predictive efficacy of MgDS on patients with advanced CKM Stages.
All statistical analyses were performed using R software (version 4.4.2). A 2-sided P < 0.05 was considered significant.
Results
Participant characteristics
A total of 18038 participants were included in the study. Baseline characteristics, as presented in Table 1 according to CKM stages, revealed that participants with advanced CKM stages were more likely to be older, male, of non-Hispanic black and white ethnicity, and with lower education and family income levels. Compared with individuals with nonadvanced CKM stages, participants with advanced CKM stages exhibited higher BMI, higher waist circumference, higher TGs, lower HDL-c, lower eGFR, higher HOMA-IR, higher HbA1c, higher serum Ca levels, and consume less magnesium. However, a notable observation was the significantly lower levels of TC and LDL-C in participants with advanced CKM stages. This finding might be attributable to the use of lipid-lowering medications. The advanced CKM group had more current smokers and non-current drinkers, more diuretic and PPI users, fewer heavy drinkers, and higher eGFR scores. Specifically, the advanced CKM group showed unfavorable MgDS levels. In contrast to the nonadvanced CKM group, participants with MgDS categories of 0 or 1 were less prevalent in the advanced CKM group, whereas those with MgDS categories of 2 or ≥ 3 were more prevalent in the advanced CKM group (all P < 0.05). The baseline characteristics of individuals after multiple imputation and excluding those with any missing values are also detailed in Table S3 and Table S5.
Baseline characteristics of participants according to CKM syndrome stages.
| Variables | Total (n=18038) | CKM Stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage 0(n=1766) | Stage 1(n=3638) | Stage 2(n=9810) | Stage 3(n=1211) | Stage 4(n=1613) | P value | Nonadvanced (n=15214) | Advanced(n=2824) | P value | ||
| Age, y | 45.86 ± 0.21 | 34.72 ± 0.35 | 39.56 ± 0.36 | 46.99 ± 0.22 | 70.91 ± 0.29 | 61.36 ± 0.39 | < 0.0001 | 43.44 ± 0.21 | 64.78 ± 0.29 | < 0.0001 |
| Sex | < 0.0001 | < 0.0001 | ||||||||
| Female | 9115 (50.90) | 1088 (63.70) | 1992 (53.31) | 4871 (48.66) | 491 (44.60) | 673 (42.08) | 7951 (51.91) | 1164 (42.98) | ||
| Male | 8923 (49.10) | 678 (36.30) | 1646 (46.69) | 4939 (51.34) | 720 (55.40) | 940 (57.92) | 7263 (48.09) | 1660 (57.02) | ||
| Race | < 0.0001 | < 0.0001 | ||||||||
| Mexican American | 3333 (8.22) | 247 (5.98) | 763 (10.73) | 1922 (8.46) | 205 (5.45) | 196 (4.29) | 2932 (8.67) | 401 (4.70) | ||
| Non-Hispanic Black | 3645 (11.05) | 267 (7.92) | 710 (11.51) | 1979 (11.07) | 301 (14.60) | 388 (12.83) | 2956 (10.73) | 689 (13.46) | ||
| Non-Hispanic White | 7838 (68.56) | 930 (74.93) | 1438 (64.32) | 4119 (68.25) | 542 (69.20) | 809 (72.30) | 6487 (68.23) | 1351 (71.19) | ||
| Other | 3222 (12.17) | 322 (11.17) | 727 (13.43) | 1790 (12.22) | 163 (10.75) | 220 (10.58) | 2839 (12.37) | 383 (10.64) | ||
| Education | < 0.0001 | < 0.0001 | ||||||||
| > High school | 9301 (59.41) | 1131 (69.22) | 2109 (64.33) | 4939 (57.97) | 459 (45.88) | 663 (46.50) | 8179 (61.11) | 1122 (46.28) | ||
| High school | 4120 (24.06) | 354 (19.80) | 748 (21.78) | 2327 (25.17) | 302 (28.04) | 389 (27.77) | 3429 (23.58) | 691 (27.87) | ||
| < High school | 4607 (16.49) | 281 (10.98) | 780 (13.88) | 2538 (16.86) | 450 (26.08) | 558 (25.72) | 3599 (15.30) | 1008 (25.85) | ||
| PIR | < 0.0001 | < 0.0001 | ||||||||
| <1.3 | 4904 (19.03) | 417 (17.96) | 933 (19.11) | 2639 (20.00) | 360 (24.55) | 555 (27.94) | 3989 (19.50) | 915 (26.73) | ||
| 1.3-3.5 | 6331 (34.11) | 587 (33.40) | 1279 (36.50) | 3386 (35.86) | 503 (45.93) | 576 (40.05) | 5252 (35.67) | 1079 (42.15) | ||
| >3.5 | 5360 (40.58) | 623 (48.64) | 1162 (44.38) | 2994 (44.14) | 236 (29.52) | 345 (32.01) | 4779 (44.83) | 581 (31.12) | ||
| BMI, kg/m2 | 28.80 ± 0.09 | 21.88 ± 0.06 | 28.28 ± 0.11 | 30.26 ± 0.12 | 29.94 ± 0.24 | 30.78 ± 0.25 | < 0.0001 | 28.59 ± 0.09 | 30.48 ± 0.19 | < 0.0001 |
| Waist circumference, cm | 98.31 ± 0.22 | 79.23 ± 0.20 | 95.76 ± 0.25 | 102.17 ± 0.28 | 105.75 ± 0.54 | 106.48 ± 0.62 | < 0.0001 | 97.34 ± 0.23 | 106.22 ± 0.45 | < 0.0001 |
| TGs, mmol/L | 1.36 ± 0.01 | 0.80 ± 0.01 | 0.92 ± 0.01 | 1.60 ± 0.01 | 1.64 ± 0.03 | 1.60 ± 0.03 | < 0.0001 | 1.32 ± 0.01 | 1.62 ± 0.02 | < 0.0001 |
| TC, mmol/L | 5.02 ± 0.01 | 4.64 ± 0.03 | 4.87 ± 0.02 | 5.21 ± 0.02 | 4.87 ± 0.04 | 4.75 ± 0.04 | < 0.0001 | 5.05 ± 0.01 | 4.79 ± 0.03 | < 0.0001 |
| HDL-c, mmol/L | 1.39 ± 0.01 | 1.60 ± 0.01 | 1.46 ± 0.01 | 1.33 ± 0.01 | 1.30 ± 0.01 | 1.29 ± 0.02 | < 0.0001 | 1.40 ± 0.01 | 1.30 ± 0.01 | < 0.0001 |
| LDL-c, mmol/L | 3.00 ± 0.01 | 2.66 ± 0.03 | 2.98 ± 0.02 | 3.14 ± 0.01 | 2.82 ± 0.03 | 2.73 ± 0.03 | < 0.0001 | 3.04 ± 0.01 | 2.76 ± 0.03 | < 0.0001 |
| eGFR, mL/min/1.73 m2 | 98.55 ± 0.28 | 107.57 ± 0.54 | 104.07 ± 0.45 | 98.64 ± 0.32 | 70.57 ± 0.79 | 81.41 ± 0.74 | < 0.0001 | 101.23 ± 0.28 | 77.54 ± 0.59 | < 0.0001 |
| HOMA-IR | 3.36 ± 0.04 | 1.31 ± 0.02 | 2.28 ± 0.04 | 3.87 ± 0.06 | 5.53 ± 0.28 | 5.09 ± 0.23 | < 0.0001 | 3.12 ± 0.05 | 5.24 ± 0.16 | < 0.0001 |
| HbA1c, % | 5.55 ± 0.01 | 5.12 ± 0.01 | 5.29 ± 0.01 | 5.62 ± 0.01 | 6.33 ± 0.05 | 6.08 ± 0.04 | < 0.0001 | 5.47 ± 0.01 | 6.17 ± 0.03 | < 0.0001 |
| Ca, mg/dL | 9.38 ± 0.01 | 9.41 ± 0.01 | 9.33 ± 0.01 | 9.38 ± 0.01 | 9.43 ± 0.02 | 9.38 ± 0.01 | < 0.0001 | 9.37 ± 0.01 | 9.40 ± 0.01 | 0.03 |
| Magnesium intake, mg/d | 299.88 ± 1.95 | 311.26 ± 4.71 | 309.63 ± 3.91 | 299.34 ± 2.21 | 263.81 ± 4.64 | 275.43 ± 5.81 | < 0.0001 | 303.54 ± 2.00 | 271.28 ± 4.23 | < 0.0001 |
| Current smoker | < 0.001 | 0.02 | ||||||||
| Yes | 3876 (21.74) | 405 (23.68) | 725 (19.59) | 2102 (21.66) | 240 (19.86) | 404 (26.49) | 3232 (21.44) | 644 (24.12) | ||
| No | 14162 (78.26) | 1361 (76.32) | 2913 (80.41) | 7708 (78.34) | 971 (80.14) | 1209 (73.51) | 11982 (78.56) | 2180 (75.88) | ||
| Current drinker | < 0.0001 | < 0.0001 | ||||||||
| Yes | 11900 (71.68) | 1321 (82.50) | 2620 (79.66) | 6513 (75.64) | 617 (57.47) | 829 (60.95) | 10454 (77.60) | 1446 (59.69) | ||
| No | 5027 (23.13) | 349 (17.50) | 786 (20.34) | 2718 (24.36) | 519 (42.53) | 655 (39.05) | 3853 (22.40) | 1174 (40.31) | ||
| Physical activity | < 0.0001 | 0.24 | ||||||||
| Active | 9154 (53.39) | 999 (68.55) | 2116 (72.94) | 4904 (67.19) | 473 (63.31) | 662 (69.22) | 8019 (68.84) | 1135 (67.14) | ||
| Inactive | 4052 (24.35) | 444 (31.45) | 733 (27.06) | 2291 (32.81) | 268 (36.69) | 316 (30.78) | 3468 (31.16) | 584 (32.86) | ||
| Diuretics use | < 0.0001 | < 0.0001 | ||||||||
| No | 15685 (88.79) | 1760 (99.62) | 3618 (99.34) | 8503 (86.98) | 803 (64.99) | 1001 (65.73) | 13881 (91.78) | 1804 (65.46) | ||
| Yes | 2351 (11.19) | 6 (0.38) | 20 (0.66) | 1307 (13.02) | 408 (35.01) | 610 (34.27) | 1333 (8.22) | 1018 (34.54) | ||
| PPI use | < 0.0001 | < 0.0001 | ||||||||
| No | 16542 (91.85) | 1724 (97.71) | 3504 (95.91) | 9002 (90.98) | 1017 (83.53) | 1295 (80.84) | 14230 (93.14) | 2312 (81.81) | ||
| Yes | 1494 (8.15) | 42 (2.29) | 134 (4.09) | 808 (9.02) | 194 (16.47) | 316 (19.16) | 984 (6.86) | 510 (18.19) | ||
| Heavy drinking | < 0.0001 | < 0.0001 | ||||||||
| No | 15502 (83.15) | 1457 (79.28) | 3087 (82.40) | 8340 (82.69) | 1122 (90.90) | 1496 (91.19) | 12884 (82.14) | 2618 (91.08) | ||
| Yes | 2536 (16.85) | 309 (20.72) | 551 (17.60) | 1470 (17.31) | 89 (9.10) | 117 (8.81) | 2330 (17.86) | 206 (8.92) | ||
| eGFR scores | < 0.0001 | < 0.0001 | ||||||||
| 0 | 11375 (63.11) | 1461 (79.31) | 2859 (73.81) | 6395 (63.17) | 196 (12.50) | 464 (31.19) | 10715 (68.05) | 660 (24.51) | ||
| 1 | 5638 (32.50) | 301 (20.56) | 771 (25.98) | 3157 (34.14) | 623 (52.55) | 786 (48.94) | 4229 (30.23) | 1409 (50.24) | ||
| 2 | 1023 (4.39) | 4 (0.13) | 8 (0.21) | 258 (2.69) | 392 (34.95) | 361 (19.87) | 270 (1.72) | 753 (25.25) | ||
| MgDS | 0.77 ± 0.01 | 0.44 ± 0.02 | 0.49 ± 0.02 | 0.79 ± 0.01 | 1.83 ± 0.04 | 1.51 ± 0.04 | < 0.0001 | 0.67 ± 0.01 | 1.62 ± 0.03 | < 0.0001 |
| MgDS categories | < 0.0001 | < 0.0001 | ||||||||
| 0 | 8545 (45.98) | 1172 (61.29) | 2351 (58.19) | 4631 (44.19) | 122 (6.98) | 269 (18.43) | 8154 (50.02) | 391 (14.34) | ||
| 1 | 6044 (35.92) | 525 (33.37) | 1092 (35.20) | 3493 (37.30) | 402 (31.80) | 532 (34.31) | 5110 (36.23) | 934 (33.42) | ||
| 2 | 2490 (13.53) | 66 (5.17) | 185 (6.27) | 1337 (14.56) | 425 (37.90) | 477 (28.30) | 1588 (11.21) | 902 (31.72) | ||
| ≥3 | 959 (4.57) | 3 (0.17) | 10 (0.34) | 349 (3.95) | 262 (23.32) | 335 (18.96) | 362 (2.54) | 597 (20.52) | ||
Abbreviations: PIR, poverty/income ratio; BMI, body mass index; TGs, triglycerides; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; HOMA-IR, homeostatic model assessment for insulin resistance; HbA1c, glycated hemoglobin A1c; Ca, serum calcium; PPI, proton pump inhibitor; MgDS, magnesium depletion score. Data are presented as mean ± SE or n (weighted %). Variable categories may not sum to 100% due to missing data.
Proportional Rose plot and Bar chart of CKM conditions by MgDS categories. (A) Rose plot showing the prevalence of CKM stages (0-4) in each MgDS category. (B) Bar chart comparing the prevalence of CKM (nonadvanced and advanced) in each MgDS category.
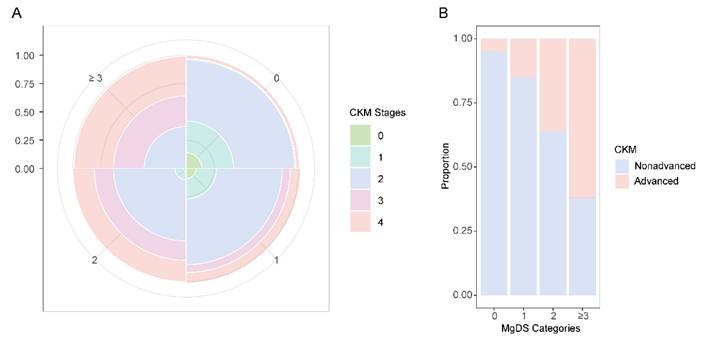
Prevalence of CKM conditions in each MgDS category
In overall participants, the prevalence of CKM stage 2 accounted for the largest proportion in MgDS levels 0, 1, and 2. Advanced CKM stages (stage 3 or 4) were the most prevalent CKM condition in high MgDS level (≥ 3). The prevalence of advanced CKM stages showed an increasing trend with rising MgDS levels (Figure 2).
Multivariable logistic regression analysis
The survey-weighted multivariable regression analyses showed a positive association between MgDS categories and the odds of advanced CKM stages. Compared with low MgDS level (MgDS = 0), the odds ratios (OR) for advanced CKM stages among participants with high MgDS levels (MgDS = 2 and ≥ 3) were 2.31 (95%CI 1.82-2.94), 4.45 (95%CI 3.32-5.98) in the minimally adjusted Model 1 (all P < 0.05). This association remained significant for the MgDS = 2 and MgDS ≥ 3 groups in the moderately adjusted Model 2 (OR 2.18, 95%CI 1.70-2.80; OR 3.68, 95%CI 2.68-5.06) and the fully adjusted Model 3 (OR 2.33, 95%CI 1.74-3.14; OR 3.41, 95%CI 2.28-5.11) (all P < 0.05) (Table 2). As demonstrated in Table 2, the odds of advanced CKM stages showed an increasing trend with elevated MgDS levels (P for trend < 0.05).
Stratified and interaction analysis
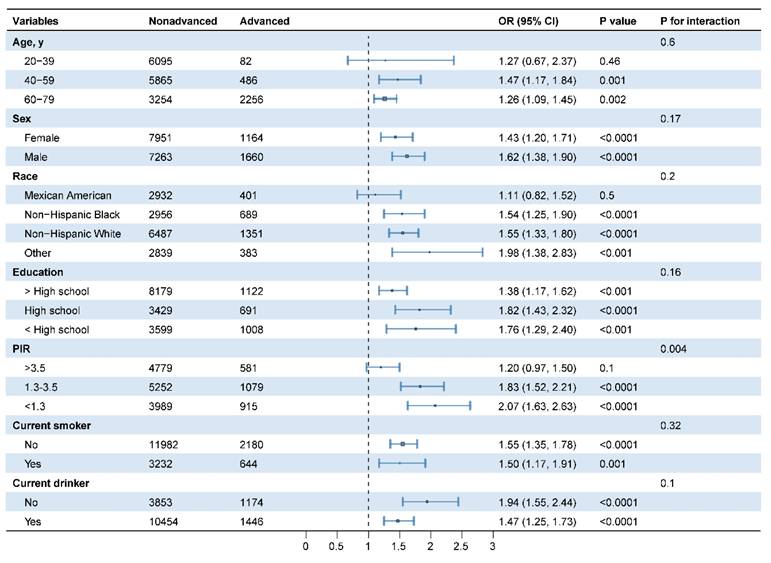
Stratified and interaction analyses were conducted to assess the potential modification of demographic and lifestyle variables (age, sex, race, education, PIR, smoking, and drinking status) on the relationship between MgDS and the incidence of advanced CKM stages (Figure 3). After adjusting for potential confounders, PIR had significant effects on the association (P for interaction < 0.05). Among the three PIR groups, participants in lower PIR groups with elevated MgDS demonstrated significantly greater odds of advanced CKM stages. In the 1.3 ≤ PIR ≤ 3.5 group, the OR was 1.83 (95%CI 1.52-2.21, P < 0.05). The effect was even stronger in the lowest PIR group (PIR < 1.3), where the odds ratio was 2.07 (95%CI 1.63-2.63, P < 0.05). Conversely, for participants in the highest PIR group (PIR > 3.5), the association between MgDS and advanced CKM stages was not statistically significant (OR 1.20, 95%CI 0.97-1.50, P = 0.1). Other variables demonstrated no significant interactions (all P for interaction > 0.05) (Figure 3).
Sensitivity analysis
We conducted two sensitivity analyses to validate the robustness of our results. First, multiple imputation techniques were utilized to address missing values, and multivariable logistic regression analysis was conducted to explore the relationship between MgDS and advanced CKM stages. In addition, we conducted an analysis excluding individuals with any missing values. The results consistent with the primary findings (Table S4 and Table S6).
Subgroup analysis for the association between MgDS and advanced CKM stages. Adjusted for BMI, TGs, HDL-c, LDL-c, HOMA-IR, HbA1c, Ca, magnesium intake, physical activity.
Multivariable logistic regression analysis of the association between MgDS categories and advanced CKM stages.
| Variable | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| MgDS categories | ||||||||
| 0 | Ref | Ref | Ref | |||||
| 1 | 1.20(0.97,1.49) | 0.09 | 1.26(1.00,1.57) | 0.05 | 1.28(0.99,1.66) | 0.06 | ||
| 2 | 2.31(1.82,2.94) | <0.0001 | 2.18(1.70,2.80) | <0.0001 | 2.33(1.74,3.14) | <0.0001 | ||
| ≥3 | 4.45(3.32,5.98) | <0.0001 | 3.68(2.68,5.06) | <0.0001 | 3.41(2.28,5.11) | <0.0001 | ||
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||||
Model 1: adjusted for age, sex, race, education, PIR.
Model 2: adjusted for age, sex, race, education, PIR, BMI, TG, HDL-c, LDL-c, HOMA-IR, HbA1c, Ca, magnesium intake.
Model 3: adjusted for age, sex, race, education, PIR, BMI, TG, HDL-c, LDL-c, HOMA-IR, HbA1c, Ca, magnesium intake, current smoker, current drinker, physical activity.
Abbreviations: OR, odds ratio; Ref, reference; PIR, poverty/income ratio; BMI, body mass index; TGs, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; HbA1c, glycated hemoglobin A1c; Ca, serum calcium; PPI, proton pump inhibitor; MgDS, magnesium depletion score.
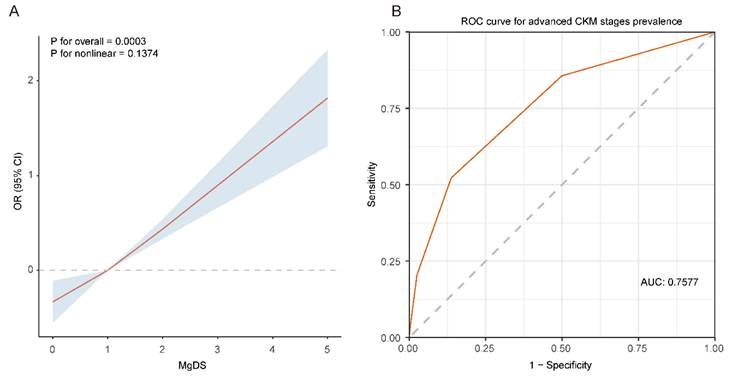
The RCS analysis and ROC curve between MgDS and risk of advanced CKM stages. (A) The RCS analysis between MgDS and risk of advanced CKM stages. The model was adjusted for age, sex, race, education, PIR, BMI, TGs, HDL-c, LDL-c, HOMA-IR, HbA1c, Ca, magnesium intake, current smoker, current drinker, physical activity. (B) The ROC curve of MgDS for predicting the risk of advanced CKM stages.
RCS analysis
To further explore the relationship between MgDS and advanced CKM stages, we conducted RCS analysis with 3 knots. The results indicated that elevated MgDS showed a positive linear relationship with the OR of advanced CKM stages after full adjustment for confounders (P for overall = 0.0003, P for nonlinear = 0.1374) (Figure 4A).
ROC analysis
ROC analysis was utilized to assess the predictive capability of MgDS on patients with advanced CKM stages (Figure 4B). The area under the ROC curve, as predicted by MgDS, was 0.7577 (95% CI 0.7515-0.7640, P < 0.05). The result revealed that MgDS had statistically significant diagnostic capability in the prediction of advanced CKM stages (AUC > 0.5). The optimal cutoff value for MgDS was 2, suggesting the potential predictive value for the detection of advanced CKM stages when the MgDS is greater than 2. The corresponding sensitivity and specificity were 52.2% and 86.3%, respectively.
Discussion
Using a nationally representative sample of US adults, our study indicated that elevated MgDS levels (MgDS ≥ 2) were associated with an increased probability of advanced CKM stages (CKM stages 3 or 4). After fully adjusting for multiple variables, the positive correlation between MgDS and advanced CKM stages remained significant. Furthermore, we observed a linear relationship between MgDS and the odds of advanced CKM stages. In addition, ROC curve revealed that MgDS had diagnostic capability in distinguishing patients with advanced CKM stages from those with nonadvanced CKM stages when the MgDS is greater than 2. These findings highlight that magnesium depletion may be a key driver of advanced CKM morbidity and revealed the predictive role of MgDS in identifying high-risk individuals for CKM syndrome.
A growing body of research has documented that hypomagnesemia is prevalent among the general population, with evidence suggesting that individuals with lower magnesium levels are at a heightened risk for major components of CKM syndrome, including obesity, diabetes, hypertension, MetS, CVD, and CKD [8, 32-37]. MgDS was recently proposed as a simpler and more precise alternative indicator for predicting magnesium deficiency. Research has indicated that an adverse MgDS acts as an independent risk component for MetS and diabetes [38, 39]. A comprehensive cross-sectional and longitudinal study revealed that individuals with elevated MgDS levels exhibited an increased likelihood of CVD, accompanied by heightened mortality risk among patients with CVD [20]. Another study from NHANES indicated that higher MgDS levels independently predict an elevated risk of CVD and longitudinal mortality among US adults with diabetic disease kidney (DKD) [40]. Moreover, one study suggested that higher MgDS is strongly associated with an elevated risk of all-cause and cardiovascular mortality in participants with hypertension [41]. Given these findings, investigating the association between MgDS and CKM syndrome is urgently needed. In this study, we revealed a positive association between MgDS and advanced CKM stages, which aligned with previous literature exploring the impact of MgDS on various components of CKM syndrome, including metabolic disorders and cardiovascular outcomes. Hence, our findings emphasize the importance of screening for magnesium status and imply that interventions aimed at improving the nutritional status of magnesium need to be carried out among magnesium-depleted populations (MgDS ≥ 2) in order to mitigate the risk of advanced CKM stages.
Subgroup analysis revealed that PIR had a notable impact on the relationship between MgDS and the occurrence of advanced CKM stages. Individuals in the lower PIR group with elevated MgDS showed more pronounced risks of advanced CKM stages. Research revealed that adults with low-income levels tend to have low intakes of magnesium-rich foods, which can contribute to magnesium-depleted nutritional status [42]. Our finding imply that we ought to prioritize the issue of magnesium deficiency and its associated risk of advanced CKM stages in low-income populations.
The exact mechanism between magnesium deficiency and advanced CKM stages is not yet clear, there are several potential explanations. First, magnesium plays an important role in heart function through its substantial vasodilatory effect [43, 44]. Magnesium deficiency promotes oxidative stress notably in endothelial cells, resulting in increased reactive oxygen species (ROS) and cytotoxicity [45]. Low magnesium-induced oxidative stress induces the endothelium to develop a state of permanent inflammation, which is marked by increased NFκB activitiy [46]. Under the condition of local inflammation, the vessel wall will recruit monocytes and trigger the proliferation and migration of vascular smooth muscle cells, resulting in atherosclerosis, thrombosis and CVD. Second, low serum magnesium levels induced chronic inflammation is associated with vascular calcification, which may result in CKD and CVD [6]. Furthermore, insulin receptors (IR) are part of the family of tyrosine kinase receptors, and the kinase function is dependent on the binding of magnesium [47]. In low magnesium conditions, diminished IR signal transduction may contribute to the development of diabetes mellitus by increasing insulin resistance. Consequently, magnesium deficiency may contribute to the advancement of CKM syndrome.
Utilizing a large sample population from NHANES, this study made a significant contribution by investigating the association between MgDS and advanced CKM stages for the first time. Our findings underscored significant disparities in the prevalence of advanced CKM stages across MgDS levels. These findings highlight the necessity for targeted screening and prevention strategies, including magnesium supplementation, among individuals at high risk of magnesium deficiency (i.e., with MgDS ≥ 2), to avert the development of advanced CKM stages. Of note, low family income populations deserve special attention and should be prioritized for intervention.
This study had several limitations. First, due to the cross-sectional nature of the design, it was not possible to establish a causal relationship between MgDS and advanced CKM stages. Second, CVD-related data was self-reported by participants in NHANES, which may slightly deviate from the actual incidence rates. Third, due to data limitations, subclinical heart failure and peripheral arterial disease were not assessed, which potentially resulted in an underestimation of advanced CKM stages. Finally, despite the overall large sample size, groups (eg, Black individuals, PPI user, participants with higher MgDS) still had limited numbers, sparse data bias is inevitable.
Conclusion
In conclusion, our study revealed that a higher MgDS level (MgDS ≥ 2) was associated with increased odds of advanced CKM stages, with notable PIR differences. Our findings underscore the burden of advanced CKM stages among adults with magnesium deficiency and suggest that addressing magnesium-depleted nutritional status might be crucial for the prevention and treatment of advanced CKM stages.
Supplementary Material
Supplementary tables.
Acknowledgements
The author thanks all the members of the NHANES study for their valuable contributions.
Funding
This work was supported by grants from the Beijing Natural Science Foundation (No. L248018) and the Chinese National Natural Science Foundation (No. 82072527) and to Jia Liu.
Author contributions
JT drafted the manuscript. JT and XD performed the statistical analysis. XR collected data. GW, WW and JL reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Data availability
Publicly available data sets were analyzed in this study. These data can be found at https://www.cdc.gov/nchs/nhanes/index.htm.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS. et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation. 2023;148:1606-35
2. Li W, Shen C, Kong W, Zhou X, Fan H, Zhang Y. et al. Association between the triglyceride glucose-body mass index and future cardiovascular disease risk in a population with Cardiovascular-Kidney-Metabolic syndrome stage 0-3: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23:292
3. Li J, Lei L, Wang W, Ding W, Yu Y, Pu B. et al. Social Risk Profile and Cardiovascular-Kidney-Metabolic Syndrome in US Adults. J Am Heart Assoc. 2024;13:e034996
4. Zhu R, Wang R, He J, Wang L, Chen H, Niu X. et al. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages by Social Determinants of Health. JAMA Netw Open. 2024;7:e2445309
5. Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS. et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation. 2023;148:1636-64
6. de Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1-46
7. Gröber U, Schmidt J, Kisters K. Magnesium in Prevention and Therapy. Nutrients. 2015;7:8199-226
8. Pitliya A, Vasudevan SS, Batra V, Patel MB, Desai A, Nethagani S. et al. Global prevalence of hypomagnesemia in type 2 diabetes mellitus - a comprehensive systematic review and meta-analysis of observational studies. Endocrine. 2024;84:842-51
9. Oka T, Hamano T, Sakaguchi Y, Yamaguchi S, Kubota K, Senda M. et al. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: a common electrolyte abnormality in chronic kidney disease. Nephrol Dial Transplant. 2019;34:1154-62
10. Wannamethee SG, Papacosta O, Lennon L, Whincup PH. Serum magnesium and risk of incident heart failure in older men: The British Regional Heart Study. Eur J Epidemiol. 2018;33:873-82
11. Kishi S, Nakashima T, Goto T, Nagasu H, Brooks CR, Okada H. et al. Association of serum magnesium levels with renal prognosis in patients with chronic kidney disease. Clin Exp Nephrol. 2024;28:784-92
12. Sitzia C, Sterlicchio M, Crapanzano C, Dozio E, Vianello E, Corsi Romanelli MM. Intra-erythrocytes magnesium deficiency could reflect cognitive impairment status due to vascular disease: a pilot study. J Transl Med. 2020;18:458
13. Gao Q, Cil O. Magnesium for disease treatment and prevention: emerging mechanisms and opportunities. Trends Pharmacol Sci. 2024;45:708-22
14. Ray E, Mohan K, Ahmad S, Wolf MTF. Physiology of a Forgotten Electrolyte-Magnesium Disorders. Adv Kidney Dis Health. 2023;30:148-63
15. Ryzen E, Elbaum N, Singer FR, Rude RK. Parenteral magnesium tolerance testing in the evaluation of magnesium deficiency. Magnesium. 1985;4:137-47
16. Fan L, Zhu X, Rosanoff A, Costello RB, Yu C, Ness R. et al. Magnesium Depletion Score (MDS) Predicts Risk of Systemic Inflammation and Cardiovascular Mortality among US Adults. J Nutr. 2021;151:2226-35
17. Mohadjer L, Curtin LR. Balancing sample design goals for the National Health and Nutrition Examination Survey. Surv Methodol. 2008;34:119-26
18. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y. et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737-49
19. Wang J, Xiao Y, Yang Y, Yin S, Cui J, Huang K. et al. Association between magnesium depletion score and the prevalence of kidney stones in the low primary income ratio: a cross-sectional study of NHANES 2007-2018. Int J Surg. 2024;110:7636-46
20. Ye L, Zhang C, Duan Q, Shao Y, Zhou J. Association of Magnesium Depletion Score With Cardiovascular Disease and Its Association With Longitudinal Mortality in Patients With Cardiovascular Disease. J Am Heart Assoc. 2023;12:e030077
21. Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433-8
22. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021; 100: S1-S276.
23. Khan SS, Matsushita K, Sang Y, Ballew SH, Grams ME, Surapaneni A. et al. Development and Validation of the American Heart Association's PREVENT Equations. Circulation. 2024;149:430-49
24. Aggarwal R, Ostrominski JW, Vaduganathan M. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages in US Adults, 2011-2020. JAMA. 2024;331:1858-60
25. Tu D, Sun J, Wang P, Xu Q, Ma C. Overall Sleep Quality Is Associated With Advanced Stages in Patients With Cardiovascular-Kidney-Metabolic Syndrome. J Am Heart Assoc. 2025;14:e038674
26. D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM. et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743-53
27. Chen Y, Wu S, Liu H, Zhong Z, Bucci T, Wang Y. et al. Role of oxidative balance score in staging and mortality risk of cardiovascular-kidney-metabolic syndrome: Insights from traditional and machine learning approaches. Redox Biol. 2025;81:103588
28. Wu S, Zhu J, Lyu S, Wang J, Shao X, Zhang H. et al. Impact of DNA-Methylation Age Acceleration on Long-Term Mortality Among US Adults With Cardiovascular-Kidney-Metabolic Syndrome. J Am Heart Assoc. 2025;14:e039751
29. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM. et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. 2013
30. Quartagno M, Carpenter JR, Goldstein H. Multiple Imputation with Survey Weights: A Multilevel Approach. Journal of Survey Statistics and Methodology. 2020;8:965-89
31. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72:558-69
32. Piuri G, Zocchi M, Della Porta M, Ficara V, Manoni M, Zuccotti GV. et al. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients. 2021;13:320
33. Wang T, Wang L, Ma N, Gu S, Jiang D, Li J. et al. Whole-blood magnesium and blood lipids are individually and jointly associated with an elevated likelihood of youngsters being overweight or obese: A matched case-control study using the propensity score. Nutrition. 2022;93:111425
34. Xu M-R, Wang A-P, Wang Y-J, Lu J-X, Shen L, Li L-X. Serum Magnesium Levels Are Negatively Associated with Obesity and Abdominal Obesity in Type 2 Diabetes Mellitus: A Real-World Study. Diabetes Metab J. 2024;48:1147-59
35. Yang J, Cao Y, Zhang H, Hu Y, Lu J, Wang R. et al. Association and dose-response relationship of plasma magnesium with metabolic syndrome in Chinese adults older than 45 years. Front Nutr. 2024;11:1346825
36. Segev A, Shechter M, Tsur AM, Belkin D, Cohen H, Sharon A. et al. Serum Magnesium Is Associated with Long-Term Survival of Non-ST-Elevation Myocardial Infarction Patients. Nutrients. 2023;15:4299
37. Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH. et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015;87:820-7
38. Wang X, Zeng Z, Wang X, Zhao P, Xiong L, Liao T. et al. Magnesium Depletion Score and Metabolic Syndrome in US Adults: Analysis of NHANES 2003 to 2018. J Clin Endocrinol Metab. 2024;109:e2324-e33
39. Tian Z, Qu S, Chen Y, Fang J, Song X, He K. et al. Associations of the magnesium depletion score and magnesium intake with diabetes among US adults: an analysis of the National Health and Nutrition Examination Survey 2011-2018. Epidemiol Health. 2024;46:e2024020
40. Zhou Z, Yao X. The kidney reabsorption-related magnesium depletion score is associated with cardiovascular disease and longitudinal mortality in diabetic kidney disease patients. Diabetol Metab Syndr. 2025;17:38
41. Song J, Zhang Y, Lin Z, Tang J, Yang X, Liu F. Higher Magnesium Depletion Score Increases the Risk of All-cause and Cardiovascular Mortality in Hypertension Participants. Biol Trace Elem Res. 2025;203:1287-96
42. Leung CW, Ding EL, Catalano PJ, Villamor E, Rimm EB, Willett WC. Dietary intake and dietary quality of low-income adults in the Supplemental Nutrition Assistance Program. Am J Clin Nutr. 2012;96:977-88
43. Altura BT, Altura BM. Endothelium-dependent relaxation in coronary arteries requires magnesium ions. Br J Pharmacol. 1987;91:449-51
44. Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart. 2001;86:212-6
45. Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett. 1992;311:187-91
46. Ferrè S, Baldoli E, Leidi M, Maier JAM. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFkB. Biochim Biophys Acta. 2010;1802:952-8
47. Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572-81
Author contact
![]() Corresponding authors: Wei Wang, Email: urodoctorwangcom, Department of Urology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing 100020, China. Jia Liu, Email: liujiacyedu.cn, Department of Endocrinology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing 100020, China.
Corresponding authors: Wei Wang, Email: urodoctorwangcom, Department of Urology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing 100020, China. Jia Liu, Email: liujiacyedu.cn, Department of Endocrinology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing 100020, China.

 Global reach, higher impact
Global reach, higher impact