3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(11):2782-2791. doi:10.7150/ijms.111558 This issue Cite
Research Paper
Development Of the VAMPCT Score for Predicting Mortality in CKD Patients with COVID-19
1. Department of Nephrology, First Medical Center of Chinese PLA General Hospital, State Key Laboratory of Kidney Diseases, National Clinical Research Center for Kidney Diseases, Beijing Key Laboratory of Medical Devices and Integrated Traditional Chinese and Western Drug Development for Severe Kidney Diseases, Beijing Key Laboratory of Digital Intelligent TCM for the Prevention and Treatment of Pan-vascular Diseases, Key Disciplines of National Administration of Traditional Chinese Medicine (zyyzdxk-2023310), Beijing 100853, China.
2. Department of Endocrine, Hebei General Hospital, Hebei 050051, China.
3. Department of Urology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
# Both authors contribute equally to this work and should be considered co-first authors.
Received 2025-2-5; Accepted 2025-4-28; Published 2025-5-31
Abstract
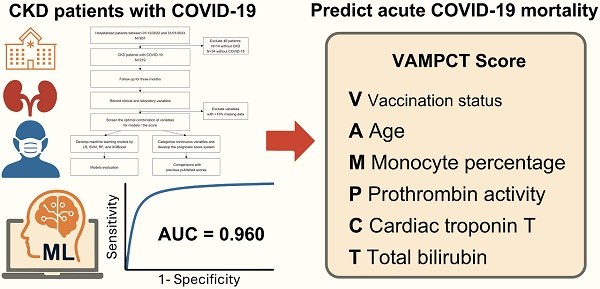
Background: Chronic kidney disease (CKD) patients with coronavirus disease 2019 (COVID-19) are at significant risk of death. However, clinical identification of high-risk individuals remains suboptimal despite the recognition of many pathophysiological and comorbidity-related risk factors. We aim to develop a clinically simple machine learning (ML)-based score to predict acute COVID-19 mortality among CKD patients.
Methods: CKD inpatients with COVID-19 were prospectively enrolled from December 2022 to January 2023 with a three-month follow-up. Feature selection from clinical and laboratory results was performed through least absolute shrinkage and selection operator and stepwise selection. Logistic regression, support vector machine (SVM), random forest, and extreme gradient boosting were applied for ML model development. A predictive score for mortality was constructed using logistic regression. We compared predictive ability between the proposed score and other published scores.
Results: 219 CKD patients were included and had a high mortality rate of 25.1%. The SVM model exhibited the best performance, with the validation area under the receiver operating characteristic curve (AUC) being 0.946 (95% CI 0.918, 0.974). The COVID-19 vaccination status, age, monocyte percentage, prothrombin activity, cardiac troponin T, and total bilirubin (“VAMPCT”) were the most relevant factors and utilized to develop the scoring system with an AUC of 0.960 (95% CI 0.935, 0.985).
Conclusion: ML models predicting three-month mortality had favorable performance for CKD patients with COVID-19. The VAMPCT mortality score provided a user-friendly approach.
Keywords: chronic kidney disease, COVID-19, cohort study, machine learning, mortality.
Introduction
According to the report from the World Health Organization, although coronavirus disease 2019 (COVID-19) no longer constitutes a public health emergency of international concern, there are still ongoing reports of new infections and deaths related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants around the world, which have also contributed to the overall COVID-19 burden with varying magnitudes [1-3]. Official data indicate that 90% of COVID-19-related in-hospital fatalities in China involved individuals with pre-existing medical conditions [4]. Chronic kidney disease (CKD) is acknowledged as a significant comorbidity that predisposes individuals to a heightened risk of contracting SARS-CoV-2 and experiencing adverse outcomes, including increased mortality rates [5, 6]. The Omicron variants sustained dominance in the global and Chinese COVID-19 landscapes. The characteristics of the COVID-19 acute phase and its impact on the CKD population in China during the Omicron wave are not well understood. Meanwhile, studies indicate that the initial three months post-infection are the peak period for mortality [7]. Considering deaths within a three-month period post-infection as COVID-19-associated provides a more precise measure of the disease's impact. Furthermore, CKD patients are at elevated risk for viral infections, with factors influencing poor outcomes from SARS-CoV-2 potentially applicable to other viral infections in this group [8].
The full automation of ML processes has streamlined the development of models that are not only simple and rapid but also easily replicable, ensuring consistency and reliability. These models have proven to be more efficient than traditional, manually crafted models, offering significant advantages in supporting clinical decision-making and the strategic deployment of healthcare resources.
Consequently, we aimed to construct and validate a predictive scoring system utilizing machine learning techniques designed to pinpoint high-risk CKD patients who may benefit from timely interventions of COVID-19, thereby enhancing their overall prognosis during the Omicron wave.
Methods
Participants and setting
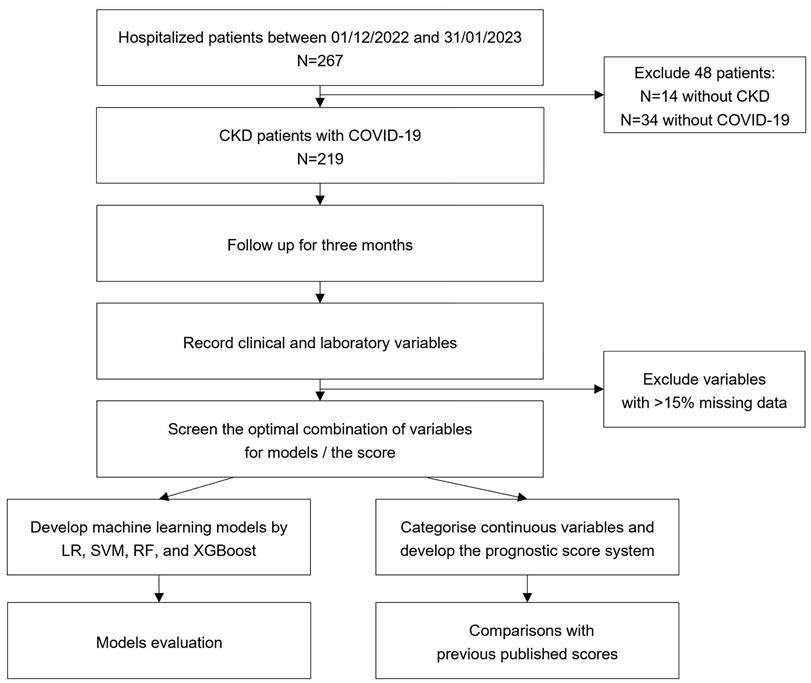
The prospective cohort study consecutively enrolled CKD inpatients with COVID-19 during the Omicron period from December 1, 2022 to January 31, 2023 at the Chinese People's Liberation Army General Hospital (PLAGH) (shown in Figure 1).
Flow chart of the study. CKD: chronic kidney disease; COVID-19: coronavirus disease 2019; LR: logistic regression; SVM: support vector machine; RF: random forest; XGBoost: extreme gradient boosting.
Data collection and variable definition
Data extraction was performed from the electronic health records within the hospital information system at the PLAGH [9]. The date of admission was designated as the index date for all enrolled patients. Comprehensive reviews of clinical charts, nursing notes, laboratory results, and radiological imaging were conducted.
Patients aged over 18 years were required to meet both diagnostic criteria for CKD (defined by the guideline of “Kidney Disease: Improving Global Outcomes” organization”) and COVID-19. Patients with extensive missing data or inability to complete follow-up were excluded. CKD is defined as abnormalities of kidney structure or function, present for a minimum of three months, with implications for health [10]. The diagnostic criteria for COVID-19 involve the presence of clinical manifestations associated with SARS-CoV-2 infection and the fulfillment of at least one of the following etiological or serological test results: a positive SARS-CoV-2 nucleic acid test, a positive SARS-CoV-2 antigen test, successful isolation and culture of SARS-CoV-2, or SARS-CoV-2-specific IgG antibody levels in the convalescent phase being fourfold or higher than those in the acute phase, which adhered to the criteria outlined in the 10th edition of the Diagnosis and Treatment Protocol for COVID-19, as issued by the National Health Commission of China [1]. In accordance with the guideline, patients acceped conservative or non-conservative treatment according to their disease severity. Conservative management included symptomatic support (e.g., hydration, oxygen therapy), while non-conservative interventions encompassed pharmacologic therapies such as glucocorticoids, Nirmatrelvir/Ritonavir, Azvudine, Baricitinib, or Tocilizumab.
The individual vaccination status was categorized into three groups: unvaccinated, partially vaccinated, and fully vaccinated. Full vaccination was defined as receiving at least one dose of the adenovirus vector vaccine, two doses of the inactivated vaccine, or three doses of the recombinant protein vaccine. CKD was identified according to the KDIGO guideline for CKD [10]. Laboratory data included a complete blood count, coagulation profile, infection-related indicators, serum biochemical tests (including renal and liver function, creatine kinase, lactate dehydrogenase (LDH), and electrolytes), and cardiac biomarkers (such as troponin, brain natriuretic peptide, and myoglobin).
This retrospective cohort study analyzed the prognostic performance of the score across these subgroups to calculate odds ratios for 3-month mortality. Interaction terms were included to evaluate whether treatment modality modified the predictive utility of the score.
Outcome
The clinical outcome was all-cause mortality confirmed by vital status at discharge, outpatient visits, or telephone follow-up during the three months after the admission. Patients were followed up and rightly censored on May 1, 2023.
Data processing and variables selection
Variables with more than 15% missing values have not been considered. Multiple imputation was used to handle missing values on candidate variables, considering them missing at random (Table S1). Numeric variables were standardized based on the mean and variance. Least absolute shrinkage and selection operator (LASSO) regression and stepwise selection regression were performed for screening features to optimize the performance of machine learning models.
Models and the score system development
The selected variables were fitted with ML algorithms including logistic regression (LR), support vector machine (SVM), random forest (RF), and extreme gradient boosting (XGBoost). To create the pragmatic mortality score, six variables that contributed the most to the outcome were further filtered out. Continuous variables were converted to dichotomous variables whose cut-off values were chosen by component smoothed functions from generalized additive modeling. The coefficients of logistic regression were converted into prognostic indexes for developing a practical score system.
Model evaluation
Discrimination was evaluated using the area under the curve (AUC) of the receiver operator characteristic (ROC). We also assessed the corresponding Youden indexes, sensitivity, specificity, positive predictive values, and negative predictive values. The calibration was evaluated by the Hosmer-Lemeshow (H-L) test and calibration plot. The model's performance was rated using accuracy, F1 score, kappa coefficient, and Brier score. Additionally, decision curve analysis (DCA) was carried out to determine the clinical utility and calculate the net benefits at different threshold probabilities. All results underwent leave-one-out cross-validation for internal validation. Sensitivity analyses were performed by using complete case data and multiple imputation with different random seeds for missing data. The prognostic performance of the predicted score across treatment subgroups was evaluated to calculate odds ratios for 3-month mortality.
Comparison with previous scores
In this study, "International Severe Acute Respiratory and Emerging Infections Consortium Coronavirus Clinical Characterization Consortium" (4C) mortality score, "Confusion, Urea, Respiratory rate, Blood pressure, and age ≥ 65 years" (CURB65) score, “Hypertension, Neutrophil count, C-reactive protein, Lymphocyte count, Lactate dehydrogenase” (HNC-LL) score, "quick Sequential Organ Failure Assessment" (qSOFA), and "Modified Early Warning Score" (MEWS) were calculated for each patient [11-14]. The mortality score generated from this dataset was compared with the above-mentioned ones.
General statistical analysis
The mean and standard deviation were used to represent normally distributed data, and independent t-tests were used to compare them. The Mann-Whitney test was used to compare non-normally distributed data that were reported as median (25%-75% interquartile range). Categorical variables were expressed as counts and percentages and tested using the chi-square test. A two-sided P <0.05 was considered statistically significant.
Statistical software
All analyses were conducted with R 4.2.0 via packages including caret version 6.0-93, mice version 3.15.0, randomForest version 4.7.1.1, e1071 version 1.7-13, xgboost version 1.7.3.1, glmnet version 4.1.6, pROC version 1.18.0, and ggplot2 version 3.4.1.
Ethical approval
The study was carried out in accordance with the Helsinki Declaration. It was authorized by the Ethics Committee of the Chinese PLAGH (S2023-111-01). All patients provided written informed consent prior to participation.
Results
Patients' characteristics
In our study, encompassing 219 participants, the majority were male (69.4%) with an average age of 59 years, and nearly half (47.5%) were 60 years of age or older (Table 1).
The average body mass index (BMI) was 23.95 kg/m². A significant portion, 63.5%, suffered from advanced CKD stages (four or five). Prior to the infection, 32.4% were on maintenance dialysis, while 5.5% had undergone kidney transplantation without dialysis. Hypertension was the predominant comorbidity at 77.2%, with cardiovascular disease (CVD) and diabetes mellitus following at 47.5% and 37.9%, respectively.
Vaccination rates against SARS-CoV-2 were suboptimal, with only 39.7% vaccinated, of which 36.0% had completed the basic vaccination schedule. The finger oxygen saturation on air of 23.3% of patients was below 90%. The median length of follow-up was 93 days. 74.9% (n = 164) of patients survived, whereas 25.1% (n = 55) deceased. The death group was older than the survivor group (76 ± 13 years vs. 53 ± 18 years, P<0.001). They displayed lower BMI (22.21 ± 3.94 kg/m2 vs. 24.53 ± 4.10 kg/m2, P<0.001), a higher proportion of combined CVD (76.4% vs. 37.8%, P<0.001), and cerebrovascular disease (18.2% vs. 6.7%, P = 0.025). The unvaccinated rate in the deceased was significantly higher at 89.1% versus 50.3% in survivors (P<0.001). At admission, systolic blood pressure (SBP) (131 ± 25 mmHg vs. 142 ± 24 mmHg, P = 0.006) and diastolic blood pressure (72 ± 13 mmHg vs. 80 ± 16 mmHg, P = 0.002) were lower in the death group than those in the survivor group. The proportion of finger oxygen saturation on air <90% (49.1% vs. 14.6%, P<0.001) was significantly higher in the death group than that in the survivor group.
Variables selection
Through subsequent cross-validation with ML algorithms, the variable combination with the best performance was selected for modeling. Eleven variables were retained: age, SBP, COVID-19 vaccination status (Vacc), CVD, red blood cell volume distribution width (RDW), hematocrit (HCT), percentage of monocytes (mono), prothrombin activity (PTA), LDH, total bilirubin (TBil), and cardiac troponin T (cTnT).
Model development and evaluation
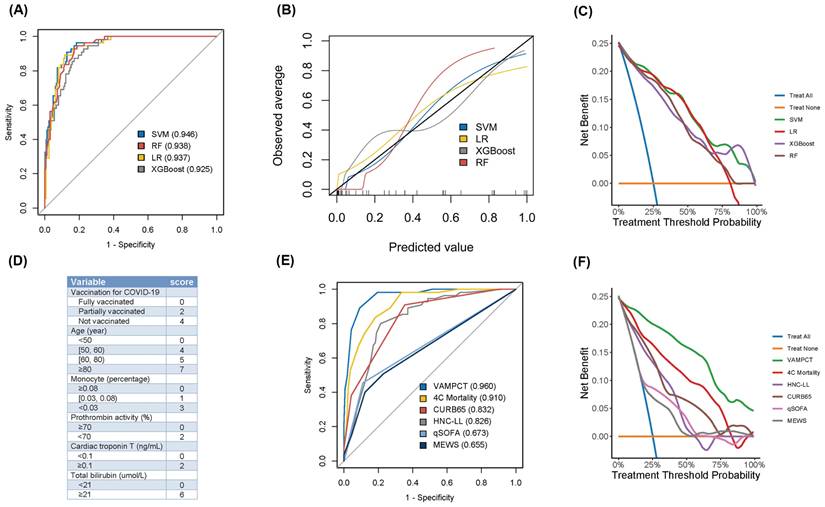
Four ML models, including SVM, LR, RF, and XGBoost, were finally developed and tested with leave-one-out cross-validation. As the ROC curves shown in Figure 2A, the SVM model yielded better discrimination to predict the mortality of patients than other ML models (Table 2). The AUC (95% CI) and the Youden index of the SVM model were 0.946 (0.918, 0.974) and 0.781, respectively. Moreover, the Brier score of the SVM model was the lowest at 0.082 among the four models. For each ML model, calibration performance was further evaluated. The P values of H-L tests for both SVM and XGBoost models were all >0.05. Graphically, the calibration plot of the SVM model fitted well with the diagonal reference line (shown in Figure 2B). Generally, the SVM model had better calibration performance than the other models. As shown in Figure 2C, DCA was applied for assessing the clinical benefits, and the SVM model performed better than the others. It still revealed net benefits when approaching the 100% threshold probability. Based on the above evaluations from three aspects, the SVM model had the best predictive performance among the four ML models when predicting the mortality of CKD patients with COVID-19.
The three-month mortality score
Given the need to use pragmatic scores at the bedside, the number of variables was reduced, and we identified six significant predictors of mortality as Vacc, age, mono, PTA, cTnT, and TBil (for short as “VAMPCT”). The continuous variables were transformed into factors with cut-off values (shown in Figure S1). Age was stratified into four categories: less than 50 years old, 50 to 60 years old, 60 to 80 years old, and 80 years old or older. The percentage of monocytes was divided into three tiers: not less than 0.08, 0.03 to 0.08, and less than 0.03. The PTA was bifurcated at the threshold of 70. Similarly, cTnT and TBil were stratified into two levels using the cut-offs of 0.1 and 21, respectively. Logistic regression was used to construct a risk score, and the regression coefficients were converted into a prognostic index by using appropriate scaling. As shown in Figure 2D, the total scores of VAMPCT ranged from 0 to 24. In the derivation cohort, the VAMPCT score showed a good discrimination of mortality within three months (AUC 0.960, 95% CI 0.935, 0.985), which was better than the existing scores (4C mortality score, CURB65 score, HNC-LL, qSOFA, and MEWS) (shown in Figure 2E-F and Table S2). DCA analysis showed that the VAMPCT score had better clinical utility across a wide range of thresholds. In general, the VAMPCT score outperformed the existing risk scores in predicting three-month mortality. According to the ROC analysis, two risk groups were defined with the optimal cut-off value determined (Table S3): low risk (0-10 score, mortality rate 3.87%) group and high risk (≥ 11 score, mortality rate 76.56%) group.
The evaluation of predictive machine learning models and scores for three-month mortality in CKD patients with COVID-19. (A) ROC analysis of four machine learning models. (B) The calibration plot of four machine learning models. (C) DCA of four machine learning models. (D) The predictive VAMPCT score. (E) ROC analysis of six predictive scores. (F) DCA analysis of six predictive scores. ROC: receiver operating characteristic; DCA: decision curve analysis; SVM: support vector machine; LR: logistic regression; XGBoost: extreme gradient boosting; RF: random forest; COVID-19: coronavirus disease 2019; 4C: Coronavirus Clinical Characterisation Consortium; HNC-LL: hypertension: neutrophil count: C-reactive protein: lymphocyte count: and lactate dehydrogenase; CURB65: confusion: urea: respiratory rate: blood pressure: and age ≥ 65 years; qSOFA: quick sequential organ failure assessment; MEWS: modified early warning score. The values in parentheses were the area under the curve.
Clinical characteristics of CKD patients with COVID-19 according to the outcomes
| Characteristic | Total (N=219) | Survivor (N=164) | Death (N=55) | P value |
|---|---|---|---|---|
| Age (year) | 59 ± 19 | 53 ± 18 | 76 ± 13 | < 0.001 |
| Sex | 0.571 | |||
| Male | 152 (69.4) | 116 (70.7) | 36 (65.5) | |
| Female | 67 (30.6) | 48 (29.3) | 19 (34.5) | |
| Body mass index (kg/m2) | 23.95 ± 4.18 | 24.53 ± 4.10 | 22.21 ± 3.94 | < 0.001 |
| CKD stages | 0.005 | |||
| CKD 1 | 20 (9.1) | 20 (12.2) | 0 (0.0) | |
| CKD 2 | 23 (10.5) | 22 (13.4) | 1 (1.8) | |
| CKD 3 | 37 (16.9) | 26 (15.9) | 11 (20.0) | |
| CKD 4 | 34 (15.5) | 23 (14.0) | 11 (20.0) | |
| CKD 5 | 105 (47.9) | 73 (44.5) | 32 (58.2) | |
| Diagnosis of CKD | <0.001 | |||
| IgA nephropathy | 23 (10.5) | 23 (14.0) | 0 (0.0) | |
| Diabetic nephropathy | 18 (8.2) | 15 (9.1) | 3 (5.5) | |
| Membranous nephropathy | 13 (5.9) | 13 (7.9) | 0 (0.0) | |
| Other CGNa | 82 (37.4) | 52 (31.7) | 30 (54.5) | |
| Renal replacementb | 83 (37.9) | 61 (37.2) | 22 (40.0) | |
| Comorbidities | ||||
| Hypertension | 169 (77.2) | 125 (76.2) | 44 (80.0) | 0.695 |
| Cardiovascular disease | 104 (47.5) | 62 (37.8) | 42 (76.4) | < 0.001 |
| Diabetes mellitus | 83 (37.9) | 57 (34.8) | 26 (47.3) | 0.135 |
| Cerebrovascular disease | 21 (9.6) | 11 (6.7) | 10 (18.2) | 0.025 |
| Cancer | 21 (9.6) | 13 (7.9) | 8 (14.5) | 0.239 |
| Vaccination for COVID-19c | < 0.001 | |||
| Unvaccinated | 129 (60.3) | 80 (50.3) | 49 (89.1) | |
| Partially vaccinated | 8 (3.7) | 6 (3.8) | 2 (3.6) | |
| Fully vaccinated | 77 (36.0) | 73 (45.9) | 4 (7.3) | |
| Admission vitals | ||||
| Body temperature (℃) | 36.5 (36.3-36.8) | 36.5 (36.3-36.7) | 36.5 (36.4-36.8) | 0.187 |
| Heart rate (beats/min) | 86 ± 15 | 85 ± 14 | 86 ± 18 | 0.669 |
| Systolic blood pressure (mmHg) | 139 ± 24 | 142 ± 24 | 131 ± 25 | 0.006 |
| Diastolic blood pressure (mmHg) | 78 ± 16 | 80 ± 16 | 72 ± 13 | 0.002 |
| Finger oxygen saturation on air < 90% | 51 (23.3) | 24 (14.6) | 27 (49.1) | < 0.001 |
| Laboratory test | ||||
| Red blood cell (1012/L) | 3.41 ± 0.94 | 3.42 ± 0.92 | 3.39 ± 0.99 | 0.807 |
| Hemoglobin (g/dL) | 10.42 ± 2.81 | 10.43 ± 2.83 | 10.40 ± 2.77 | 0.945 |
| RDW (%) | 13.99 ± 1.98 | 13.62 ± 1.65 | 15.13 ± 2.43 | < 0.001 |
| White blood cell (109/L) | 6.50 (4.81-9.39) | 6.08 (4.58-8.32) | 7.54 (6.08-11.80) | < 0.001 |
| Neutrophil (percentage) | 0.74 ± 0.14 | 0.70 ± 0.13 | 0.84 ± 0.12 | < 0.001 |
| Lymphocyte (percentage) | 0.16 ± 0.11 | 0.19 ± 0.11 | 0.09 ± 0.07 | < 0.001 |
| Monocyte (percentage) | 0.08 ± 0.04 | 0.09 ± 0.03 | 0.06 ± 0.04 | < 0.001 |
| Platelet (109/L) | 190.32 ± 85.19 | 201.49 ± 87.68 | 157.04 ± 67.68 | 0.001 |
| Serum albumin (g/L) | 31.42 ± 6.64 | 31.93 ± 7.17 | 29.91 ± 4.44 | 0.051 |
| Blood urea (mmol/L) | 17.45 (10.50-27.51) | 15.73 (8.72-24.41) | 25.15 (16.45-39.74) | < 0.001 |
| Serum creatinine (μmol/L) | 403.00 (158.00-761.13) | 365.10 (130.2-796.5) | 457.2 (219.6,723.0) | 0.234 |
| eGFR (mL/min/1.73 m2) | 11.25 (5.60-42.20) | 13.68 (5.69-48.34) | 9.19 (5.36-21.33) | 0.016 |
| C-reactive protein (mg/dL) | 1.53 (0.16-6.04) | 0.39 (0.10-2.67) | 8.35 (2.81-11.77) | < 0.001 |
| Interleukin-6 (pg/mL) | 13.93 (3.02-63.35) | 6.04 (2.29-30.25) | 103.95 (27.99-192.62) | < 0.001 |
| Lactate dehydrogenase (U/L) | 232.80 (189.70-336.50) | 217.90 (172.48-272.10) | 339.50 (251.30-438.25) | < 0.001 |
| Prothrombin activity (%) | 92.45 ± 22.30 | 98.66 ± 18.95 | 73.83 ± 21.32 | < 0.001 |
| APTT (s) | 37.50 (34.18-43.60) | 36.80 (33.80-41.85) | 40.55 (36.95-46.90) | < 0.001 |
| Plasma fibrinogen (g/L) | 4.87 ± 1.70 | 4.72 ± 1.57 | 5.34 ± 2.00 | 0.019 |
| D-dimer (μg/mL) | 1.34 (0.58-2.54) | 1.02 (0.44-2.02) | 2.65 (1.59-5.49) | < 0.001 |
| BNP (pg/mL) | 5196.00 (573.15-21150.50) | 3773.50 (359.40-16038.50) | 10630.00 (2031.00-28398.50) | 0.002 |
| Myoglobin (ng/mL) | 171.65 (70.28-338.00) | 120.10 (58.90-221.00) | 259.60 (168.45-639.45) | < 0.001 |
| Cardiac troponin T (ng/mL) | 0.06 (0.02-0.12) | 0.04 (0.01-0.09) | 0.11 (0.07-0.15) | < 0.001 |
| Time from onset to admission (day) | 14 (7-32) | 18 (10-38) | 7 (2-14) | < 0.001 |
| Length of hospital stay (day) | 15 (8-32) | 16 (8-31) | 13 (6-35) | 0.331 |
Data are expressed as number (%), mean ± standard deviation, or median (interquartile range).
CKD: chronic kidney disease; COVID-19: coronavirus disease 2019; CGN: chronic glomerulonephritis; RDW: red blood cell volume distribution width; eGFR: estimated glomerular filtration rate; APTT: activated partial thromboplastin time; BNP: brain natriuretic peptide.
a: Other CGN: minimal change disease (9, 4.1%), anti-neutrophil cytoplasmic antibodies-associated glomerulonephritis (4, 1.8%), focal segmental glomerulosclerosis (3, 1.4%), lupus nephritis (2, 0.9%), C3 glomerulopathies (1, 0.5%), Henoch-Schönlein purpura nephritis (1, 0.5%), hypertensive nephropathy (1, 0.5%), multiple myeloma-associated nephropathy (1, 0.5%), idiopathic glomerular nodular sclerosis (1, 0.5%), polycystic kidney (1, 0.5%), and type of uncertain etiology (58, 26.5%).
b: Renal replacement: hemodialysis (53, 24.2%), peritoneal dialysis (18, 8.2%), and kidney transplantation (12, 5.5%).
c: The number of valid cases was 214, of which 159 patients survived and 55 patients died during the follow-up.
The assessment of machine learning models for CKD patients with COVID-19
| Model | AUC (95% CI) | Youden | Accuracy | Sensitivity | Specificity | PPV | NPV | F1 | Kappa | Brier | H-L test* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | 0.937 (0.905, 0.968) | 0.775 | 0.886 | 0.891 | 0.884 | 0.721 | 0.960 | 0.797 | 0.719 | 0.090 | <0.001 |
| SVM | 0.946 (0.918, 0.974) | 0.781 | 0.881 | 0.909 | 0.872 | 0.704 | 0.966 | 0.794 | 0.712 | 0.082 | 0.968 |
| RF | 0.938 (0.908, 0.968) | 0.757 | 0.854 | 0.927 | 0.829 | 0.646 | 0.971 | 0.761 | 0.661 | 0.100 | 0.048 |
| XGBoost | 0.925 (0.892, 0.959) | 0.702 | 0.840 | 0.873 | 0.829 | 0.632 | 0.951 | 0.733 | 0.623 | 0.100 | 0.430 |
ML: machine learning; CKD: chronic kidney disease; COVID-19: coronavirus disease 2019; AUC: area under the curve; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value; H-L: Hosmer-Lemeshow; LR: least absolute shrinkage and selection operator regression; SVM: support vector machine; RF: random forest; XGBoost: extreme gradient boosting.
*: P value for the Hosmer-Lemeshow test.
Sensitivity analysis
In the development of ML models, analyses with complete-data instances and alternative imputed cases produced findings comparable to those from the primary imputed dataset (Table S4). In the development of predictive scores, the analysis of forest plots with complete-data cases, distinct imputed instances, and in-hospital outcomes revealed significant P values and coefficients that were similar to the primary analysis (Figure S2).
Subgroup analysis
As shown in Figure S3, subgroup analysis based on treatment modality demonstrated that the VAMPCT score effectively predicted three-month mortality in CKD patients with COVID-19 across both subgroups. In the conservative treatment-only group, the OR was 3.04 (95% CI: 1.68-8.71, P = 0.006), while in the group receiving at least one non-conservative treatment, the OR was 2.73 (95% CI: 1.97-4.21, P < 0.001). However, no statistically significant interaction was observed between treatment modality and the predictive performance of the VAMPCT score (P = 0.804), suggesting that its prognostic utility remained consistent regardless of treatment strategy.
Discussion
The relentless global spread and mutational evolution of SARS-CoV-2 have posed profound threats to both human health and the social economy. In China, the validated genome sequences of SARS-CoV-2 have all been Omicron variants since December 2022 [15]. Notably, infections with the Omicron variant have been associated with reduced hospitalization and mortality rates compared to earlier variants of concern [16]. According to the latest epidemiological survey, there were 82 million adults with CKD in China [17]. A recent meta-analysis of 12 studies revealed that the mortality rate among CKD patients with COVID-19 was alarmingly 5.81 times higher than among those without infection [18]. Highlighting the urgency of early identification of CKD patients at risk of severe outcomes is essential. This study, through an analysis of acute phase infection characteristics and subsequent follow-up of CKD patients, aimed to pinpoint risk factors and formulate a predictive model for mortality of COVID-19 during the Omicron wave.
In our study, all-cause mortality among patients with CKD at three months after COVID-19 was 25.1%, which varies from different studies. According to a multicenter cohort study, the 12-week mortality rate of COVID-19 patients with CKD was 41.5% [19]. In Turkish, the mortality of CKD patients at three months after the diagnosis of COVID-19 was 5.2% [20]. Several explanations may elucidate these variances. Principally, our study's patient population was largely affected by the Omicron variant, which is characterized by a reduced severity and mortality profile relative to its predecessors [21]. Additionally, racial disparities could play a pivotal role in post-COVID-19 mortality, attributed to a spectrum of factors including distinct comorbidities and divergent biochemical progressions [22, 23].
In our analysis, eleven predictors were meticulously selected and applied through machine learning algorithms, capturing a comprehensive profile of COVID-19's impact. These predictors encompassed indicators of cardiac injury (cTnT and LDH), coagulation dysfunction (PTA), erythrocyte abnormalities (RDW), and the involvement of the immune system, including COVID-19 vaccination status and monocyte percentage. These parameters are not only routinely measured but also corroborate established risk factors for COVID-19 mortality as identified in previous studies [24-26]. Within our cohort, age emerged as the most significant predictor of mortality. A wealth of evidence supports the association between advanced age and adverse outcomes in COVID-19 patients with CKD [27, 28]. The interplay of a milder inflammatory response with aging, slower viral clearance, and the diminished compensatory capacity of the remaining glomeruli likely underpins this association [29-31]. Frailty, a prevalent geriatric syndrome, is strongly associated with aging and portends elevated mortality in CKD patients, particularly when compounded by COVID-19. Mechanistically, age-related senescence involves subcellular/cellular perturbations—inflammaging, mitochondrial dysfunction, cellular senescence, and dysregulated nutrient-sensing pathways—culminating in multisystem physiological decline and clinical frailty [32]. In CKD patients, frailty and COVID-19 synergistically amplify proinflammatory cascades, further impairing antiviral immunity while exacerbating hyperinflammation-driven organ injury, thereby increasing severe disease and mortality risks [33]. Frailty also compromises tolerance to SARS-CoV-2-targeted antivirals (e.g., nirmatrelvir/ritonavir), necessitating dose modifications or alternative regimens that may undermine therapeutic efficacy [34].
Incorporating cardiac biomarkers into the scoring system is critical, given the high prevalence of cardiovascular comorbidities (e.g., hypertension, diabetes, coronary artery disease) and compounded cardiorenal risks in CKD patients [35]. Meanwhile, COVID-19 exacerbates these risks through direct myocardial injury (ACE2-mediated viral entry) and systemic hyperinflammation, increasing acute complications like myocarditis and thrombosis. Previous clinical studies have implied that COVID-19 leads to diverse cardiovascular complications [36]. Biomarkers such as troponin refine prognostic accuracy by quantifying these interactions, enabling early intervention to mitigate mortality. Thus, cardiovascular-integrated scoring addresses the unique pathophysiology of CKD-COVID-19 overlap, improving both risk prediction and personalized management.
Vaccination has been heralded as a pivotal preventive measure in mitigating the severity and reducing fatalities from COVID-19 [37]. Our findings underscore vaccination status as the most potent protective factor, a consensus echoed by prior research. A multicenter study highlighted that the relative risk of death for vaccinated individuals 90 days post-COVID-19 was a fifth of that for their unvaccinated counterparts [38]. Similarly, in the hemodialysis population, vaccination has been linked to attenuated disease severity and lower mortality rates attributable to COVID-19 [39].
Advanced machine learning (ML) techniques have unlocked the potential to uncover subtle patterns within the intricate and high-dimensional landscape of clinical data. In terms of the AUC, our ML models demonstrated exceptional performance, a testament to the effectiveness of feature selection as well as the meticulous training and tuning processes employed. When considering calibration and clinical applicability, support vector machine (SVM) models emerged with a more advantageous overall performance, a finding that aligns with reports on COVID-19 patient outcomes [40, 41]. A recent meta-analysis has pointed out that the algorithm used, the population studied, the study design, and the dataset source all exert influence on the pooled estimate of model performance [42]. With clinical practicality in mind, we distilled six impactful indicators from those identified by ML to develop the "VAMPCT" scoring system. This scoring system offers predictive discrimination comparable to the SVM model, coupled with enhanced specificity, thereby facilitating its utility in clinical decision-making.
Despite the robust findings of our study, several limitations warrant acknowledgment. Firstly, the data were sourced from a single hospital, and the modest sample size may constrain the robustness of the machine learning model scoring and the generalizability of our results to other geographic regions or ethnic groups, where variations in healthcare practices, genetic predispositions, and COVID-19 strain prevalence could influence prognostic accuracy. Prospective validation in multiethnic, multinational cohorts is required to confirm its broader applicability. Secondly, our analysis relied on multiple imputation under the assumption of data missing at random, which may not accurately reflect the true distribution patterns; this assumption could introduce bias. Thirdly, our findings may be influenced by residual confounding from unmeasured factors (e.g., socioeconomic status, lifestyles, and behaviors) and imperfectly modeled nonlinear/interaction effects. While sensitivity analyses supported robustness, future prospective studies with granular phenotyping are needed to fully address these limitations. These limitations should be considered when interpreting the study outcomes and when planning subsequent research to address these gaps.
Conclusion
In this study, we developed predictive models for three-month mortality in CKD patients with COVID-19, identifying the SVM model as the most effective. We also introduced the VAMPCT score to facilitate early prognostic evaluation during the acute phase of the disease. Against the backdrop of the Omicron variant's sustained dominance in the global and Chinese COVID-19 landscapes, our research offers initial observations regarding the mortality associated with Omicron infection in CKD patients. It contributes to paving the way for the advancement of more refined and prognostically relevant clinical tools.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This work was supported by the National Key Research and Development Program of China [2022YFC3602000], the National Natural Science Foundation of China [No. 82274327, No. 32100631, and No. 32141005], and the Young Elite Scientists Sponsorship Program by the China Association for Science and Technology [YESS20210056]. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Ethics Statement
The study was carried out in accordance with the Helsinki Declaration of 1975, as revised in 2013. Its protocol was reviewed and approved by the Ethics Committee of the Chinese PLAGH, approval number [S2023-111-01]. All patients provided informed consent prior to participation in the study.
Data Availability
The data that support the findings of this study cannot be openly shared due to the privacy of research participants but are available from the corresponding author (Ping Li).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ikejezie J, Miglietta A, Hammermeister Nezu I, Adele S, Higdon MM, Feikin D. et al. Informing the pandemic response: the role of the WHO's COVID-19 Weekly Epidemiological Update. BMJ Glob Health. 2024 9
2. Peramaiyan R, Anthony J, Varalakshmi S, Sekar AK, Ali EM, A AHS. et al. Comparison of the role of vitamin D in normal organs and those affected by COVID-19. Int J Med Sci. 2025;22:240-51
3. Al-Saeedi F, Rajendran P, Tipre D, Aladwani H, Alenezi S, Alqabandi M. et al. The effect of COVID-19 on nuclear medicine and radiopharmacy activities: A global survey. Sci Rep. 2023;13:10489
4. Transcript of press conference under The Joint Prevention and Control Mechanism of the State Council on January 14, 2023. National Health Commission of the People's Republic of China; 2023
5. Mahalingasivam V, Su G, Iwagami M, Davids MR, Wetmore JB, Nitsch D. COVID-19 and kidney disease: insights from epidemiology to inform clinical practice. Nat Rev Nephrol. 2022;18:485-98
6. Wijewickrama ES, Abdul Hafidz MI, Robinson BM, Johnson DW, Liew A, Dreyer G. et al. Availability and prioritisation of COVID-19 vaccines among patients with advanced chronic kidney disease and kidney failure during the height of the pandemic: a global survey by the International Society of Nephrology. BMJ Open. 2022;12:e065112
7. Carriazo S, Mas-Fontao S, Seghers C, Cano J, Goma E, Avello A. et al. Increased 1-year mortality in haemodialysis patients with COVID-19: a prospective, observational study. Clin Kidney J. 2022;15:432-41
8. Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786-802
9. Li P, Xie C, Pollard T, Johnson AEW, Cao D, Kang H. et al. Promoting Secondary Analysis of Electronic Medical Records in China: Summary of the PLAGH-MIT Critical Data Conference and Health Datathon. JMIR Med Inform. 2017;5:e43
10. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024; 105: S117-s314.
11. Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339
12. Xiao LS, Zhang WF, Gong MC, Zhang YP, Chen LY, Zhu HB. et al. Development and validation of the HNC-LL score for predicting the severity of coronavirus disease 2019. EBioMedicine. 2020;57:102880
13. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A. et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama. 2016;315:762-74
14. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. Qjm. 2001;94:521-6
15. Epidemic situation of COVID-19 in China. Chinese Center for Disease Control and Prevention; 2023
16. Fan Y, Li X, Zhang L, Wan S, Zhang L, Zhou F. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7:141
17. Wang L, Xu X, Zhang M, Hu C, Zhang X, Li C. et al. Prevalence of Chronic Kidney Disease in China: Results from the Sixth China Chronic Disease and Risk Factor Surveillance. JAMA Intern Med. 2023;183:298-310
18. Cai R, Zhang J, Zhu Y, Liu L, Liu Y, He Q. Mortality in chronic kidney disease patients with COVID-19: a systematic review and meta-analysis. Int Urol Nephrol. 2021;53:1623-9
19. Appelman B, Oppelaar JJ, Broeders L, Wiersinga WJ, Peters-Sengers H, Vogt L. Mortality and readmission rates among hospitalized COVID-19 patients with varying stages of chronic kidney disease: a multicenter retrospective cohort. Sci Rep. 2022;12:2258
20. Karadag S, Ozturk S, Arici M, Gorgulu N, Akcali E, Pembegul I. et al. Post-COVID-19 outcomes of non-dialysis dependent chronic kidney disease patients: a national, multicenter, controlled study. Int Urol Nephrol. 2023;55:399-408
21. Liu Y, Yu Y, Zhao Y, He D. Reduction in the infection fatality rate of Omicron variant compared with previous variants in South Africa. Int J Infect Dis. 2022;120:146-9
22. Siddiq S, Ahmed S, Akram I. Clinical outcomes following COVID-19 infection in ethnic minority groups in the UK: a systematic review and meta-analysis. Public Health. 2022
23. Wan YI, Puthucheary ZA, Pearse RM, Prowle JR. Characterising biological mechanisms underlying ethnicity-associated outcomes in COVID-19 through biomarker trajectories: a multicentre registry analysis. Br J Anaesth. 2023
24. Klén R, Purohit D, Gómez-Huelgas R, Casas-Rojo JM, Antón-Santos JM, Núñez-Cortés JM. et al. Development and evaluation of a machine learning-based in-hospital COVID-19 disease outcome predictor (CODOP): A multicontinental retrospective study. Elife. 2022 11
25. Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad Med J. 2022;98:422-7
26. Wu G, Zhou S, Wang Y, Lv W, Wang S, Wang T. et al. A prediction model of outcome of SARS-CoV-2 pneumonia based on laboratory findings. Sci Rep. 2020;10:14042
27. D'Marco L, Puchades MJ, Romero-Parra M, Gimenez-Civera E, Soler MJ, Ortiz A. et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J. 2020;13:297-306
28. Pilgram L, Eberwein L, Jensen BO, Jakob CEM, Koehler FC, Hower M. et al. SARS-CoV-2 infection in chronic kidney disease patients with pre-existing dialysis: description across different pandemic intervals and effect on disease course (mortality). Infection. 2023;51:71-81
29. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269-70
30. Fu Y, Han P, Zhu R, Bai T, Yi J, Zhao X. et al. Risk factors for viral RNA shedding in COVID-19 patients. Eur Respir J. 2020 56
31. Gekle M. Kidney and aging - A narrative review. Exp Gerontol. 2017;87:153-5
32. Kim DH, Rockwood K. Frailty in Older Adults. N Engl J Med. 2024;391:538-48
33. Sablerolles RSG, Lafeber M, van Kempen JAL, van de Loo BPA, Boersma E, Rietdijk WJR. et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021;2:e163-e70
34. Rahman S, Singh K, Dhingra S, Charan J, Sharma P, Islam S. et al. The Double Burden of the COVID-19 Pandemic and Polypharmacy on Geriatric Population - Public Health Implications. Ther Clin Risk Manag. 2020;16:1007-22
35. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-72
36. Liu F, Liu F, Wang L. COVID-19 and cardiovascular diseases. J Mol Cell Biol. 2021;13:161-7
37. Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20:200
38. Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The Protective Effect of Coronavirus Disease 2019 (COVID-19) Vaccination on Postacute Sequelae of COVID-19: A Multicenter Study from a Large National Health Research Network. Open Forum Infect Dis. 2022;9:ofac228
39. Ao G, Li A, Wang Y, Tran C, Gao M, Chen M. The effect of SARS-CoV-2 double vaccination on the outcomes of hemodialysis patients with COVID-19: A meta-analysis. J Infect. 2023;86:e43-e5
40. Ballı S. Data analysis of Covid-19 pandemic and short-term cumulative case forecasting using machine learning time series methods. Chaos Solitons Fractals. 2021;142:110512
41. Wollenstein-Betech S, Cassandras CG, Paschalidis IC. Personalized predictive models for symptomatic COVID-19 patients using basic preconditions: Hospitalizations, mortality, and the need for an ICU or ventilator. Int J Med Inform. 2020;142:104258
42. Chen R, Chen J, Yang S, Luo S, Xiao Z, Lu L. et al. Prediction of prognosis in COVID-19 patients using machine learning: A systematic review and meta-analysis. Int J Med Inform. 2023;177:105151
Author contact
![]() Corresponding author: Guangyan Cai, caiguangyancom; Xiangmei Chen, xmchen301com; Ping Li, lipingcom.cn.
Corresponding author: Guangyan Cai, caiguangyancom; Xiangmei Chen, xmchen301com; Ping Li, lipingcom.cn.

 Global reach, higher impact
Global reach, higher impact