3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(11):2738-2756. doi:10.7150/ijms.107479 This issue Cite
Review
Expert Consensus on the Diagnosis and Management of Carotid Atherosclerotic Plaque: Pathophysiology, Clinical Management, and Preventive Approaches
1. Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053
2. The Sixth Medical Center of PLA General Hospital, Beijing, 100048
3. Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203
4. Beijing University of Chinese Medicine, Beijing, 100029
5. Hubei University of Chinese Medicine, Hubei, Wuhan, 430065
6. State Key Laboratory of Traditional Chinese Medicine Syndrome/Department of Cardiovascular Surgery, Guangdong Provincial Hospital of Chinese Medicine, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, the Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou 510120, Guangdong, China.
7. Dongfang Hospital, Beijing University of Chinese Medicine, 100078
† These authors contributed equally to this article.
Received 2024-11-23; Accepted 2025-4-15; Published 2025-5-30
Abstract
To standardize and harmonize pharmacist-led cholesterol-lowering medication therapy management (MTM) for patients with carotid atherosclerosis and plaque, the Expert Consensus on Lipid-Lowering Pharmacotherapy Management in Patients with Carotid Atherosclerosis and Plaque was developed under the leadership of the PLA General Hospital. This consensus establishes a systematic framework spanning the full-cycle management process: data collection, analytical evaluation, intervention implementation, and long-term follow-up, supported by standardized protocols, documentation forms, and assessment tools. It prioritizes seven evidence-based evaluation domains: therapeutic efficacy, drug selection, dosing appropriateness, adverse reactions, drug-drug/food interactions, cost-effectiveness, and medication adherence. By integrating practical workflows with clinical decision-support tools, the consensus aims to optimize therapeutic outcomes, mitigate safety risks, and provide actionable guidance for healthcare professionals managing this high-risk population
Keywords: Consensus, Carotid atherosclerosis with plaque, Cholesterol, Medication therapy management
1. Introduction
Elevated low-density lipoprotein cholesterol (LDL-C) constitutes a central pathogenic mechanism driving plaque progression and increased ischemic stroke risk in carotid atherosclerosis with plaque formation [1]. Effective cholesterol-lowering therapy demonstrates significant clinical benefits through reducing high-risk plaque features (e.g., hypoechoic morphology, superficial ulceration), decreasing recurrence rates of cerebrovascular events and mortality, while improving patients' quality of life [2, 3]. The 2018 U.S. Cholesterol Management Guidelines emphasize multidisciplinary collaboration as an effective strategy for cholesterol optimization, specifically highlighting pharmacist interventions to enhance therapeutic adherence [1]. Meta-analyses corroborate that pharmacist-led interventions significantly improve both therapeutic efficacy and medication adherence in lipid-lowering therapies [4, 5]. The National Health Commission of China's Guidelines for Accelerating High-Quality Pharmaceutical Services Development (2019) explicitly mandates pharmacists' proactive role in chronic disease management through medication follow-up and therapy optimization. Therefore, pharmacists should actively engage in cholesterol management protocols to ensure medication safety and resolve drug therapy-related issues through professional pharmaceutical care.
Currently, medical community lacks standardized pharmaceutical care protocols for cholesterol-lowering therapy management in patients with carotid atherosclerosis and plaque, while significant disparities in theoretical knowledge and clinical competencies exist among pharmacists across different tiers of healthcare institutions. These limitations impede the standardized, homogeneous implementation of cholesterol-lowering pharmacotherapy management for this patient population. To address this critical gap, the PLA General Hospital has led the development of the Expert consensus on the management of cholesterol-lowering drug therapy in patients with carotid atherosclerosis and plaque disease (hereinafter referred to as "the Consensus"), which underwent rigorous review by pharmaceutical and medical experts from eight nationally representative healthcare institutions.
This Consensus focuses on comprehensive lipid profile optimization and plaque stabilization management in carotid atherosclerosis patients. It systematically outlines standardized pharmaceutical care processes for pharmacists, including baseline data collection, therapeutic regimen analysis, individualized intervention implementation, and longitudinal therapeutic monitoring. The drafting committee structured the Consensus around medication therapy management (MTM) workflows, specifically addressing clinical challenges in evaluating cholesterol-lowering regimens, such as efficacy assessment, drug selection criteria, dosage optimization, adverse effect surveillance, and drug interaction management.
The development process incorporated extensive literature reviews of current international guidelines, recent expert consensus statements, global prescribing information, and authoritative pharmacotherapy databases (UpToDate®, Lexicomp®). Clinical recommendations were further contextualized with China's healthcare policies, including the National Essential Medicines Policy, National Centralized Drug Procurement Policy, and National Medical Insurance Drug Negotiation Policy. By integrating evidence-based medicine with practical clinical experience, this Consensus aims to establish China's first standardized MTM framework for carotid atherosclerosis management. Its implementation is expected to enhance pharmacists' competencies in lipid management and plaque stabilization, optimize therapeutic outcomes through policy-aligned interventions, and reduce medication-related risks through systematic adverse effect monitoring.
1.1 Epidemiology
The epidemiological characteristics of carotid atherosclerotic plaques exhibit marked population heterogeneity and regional disparities. The China Kadoorie Biobank (CKB) study reported an average carotid intima-media thickness (cIMT) of 0.70 mm among 24,822 adults across 10 Chinese regions, with a 0.08 mm increase per decade [6]. Carotid plaques were detected in 31% of adults, showing a sharp prevalence escalation from 6% in those aged 40-49 to 58% among 70-89-year-olds, alongside interregional variations of 11%-50%. In northeastern China, the crude prevalence reached 42.1% among individuals over 40, with plaques accounting for 40.0% and carotid stenosis at 3.6%, compounded by suboptimal risk factor control (e.g., only 4.7% hypertension management) [7]. Compared to European populations, Chinese adults demonstrated thicker cIMT (0.70 vs 0.66 mm, p < 0.001) despite comparable overall plaque burden [6].
Key risk factors included hypertension, elevated LDL-C, diabetes, and smoking (34% plaque prevalence in smokers vs 23% in non-smokers) [6]. Blood pressure control emerged as particularly critical, with systolic pressure ≥160 mmHg conferring double the plaque risk compared to < 120 mmHg (39% vs 19%). Notably, young stroke patients (32-50 years) without conventional risk factors still showed significantly elevated cIMT progression versus controls [8]. Metabolic analyses revealed positive correlations between insulin resistance and plaque area, while higher BMI displayed paradoxical protective trends [9]. High-risk subgroups exhibited stark profiles: 74.3% of stage 3-5 chronic kidney disease patients had detectable plaques, with each 1 pmol/L serum sclerostin increase elevating plaque risk by 2.6% [10]. Inflammatory mechanisms showed TLR4 overexpression tripled cerebrovascular event risk, and fibrinogen > 407 mg/dL quintupled thrombotic risk alongside fibrous cap thinning [11].
Geographical variations manifested prominently, with 12.5% moderate-severe stenosis and 76% stable hyperechoic plaques in sub-Saharan African stroke patients, contrasting with China's northern regions demonstrating 31.0% prevalence [6]. Gender-specific phenotypes emerged: males predominantly developed structurally complex plaques compared to females' lipid-rich vulnerable lesions. These population-specific patterns underscore the imperative for precision risk factor management and optimized healthcare resource allocation in China.
2. Pathophysiological Mechanisms of Carotid Atherosclerotic Plaque
The pathophysiological mechanisms of carotid atherosclerotic plaques involve complex interactions among hemodynamics, inflammatory responses, and vascular remodeling. Below is a systematic synthesis based on current research evidence, as listed in Table 1.
2.1 Hemodynamics and Endothelial Dysfunction
Atherosclerosis preferentially develops in curved and branching arterial regions, where endothelial cells are exposed to disturbed flow characterized by low-magnitude oscillatory shear stress. Flow dynamics regulate endothelial structure, function, transcriptome, epigenome, and metabolism through mechanosensors and mechanotransduction pathways [12-15]. Wall shear stress (WSS) is a critical mechanical factor in plaque formation. Normal laminar shear stress (10-70 dyn/cm²) suppresses inflammation and smooth muscle proliferation via endothelial nitric oxide (NO) pathway activation. In contrast, low/oscillatory shear stress (< 4 dyn/cm²) induces endothelial cell gap widening, increased permeability, and promotes low-density lipoprotein (LDL) infiltration and monocyte adhesion [16-19]. Clinical studies demonstrate that hemodynamically significant stenosis (e.g., at the carotid sinus) correlates with increased white matter hyperintensity volume, suggesting synergistic effects of hypoperfusion and endothelial dysfunction [16, 18]. Moreover, disturbed flow transmits low-magnitude oscillatory shear stress to endothelial cells, inducing in situ reprogramming, which promotes endothelial inflammation, endothelial-to-mesenchymal transition, endothelial-to-immune-like cell transformation, and metabolic alterations, thereby accelerating atherosclerosis [20, 21].
2.2 Inflammatory Response and Plaque Formation
Compared with other arteries, particularly the femoral artery, human carotid atherosclerotic lesions exhibit a higher inflammatory burden, with an increased prevalence of anti-inflammatory foam cell-like macrophages, TREM2-positive macrophages, and LYVE1-positive resident macrophages [22, 23]. Under low shear stress, endothelial cells upregulate vascular cell adhesion molecule-1 (VCAM-1) and monocyte chemoattractant protein-1 (MCP-1), promoting macrophage infiltration and foam cell formation. Plaque composition analysis reveals that embolic ischemic patients exhibit higher T-cell density, macrophage area, and matrix metalloproteinase-9 (MMP-9) expression in plaques compared to hemodynamic ischemic patients, indicating intensified inflammation-driven plaque instability [24, 25].
2.3 Mechanical Mechanisms of Plaque Instability
High time-averaged wall shear stress (TAWSS > 60 dyn/cm²) is associated with recent symptomatic carotid stenosis, potentially inducing fibrous cap rupture or surface thrombosis via mechanical stress. Computational fluid dynamics (CFD) simulations show that symptomatic plaques exhibit significantly higher local maximum WSS than asymptomatic plaques (116.65 vs. 68.28 dyn/cm²), with reduced relative residence time (RRT), reflecting flow disturbances that exacerbate plaque erosion risk [26, 27]. Additionally, mechanical stress influences vascular macrophage biomechanics, enhancing matrix metalloproteinase activity and inducing immediate-early gene responses to mechanical deformation. In human monocytes/macrophages and THP-1 cells, biomechanical strain induces class A scavenger receptor expression, a critical lipoprotein receptor in atherogenesis, further promoting plaque formation [28].
2.4 Vascular Remodeling and Hemodynamic Adaptation
Carotid bifurcation geometry influences local flow patterns. Smaller luminal expansion (reduced FlareA) is independently associated with vulnerable plaques (lipid-rich or intraplaque hemorrhage; OR=0.45) [29, 30]. Compensatory vascular dilation maintains luminal area in early stages, but persistent low shear stress leads to negative remodeling, characterized by intimal thickening and smooth muscle migration [31, 32]. In smooth muscle cells, C-terminal β-catenin signaling promotes vascular remodeling by activating the S1pr1 promoter and inducing S1PR1 expression, facilitating neointimal formation following arterial injury and contributing to atherogenesis [33].
2.5 Immune Microenvironment and Hemodynamic Interactions
Alterations in the immune microenvironment are fundamental pathological mechanisms by which external factors influence plaque formation and progression. CFD combined with mass cytometry reveals increased neutrophil infiltration in high oscillatory shear index (OSI) regions, while high WSS regions are dominated by anti-inflammatory M2 macrophages, suggesting a flow-dependent immune polarization that contributes to atherosclerotic plaque progression [34, 35]. Additionally, complement system activation exacerbates endothelial inflammation in low shear stress regions, whereas physiological shear stress attenuates this effect by upregulating complement-inhibitory proteins [16, 18].
2.6 Clinical Implications and Therapeutic Targets
Plaque stability assessment requires integration of hemodynamic parameters (e.g., WSS, OSI) and morphological features (e.g., luminal expansion, lipid core percentage) [26, 27, 29, 30]. Emerging technologies like arterial wall magnetic resonance imaging (MRI) and adaptive pulse wave imaging (PWI) enable simultaneous evaluation of plaque biomechanics and hemodynamic status, guiding personalized treatment [26, 27, 36]. Future research should clarify spatiotemporal relationships between local hemodynamics and immune responses to develop precision therapies targeting mechanotransduction pathways [ 37].
3. Classification of plaques
3.1 Plaque Classification-By Degree of Stenosis Based on the North American Symptomatic Carotid Endarterectomy)
3.1.1 Trial (NASCET) criteria
1) Mild stenosis (< 50%): Minimal hemodynamic impact, low stroke risk.
2) Moderate stenosis (50%-69%): Requires combined assessment of plaque stability for stroke risk stratification.
3) Severe stenosis (≥ 70%): Significant hemodynamic compromise; elevated stroke risk, particularly with vulnerable plaque features.
3.2 By Plaque Stability
Stable plaque: Dominated by calcification and fibrous components with intact fibrous caps. Calcified plaques on CT correlate with reduced symptomatic risk (OR = 0.047, p = 0.03) [38].
Vulnerable plaque: Pathological features include thin fibrous caps, large lipid cores, intraplaque hemorrhage, or neovascularization. Hypoechoic plaques on ultrasonography are strongly associated with stroke risk (sensitivity 92.1%, specificity 95.6%) [39].
3.3 By Clinical Presentation
1) Symptomatic plaque: Ipsilateral ischemic events within 6 months necessitate aggressive intervention.
2) Asymptomatic plaque: Annual stroke risk of 0.5%-1.0%, but high-risk features (e.g., rapid progression, microembolic signals on TCD, silent infarcts) warrant escalated management.
4. Risk Assessment Framework
4.1 Imaging Modalities
1) Ultrasonography: Evaluates stenosis and plaque echogenicity; hypoechoic regions (>40% plaque area) and irregular surfaces predict vulnerability. Machine learning models improve classification accuracy (AUC ≈ 0.95) [40].
2) CT Angiography (CTA): Detects calcification; calcified plaques show a 21-fold lower risk of symptomatic presentation (p = 0.03).
Classification of Carotid Atherosclerotic Plaques and Risk Assessment
| Mechanism Category | Key Findings | Clinical Significance |
|---|---|---|
| Hemodynamics & Endothelial Dysfunction | 1.Low WSS (<4 dyn/cm²) induces endothelial permeability ↑, promoting LDL infiltration [18, 19] 2.Hemodynamic stenosis correlates with white matter hyperintensity volume [18] | Indicates synergistic effects of hypoperfusion and endothelial dysfunction |
| Inflammation & Plaque Formation | 1.Low WSS upregulates VCAM-1/MCP-1 → macrophage infiltration [25] 2.Embolic plaques show T-cell density and MMP-9 expression↑ [25] | Highlights inflammatory contribution to plaque instability |
| Mechanical Plaque Destabilization | High TAWSS (>60 dyn/cm²) correlates with symptomatic stenosis<br>- Symptomatic plaques show higher WSS (116.65 vs 68.28 dyn/cm²) [27] | CFD reveals flow disturbance as erosion risk factor |
| Vascular Remodeling | 1.Bifurcation geometry affects flow patterns 2.Reduced FlareA correlates with vulnerable plaques [30] | Negative remodeling manifests as intimal thickening |
| Immune-Hemodynamic Interactions | 1.High OSI zones: neutrophil infiltration↑ 2.High WSS zones: M2 macrophage polarization [35] 2.Complement activation in low WSS regions [35] | Demonstrates flow-pattern dependent immune modulation |
| Clinical Assessment & Intervention | 1.Requires combined WSS/OSI and morphological evaluation 2.Novel MRI/PWI enables simultaneous plaque mechanics-flow assessment [27, 36] | Guides personalized therapy; Future focus: mechano-signaling targeted strategies |
3) Magnetic Resonance Imaging (MRI): High-resolution plaque imaging identifies fibrous cap integrity and intraplaque hemorrhage, achieving 94% specificity for vulnerable plaques [41].
4.2 Multimodal Risk Stratification
1) High-risk morphological features: Ulceration, irregular surface, or hypoechoic components.
2) Hemodynamic parameters: Ipsilateral cerebrovascular reserve < 1.5 indicates hypoperfusion, tripling stroke risk [40, 42].
3) Systemic atherosclerotic burden: Carotid plaque score ≥ 2 (bilateral/multiple plaques) correlates with coronary artery calcification (r = 0.62, p < 0.001) [43].
4.3 Dynamic Monitoring Strategies
For asymptomatic stenosis: Biannual ultrasonography to track plaque progression (annual growth > 15% indicates high risk). Emerging molecular imaging (e.g., VCAM-1-targeted MRI probes) quantifies plaque inflammation to guide statin therapy [44, 45].
4.4 Clinical Pathway Recommendations
1) Low-risk plaques (stable with < 50% stenosis): Intensive lifestyle interventions and LDL-C control < 2.6 mmol/L.
2) Intermediate-risk plaques (vulnerable or 50%-69% stenosis): LDL-C target < 1.8 mmol/L with antiplatelet therapy.
3) High-risk plaques (symptomatic/vulnerable with ≥ 70% stenosis): Vascular reconstruction (CEA/CAS) combined with aggressive lipid-lowering (≥ 50% LDL-C reduction).
4) Future Directions: Validate AI-assisted diagnostic systems (e.g., quadruple-path neural networks) for risk stratification, and explore integrated models combining multi-omics biomarkers and imaging features.
5. Diagnostic method
5.1 Ultrasonography
5.1.1 Conventional Ultrasound (US)
1) Advantages: As a first-line screening tool, US noninvasively evaluates carotid intima-media thickness (IMT) and plaque morphology (location, size, echogenicity). IMT ≥ 1.5 mm defines plaque formation. It is sensitive to calcified plaques (high echogenicity) but limited in differentiating intraplaque hemorrhage (IPH) from lipid-rich necrotic core (LRNC) [46, 47].
2) Clinical Application: Recommended for annual follow-up in patients with mild to moderate stenosis (< 70%). Severe stenosis (≥ 70%) requires further multimodal imaging.
5.1.2 Contrast-Enhanced Ultrasound (CEUS)
1) Advantages: Microbubble contrast agents enhance neovascularization imaging, detecting intraplaque angiogenesis and ulceration with > 80% sensitivity [46, 47].
2) Limitations: Operator-dependent and prone to calcification artifacts; requires validation with complementary techniques.
5.1.3 Intravascular Ultrasound (IVUS)
1) Advantages: Quantifies plaque burden and identifies LRNC (hypoechoic regions) and calcification, though limited in assessing fibrous cap thickness [48, 49].
2) Clinical Application: Used for precise plaque morphology evaluation prior to endovascular interventions.
5.1.4 Transcranial Doppler Ultrasound (TCD)
1) Advantages: Real-time hemodynamic monitoring and microembolic signal (HITS) detection for stroke risk stratification.
2) Limitations: Requires adequate acoustic bone windows; best combined with other imaging modalities.
5.2 CT Imaging
5.2.1 Computed Tomography (CT)
1) CT Angiography (CTA)
2) Advantages: High spatial resolution visualizes luminal stenosis, calcified plaques (CT attenuation > 130 HU), and ulceration (depth > 1 mm) [50, 51].
3) Clinical Application: Preferred for evaluating complex vascular anatomy and calcified plaques.
5.2.2 Gemstone Spectral CT (GSCT)
1) Advantages: Differentiates LRNC (rising curve), IPH (attenuated curve), and calcification (descending curve) via spectral analysis, reducing metal artifacts.
2) Limitations: Radiation exposure considerations; ideal for detailed calcification analysis.
5.3 Magnetic Resonance Imaging (MRI)
5.3.1 High-Resolution MRI (HR-MRI)
1) Advantages: Superior soft-tissue contrast identifies vulnerable plaque features: thin fibrous cap (FC < 0.7 mm), IPH (T1WI hyperintensity), and LRNC (non-enhancing on T1WI) [52].
2) Clinical Application: Recommended for compositional plaque assessment and risk stratification in symptomatic stenosis.
5.3.2 MR Vessel Wall Imaging (MR-VWI)
1) Advantages: 3D techniques (e.g., 3D-MERGE, SNAP) quantify plaque volume and components, enabling craniocervical joint imaging.
2) Recommendation: Annual monitoring for plaque progression in moderate-to-severe stenosis.
5.4 Nuclear Medicine Imaging
Positron Emission Tomography (PET)
1) Advantages: 18F-FDG (inflammatory activity) and 18F-NaF (microcalcifications) tracers detect plaque vulnerability. The SCAIL score (integrating stenosis and inflammation) predicts stroke recurrence (specificity 90%) [53].
2) Limitations: High cost; reserved for research or high-risk metabolic profiling.
5.5 Digital Subtraction Angiography (DSA)
1) Advantages: Gold standard for comprehensive evaluation of stenosis, collateral circulation, and plaque morphology.
2) Limitations: Invasive risks (e.g., plaque disruption, vascular injury); reserved for pre-interventional planning or complex cases.
5.6 Artificial Intelligence (AI)
1) Advantages: Machine learning algorithms (e.g., SNAP-based segmentation) automate plaque component detection (IPH, LRNC, FC), reducing observer variability.
2) Future Directions: Multimodal imaging combined with AI enhances risk stratification and personalized management.
5.7 Summary
Diagnostic strategies should integrate clinical needs and technical strengths, as listed in Table 2.
1) Screening and Follow-up: Begin with US, supplemented by CTA or MRI for plaque characterization.
2) High-Risk Plaque Evaluation: Prioritize HR-MRI or PET/CT for metabolic and morphologic profiling.
3) Pre-Interventional Planning: Combine IVUS, DSA, and CTA for precision.
Diagnostic Methods for Carotid Atherosclerotic Plaques
| Imaging Modality | Technique | Advantages | Limitations | Clinical Applications |
|---|---|---|---|---|
| I. Ultrasound | (1) Conventional US | Non-invasive assessment of IMT (≥1.5mm defines plaque), plaque location/size/echogenicity; sensitive for calcification | Limited differentiation between IPH and LRNC | Annual follow-up for mild-moderate stenosis (<70%); severe stenosis (≥70%) requires combined imaging |
| (2) Contrast-Enhanced US (CEUS) | Microbubble-enhanced visualization of neovascularization/ulceration (sensitivity >80%) | Operator-dependent; calcification artifacts | Requires validation with other techniques | |
| (3) Intravascular US (IVUS) | Quantitative plaque burden assessment; identifies LRNC/calcification | Limited evaluation of fibrous cap thickness | Pre-interventional plaque morphology assessment | |
| (4) Transcranial Doppler (TCD) | Real-time monitoring of cerebral hemodynamics/microemboli (HITS) | Requires adequate acoustic bone window | Combined use for stroke risk assessment | |
| II. CT Imaging | (1) CTA | High spatial resolution for stenosis/calcification/ulcer (depth >1mm); calcification CT value >130 HU | - | Evaluation of complex vascular anatomy and calcified plaques |
| (2) Spectral CT (GSCT) | Spectral curves distinguish LRNC (rising), IPH (attenuating), calcification (falling); reduces metal artifacts | Radiation exposure | Detailed analysis of calcified plaques | |
| III. MRI | (1) HR-MRI | Superior soft-tissue contrast for vulnerable plaque features: thin fibrous cap (FC<0.7mm), IPH (T1WI hyperintensity), LRNC (T1WI non-enhancement) | - | Plaque composition analysis and risk stratification in symptomatic carotid stenosis |
| (2) MR-VWI | 3D plaque volume/component quantification (3D-MERGE/SNAP sequences) | - | Annual monitoring for moderate-severe stenosis progression | |
| IV. Nuclear Imaging | PET | 18F-FDG/18F-NaF detects inflammation/microcalcification; SCAIL score (specificity 90%) | High cost | Research/high-risk patient metabolic evaluation |
| V. DSA | DSA | Gold standard for stenosis/collateral circulation/plaque morphology | Invasive risks (plaque dislodgement, vascular injury) | Pre-interventional assessment or complex cases |
| VI. AI Technology | Machine Learning Algorithms | Automated plaque segmentation (SNAP sequences) for IPH/LRNC/FC identification; reduces human error | - | Multimodal imaging integration improves risk assessment efficiency and personalized care |
6. Treatment
6.1 General treatment
General management primarily involves lifestyle modifications. A healthy lifestyle includes a balanced diet, regular physical activity, weight control, smoking cessation, and limited alcohol consumption, all of which contribute to reducing cardiovascular risk factors such as hypertension and hyperlipidemia [54, 55]. Lifestyle modifications play a crucial role in the prevention and management of carotid atherosclerotic plaques and stroke.
6.1.1 Balanced Diet
A well-structured diet is a key component of lifestyle management. The Mediterranean diet has been shown to reduce the risk of cardiovascular events, particularly stroke. This diet emphasizes the consumption of fresh vegetables and fruits (especially green leafy vegetables), whole grains, and fish (preferably those rich in omega-3 fatty acids). It recommends limited intake of red meat and poultry, substitution of high-fat dairy products with low-fat or skimmed dairy, and the inclusion of olive oil, nuts, and moderate amounts of red wine [56-58]. Increasing the intake of whole grains, legumes, and tubers; fresh vegetables and fruits; fish; and moderate amounts of eggs, soy products, dairy, and nuts is beneficial [59-64]. Additionally, moderate tea consumption has been associated with cardiovascular benefits [65-67]. Reducing saturated fat intake, limiting salt consumption (< 5 g/day), minimizing processed meats, controlling cholesterol intake, and avoiding sugar-sweetened beverages and trans fats contribute to reducing the risk of cardiometabolic diseases [68-70].
A diet primarily based on grains is the foundation of balanced nutrition. General dietary recommendations for the population include daily intake of 250-400 g of grains and tubers, including 50-150 g of whole grains and legumes and 50-100 g of tubers. Daily fresh vegetable intake should be ≥ 500 g, with dark-colored vegetables comprising at least half, and fresh fruit intake should be 200-350 g, as fruit juice cannot replace whole fruit. Weekly fish intake should be ≥300 g, prepared using non-frying methods such as boiling or steaming to preserve nutrients [71].
Daily meat consumption should range between 40-75 g, with limited intake of red meat (e.g., pork, beef, lamb). Egg consumption should be 3-6 per week. For individuals with hypercholesterolemia and those at high cardiovascular risk, daily cholesterol intake should be < 300 mg (approximately the amount in one egg yolk) [72-74]. If other high-cholesterol foods such as organ meats, red meat, or shrimp are consumed, egg intake should be reduced accordingly. Other recommendations include a daily soybean intake of 25 g, a weekly nut intake of 50-70 g, and a daily dairy intake of 150-300 g. Sugar-sweetened beverages should be avoided. Moderate tea consumption is recommended, with a monthly tea intake of 50-250 g, preferably green tea, while avoiding excessive consumption of strong tea over long periods.
6.1.2 Moderate Exercise
The detrimental effects of a sedentary lifestyle on health have been well-documented in multiple studies, underscoring the importance of minimizing prolonged sitting [75]. Regular and sustained physical activity has been associated with a reduced risk of cardiovascular events. In asymptomatic patients undergoing carotid endarterectomy, consistent exercise serves as a crucial factor in preventing cerebrovascular disease and mortality [76]. The type and intensity of exercise should be tailored to individual needs and follow a progressive approach [77]. Adults are recommended to engage in brisk walking, jogging, swimming, or cycling, accumulating at least 150 minutes of moderate-intensity or 75 minutes of high-intensity physical activity per week. For older adults, activities such as Tai Chi may be beneficial [78].
6.1.3 Weight Management
Obesity (BMI ≥ 30 kg/m2; Asia: BMI ≥ 27.5 kg/m2) and overweight status (25.0 kg/m2 ≤ BMI < 30 kg/m2; Asia: 23.0 kg/m2 ≤ BMI < 27.5 kg/m2) are associated with an increased risk of cardiovascular and cerebrovascular diseases compared to individuals with normal weight [79]. In particular, visceral obesity has been identified as a novel risk factor for atherosclerosis and cardiovascular disease [80]. Weight control through caloric restriction and increased physical activity has been shown to mitigate cardiovascular event risk [81]. Recommended daily caloric intake for weight management is 6,276-7,530 kJ/day (1,500-1,800 kcal/day) for men and 5,020-6,276 kJ/day (1,200-1,500 kcal/day) for women [82].
6.2 Drug treatment
Early studies suggested that surgical intervention provided a significant advantage over medical therapy alone in patients with asymptomatic carotid atherosclerotic plaques [83]. However, with advancements in pharmacological treatments, recent evidence indicates that medical therapy is becoming equally effective as vascular reconstruction [84]. In patients with asymptomatic carotid stenosis, carotid endarterectomy (CEA) or carotid artery stenting (CAS) does not provide a significant advantage over optimal medical therapy in preventing ischemic stroke within one year [85], nor does it differ in long-term mortality risk [86]. Therefore, medical therapy should be implemented in both symptomatic and asymptomatic carotid stenosis [87]. The three pillars of pharmacological management include antiplatelet therapy, antihypertensive therapy, and lipid-lowering therapy. All patients diagnosed with carotid atherosclerotic disease should receive antiplatelet agents and lipid-lowering drugs, regardless of their serum cholesterol levels.
6.2.1 Antiplatelet
Antiplatelet therapy inhibits platelet adhesion and aggregation, preventing thrombus formation and mitigating the progression of vascular occlusive disease. Although randomized controlled trials have not confirmed that antiplatelet therapy reduces stroke risk in asymptomatic carotid stenosis, long-term use of low-dose aspirin has been shown to reduce myocardial infarction and vascular mortality. Furthermore, perioperative low-dose aspirin therapy can lower the risk of stroke [87-90]. Consequently, all patients with carotid stenosis should receive antiplatelet therapy. Commonly used agents include enteric-coated aspirin, clopidogrel, and ticagrelor. The main adverse effects are gastrointestinal symptoms and an increased risk of bleeding. Some studies have also reported a positive correlation between antiplatelet therapy and a higher incidence of intraplaque hemorrhage (IPH), with prolonged antiplatelet use associated with increased IPH frequency. Furthermore, daily doses exceeding 1.0 mg have been linked to a higher occurrence of IPH [91].
6.2.2 Lipid lowering
Lipid-lowering therapy is the cornerstone of managing carotid atherosclerotic plaques. The protective effects of statins extend beyond patients with hypercholesterolemia [92-94]. In a subgroup of 1,007 patients with an average carotid stenosis of 51%, high-intensity lipid-lowering therapy led to a remarkable 33% reduction in the risk of any stroke. Beyond stabilizing plaques, lipid-lowering therapy can potentially "reverse" plaques, reducing their volume, modifying their composition, and suppressing inflammatory responses. This multifaceted approach inhibits the progression of coronary atherosclerosis and reduces cardiovascular events [95-97].
The primary goal of lipid-lowering therapy is to reduce low-density lipoprotein cholesterol (LDL-C) levels. Statins are the mainstay of treatment and should be taken consistently over the long term. All patients with carotid stenosis should receive statin therapy. For patients with unstable plaques or a high risk of stroke, intensive lipid-lowering therapy is recommended, targeting LDL-C levels below 1.8 mmol/L or achieving at least a 50% reduction in LDL-C levels if baseline levels range from 1.8 to 3.5 mmol/L [98]. If statin monotherapy is insufficient, ezetimibe or other lipid-lowering agents may be added [99].
The evaluation of cholesterol-lowering drug selection encompasses two critical dimensions: unjustified statin non-use assessment (rationality of avoiding statins) and contraindication assessment (appropriateness of drug selection in cases of contraindications or special physiological states).
① Unjustified Statin Non-Use Assessment
Current international guidelines [100-102] unequivocally designate statins as first-line therapy for carotid atherosclerosis with plaque. Statins should be prioritized and maintained in treatment regimens unless contraindications or intolerance exist. Notably, in patients with severe carotid stenosis (> 70%), high-intensity statin initiation warrants caution regarding intraplaque hemorrhage risk, recommending halved starting doses with neurological symptom monitoring [102].
Stating intolerance is defined by meeting all four criteria [103]
a) Clinical manifestations: Symptomatic adverse reactions or abnormal laboratory markers.
b) Drug exposure: Intolerance to ≥ 2 statins, with at least one administered at the minimum daily dose.
c) Temporal causality: Adverse events occurring post-initiation/dose escalation, resolving upon discontinuation and recurring upon rechallenge.
d) Exclusion of confounders: Ruling out comorbidities, drug interactions, or other causative factors.
For patients failing to achieve LDL-C targets with statin monotherapy, combination therapy with non-statin agents (e.g., cholesterol absorption inhibitors, PCSK9 inhibitors) is recommended. Expected LDL-C reductions are stratified as shown in Table 3:
Stations: Dose-dependent reductions per Table 3
Cholesterol absorption inhibitors: 15-22% reduction monotherapy; additive 21-27% reduction when combined with statins. PCSK9 inhibitors: 47-57% reduction monotherapy; synergistic 44-75% reduction in combination regimens.
Expected LDL-C Reduction Rates of Different Types and Doses of Statins
| Drug class | Low intensity | Moderate intensity | High intensity |
|---|---|---|---|
| Amount of LDL-Ca lowering | < 30% | 30 to 49% | ≥ 50% |
| Atorvastatin | - | 10 to 20 mg | 40 to 80 mg |
| Rosuvastatin | - | 5 to 10 mg | 20 to 40 mg |
| Lovastatin | 20 mg | 40 to 80 mg | - |
| Pravastatin | 20 mg | 40 to 80 mg | - |
| Fluvastatin | 40 mg | 80 mg | - |
| Simvastatin | 10 mg | 20 to 40 mg | - |
| Pitavastatin | 1 | 1 to 4 mg | - |
aLCL-C: low-density lipoprotein cholesterol; "-" indicates no available data.
② Contraindication Assessment
Drug selection must account for contraindications and physiological constraints (e.g., pregnancy, hepatic/renal impairment, geriatric status) as detailed in Table 4. Therapeutic decisions should integrate patient-specific characteristics and prescribing restrictions from official drug labels.
Evaluation Recommendations
Unjustified statin non-use: Identified when statins are omitted despite absence of contraindications or intolerance.
Contraindication violation: Determined when contraindicated agents are prescribed without appropriate clinical justification.
Dosage and Administration Evaluation
The evaluation of cholesterol-lowering drug regimens requires comprehensive understanding of dosing parameters including standard/maximum doses, administration frequency, timing, and treatment duration. Key principles include:
① Dosage Compliance
Prescribed doses must adhere to drug labeling specifications, particularly in patients with hepatic or renal impairment (see Table 5 for dose adjustment criteria).
② Administration Protocol
Short-acting statins (simvastatin, lovastatin, pitavastatin, fluvastatin, pravastatin): Administered at bedtime to align with circadian cholesterol biosynthesis patterns.
Long-acting statins (atorvastatin, rosuvastatin) and extended-release formulations: Fixed daily timing without circadian restriction.
PCSK9 inhibitors: Subcutaneous injection technique requires rotation between the upper arm (lateral aspect), abdomen (> 5 cm from umbilicus), and thigh, avoiding areas of ecchymosis or induration. Post-injection monitoring mandates carotid ultrasound at 1 month to assess initial plaque response.
③ Treatment Duration
Cholesterol-lowering therapies typically require indefinite continuation without self-directed dose reduction or discontinuation.
Evaluation Recommendations
Regimens are deemed inappropriate if:
Single dose exceeds labeled maximum thresholds.
Unsupervised dose reduction or therapy cessation occurs.
Administration methods deviate from evidence-based protocols.
drugs under different pathophysiological conditions
| Drug class | Children(age/year) | Pregnancy | Lactation | Elderly | Hepatic Impairment | Renal Impairment | |||
|---|---|---|---|---|---|---|---|---|---|
| <10 | >10 | Mild | Moderate | Severe | |||||
| Atorvastatin | △ | √ | × | × | √ | ×# | √ | √ | √ |
| Rosuvastatin | △ | √ | × | × | √ | ×# | √ | √ | × |
| Lovastatin | △ | √ (< 40 mg/day) | × | × | √ | ×# | √ | √ | △ |
| Pravastatin | △ | √ (< 40 mg/day) | × | × | √ | ×# | √ | √ | △ |
| Fluvastatin | △ | √ | × | × | √ | ×# | √ | √ | × |
| Simvastatin | △ | √ | × | × | √ | ×# | √ | √ | △ |
| Pitavastatin | △ | √ | × | × | √ | ×# | √ | △ | △ |
| Ezetimibe | △ | √ | △ | △ | √ | ×# | √ | √ | √ |
| Alirocumab | √ | √ | △ | × | √ | △* | √ | √ | △* |
| Evolocumab | △ | △ | △ | × | √ | △* | √ | √ | √ |
×: Contraindicated; △: Use with caution; requires thorough risk-benefit assessment by physicians and careful review of prescribing information; √: Safe for routine use; #: Contraindicated in active liver disease, decompensated cirrhosis, acute liver failure, or unexplained persistent elevation of transaminases (≥3× upper limit of normal); *: Limited data in severe hepatic impairment; use with caution; +: Limited data in corresponding degrees of renal impairment; use with caution.
Adverse Reaction Evaluation
The assessment of adverse reactions in cholesterol-lowering therapy involves identifying and managing both existing and potential medication-related risks to ensure therapeutic safety. Adverse effects associated with lipid-lowering agents, particularly statins, primarily stem from two aspects: ① the intrinsic harm of the reactions themselves and ② overestimation of harm leading to unnecessary treatment cessation. While most patients tolerate cholesterol-lowering therapies well, a minority may experience adverse events requiring prompt identification and intervention. Conversely, excessive concern over adverse effects often results in premature discontinuation, inadvertently increasing the risk of ischemic stroke and carotid plaque progression. Clinical studies indicate that nearly half of patients discontinue statins during long-term treatment, with safety concerns being a key contributing factor [104, 105]. A recent meta-analysis of 4 million patients in primary and secondary cardiovascular prevention revealed that true statin intolerance occurs in fewer than 10% of cases [106-108], underscoring the urgent need to address disproportionate patient anxiety regarding medication safety. Common adverse reactions and their clinical features are summarized in Table 6 [29, 109], while table 6 outlines evidence-based protocols for monitoring and managing statin-related hepatic, muscular, and glycemic safety.
During adverse reaction management, causality assessment is critical and may utilize standardized tools such as the National Medical Products Administration Adverse Reaction Evaluation Criteria [110] or the Statin-Associated Muscle Symptom Clinical Indexto determine the likelihood of drug-related causality. These evaluations inform clinical decision-making by differentiating true adverse drug reactions from coincidental symptoms.
Dosing and drug Administration of lipid-lowering drugs
| Drug class | Typical Dose | Maximum Dose | Administration | Food Effect | ||
|---|---|---|---|---|---|---|
| Adult | Children (aged ≥10 years) | Adult | Children (aged ≥10 years) | |||
| Atorvastatin | 10 to 20 mg/d | 5 to 10 mg/d | 80 mg/d | 20 mg/d | Take any time | None |
| Rosuvastatin | 5 to 10 mg/d | 5 mg/d | 20 mg/d | 20 mg/d | Take any time | None |
| Lovastatin | 40 mg/d | 10 mg/d | 80 mg/d | 40 mg/d | With dinner | Increased absorption |
| Pravastatin | 40 mg/d | 10 mg/d | 40 mg/d | 40 mg/d (aged 14 to 18 years) 20 mg/d (aged 8 to 13 years) | At bedtime | absorption |
| Fluvastatin | 80 mg/d | 20 mg/d | 80 mg/d | 80 mg/d | At bedtime | Negligible |
| Simvastatin | 20 to 40 mg/d | 10 mg/d (aged ≥10 years) 5 mg/d (aged 10 years) | 80 mg/d | 40 mg/d | At bedtime | None |
| Pitavastatin | 2 to 4 mg/d | - | 4 mg/d | - | Take any time | Decreases |
| Ezetimibe | 10 mg/d | 10 mg/d | 10 mg/d | 10 mg/d | Take any time | None |
| Alirocumab | 75mg, every 2 weeks | - | 150 mg, every 2 weeks or 300 mg, every 4 weeks | - | subcutaneous injection | None |
| Evolocumab | 140 mg, every 2 weeks | - | 420 mg, every 4 weeks | - | subcutaneous injection | None |
Major side effects of lipid-lowering drugs
| Drug class | Major side effects |
|---|---|
| Statins | Musculoskeletal complications (frequency varies by agent): Myalgia (most common); Myopathy (1-5% in clinical trials); Rhabdomyolysis (rare, <0.1%); Hepatocellular effects: Asymptomatic transaminase elevation (ALT/AST >3× ULN in 0.5-2%); Alkaline phosphatase increase (dose-dependent); Gastrointestinal disturbances: Nausea, diarrhea (5-10%); Constipation, dyspepsia. Neurologic manifestations: Headache (2-5%); Sleep pattern disruption. |
| Cholesterol absorption inhibitor | Clinical manifestations: Typically, mild and transient, including: Headache (incidence 5-8%); Asthenia/fatigue (3-6%); Gastrointestinal disturbances (nausea, diarrhea; 4-7%); Myalgia (2-4%); Asymptomatic transaminase elevation (ALT/AST >1.5× ULN in 1-3%). Overall adverse event rates were generally comparable to placebo groups in clinical trials (p>0.05) |
| PCSK9 inhibitors | Characteristic reactions: Injection-site reactions (pain, erythema, pruritus; 10-15%); Systemic flu-like symptoms (myalgia, pyrexia; 5-8%); Hypersensitivity manifestations (rash, urticaria; <1%). Frequency and severity of adverse effects demonstrated non-inferiority to placebo controls across Phase III studies |
| Adenosine triphosphate citrate lyase inhibitor | Hyperuricemia: May require serum uric acid monitoring (≥7.0 mg/dL in 3-8% of cases). Myalgia/Arthralgia: Mild-to-moderate muscle pain (5-12% incidence); Joint stiffness (3-7%), typically dose-dependent. Muscle spasms: Predominantly nocturnal (2-4%). Tendon rupture: Rare but serious (<0.3%), predominantly affecting Achilles tendon. Aspartate aminotransferase (AST) elevation: ≥3× ULN in 1.5-4% of patients; Requires monitoring (baseline + q12 weeks). |
Clinical Recommendations: Adverse reaction assessments should integrate symptomatic manifestations, laboratory findings (e.g., liver enzymes, creatine kinase, glucose levels), and characteristic drug-specific toxicity profiles to guide timely interventions. Clinicians are advised to prioritize patient education to mitigate unwarranted treatment discontinuation while maintaining rigorous biochemical and clinical surveillance aligned with guideline recommendations.
Interaction Evaluation
The evaluation of drug-drug and drug-food interactions in cholesterol-lowering therapy focuses on identifying risks of clinically significant pharmacokinetic or pharmacodynamic interactions and providing actionable mitigation strategies. Stains exhibit two predominant interaction mechanisms:
1. Metabolic Inhibition: Concomitant use with CYP3A4 inhibitors (e.g., macrolides, azole antifungals) reduces the metabolism of CYP3A4-dependent statins (simvastatin, lovastatin), leading to elevated systemic exposure and toxicity risks.
2. Transporter Inhibition: Drugs inhibiting organic anion-transporting polypeptide 1B1 (OATP1B1), such as cyclosporine or gemfibrozil, impair hepatic statin uptake, increasing plasma concentrations and myopathy risks.
3. Cholesterol absorption inhibitors (e.g., ezetimibe) demonstrate limited interactions but require avoidance of gemfibrozil co-administration due to heightened risks of myotoxicity and cholelithiasis. Special caution is warranted for CYP2C19-metabolized statins (e.g., rosuvastatin), which may attenuate clopidogrel's antiplatelet efficacy, elevating stent thrombosis risks. PCSK9 inhibitors currently show no clinically relevant drug-food interactions. Clinically actionable interactions and management recommendations, derived from drug labeling and Lexicomp® database analyses, are summarized in Table 7.
Common clinically significant drug/food interactions with cholesterol-lowering drugs
| Drug / Food | Atorvastatin | Rosuvastatin | Lovastatin | Pravastatin | Fluvastatin | Simvastatin | Pitavastatin | Ezetimibe |
|---|---|---|---|---|---|---|---|---|
| Antimicrobials | ||||||||
| Erythromycin | √ | √ | × | (40) | √ | × | (1) | √ |
| Itraconazole | (20) | √ | × | √ | √ | × | √ | √ |
| Ketoconazole | √ | √ | × | √ | √ | × | √ | √ |
| Fluconazole | √ | √ | √ | √ | (40) | √ | ○ | √ |
| Rifampin/Rifampicin | √ | ○ | √ | √ | √ | √ | (2) | √ |
| Clarithromycin | (20) | √ | × | (40) | √ | × | ○ | √ |
| Voriconazole | √ | √ | × | √ | √ | × | √ | √ |
| Fusidic acid | × | × | × | × | × | × | √ | |
| Posaconazole | √ | × | √ | √ | × | √ | √ | |
| Daptomycin | √ | √ | √ | √ | √ | × | √ | √ |
| Cardiovascular Agents | ||||||||
| Verapamil | ○ | √ | (20) | √ | √ | (10) | √ | √ |
| Amiodarone | √ | √ | (40) | √ | √ | (20) | √ | √ |
| Gemfibrozil | × | × | × | × | × | × | × | × |
| Diltiazem | ○ | √ | (20) | √ | √ | (10) | √ | √ |
| Dronedarone | ○ | √ | (20) | √ | √ | (10) | √ | √ |
| Amlodipine | ○ | √ | ○ | √ | √ | (10) | √ | √ |
| Other Drugs | ||||||||
| Danazol | √ | √ | (20) | √ | √ | × | √ | √ |
| Regorafenib | √ | (5) | √ | √ | √ | √ | √ | √ |
| Cyclosporine | × | × | × | (20) | (40) | × | × | √ |
| Darolutamide | √ | × | √ | √ | √ | √ | √ | √ |
| Foods | ||||||||
| Grapefruit juice | [1.2] | √ | × | √ | √ | × | √ | √ |
| Red yeast rice | × | × | × | × | × | × | × | √ |
×Contraindicated: Avoid concomitant use.√Standard-dose combination allowed: No dose adjustment required.( )Maximum statin dose when combined: [value] mg/day (e.g., "Simvastatin (20)" indicates 20 mg/day maximum).[ ]
Maximum grapefruit juice intake: [value] L/day (applies to statins metabolized by CYP3A4).○Use with caution: Requires thorough risk-benefit assessment by physicians and strict adherence to prescribing information.
Assessment Recommendations
• Interaction Evaluation: Deem an interaction clinically significant if inappropriate drug combinations persist without mitigation.
• Cost-Effectiveness Evaluation: Incorporate regional healthcare economics and individualized patient factors (e.g., insurance coverage, out-of-pocket costs) into therapeutic decision-making.
Adherence Evaluation
Adherence to cholesterol-lowering therapy critically determines clinical outcomes in patients with carotid atherosclerosis and plaque, particularly influencing stroke risk and plaque stability. Studies demonstrate that a 10% improvement in medication adherence correlates with a 12% reduction in carotid plaque-related ischemic stroke incidence [111-115]. However, suboptimal adherence remains prevalent, driven by concerns over adverse drug reactions or asymptomatic dose omissions [105, 116, 117]. Adherence assessments are imperative for patients exhibiting inadequate therapeutic responses.
6.2.3 Surgery
Carotid revascularization includes carotid endarterectomy (CEA) and carotid artery stenting (CAS). CEA remains the gold standard surgical treatment for carotid artery disease, significantly reducing mortality and disability rates.
CAS serves as a minimally invasive and effective alternative, with comparable long-term outcomes to CEA [118-122]. However, CAS is associated with a lower periprocedural risk [119, 123, 124]. The two procedures are complementary, and the decision to pursue revascularization should consider the degree of stenosis, symptom status, and the institution's perioperative risk management capabilities [120, 125, 126]. For patients with mild carotid stenosis, medical therapy is recommended (i.e., <50% stenosis in symptomatic patients and <60% in asymptomatic patients). Patients with symptomatic moderate stenosis should be considered for CEA, while those with severe stenosis are advised to undergo CEA. For selected patients aged ≥75 years with ≥60% asymptomatic carotid stenosis, an expected survival of at least five years, and a high stroke risk despite optimal medical therapy, CEA may be recommended following a multidisciplinary evaluation of risks and benefits. CAS may be considered as an alternative to CEA in symptomatic carotid stenosis, particularly in patients younger than 70 years [99, 125, 127].
6.2.4 Traditional Chinese Medicine
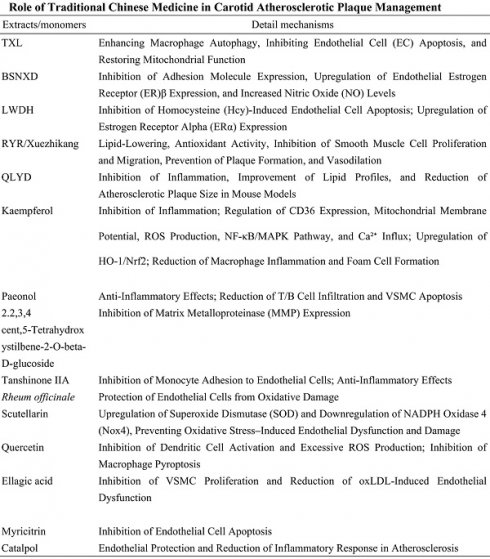
Traditional Chinese Medicine (TCM) is widely used in China and many Asian countries for the treatment of carotid atherosclerotic plaques. Numerous studies have demonstrated the efficacy of integrating TCM with Western medicine in managing carotid atherosclerosis. Clinically, TCM may be used either alone or in combination with Western therapies, particularly benefiting patients who are intolerant or resistant to statin therapy, as listed in Table 8 [128-134].
Tongxinluo (TXL)
Tongxinluo (TXL) is a traditional herbal compound comprising ginseng, leech, frankincense, and borneol. The primary active components include paeoniflorin and ginsenosides. TXL inhibits the progression of atherosclerotic plaques and stabilizes existing plaques by enancing macrophage autophagy, suppressing endothelial cell (EC) apoptosis, and restoring mitochondrial function. These mechanisms are critical in the treatment of atherosclerosis [135-137].
Bu-Shen-Ning-Xin Decoction (BSNXD)
Bu-Shen-Ning-Xin Decoction (BSNXD) is a traditional Chinese patent medicine used for decades to prevent estrogen deficiency-induced atherosclerosis. Wang et al. reported that BSNXD administration mitigated early atherosclerotic pathological damage by suppressing adhesion molecule expression, upregulating endothelial estrogen receptor β (ERβ) expression, and increasing serum nitric oxide (NO) levels in ovariectomized rabbits. Notably, BSNXD did not affect serum lipid profiles, endometrial thickness, or hepatic lipid deposition. ERβ modulates target gene expression to protect endothelial cells, with its expression in the endothelium associated with atherosclerosis in postmenopausal women [138-140].
Liuwei Dihuang (LWDH)
Liuwei Dihuang (LWDH) is a classic herbal formula with over 1,000 years of history, comprising six herbs: Rehmannia root, Cornus officinalis fruit, Dioscorea tuber, Poria cocos sclerotium, Chinese yam rhizome, and Paeonia root bark. LWDH inhibits homocysteine (Hcy)-induced endothelial apoptosis by modulating the GPR30 pathway, significantly reducing plaque formation in postmenopausal atherosclerotic animal models. Additionally, LWDH suppresses DNMT1-dependent ERα methylation, enhancing estrogen receptor α (ERα) expression to prevent atherosclerosis [141-145].
Role of Traditional Chinese Medicine in Carotid Atherosclerotic Plaque Management
| Extracts/monomers | Detail mechanisms | Refs. |
|---|---|---|
| TXL | Enhancing Macrophage Autophagy, Inhibiting Endothelial Cell (EC) Apoptosis, and Restoring Mitochondrial Function | [135, 136] |
| BSNXD | Inhibition of Adhesion Molecule Expression, Upregulation of Endothelial Estrogen Receptor (ER)β Expression, and Increased Nitric Oxide (NO) Levels | [138] |
| LWDH | Inhibition of Homocysteine (Hcy)-Induced Endothelial Cell Apoptosis; Upregulation of Estrogen Receptor Alpha (ERα) Expression | [141, 142] |
| RYR/Xuezhikang | Lipid-Lowering, Antioxidant Activity, Inhibition of Smooth Muscle Cell Proliferation and Migration, Prevention of Plaque Formation, and Vasodilation | [146, 150, 152] |
| QLYD | Inhibition of Inflammation, Improvement of Lipid Profiles, and Reduction of Atherosclerotic Plaque Size in Mouse Models | [154, 155, 157] |
| Kaempferol | Inhibition of Inflammation; Regulation of CD36 Expression, Mitochondrial Membrane Potential, ROS Production, NF-κB/MAPK Pathway, and Ca²⁺ Influx; Upregulation of HO-1/Nrf2; Reduction of Macrophage Inflammation and Foam Cell Formation | [162, 163, 166] |
| Paeonol | Anti-Inflammatory Effects; Reduction of T/B Cell Infiltration and VSMC Apoptosis | [167, 168] |
| 2.2,3,4 cent,5-Tetrahydroxystilbene-2-O-beta-D-glucoside | Inhibition of Matrix Metalloproteinase (MMP) Expression | [169] |
| Tanshinone IIA | Inhibition of Monocyte Adhesion to Endothelial Cells; Anti-Inflammatory Effects | [172] |
| Rheum officinale | Protection of Endothelial Cells from Oxidative Damage | [174] |
| Scutellarin | Upregulation of Superoxide Dismutase (SOD) and Downregulation of NADPH Oxidase 4 (Nox4), Preventing Oxidative Stress-Induced Endothelial Dysfunction and Damage | [176] |
| Quercetin | Inhibition of Dendritic Cell Activation and Excessive ROS Production; Inhibition of Macrophage Pyroptosis | [177, 178] |
| Ellagic acid | Inhibition of VSMC Proliferation and Reduction of oxLDL-Induced Endothelial Dysfunction | [185] |
| Myricitrin | Inhibition of Endothelial Cell Apoptosis | [186, 187] |
| Catalpol | Endothelial Protection and Reduction of Inflammatory Response in Atherosclerosis | [189, 190] |
Red Yeast Rice (RYR)
Red Yeast Rice (RYR) is a traditional Chinese medicinal product made by fermenting Monascus purpureus with rice. RYR effectively reduces low-density lipoprotein cholesterol (LDL-C) levels and other cardiovascular risk factors, demonstrating efficacy and safety in carotid atherosclerosis management [146-149]. Xuezhikang, a red yeast rice extract containing 13 monoterpenoid compounds and other bioactive ingredients, has been evaluated in a meta-analysis of 20 randomized controlled trials. Daily doses of 1200-4800 mg, over 6-168 weeks, were associated with LDL-C reduction of approximately -1.02 mmol/L (range, -1.20 to -0.83), triglyceride reduction by -0.26 mmol/L (-0.35 to -0.17), total cholesterol (TC) reduction by -1.0 mmol/L (-1.23 to -0.77), and HDL-C elevation by 0.07 mmol/L (0.03-0.11). The LDL-C reduction with RYR is comparable to low-dose statins, such as pravastatin 40 mg, simvastatin 10 mg, and lovastatin 20 mg [150, 151]. The anti-atherosclerotic effects of Xuezhikang are attributed to lipid-lowering properties, antioxidant activity, inhibition of vascular smooth muscle cell (VSMC) proliferation and migration, plaque formation prevention, and vasodilation [152, 153].
Scutellariae Radix-Coptidis Rhizoma (QLYD)
Scutellariae Radix-Coptidis Rhizoma (QLYD) represents a core herbal combination in traditional Chinese medicine (TCM) formulations for the treatment of atherosclerosis. This combination exhibits immunomodulatory, anti-inflammatory, and glucose-lipid metabolism regulatory effects [154-156]. Studies have demonstrated that QLYD prevents and treats atherosclerosis by inhibiting inflammation, improving lipid profiles, and reducing plaque area in atherosclerotic mouse models [157-159].
Kaempferol
Kaempferol, a naturally occurring flavonoid found in various medicinal herbs, exhibits broad biological activities, including anti-inflammatory, antioxidant, cardioprotective, and neuroprotective effects [160, 161]. Epidemiological studies suggest an inverse relationship between dietary kaempferol intake and cardiovascular disease risk [160, 162]. In high-fat diet (HFD)-fed ApoE-/- mice, kaempferol improves vascular morphology, lipid profiles, and suppresses inflammatory responses [163-165]. Mechanistically, kaempferol mitigates atherosclerosis by inhibiting the Piezo1 channel and Ca²⁺ influx, regulating mitochondrial membrane potential, and reducing CD36-mediated mitochondrial reactive oxygen species (ROS) production. These effects subsequently modulate the NF-κB/MAPK and HO-1/Nrf2 signaling pathways, reducing macrophage inflammation and foam cell formation [166].
Paeonol (PAE)
Paeonol (PAE)is a phenolic compound extracted from the cortex or roots of Paeonia lactiflora Pallas, known for its anti-atherosclerotic properties. PAE exerts anti-inflammatory effects by inhibiting ox-LDL-induced miR-21 expression and TNF-α release while restoring ox-LDL-mediated suppression of PTEN expression in rat vascular endothelial cells (VECs) in vitro [167]. In ApoE-/- mice, PAE significantly suppresses atherosclerotic progression and stabilizes vulnerable plaques by reducing T/B cell infiltration and vascular smooth muscle cell (VSMC) apoptosis [168].
2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside (TSG)
TSG, extracted from Polygonum multiflorum, reduces atherosclerosis by inhibiting matrix metalloproteinase (MMP) expression and lowering lipid levels, thus preventing plaque development [169].
Tanshinone IIA
Tanshinone IIA, a diterpenoid compound derived from Salvia miltiorrhiza (Danshen), is used extensively in TCM for cardiovascular diseases. Tanshinone IIA exhibits antioxidant, anti-inflammatory, and anti-angiogenic properties [170, 171]. It mitigates atherosclerosis by inhibiting THP-1 monocyte adhesion to TNF-α-stimulated human vascular endothelial cells, reducing TNF-α-induced fractalkine/CX3CL1 mRNA expression and soluble fractalkine levels. Additionally, Tanshinone IIA suppresses TNF-α-induced NF-κB nuclear translocation, thereby reducing monocyte-endothelial adhesion and exerting anti-inflammatory effects [172, 173].
Rheum officinale
Emodin, extracted from Rheum officinale, protects endothelial cells from oxidative injury by downregulating Bid mRNA and caspase-3, -8, and -9 mRNA expression, offering protection against atherosclerosis [174, 175].
Scutellarin
Scutellarin, a major bioactive component of Erigeron breviscapus (Vant.), prevents oxidative stress-induced endothelial dysfunction and endothelial cell injury by upregulating superoxide dismutase (SOD) and downregulating NADPH oxidase 4 (Nox4). Scutellarin's antioxidant properties contribute to the prevention of atherosclerosis in vivo [176].
Quercetin
Quercetin, a naturally occurring antioxidant present in various medicinal herbs, inhibits dendritic cell activation and excessive ROS production by modulating the PI3K/AKT signaling pathway. It mitigates atherosclerosis in high-fructose-fed models [177]. Additionally, quercetin activates the Nrf2 pathway by competitively binding to the Arg483 site of KEAP1, inhibiting macrophage pyroptosis and providing protective effects against atherosclerosis [178, 179].
Ellagic Acid (EA)
Ellagic Acid (EA) is a naturally occurring phenolic compound found abundantly in medicinal herbs, with the highest concentration in raspberries [180]. EA mitigates oxidative stress by reducing plasma and macrophage lipid peroxidation and preventing endothelial inflammation [181-184]. Mechanistically, EA inhibits platelet-derived growth factor receptor beta (PDGFR-β) tyrosine phosphorylation, reduces intracellular reactive oxygen species (ROS) production, and suppresses downstream activation of extracellular signal-regulated kinase 1/2 (ERK1/2). This blockade prevents the expression of cyclin D1 in vascular smooth muscle cells (VSMCs), inhibiting their proliferation and alleviating atherosclerosis [185]. Additionally, EA protects endothelial cells by inhibiting NADPH oxidase-induced superoxide overproduction, downregulating inducible nitric oxide synthase (iNOS) to suppress nitric oxide (NO) release, enhancing cellular antioxidant defenses, and attenuating oxLDL-induced LOX-1 upregulation and eNOS downregulation.
Myricitrin
Myricitrin, a natural flavonoid isolated from the root bark of Myrica cerifera, exhibits potent antioxidant effects. Myricitrin inhibits ROS-induced endothelial cell apoptosis by modulating antioxidant enzyme activity and the expression of apoptosis-related genes, effectively suppressing early atherosclerotic plaque formation. Moreover, myricitrin reduces lipid peroxidation, blocks NO release, and maintains mitochondrial membrane potential, collectively inhibiting endothelial cell apoptosis and reducing lesion size in ApoE-/- mice [186]. Additionally, myricitrin preserves endothelial cell integrity by optimizing the balance between pro- and anti-apoptotic proteins, including Bax, Bad, XIAP, cIAP-2, and survivin, thereby protecting against oxLDL-induced apoptosis [187, 188].
Catalpol
Catalpol, an active compound extracted from Rehmannia glutinosa, possesses anti-inflammatory, antioxidant, and insulin-sensitizing properties. Catalpol protects endothelial cells and mitigates atherosclerosis by reducing homocysteine (HCY)-induced endoplasmic reticulum (ER) stress, suppressing NADPH oxidase 4 (NOX4) expression, and blocking the NF-κB/p65 signaling pathway [189]. Furthermore, catalpol exhibits anti-inflammatory effects by modulating estrogen receptor alpha (ERα), reducing pro-inflammatory cytokines (C-reactive protein, TNF-α, and IL-1β), and increasing anti-inflammatory cytokine IL-10 levels, thereby attenuating the inflammatory response in atherosclerosis [190-192].
Funding
Non communicable Chronic Diseases-National Science and Technology Major Project (No. 2024ZD0528200).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e143
2. Romaguera R, Gomez-Lara J, Gomez-Hospital JA. Letter by Romaguera et al Regarding Article, "Newer Generation Ultrathin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease: Meta-Analysis of Randomized Trials". Circulation. 2019;139:2081-2
3. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F. et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-99
4. Rattanavipanon W, Chaiyasothi T, Puchsaka P, Mungkornkaew R, Nathisuwan S, Veettil SK. et al. Effects of pharmacist interventions on cardiovascular risk factors and outcomes: An umbrella review of meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2022;88:3064-77
5. Dixon DL, Khaddage S, Bhagat S, Koenig RA, Salgado TM, Baker WL. Effect of pharmacist interventions on reducing low-density lipoprotein cholesterol (LDL-C) levels: A systematic review and meta-analysis. J Clin Lipidol. 2020;14:282-92.e4
6. Clarke R, Du H, Kurmi O, Parish S, Yang M, Arnold M. et al. Burden of carotid artery atherosclerosis in chinese adults: implications for future risk of cardiovascular diseases. European Journal of Preventive Cardiology. 2017;24:647-56
7. Xing L, Li R, Zhang S, Li D, Dong B, Zhou H. et al. High burden of carotid atherosclerosis in rural northeast China: a population-based study. Front Neurol. 2021;12:597992
8. Oliviero U, Orefice G, Coppola G, Scherillo G, Ascione S, Casaburi C. et al. Carotid atherosclerosis and ischemic stroke in young patients. International Angiology: A Journal of the International Union of Angiology. 2002;21:117-22
9. Panayiotou AG, Kouis P, Griffin M, Nicolaides AN. Comparison between insulin resistance indices and carotid and femoral atherosclerosis: A cross-sectional population study. International Angiology: A Journal of the International Union of Angiology. 2015;34:437-44
10. Zhao B, Chen A, Wang H, Cui J, Sun Y, Xu L. et al. The relationship between sclerostin and carotid artery atherosclerosis in patients with stage 3-5 chronic kidney disease. Int Urol Nephrol. 2020;52:1329-36
11. Katsargyris A, Theocharis SE, Tsiodras S, Giaginis K, Bastounis E, Klonaris C. Enhanced TLR4 endothelial cell immunohistochemical expression in symptomatic carotid atherosclerotic plaques. Expert Opin Ther Targets. 2009;14:1-10
12. Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159-61
13. VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12-22
14. Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng. 2003;5:79-118
15. Fang Y, Wu D, Birukov KG. Mechanosensing and mechanoregulation of endothelial cell functions. Compr Physiol. 2019;9:873-904
16. Yang W, Jung KH, Park KI, Chung M, Ha J, Lee EJ. et al. Pathophysiological link between carotid atherosclerosis and cerebral white matter lesions. Sci Rep. 2025;15:6619
17. Harloff A. Carotid plaque hemodynamics. Interv Neurol. 2012;1:44-54
18. Kwok CHR, Park JC, Joseph SZ, Foster JK, Green DJ, Jansen SJ. Cognition and Cerebral Blood Flow After Extracranial Carotid Revascularization for Carotid Atherosclerosis: A Systematic Review. Clin Ther. 2023;45:1069-76
19. Zuo S, Kong D, Wang C, Liu J, Wang Y, Wan Q. et al. CRTH2 promotes endoplasmic reticulum stress-induced cardiomyocyte apoptosis through m-calpain. EMBO Mol Med. 2018;10:e8237
20. Andueza A, Kumar S, Kim J, Kang D-W, Mumme HL, Perez JI. et al. Endothelial reprogramming by disturbed flow revealed by single-cell RNA and chromatin accessibility study. Cell Rep. 2020;33:108491
21. Tamargo IA, Baek KI, Kim Y, Park C, Jo H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat Rev Cardiol. 2023;20:738-53
22. Poredoš P, Cevc M, Blinc A. Characteristics of atherosclerosis in femoropopliteal artery and its clinical relevance. Atherosclerosis. 2021;335:31-40
23. Slysz J, Sinha A, DeBerge M, Singh S, Avgousti H, Lee I. et al. Single-cell profiling reveals inflammatory polarization of human carotid versus femoral plaque leukocytes. JCI Insight. 2023 8
24. Kunte H, Kunte G, Busch MA, Weichert W, Rückert RI, Harms L. Differences in carotid plaque content of macrophages, T cells and MMP-9 between patients with embolic and hemodynamic cerebral ischemia due to symptomatic carotid stenosis. Atherosclerosis. 2010;211:456-60
25. Kazandjian C, Settembre N, Lareyre F, Kretz B, Soudry-Faure A, Béjot Y, Malikov S, Hassen-Khodja R, Jean-Baptiste E, Steinmetz E. Cerebral Infarct Topography and Early Outcome after Surgery for Symptomatic Carotid Stenosis: A Multicentre Study. Cerebrovasc Dis. 2017;44:291-6
26. Zhang X, Jiao Z, Hua Z, Cao H, Liu S, Zhang L. et al. Localized Elevation of Wall Shear Stress Is Linked to Recent Symptoms in Patients with Carotid Stenosis. Cerebrovasc Dis. 2023;52:283-92
27. Zhang X, Jiao Z, Hua Z, Cao H, Liu S, Zhang L. et al. Localized elevation of wall shear stress is linked to recent symptoms in patients with carotid stenosis. Cerebrovasc Dis. 2022;52:283-92
28. Sakamoto H, Aikawa M, Hill CC, Weiss D, Taylor WR, Libby P. et al. Biomechanical strain induces class a scavenger receptor expression in human monocyte/macrophages and THP-1 cells: A potential mechanism of increased atherosclerosis in hypertension. Circulation. 2001;104:109-14
29. Jiang P, Chen Z, Hippe DS, Watase H, Sun B, Lin R. et al. Association Between Carotid Bifurcation Geometry and Atherosclerotic Plaque Vulnerability: A Chinese Atherosclerosis Risk Evaluation Study. Arterioscler Thromb Vasc Biol. 2020;40:1383-91
30. Ding L, Lu S, Zhou Y, Lyu D, Ouyang C, Ma Z. et al. The 3' Untranslated Region Protects the Heart from Angiotensin II-Induced Cardiac Dysfunction via AGGF1 Expression. Mol Ther. 2020;28:1119-32
31. Kiyatkin ME, Zuver AM, Gaudig A, Javaid A, Mabasa M, Royzman E. et al. Carotid artery structure and hemodynamics and their association with adverse vascular events in left ventricular assist device patients. J Artif Organs. 2021;24:182-90
32. Kiyatkin ME, Zuver AM, Gaudig A, Javaid A, Mabasa M, Royzman E. et al. Carotid artery structure and hemodynamics and their association with adverse vascular events in left ventricular assist device patients. J Artif Organs: Off J Jpn Soc Artif Organs. 2021;24:182-90
33. Oliveira-Paula GH, Liu S, Maira A, Ressa G, Ferreira GC, Quintar A. et al. The β-catenin C terminus links wnt and sphingosine-1-phosphate signaling pathways to promote vascular remodeling and atherosclerosis. Sci Adv. 2024;10:eadg9278
34. Ya X, Ma L, Li H, Ge P, Zheng Z, Mou S. et al. Exploring the relationship between hemodynamics and the immune microenvironment in carotid atherosclerosis: Insights from CFD and CyTOF technologies. J Cereb Blood Flow Metab. 2024;44:1733-44
35. Wang X, Ling G, Wei Y, Li W, Zhang Y, Tan N. et al. Activation of ULK1 to trigger FUNDC1-mediated mitophagy in heart failure: Effect of Ginsenoside Rg3 intervention. Phytomedicine. 2023;120:155042
36. Karageorgos GM, Apostolakis IZ, Nauleau P, Gatti V, Weber R, Konofagou EE. Atherosclerotic plaque mechanical characterization coupled with vector Doppler imaging in atherosclerotic carotid arteries in-vivo. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:6200-3
37. Canton G, Chiu B, Chen H, Chen Y, Hatsukami TS, Kerwin WS. et al. A framework for the co-registration of hemodynamic forces and atherosclerotic plaque components. Physiol Meas. 2013;34:977-90
38. Nandalur KR, Baskurt E, Hagspiel KD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR Am J Roentgenol. 2005;184:295-8
39. Araki T, Jain PK, Suri HS, Londhe ND, Ikeda N, El-Baz A. et al. Stroke Risk Stratification and its Validation using Ultrasonic Echolucent Carotid Wall Plaque Morphology: A Machine Learning Paradigm. Comput Biol Med. 2017;80:77-96
40. Ma W, Cheng X, Xu X, Wang F, Zhou R, Fenster A. et al. Multilevel Strip Pooling-Based Convolutional Neural Network for the Classification of Carotid Plaque Echogenicity. Comput Math Methods Med. 2021;2021:3425893
41. Young VE, Sadat U, Gillard JH. Noninvasive carotid artery imaging with a focus on the vulnerable plaque. Neuroimaging Clin N Am. 2011;21:391-405 xi-xii
42. Kim GH, Youn HJ. Is Carotid Artery Ultrasound Still Useful Method for Evaluation of Atherosclerosis? Korean Circ J. 2017;47:1-8
43. Oliveira MS, Torquato BGS, Soares MH, Monteiro M, Juliano GR, Aguiar LS. et al. Macroscopic Evaluation of Atherosclerosis in the Arteries: An Autopsy Assessment Tool. Arq Bras Cardiol. 2021;116:1119-26
44. Chan JMS, Park SJ, Ng M, Chen WC, Chan WY, Bhakoo K. et al. Translational Molecular Imaging Tool of Vulnerable Carotid Plaque: Evaluate Effects of Statin Therapy on Plaque Inflammation and American Heart Association-Defined Risk Levels in Cuff-Implanted Apolipoprotein E-Deficient Mice. Transl Stroke Res. 2024;15:110-26
45. Park SJ, Chan WY, Ng M, Chung YC, Chong TT, Bhakoo K, Chan JMS. Development of Molecular Magnetic Resonance Imaging Tools for Longitudinal Tracking of Carotid Atherosclerotic Disease Using Fast Imaging with Steady-State Precession. Transl Stroke Res. 2023;14:357-63
46. Lukanova DV, Nikolov NK, Genova KZ, Stankev MD, Georgieva EV. The Accuracy of Noninvasive Imaging Techniques in Diagnosis of Carotid Plaque Morphology. Open Access Maced J Med Sci. 2015;3:224-30
47. Cao Y, Li Q, Yang Y, Ke Z, Chen S, Li M. et al. Cardioprotective Effect of Stem-Leaf Saponins from Panax notoginseng on Mice with Sleep Derivation by Inhibiting Abnormal Autophagy Through PI3K/Akt/mTOR Pathway. Frontiers in cardiovascular medicine. 2021;8:694219
48. Laksono S, Kusharsamita H. Unravelling the role of carotid atherosclerosis in predicting cardiovascular disease risk: A review. ARYA Atheroscler. 2024;20:52-9
49. Li FJ, Abudureyimu M, Zhang ZH, Tao J, Ceylan AF, Lin J. et al. Inhibition of ER stress using tauroursodeoxycholic acid rescues obesity-evoked cardiac remodeling and contractile anomalies through regulation of ferroptosis. Chem Biol Interact. 2024;398:111104
50. Ibrahimi P, Jashari F, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: how useful is the imaging? Atherosclerosis. 2013;231:323-33
51. Yu W, Qin X, Zhang Y, Qiu P, Wang L, Zha W. et al. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther. 2020;10:752-69
52. Kerwin WS, Miller Z, Yuan C. Imaging of the high-risk carotid plaque: magnetic resonance imaging. Semin Vasc Surg. 2017;30:54-61
53. Zhu G, Hom J, Li Y, Jiang B, Rodriguez F, Fleischmann D. et al. Carotid plaque imaging and the risk of atherosclerotic cardiovascular disease. Cardiovasc Diagn Ther. 2020;10:1048-67
54. Wang Q, Ni S, Ling L, Wang S, Xie H, Ren Z. Ginkgolide B Blocks Vascular Remodeling after Vascular Injury via Regulating Tgfβ1/Smad Signaling Pathway. Cardiovasc Ther. 2023;2023:8848808
55. Zhu Y, Lai Y, Hu Y, Fu Y, Zhang Z, Lin N. et al. The mechanisms underlying acute myocardial infarction in chronic kidney disease patients undergoing hemodialysis. Biomed Pharmacother. 2024;177:117050
56. Almevall AD, Wennberg P, Liv P, Nyman E, Lindvall K, Norberg M. et al. Midlife Mediterranean Diet is Associated with Subclinical Carotid Atherosclerosis in Late Midlife. Eur J Prev Cardiol. 2025
57. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ. et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140:e563-e95
58. Zhu ML, Yu YN, Song YT, Wang CY, Miao Z, Chen BL. et al. Cardioprotective role of A-cycloglycosylated derivative of Rubiadin in diabetic cardiomyopathy in rats. Int Immunopharmacol. 2023;118:110008
59. Viguiliouk E, Glenn AJ, Nishi SK, Chiavaroli L, Seider M, Khan T. et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv Nutr. 2019;10:S308-S19
60. Wang DD, Li Y, Bhupathiraju SN, Rosner BA, Sun Q, Giovannucci EL. et al. Fruit and Vegetable Intake and Mortality: Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation. 2021;143:1642-54
61. Jayedi A, Zargar MS, Shab-Bidar S. Fish consumption and risk of myocardial infarction: A systematic review and dose-response meta-analysis suggests a regional difference. Nutr Res. 2019;62:1-12
62. Wang S, Liu Y, Cai H, Li Y, Zhang X, Liu J. et al. Decreased risk of all-cause and heart-specific mortality is associated with low-fat or skimmed milk consumption compared with whole milk intake: a cohort study. Clin Nutr. 2021;40:5568-75
63. Zhuang P, Wu F, Mao L, Zhu F, Zhang Y, Chen X. et al. Egg and cholesterol consumption and mortality from cardiovascular and different causes in the United States: A population-based cohort study. PLoS Med. 2021;18:e1003508
64. Becerra-Tomás N, Paz-Graniel I, W C Kendall C, Kahleova H, Rahelić D, Sievenpiper JL. et al. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr Rev. 2019;77:691-709
65. Chung M, Zhao N, Wang D, Shams-White M, Karlsen M, Cassidy A. et al. Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of population-based studies. Adv Nutr. 2020;11:790-814
66. Lei M, Liu Q, Nie J, Huang R, Mei Y, Pan D. et al. Impact and Mechanisms of Action of BDNF on Neurological Disorders, Cancer, and cardiovascular diseases. CNS Neurosci Ther. 2024;30:e70138
67. Meng XW, He CX, Chen X, Yang XS, Liu C. The extract of Gnaphalium affine D. Don protects against H(2)O(2)-induced apoptosis by targeting PI3K/AKT/GSK-3β signaling pathway in cardiomyocytes. J Ethnopharmacol. 2021;268:113579
68. Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC. et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315
69. Queiroz I, Defante MLR, Tavares A, Antunes V, de Mesquita CF, Barbosa LM. et al. High consumption of artificially sweetened beverages and associated risk of cardiovascular events: a systematic review and meta-analysis. Curr Probl Cardiol. 2025;50:102837
70. Zhang P, Cheng X, Sun H, Li Y, Mei W, Zeng C. Atractyloside Protect Mice Against Liver Steatosis by Activation of Autophagy via ANT-AMPK-mTORC1 Signaling Pathway. Front Pharmacol. 2021;12:736655
71. Zuo W, Xu K, Zhang W, Wang Y, Gu C, Bai Y. et al. Heavy metal distribution and uptake by maize in a mudflat soil amended by vermicompost derived from sewage sludge. Environ Sci Pollut Res Int. 2019;26:30154-66
72. Han C, Liu F, Yang X, Chen J, Li J, Cao J. et al. Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: the China-PAR project. Sci China Life Sci. 2018;61:504-14
73. Li T, Tu P, Bi J, Sun Y, Yu D, Wang J. et al. LncRNA Miat knockdown alleviates endothelial cell injury through regulation of miR-214-3p/Caspase-1 signalling during atherogenesis. Clin Exp Pharmacol Physiol. 2021;48:1231-8
74. Kan H, Wang P, Yang Y, Jia H, Liu A, Wang M. et al. Apigenin inhibits proliferation and differentiation of cardiac fibroblasts through AKT/GSK3β signaling pathway. J Ethnopharmacol. 2024;334:118518
75. Chen T, Chen S, Honda T, Kishimoto H, Nofuji Y, Narazaki K. Associations of objectively measured physical activity and sedentary time with all-cause mortality in japanese older adults: A 10-year prospective study. Br J Sports Med. 2025: bjsports-2024-108258.
76. Mury P, Mura M, Della-Schiava N, Chanon S, Vieille-Marchiset A, Nicaise V. et al. Association between physical activity and sedentary behaviour on carotid atherosclerotic plaques: An epidemiological and histological study in 90 asymptomatic patients. Br J Sports Med. 2019;54:469-74
77. Veronese N, Burgio MI, Mandalà C, Saguto D, Dominguez LJ, Barbagallo M. et al. Association of chronic exercise with markers of cardiometabolic health: a systematic review and meta-analysis. Ageing Res Rev. 2025;104:102645
78. Hejazi K, Iraj ZA, Saeidi A, Hackney AC, Laziri F, Suzuki K. et al. Differential effects of exercise training protocols on blood pressures and lipid profiles in older adults patients with hypertension: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2025;131:105737
79. Koskinas KC, Van Craenenbroeck EM, Antoniades C, Blüher M, Gorter TM, Hanssen H. et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur Heart J. 2024;45:4063-98
80. Neeland IJ, Ross R, Després J-P, Matsuzawa Y, Yamashita S, Shai I. et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet, Diabetes Endocrinol. 2019;7:715-25
81. Neeland IJ, Poirier P, Després J-P. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391-406
82. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA. et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985-3023
83. Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J. et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074-84
84. Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y. et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8:e721-e9
85. Guirguis-Blake JM, Webber EM, Coppola EL. Screening for asymptomatic carotid artery stenosis in the general population: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325:487
86. Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A. et al. European stroke organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. 2021;6:I-XLVII
87. Hassani S, Fisher M. Management of atherosclerotic carotid artery disease: A brief overview and update. Am J Med. 2022;135:430-4
88. King A, Shipley M, Markus H, Investigators A. The effect of medical treatments on stroke risk in asymptomatic carotid stenosis. Stroke. 2013;44:542-6
89. Gu Y, Xia H, Chen X, Li J. Curcumin Nanoparticles Attenuate Lipotoxic Injury in Cardiomyocytes Through Autophagy and Endoplasmic Reticulum Stress Signaling Pathways. Front Pharmacol. 2021;12:571482
90. Zhou C, Yang Y, Hu L, Meng X, Guo X, Lei M. et al. Effects of miR-143 regulation on cardiomyocytes apoptosis in doxorubicin cardiotoxicity based on integrated bioinformatics analysis. Toxicol In vitro. 2023;93:105662
91. Mujaj B, Bos D, Muka T, Lugt Avd, Ikram MA, Vernooij MW. et al. Antithrombotic treatment is associated with intraplaque haemorrhage in the atherosclerotic carotid artery: A cross-sectional analysis of the rotterdam study. Eur Heart J. 2018;39:3369-76
92. Zhao J, Zhang X, Dong L, Wen Y, Cui L. The many roles of statins in ischemic stroke. Curr Neuropharmacol. 2015;12:564-74
93. Zhao C, Zhu X, Tan J, Mei C, Cai X, Kong F. Lipid-based nanoparticles to address the limitations of GBM therapy by overcoming the blood-brain barrier, targeting glioblastoma stem cells, and counteracting the immunosuppressive tumor microenvironment. Biomed Pharmacother. 2024;171:116113
94. Chen Y, Xie C, Lei Y, Ye D, Wang L, Xiong F. et al. Theabrownin from Qingzhuan tea prevents high-fat diet-induced MASLD via regulating intestinal microbiota. Biomed Pharmacother. 2024;174:116582
95. Park S-J, Kang S-J, Ahn J-M, Chang M, Yun S-C, Roh JH. et al. Effect of statin treatment on modifying plaque composition: A double-blind, randomized study. Journal of the American College of Cardiology. 2016;67:1772-83
96. Räber L, Taniwaki M, Zaugg S, Kelbæk H, Roffi M, Holmvang L. et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): a serial intravascular ultrasonography study. Eur Heart J. 2015;36:490-500
97. Chen M, Gao M, Wang H, Chen Q, Liu X, Mo Q. et al. Jingangteng capsules ameliorate liver lipid disorders in diabetic rats by regulating microflora imbalances, metabolic disorders, and farnesoid X receptor. Phytomedicine. 2024;132:155806
98. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL. et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315-81
99. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D. et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467
100. Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Räber L, Mach F. et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39:1172-80
101. Stevens W, Peneva D, Li JZ, Liu LZ, Liu G, Gao R. et al. Estimating the future burden of cardiovascular disease and the value of lipid and blood pressure control therapies in China. BMC Health Serv Res. 2016;16:175
102. Bi L, Yi J, Wu C, Hu S, Zhang X, Lu J. et al. Atherosclerotic Cardiovascular Disease Risk and Lipid-Lowering Therapy Requirement in China. Frontiers in cardiovascular medicine. 2022;9:839571
103. Li J-J, Liu H-H, Wu N-Q, Yeo KK, Tan K, Ako J. et al. Statin intolerance: an updated, narrative review mainly focusing on muscle adverse effects. Expert Opin Drug Metab Toxicol. 2020;16:837-51
104. Grundy SM, Vega GL. Statin Intolerance and Noncompliance: An Empiric Approach. Am J Med. 2022;135:318-23
105. Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F. et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175-82
106. Bytyçi I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A. et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43:3213-23
107. Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131-41
108. Wang J, Chang X, Li C, Gao J, Guo Z, Zhuang H. et al. DNA-PKcs-Driven YAP1 Phosphorylation and Nuclear Translocation: a Key Regulator of Ferroptosis in Hyperglycemia-Induced Cardiac Dysfunction in Type 1 Diabetes. Adv Sci (Weinh). 2025: e2412698.
109. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-88
110. Li M, Jin M, Peng H, Wang H, Shen Q, Zhang L. Current Status and Future Prospects of TROP-2 ADCs in Lung Cancer Treatment. Drug Des Devel Ther. 2024;18:5005-21
111. Schiele F, Quignot N, Khachatryan A, Gusto G, Villa G, Kahangire D. et al. Clinical impact and room for improvement of intensity and adherence to lipid lowering therapy: Five years of clinical follow-up from 164,565 post-myocardial infarction patients. International Journal of Cardiology. 2021;332:22-8
112. Jin J, Huang C, Zhu C, Feng W, He A, Li T. et al. Pharmacokinetics, Bioequivalence, and Safety of Esomeprazole Magnesium Enteric-Coated Capsules in Healthy Chinese Subjects. Clin Pharmacol Drug Dev. 2023;12:691-8
113. Lai Q, Hu L, Zhang W, Jiang Z, Zeng C, Hu J. Single-Cell RNA sequencing highlights the regulatory role of T cell marker genes Ctla4, Ccl5 and Tcf7 in corneal allograft rejection of mouse model. International immunopharmacology. 2023;117:109911
114. Hu L, Wu X, Peng J, Yan B, Li J, Guo Y. et al. Subchronic oral toxic effects of 2,4-dinitroaniline in wistar rats: A comprehensive toxicity evaluation. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 2024;191:114846
115. Gao Z-D, Yan H-D, Wu N-H, Yao Q, Wan B-B, Liu X-F. et al. Mechanistic insights into the amelioration effects of lipopolysaccharide-induced acute lung injury by baicalein: An integrated systems pharmacology study and experimental validation. Pulm Pharmacol Ther. 2022;73-74:102121
116. Xing Y, Liu J, Hao Y, Liu J, Huo Y. et al. Prehospital statin use and low-density lipoprotein cholesterol levels at admission in acute coronary syndrome patients with history of myocardial infarction or revascularization: Findings from the Improving Care for Cardiovascular Disease in China (CCC) project. Am Heart J. 2019 Jun:212:120-128
117. Ding L. The emerging role and clinicopathological significance of MFSD12 in cancer and lysosomal storage diseases. Frontiers in pharmacology. 2024;15:1398320
118. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC. et al. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016;374:1011-20
119. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W. et al. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med. 2016;374:1021-31
120. Kan H, Wang P, Yang Y, Jia H, Liu A, Wang M. et al. Apigenin inhibits proliferation and differentiation of cardiac fibroblasts through AKT/GSK3β signaling pathway. Journal of ethnopharmacology. 2024;334:118518
121. Zhou Q, Guo X, Chen T, Liu Y, Ji H, Sun Y. et al. The neuroprotective role of celastrol on hippocampus in diabetic rats by inflammation restraint, insulin signaling adjustment, Aβ reduction and synaptic plasticity alternation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2024;179:117397
122. Xiao S, Chen L, Chen Z, Li Q. Therapeutically Harnessing Tumor Cell-Derived Extracellular Vesicles for Multiple Myeloma: Recent Advances and Future Perspectives. Pharmaceutics. 2024;16:1439
123. Hong C, Liu Y, Shi D, Liu C, Zou S, Guo M. et al. Radiofrequency-responsive black phosphorus nanogel crosslinked with cisplatin for precise synergy in multi-modal tumor therapies. J Control Release. 2024;373:853-66
124. Chang X, Li Y, Liu J, Wang Y, Guan X, Wu Q. et al. ß-tubulin contributes to Tongyang Huoxue decoction-induced protection against hypoxia/reoxygenation-induced injury of sinoatrial node cells through SIRT1-mediated regulation of mitochondrial quality surveillance. Phytomedicine. 2023;108:154502
125. Meschia JF, Klaas JP, Brown RD, Brott TG. Evaluation and Management of Atherosclerotic Carotid Stenosis. Mayo Clin Proc. 2017;92:1144-57
126. Zou R, Shi W, Chang X, Zhang M, Tan S, Li R. et al. The DNA-dependent protein kinase catalytic subunit exacerbates endotoxemia-induced myocardial microvascular injury by disrupting the MOTS-c/JNK pathway and inducing profilin-mediated lamellipodia degradation. Theranostics. 2024;14:1561-82
127. Chang X, Zhou S, Liu J, Wang Y, Guan X, Wu Q. et al. Zishenhuoxue decoction-induced myocardial protection against ischemic injury through TMBIM6-VDAC1-mediated regulation of calcium homeostasis and mitochondrial quality surveillance. Phytomedicine. 2024;132:155331
128. Su W, Xie X, Zhao J, Fan Q, Dong N, Li Q. et al. Comparative efficacy of Chinese patent medicines in patients with carotid atherosclerotic plaque: a Bayesian network meta- analysis. Chin Med. 2023;18:152
129. Dai C, Zhen F, Yu L, Xin S. Puerarin alleviates oxaliplatin-induced neuropathic pain by promoting Nrf2/GPX4-mediated antioxidative response. PloS one. 2024;19:e0308872
130. Chen Y, Xie C, Lei Y, Ye D, Wang L, Xiong F. et al. Theabrownin from Qingzhuan tea prevents high-fat diet-induced MASLD via regulating intestinal microbiota. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2024;174:116582
131. Chang X, Zhang Q, Huang Y, Liu J, Wang Y, Guan X. et al. Quercetin inhibits necroptosis in cardiomyocytes after ischemia-reperfusion via DNA-PKcs-SIRT5-orchestrated mitochondrial quality control. Phytother Res. 2024
132. Ferreira-Silva M, Faria-Silva C, Carvalheiro MC, Simões S, Marinho HS, Marcelino P. et al. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics. 2022 14
133. Mosbah IB, Zaouali MA, Martel C, Bjaoui M, Abdennebi HB, Hotter G. et al. IGL-1 solution reduces endoplasmic reticulum stress and apoptosis in rat liver transplantation. Cell Death & Disease. 2012;3:e279
134. Sadoshima J. The role of autophagy during ischemia/reperfusion. Autophagy. 2008;4:402-3
135. Qi Y, Liu W, Yan X, Zhang C, Zhang C, Liu L. et al. Tongxinluo May Alleviate Inflammation and Improve the Stability of Atherosclerotic Plaques by Changing the Intestinal Flora. Front Pharmacol. 2022;13:805266
136. Li T-T, Wang Z-B, Li Y, Cao F, Yang B-Y, Kuang H-X. The mechanisms of traditional Chinese medicine underlying the prevention and treatment of atherosclerosis. Chin J Nat Med. 2019;17:401-12
137. Zhu ZY, Liu YD, Gong Y, Jin W, Topchiy E, Turdi S. et al. Mitochondrial aldehyde dehydrogenase (ALDH2) rescues cardiac contractile dysfunction in an APP/PS1 murine model of Alzheimer's disease via inhibition of ACSL4-dependent ferroptosis. Acta Pharmacol Sin. 2022;43:39-49
138. Wang L, Qiu XM, Hao Q, Li DJ. Anti-inflammatory effects of a Chinese herbal medicine in atherosclerosis via estrogen receptor β mediating nitric oxide production and NF-κB suppression in endothelial cells. Cell Death Dis. 2013;4:e551
139. Gu Z, Zhao H, Song Y, Kou Y, Yang W, Li Y. et al. PEGylated-liposomal astaxanthin ameliorates Aβ neurotoxicity and Alzheimer-related phenotypes by scavenging formaldehyde. J Control Release. 2024;366:783-97
140. Li D, Yang Y, Wang S, He X, Liu M, Bai B. et al. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biology. 2021;46:102089
141. Chen Q, Zhang Y, Meng Q, Wang S, Yu X, Cai D. et al. Liuwei Dihuang prevents postmenopausal atherosclerosis and endothelial cell apoptosis via inhibiting DNMT1-medicated ERα methylation. J Ethnopharmacol. 2020;252:112531
142. Jing Y, Cai D, Chen Q, Xiong Q, Hu T, Yao Y. et al. Liuwei Dihuang soft capsules attenuates endothelial cell apoptosis to prevent atherosclerosis through GPR30-mediated regulation in ovariectomized ApoE-deficient mice. J Ethnopharmacol. 2017;208:185-98
143. Ren L, Yang H, Wang H, Qin S, Zhan X, Li H. et al. Tryptanthrin suppresses multiple inflammasome activation to regulate NASH progression by targeting ASC protein. Phytomedicine. 2024;131:155758
144. Hinton A, Claypool SM, Neikirk K, Senoo N, Wanjalla CN, Kirabo A. et al. Mitochondrial Structure and Function in Human Heart Failure. Circulation Research. 2024;135:372-96
145. Cheng Q-Q, Wan Y-W, Yang W-M, Tian M-H, Wang Y-C, He H-Y. et al. Gastrodin protects H9c2 cardiomyocytes against oxidative injury by ameliorating imbalanced mitochondrial dynamics and mitochondrial dysfunction. Acta pharmacologica Sinica. 2020;41:1314-27
146. Shi M, Sun T, Zhang C, Ma Y, Pang B, Cao L. et al. Effects of the combination of red yeast rice-containing commercial Chinese polyherbal preparation with statins for dyslipidemia: a systematic review and meta-analysis. Front Pharmacol. 2024;15:1398934
147. Liao W, Rao Z, Wu L, Chen Y, Li C. Cariporide Attenuates Doxorubicin-Induced Cardiotoxicity in Rats by Inhibiting Oxidative Stress, Inflammation and Apoptosis Partly Through Regulation of Akt/GSK-3β and Sirt1 Signaling Pathway. Front Pharmacol. 2022;13:850053
148. Zha W, Zhao Q, Xiao Y, Gan Y, Wei J, Yu M. et al. Mitochonic acid 5 rescues cardiomyocytes from doxorubicin-induced toxicity via repressing the TNF-α/NF-κB/NLRP3-mediated pyroptosis. Int Immunopharmacol. 2023;123:110736
149. Liu D-Q, Chen S-P, Sun J, Wang X-M, Chen N, Zhou Y-Q. et al. Berberine protects against ischemia-reperfusion injury: A review of evidence from animal models and clinical studies. Pharmacological research. 2019;148:104385
150. Rahmani P, Melekoglu E, Tavakoli S, Malekpour Alamdari N, Rohani P, Sohouli MH. Impact of red yeast rice supplementation on lipid profile: a systematic review and meta-analysis of randomized-controlled trials. Expert Rev Clin Pharmacol. 2023;16:73-81
151. Yang B-L, Long Y-Y, Lei Q, Gao F, Ren W-X, Cao Y-L. et al. Lethal pulmonary thromboembolism in mice induced by intravenous human umbilical cord mesenchymal stem cell-derived large extracellular vesicles in a dose- and tissue factor-dependent manner. Acta pharmacologica Sinica. 2024;45:2300-12
152. Yang C, Wu Y, Qian J, Li J-J. A systematic, updated review of Xuezhikang, a domestically developed lipid-lowering drug, in the application of cardiovascular diseases. Acta Pharm Sin B. 2024;14:4228-42
153. Zhou X, Wang H, Yan B, Nie X, Chen Q, Yang X. et al. Ferroptosis in Cardiovascular Diseases and Ferroptosis-Related Intervention Approaches. Cardiovasc Drugs Ther. 2024
154. Yun C, Ji X, Chen Y, Zhao Z, Gao Y, Gu L. et al. Ultrasound-assisted enzymatic extraction of Scutellaria baicalensis root polysaccharide and its hypoglycemic and immunomodulatory activities. Int J Biol Macromol. 2023;227:134-45
155. Kwon O-J, Noh J-W, Lee B-C. Mechanisms and Effect of Coptidis Rhizoma on Obesity-Induced Inflammation: In silico and In vivo Approaches. Int J Mol Sci. 2021;22:8075
156. Cheng R, Fujinaga M, Yang J, Rong J, Haider A, Ogasawara D. et al. A novel monoacylglycerol lipase-targeted (18)F-labeled probe for positron emission tomography imaging of brown adipose tissue in the energy network. Acta Pharmacol Sin. 2022;43:3002-10
157. Ji L, Song T, Ge C, Wu Q, Ma L, Chen X. et al. Identification of bioactive compounds and potential mechanisms of scutellariae radix-coptidis rhizoma in the treatment of atherosclerosis by integrating network pharmacology and experimental validation. Biomed Pharmacother. 2023;165:115210
158. Zhang J, Hu X, Zheng G, Yao H, Liang H. In vitro and in vivo antitumor effects of lupeol-loaded galactosylated liposomes. Drug Deliv. 2021;28:709-18
159. Zhao Y, Yang Z, Fang C, Xiao D, Shi Y, Lin Y. et al. A single-center observational study on the expression of circulating interleukin-20 levels and predicting outcomes in human chronic heart failure: A 2-year follow-up cohort study: Higher IL-20 levels suggest poorer outcomes in CHF patients. Clin Chim Acta. 2020;510:5-10
160. Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM. et al. Kaempferol and inflammation: From chemistry to medicine. Pharmacol Res. 2015;99:1-10
161. Ren Z, Zhang Z, Ling L, Liu X, Wang X. Drugs for treating myocardial fibrosis. Front Pharmacol. 2023;14:1221881
162. Dabeek WM, Marra MV. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients. 2019;11:2288
163. Feng Z, Wang C, Yue n, Jin n, Meng Q, Wu J. et al. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm Biol. 2021;59:1106-16
164. Qian Y, Zhou S, Li J, Ma M, Chen H, Cao Y. et al. Discovery of 4-((3,4-dichlorophenyl)amino)-2-methylquinolin-6-ol derivatives as EGFR and HDAC dual inhibitors. European Journal of Pharmacology. 2023;960:176114
165. Vinten-Johansen J. Postconditioning and controlled reperfusion: the nerve of it all. Anesthesiology. 2009;111:1177-9
166. Cui Z, Li S, Chang J, Zang E, Liu Q, Zhou B. et al. The pharmacophylogenetic relationships of two edible medicinal plants in the genus Artemisia. Front Plant Sci. 2022;13:949743
167. Liu Y-r, Chen J-j, Dai M. Paeonol protects rat vascular endothelial cells from ox-LDL-induced injury in vitro via downregulating microRNA-21 expression and TNF-α release. Acta Pharmacol Sin. 2014;35:483-8
168. Liang Y, Dong L, Yan J, Yang Y, Liu Y, Wu H. et al. Paeonol attenuates atherosclerosis by regulating vascular smooth muscle cells apoptosis and modulating immune cells infiltration through reducing LTβR expression. Phytomedicine. 2024;135:156196
169. Zhang W, Wang C-H, Li F, Zhu W-Z. 2,3,4',5-Tetrahydroxystilbene-2-O-beta-D-glucoside suppresses matrix metalloproteinase expression and inflammation in atherosclerotic rats. Clin Exp Pharmacol Physiol. 2008;35:310-6
170. Tsai M-Y, Yang R-C, Wu H-T, Pang J-HS, Huang S-T. Anti-angiogenic effect of Tanshinone IIA involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cells. Cancer Lett. 2011;310:198-206
171. Wang C, Hu L, Guo S, Yao Q, Liu X, Zhang B. et al. Phosphocreatine attenuates doxorubicin-induced cardiotoxicity by inhibiting oxidative stress and activating TAK1 to promote myocardial survival in vivo and in vitro. Toxicology. 2021;460:152881
172. Chang C-C, Chu C-F, Wang C-N, Wu H-T, Bi K-W, Pang J-HS. et al. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine. 2014;21:207-16
173. Yu YN, Ren YY, Shao ZL, Chen BL, Cui BY, Chao CY. et al. Perillaldehyde improves diabetic cardiomyopathy by upregulating miR-133a-3p to regulate GSK-3β. Eur J Pharmacol. 2023;953:175836
174. Zhong X-F, Huang G-D, Luo T, Deng Z-Y, Hu J-N. Protective effect of rhein against oxidative stress-related endothelial cell injury. Mol Med Rep. 2012;5:1261-6
175. Ding J, He L, Yang L, Cheng L, Zhao Z, Luo B. et al. Novel Nanoprobe with Combined Ultrasonography/Chemical Exchange Saturation Transfer Magnetic Resonance Imaging for Precise Diagnosis of Tumors. Pharmaceutics. 2023;15:2693
176. Mo J, Yang R, Li F, Zhang X, He B, Zhang Y. et al. Scutellarin protects against vascular endothelial dysfunction and prevents atherosclerosis via antioxidation. Phytomedicine. 2018;42:66-74
177. Chang X, Zhang Q, Huang Y, Liu J, Wang Y, Guan X. et al. Quercetin inhibits necroptosis in cardiomyocytes after ischemia-reperfusion via DNA-PKcs-SIRT5-orchestrated mitochondrial quality control. Phytother Res. 2024;38:2496-517
178. Luo X, Weng X, Bao X, Bai X, Lv Y, Zhang S. et al. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022;57:102511
179. Rao X, Wu X, Hu J, Huang Z. Pharmacokinetics and Bioequivalence of Two Formulations of Montelukast Sodium Tablets in Healthy Chinese Volunteers Under Fasting and Fed Conditions. Clin Pharmacol Drug Dev. 2025;14:161-6
180. Wada L, Ou B. Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem. 2002;50:3495-500
181. Lee W-J, Ou H-C, Hsu W-C, Chou M-M, Tseng J-J, Hsu S-L. et al. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010;52:1290-300
182. Zhu M-L, Fan J-X, Guo Y-Q, Guo L-J, Que H-D, Cui B-Y. et al. Protective effect of alizarin on vascular endothelial dysfunction via inhibiting the type 2 diabetes-induced synthesis of THBS1 and activating the AMPK signaling pathway. Phytomedicine. 2024;128:155557
183. Gelmetti V, De Rosa P, Torosantucci L, Marini ES, Romagnoli A, Di Rienzo M. et al. PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy. 2017;13:654-69
184. He Q, Chang X, Zhang H, Hao Q, Zhi J, Shi H. et al. Nuclear damage-induced DNA damage response coupled with IFI16-driven ECM remodeling underlies dilated cardiomyopathy. Theranostics. 2025;15:5998-6021
185. Rani UP, Kesavan R, Ganugula R, Avaneesh T, Kumar UP, Reddy GB. et al. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J Nutr Biochem. 2013;24:1830-9
186. Sun G-B, Qin M, Ye J-x, Pan R-l, Meng X-b, Wang M. et al. Inhibitory effects of myricitrin on oxidative stress-induced endothelial damage and early atherosclerosis in ApoE-/- mice. Toxicol Appl Pharmacol. 2013;271:114-26
187. Qin M, Luo Y, Meng X-b, Wang M, Wang H-w, Song S-y. et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol. 2015;70:23-34
188. Li Y, Huang D, Jia L, Shangguan F, Gong S, Lan L. et al. LonP1 Links Mitochondria-ER Interaction to Regulate Heart Function. Research (Wash D C). 2023;6:0175
189. Hu H, Wang C, Jin Y, Meng Q, Liu Q, Liu Z. et al. Catalpol Inhibits Homocysteine-induced Oxidation and Inflammation via Inhibiting Nox4/NF-κB and GRP78/PERK Pathways in Human Aorta Endothelial Cells. Inflammation. 2019;42:64-80
190. Chen Q, Qi X, Zhang W, Zhang Y, Bi Y, Meng Q. et al. Catalpol Inhibits Macrophage Polarization and Prevents Postmenopausal Atherosclerosis Through Regulating Estrogen Receptor Alpha. Front Pharmacol. 2021;12:655081
191. Wu Y, Chen Q, Wen B, Wu N, He B, Chen J. Berberine Reduces Aβ(42) Deposition and Tau Hyperphosphorylation via Ameliorating Endoplasmic Reticulum Stress. Front Pharmacol. 2021;12:640758
192. Chang C, He F, Ao M, Chen J, Yu T, Li W. et al. Inhibition of Nur77 expression and translocation by compound B6 reduces ER stress and alleviates cigarette smoke-induced inflammation and injury in bronchial epithelial cells. Front Pharmacol. 2023;14:1200110
Author contact
![]() Corresponding authors: Xiaoping Fan, email: fanxiaopingedu.cn, Sang-Bing Ong, email: sangbingongedu.hk, Qingyong He, email: heqingyonggcom, Hao Zhou, email: zhouhaoorg
Corresponding authors: Xiaoping Fan, email: fanxiaopingedu.cn, Sang-Bing Ong, email: sangbingongedu.hk, Qingyong He, email: heqingyonggcom, Hao Zhou, email: zhouhaoorg

 Global reach, higher impact
Global reach, higher impact