3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(11):2686-2699. doi:10.7150/ijms.110452 This issue Cite
Research Paper
The screening assessment of pro-inflammatory, anti-inflammatory, Th1, Th2 and Th17 cytokines in saliva of patients with ischemic stroke
1. Department of Risk Group Dentistry, Pediatric Dentistry, Poznan University of Medical Sciences, Poznan, Poland
2. Department of Risk Group Dentistry, Chair of Pediatric Dentistry, Poznan University of Medical Sciences, Poznan, Poland
3. Students Scientific Club “Biochemistry of Civilization Diseases” at the Department of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Poland;
4. Experimental Dentistry Laboratory, Department of Conservative Dentistry, Medical University of Bialystok, Bialystok, Poland
5. Department of Occupational Therapy, Poznan University of Medical Sciences, Poznan, Poland
6. Department of Rehabilitation, Greater Poland Cancer Centre, Poznan, Poland
7. Neurorehabilitation Ward, Greater Poland Provincial Hospital, 60-480 Poznan, Poland;
8. Department of Family Medicine, Poznan University of Medical Sciences, Poznan, Poland;
9. Department of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Bialystok, Poland
Received 2025-1-14; Accepted 2025-4-8; Published 2025-5-28
Abstract
Introduction: Ischemic stroke leads to hypoxia of brain structures, causes inflammation and tissue necrosis. Since the blood-brain barrier is damaged, inflammatory mediators can enter the bloodstream and saliva. This case-control study examines pro-inflammatory, anti-inflammatory, Th1, and Th2 cytokines in the unstimulated saliva of stroke patients and explores the association between these biomarkers and clinical status.
Patients and methods: The study group included 22 patients with ischemic stroke, while the control group included 22 healthy individuals that were matched for age, sex, dental, periodontal and oral hygiene status to the study group. Unstimulated saliva was collected from each patient. In each sample, the inflammatory profile was determined using the multiplex ELISA method. Due to the lack of normal distribution, the Mann-Whitney U test was used for comparisons. The research was approved by the Bioethics Committee of the Poznan University of Medical Sciences (59/19, 890/19, 504/21).
Results: In the unstimulated saliva of stroke patients, significantly higher levels of pro-inflammatory cytokines (IL-1β (p=0.0003), TNF-α (p≤0.0001), TNF-β; (p≤0.0001)), anti-inflammatory cytokines (IL-1ra (p≤0.0001), TRAIL (p=0.0206)), Th1 cytokines (IFN-γ (p≤0.0001), IL-2Rα (p=0.0021) and IL-12 (p40) (p≤0.0001)) and Th2 cytokines (IL-6 (p=0.0023)) were found compared to healthy participants. Interestingly, Addenbrooke's Cognitive Examination Revised (ACE-R) correlated negatively with TNF-α (p=0.01, r=-0.53), TNF-β (p=0.02, r=-0.60) and IFN-γ (p=0.02, r=-0.50). The Functional Independence Measure (FIM) exhibited a positive association with IL-6 (p=0.003, r=0.62) and BI (p=0.001, r=0.66). The positive correlation was found between Sitting Balance Scale (SBS) and IL-6 (p=0.02, r=0.52).
Conclusions: Stroke patients exhibit altered salivary composition characterized by increased secretion of inflammatory mediators. The obtained results do not indicate the dominance of any of the branches of the immune response. The concentration of salivary TNF-α, TNF-β, IFN-γ and IL-12 significantly distinguish patients with ischemic stroke from healthy individuals. Although validation of the results in a larger patient population is necessary, salivary cytokines show potential as diagnostic biomarkers.
Keywords: biomarkers, saliva, stroke, cytokines
Introduction
Stroke is a civilization disease that poses a very serious social problem. Epidemiological studies indicate that stroke is the second leading cause of death [1] and the third cause of disability in developed and developing countries [2]. Therefore, new therapeutic and diagnostic methods are being explored to improve outcomes for stroke patients.
Approximately 87% of strokes result from blocked blood supply, leading to ischemic stroke [3]. The damage occurs in the part of the brain that is supplied by the affected vessel. Reduced blood flow deprives neurons of oxygen and nutrients, ultimately leading to their necrosis [4]. Ischemia triggers a cascade of processes that occur at the cellular level, including electrolyte imbalances, acidosis, ATP depletion, excessive glutamate release and free radical overproduction. As a consequence, apoptosis, excitotoxicity, edema and inflammation of the brain occur [5]. The primary goal of inflammation is to clear dead cells and facilitate synapse regeneration through cytokine secretion [6]. However, long-term inflammation can cause brain damage, which further worsens the effects of stroke [7]. Inflammation is induced by the release of high-mobility group box 1 (HMGB1) proteins from necrotic cells, which bind to receptors for advanced glycation end products (RAGE) [8, 9]. The HMGB1-RAGE pathway plays an important role in local activation of the inflammatory response in microglia [9, 10]. The inflammatory response is amplified by neutrophils, macrophages, and lymphocytes arriving from the systemic circulation, as well as dendritic cells accumulating at the periphery of the ischemic region [6]. The activity of these cells triggers inflammation and endothelial cell apoptosis in the brain, increasing blood-brain barrier (BBB) permeability [11,12]. Damage to the BBB allows inflammatory mediators to move from the brain into the bloodstream [13].
Saliva is a plasma filtrate, and due to the salivary glands' rich vascularization, it allows numerous blood biomarkers to enter the saliva [14]. The use of this diagnostic material offers numerous benefits, such as easy and non-invasive sample collection, as well as convenient transport and storage [15]. In our previous studies, we demonstrated the diagnostic potential of salivary tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 10 (IL-10) and xanthine oxidase (XO) in the differential diagnosis of stroke patients. Salivary TNF-α correlated with cognitive decline and the severity of functional impairments [16, 17]. However, other inflammatory biomarkers have not been assessed in the saliva of stroke patients. It is well known that cytokines Th1, Th2, and Th17 play an important role in stroke. This study aims to characterize the salivary cytokine profile in stroke patients and explore the relationship between these biomarkers and clinical parameters.
Patients and Methods
Ethics Committee
The research was approved by the Bioethics Committee of the Poznan University of Medical Sciences (59/19, 890/19, 504/21) confirming the principles of the Declaration of Helsinki on human experimentations. Each patient signed an informed consent form after receiving detailed information about the purpose and scope of the study.
Study Group
The patients qualified for this study were staying at a Neurorehabilitation Ward at the Greater Poland Provincial Hospital (Kiekrz, Poland), which provides comprehensive rehabilitation assistance for individuals with neurological problems. Patients were enrolled into the study between August 2021 and May 2022.
The inclusion criteria required radiological confirmation of ischemic stroke based on the World Health Organization (WHO) standards [18-20], the first stroke in a patient's life, age over 18 years, ability to give informed consent for the study and saliva sample collection, and the ability to provide a saliva sample. The exclusion criteria included a history of second or subsequent stroke, autoimmune (e.g., diabetes, Hashimoto's disease, psoriasis, rheumatoid arthritis, systemic sclerosis) or psychiatric diseases (e.g., Alzheimer's disease, Parkinson's disease), prior acute coronary events, thromboembolic events (such as limb thrombosis or pulmonary embolism), smoking, inability to give informed consent, or to provide a saliva sample. A general medical history, including information on chronic diseases, smoking, education and lifestyle, was collected from each patient.
Participation in the study was voluntary, and each patient provided written informed consent. A total of 107 stroke patients were initially recruited for the study within the first two days of admission to the ward (between 10-13 days after stroke). Subsequently, 13 participants were excluded due to poor general condition, 21 declined to participate, and 3 individuals, despite signing the consent form, objected to saliva sample collection. After applying the inclusion and exclusion criteria, the number of participants was reduced to 44. One person withdrew from the study after saliva collection. Eleven participants were unable to provide saliva samples, and 7 did not attend the oral examination (Fig 1).
The clinical characteristics of the participants and control group are presented in the supplementary material (Table S1).
Control Group
The control group included 22 generally healthy individuals - volunteers attending dental check-ups at the Department of Restorative Dentistry of the Medical University of Bialystok (Bialystok, Poland). Participants were matched for age, gender, dental status, periodontal status, and oral hygiene to correspond with the study group. Each participant received a health certificate from a doctor before joining the study. A medical history was taken from each patient, including information on chronic diseases, education, smoking and lifestyle. The control group members followed a regular, balanced diet without any restrictions and received standard recommendations regarding physical activity. Participants over the age of 18, able to give informed consent, as well as collect and provide a saliva sample were included in the study. The exclusion criteria included: being under 18 years old, smoking, inability to provide informed consent, and inability to collect and provide a saliva sample.
Patient selection flow chart.
Saliva Collection
Patients were enrolled into the study within the first 2 days after admission to the ward, between 10 and 13 days following the stroke incident. Patients were informed that they should refrain from eating, drinking, oral hygiene procedures, and vigorous physical activity for at least 8 hours prior to sample collection. Unstimulated saliva collection took place in the morning between 8 and 10 a.m., in a quiet and isolated room. Prior to saliva collection, patients had about 5 minutes to acclimate to the room, then they were asked to rinse their mouth twice with distilled water at room temperature to minimize sample contamination. Unstimulated saliva was collected using the spitting method. Patients were instructed to lean forward slightly and spit saliva into a test tube. Saliva was collected into a sterile calibrated tube, which was placed in a cup of ice during the procedure. The duration of saliva collection was 10 minutes. The saliva samples were then centrifuged at the specified parameters: at a temperature of +4 °C, for 20 minutes, 3000×g (MPW 351, MPW Med. Instruments, Warsaw, Poland). The supernatant fluid was preserved for analysis and stored at -80°C for no longer than 3 months [21, 22].
Oral Examination
An oral examination was performed after the saliva samples were collected. Patients were invited to a separate room, where according to WHO criteria, an artificial light source, a dental mirror, and a probe were used. The oral examination was conducted by a dentist who sat opposite the patient. A dental chart was created for each patient. An assessment was made of the number of teeth with caries (DT), those with fillings due to caries (FT), and extracted teeth due to caries (MT). This information was used to calculate the DMFT index (Decay-Missing-Filled Teeth index), which is the sum of the above components. Oral hygiene was assessed using the Plaque Index (PI) and gingival status with the use of the Gingival Index (GI).
PI was calculated based on the following criteria:
0 - no plaque.
1 - plaque visible only after probing.
2 - moderate plaque visible to the naked eye.
3 - large amount of dental plaque [23].
GI was assessed based on the scale:
0 - no gingival inflammation.
1 - gingival swelling and redness.
2 - gingival swelling, redness, and bleeding on probing.
3 - swelling, redness, and spontaneous gingival bleeding [23].
Before the oral examination, calibration and training of dentists (D.F. and K.G.) were done by another experienced dental specialist (A.Z.). The online Cohen Kappa calculator was used to assess intra-examiner and inter-examiner agreement. The intra- and inter-examiner agreement for PI and GI was assessed in 10 subjects (k > 0.91).
Salivary Inflammatory Profile
The salivary biomarkers were divided into 5 groups:
- Pro-inflammatory cytokines: IL-1α (interleukin 1 alpha), IL-1β (interleukin 1 beta), IL-7 (interleukin 7), IL-16 (interleukin 16), IL-18 (interleukin 18), TNF-α (tumor necrosis factor alpha), TNF-β (tumor necrosis factor beta).
- Anti-inflammatory cytokines: IL-1ra (interleukin 1 receptor antagonist), IL-10 (interleukin 10), IL-13 (interleukin 13), TRAIL (tumor necrosis factor-related apoptosis-inducing ligand).
- Th1 cytokines: IFN-α2 (interferon-alpha 2), IFN-γ (interferon gamma), IL-2 (interleukin 2), IL-2Rα/CD25 (interleukin 2 receptor alpha / cluster of differentiation 25), IL-12 (p40): (interleukin 12 homodimer), IL-12 (p70): (interleukin 12 homodimer), IL-15 (interleukin 15).
- Th2 cytokines: IL-4 (interleukin 4), IL-5 (interleukin 5), IL-6 (interleukin 6), IL-9 (interleukin 9).
- Th17 cytokines: IL-17 (interleukin 17).
The concentration of salivary cytokines was measured using the Bio-Plex Pro Human Cytokine Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Bio-Plex technology uses magnetic beads to which antibodies directed against the desired biomarkers are attached. Initially, the saliva samples come into contact with the magnetic beads to induce a reaction of antibodies with the marker of interest. This is followed by a series of rinses to remove the unbound proteins. Next, a biotinylated detection antibody is added, forming a sandwich compound, which after the addition of streptavidin-phycoerythrin (SA-PE) conjugate, becomes the final complex. The results are read using a dedicated plate reader kit: the Bio-Plex 200 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The level of salivary biomarkers was standardized to the content of total protein assessed by the bicinchoninic acid method using the Thermo Scientific PIERCE BCA Protein Assay kit (Rockford, IL, USA).
Functional Status
The functional status of stroke patients was assessed based on:
- Addenbrooke's Cognitive Examination Revised (ACE-R): It is used to determine the cognitive status of patients. Based on the assessment of 5 cognitive domains the patients can receive a total score of 100 points. Higher scores indicate better cognitive abilities [24, 25].
- Barthel Index (BI) of ADL (activities of daily living): Evaluates patients' ability to perform basic daily activities independently. This scale contains 10 items with different weights measuring the ability to perform daily activities. The maximum score of 20 points indicates complete independence [26].
- Functional Independence Measure (FIM): Assesses the amount of assistance a patient needs to perform basic daily activities. The scale includes 18 activities divided into motor and cognitive subgroups. Each activity is rated on a scale of 1 to 7 points. The results show the degree of dependence of patients in performing tasks, with 120-126 points indicating complete independence [27].
Sitting Balance Scale (SBS): Evaluates postural balance while sitting. The assessment of balance is performed in 11 situations, each scored from 0 to 4, with higher scores indicating better balance [28].
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 10 (GraphPad Software, La Jolla, CA, USA) and Past 4.13 (Øyvind Hammer). The Kolmogorov-Smirnov test was used to check the distribution of results. Due to the lack of normal distribution, the Mann-Whitney U test was used for comparisons between two groups. The Spearman rank-order correlation coefficient (Spearman's correlation) was used to check the correlation between salivary markers and clinical data. Receiver Operating Characteristic (ROC) analysis was used to assess the diagnostic utility of salivary cytokines. AUC (area under the curve) and optimal cut-off values were determined for each parameter, ensuring high sensitivity and specificity. The assumed statistical significance was p < 0.05. The size of the study and control groups was determined based on the results of salivary IL-1β and IFN-γ from a pilot study. The ClinCalc online calculator was used, with the power of the statistical test set at 0.8 (α = 0.05). The minimum number of subjects in each group was 18.
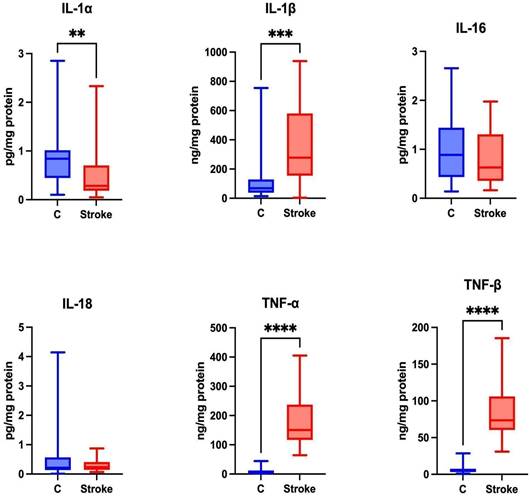
Content of proinflammatory cytokines in non-stimulated saliva of stroke patients compared to healthy controls. IL-1α: interleukin 1alpha; IL-1β: interleukin 1beta; IL-16: interleukin 16; IL-18: interleukin 18; TNF-α: tumor necrosis factor alpha; TNF-β: tumor necrosis factor beta; **—p < 0.01; ***—p < 0.001; ****—p < 0.0001.
Results
Pro-inflammatory cytokines
Pro-inflammatory cytokines trigger brain inflammation [29], influencing the severity of stroke and leading to worse mental and physical functioning in stroke patients [30]. Statistically higher levels of IL-1β, TNF-α and TNF-β were observed in the unstimulated saliva of the study group compared to the control group (p=0.0003; p ≤ 0.0001; p ≤ 0.0001, respectively). A significantly lower level of IL-1α was observed in the unstimulated saliva of the study group compared to the control group (p = 0.0024). No statistically significant differences were found between the study and control groups for IL-16 (p = 0.51) and IL-18 (p = 0.7361) (Figure 2).
The concentration of IL-7 in unstimulated saliva was below the level of detection.
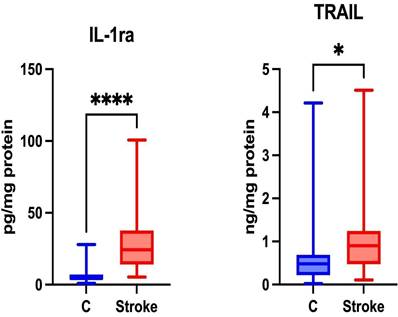
Content of anti-inflammatory cytokines in non-stimulated saliva of stroke patients compared to healthy controls. IL-1ra: interleukin 1 receptor antagonist; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; *—p < 0.05; ****—p < 0.0001.
Anti-inflammatory Cytokines
The expression of anti-inflammatory cytokines is driven by their role in modulating the immune response, either through cytokine receptors or by involving inhibitors that regulate inflammation [31]. Anti-inflammatory therapies help reduce brain damage resulting from ischemia [32]. Significantly higher levels of IL-1ra and TRAIL were noted in the unstimulated saliva of patients in the study group compared to the control group (p ≤ 0.0001; p = 0.0206; respectively) (Figure 3).
The concentrations of IL-10 and IL-13 in saliva were below the level of detection.
Th1 Cytokines
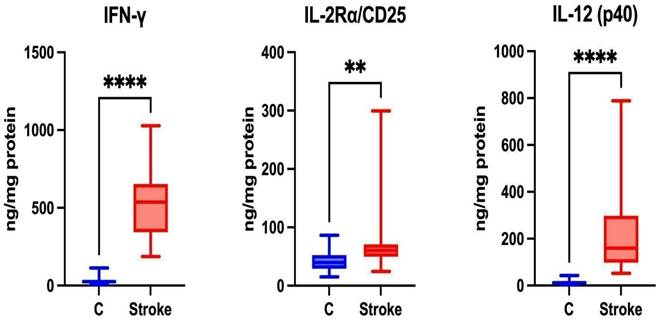
Th1 cells are associated with the secretion of pro-inflammatory Th1 cytokines such as IFN-γ, IL-2Rα/CD25, and IL-12 [33]. Th1 lymphocytes participate in the cellular immune response [34]. Statistically higher levels of IFN-γ, IL-2Rα/CD25 and IL-12 (p40) were noted in the unstimulated saliva of patients in the study group compared to the control group (p ≤ 0.0001; p=0.0021; p ≤ 0.0001, respectively) (Figure 4).
The concentrations of IFN-α2, IL-2, IL-12 (p70), and IL-15 were below the level of detection.
Th2 Cytokines
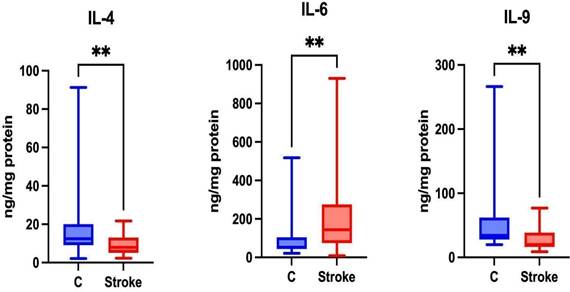
Th2 cells are responsible for the secretion of Th2 cytokines such as IL-4, IL-6, and IL-9 [35]. Th2 lymphocytes are associated with the humoral immune response [34]. Significantly higher levels of IL-6 were noted in the unstimulated saliva of patients in the study group compared to the control group (p = 0.0023). Significantly lower levels of IL-4 and IL-9 were also noted in the unstimulated saliva of patients in the study group compared to the control group (p = 0.0059; p = 0.0059, respectively) (Figure 5).
The concentration of IL-5 in unstimulated saliva was undetectable.
Th17 Cytokines
Th17 cells are associated with autoimmune diseases [36], and during a stroke, they have the ability to secrete pro-inflammatory Th17 cytokines that exacerbate neuroinflammation [33].
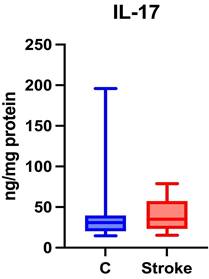
No statistically significant differences were found between the study group and the control group regarding salivary IL-17 levels (p = 0.4219) (Figure 6).
Content of Th1 cytokines in non-stimulated saliva of stroke patients compared to healthy controls. IFN-γ: interferon gamma; IL-2Rα/CD25: interleukin 2 receptor alpha/cluster of differentiation 25; IL-12 (p40): interleukin 12 homodimer; **—p < 0.01; ****—p < 0.0001.
Content of Th2 cytokines in non-stimulated saliva of stroke patients compared to healthy controls. IL-4: interleukin 4; IL-6: interleukin 6; IL-9: interleukin 9; **—p < 0.01.
Content of Th17 cytokines in non-stimulated saliva of stroke patients compared to healthy controls. IL-17: interleukin 17.
Correlations
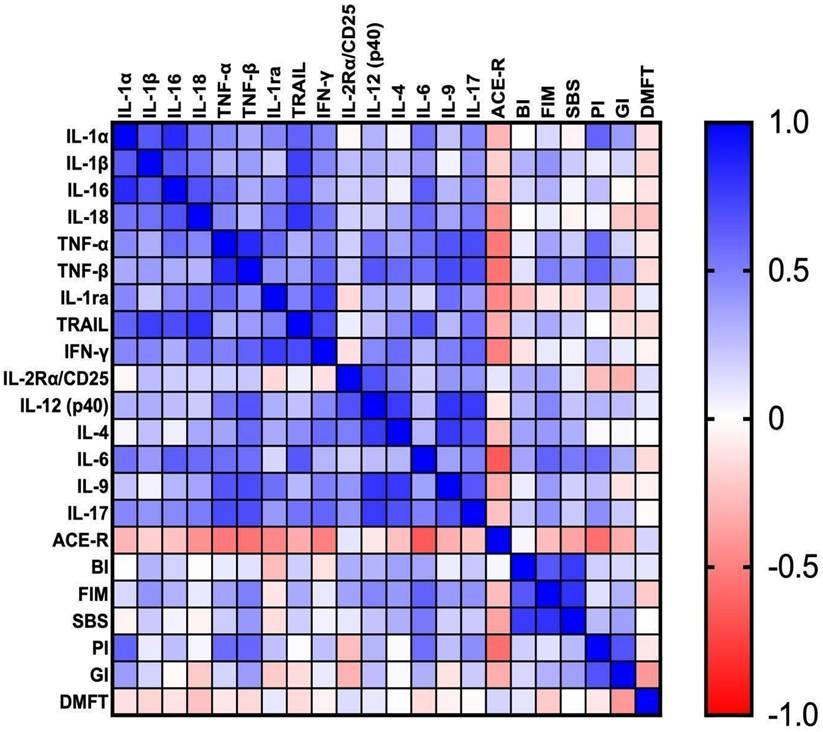
Correlations between the parameters are presented in Figure 7.
Salivary IL-1α correlated positively with IL1β (p = 0.001, r = 0.66) and TRAIL (p = 0.004, r = 0.61). We observed positive correlations between IL-1β and IL-16 (p = 0.001, r = 0.66). IL-16 correlated positively with IL-18 (p = 0.001, r = 0.68) and IL-6 (p = 0.002, r = 0.64). Positive correlation was also noted between TNF-α and IL-9 (p = 0.001, r = 0.67). TNF-β correlated positively with IFN-γ (p = 0.005, r = 0.61), IL-12 (p = 0.002, r = 0.67), IL-9 (p = 0.001, r = 0.71) and IL-17 (p = 0.001, r = 0.7). Moreover, TRAIL was positively associated with IL-6 (p = 0.002, r = 0.66), whereas the positive association was observed between IFN-γ and IL-17 (p = 0.003; r = 0.61). We observed positive correlation between IL-2Rα and IL-12 (p = 0.001; r = 0.68). IL-17 presented a positive correlation with IL-4 (p = 0.001, r = 0.68) and IL-9 (p = 0.002; r = 0.66).
IL-1α presented a correlation with IL-18 (p = 0.01, r = 0.55), TNF-α (p = 0.04, r = 0.46), IL-1ra (p = 0.04, r = 0.47), IFN-γ (p = 0.03, r = 0.48), IL-6 (p = 0.01, r = 0.55) and IL-17 (p = 0.03; r = 0.47). IL-1β presented an association with IL-18 (p = 0.01, r = 0.56) and IFN-γ (p = 0.03, r = 0.48). IL-16 presented a correlation with TNF-α (p = 0.01, r = 0.57), IL-1ra (p = 0.04, r = 0.45) and IL-17 (p = 0.03, r = 0.46). IL-18 correlated with TNF-α (p = 0.03, r = 0.47), IL-1ra (p = 0.01, r = 0.55), IFN-γ (p = 0.01, r = 0.57), IL-6 (p = 0.01, r = 0.58) and IL-17 (p = 0.01, r = 0.52). TNF-α correlated with IL-1ra (p = 0.01, r = 0.59), IFN-γ (p = 0.02, r = 0.49), IL-12 (p = 0.01, r = 0.54) and IL-6 (p = 0.01, r = 0.57). TNF-β was associated with IL-4 (p = 0.01; r = 0.58) and IL-6 (p = 0.01, r = 0.57). IL-1ra presented a correlation with TRAIL (p = 0.02, r = 0.51) and IL-9 (p = 0.01, r = 0.56). Moreover, TRAIL was associated with IL-4 (p = 0.047, r = 0.45) and IL-17 (p = 0.01, r = 0.55). IFN-γ presented an association with IL-12 (p = 0.04, r = 0.47), IL-4 (p = 0.01, r = 0.58) and IL-9 (p = 0.03, r = 0.495). There was a correlation between IL-2Rα and IL-4 (p = 0.02, r = 0.51). The correlation was also noted between IL-6 and IL-17 (p = 0.02, r = 0.50).
We found a negative correlation between ACE-R and salivary TNF-α (p = 0.01, r = -0.53), TNF-β (p = 0.02, r = -0.60) and IFN-γ (p = 0.02, r = -0.50). On the other hand, FIM presented a positive association with IL-6 (p = 0.003, r = 0.62) and BI (p = 0.001, r = 0.66). The positive correlation was also noted between SBS and IL-6 (p = 0.02, r = 0.52). PI correlated positively with IL-1α (p = 0.02, r = 0.61), TNF-α (p = 0.03, r = 0.58), TNF-β (p = 0.03, r = 0.6), IL-6 (p = 0.04, r = 0.57) and ACE-R (p = 0.03, r = -0.57). Interestingly, GI correlated positively only with PI (p = 0.01, r = 0.67).
A significant correlation was noted between ACE-R and IL-18 (p = 0.04; r = -0.43), IL-1ra (p = 0.03, r = -0.47) and IL-6 (p = 0.001, r = -0.25). Furthermore, FIM was associated with TNF-β (p = 0.027, r = 0.51), IL-12 (p = 0.03, r = 0.48) and IL-17 (p = 0.048, r = 0.43).
Correlations between the studied parameters. ACE-R: Addenbrooke's Cognitive Examination Revised; BI: Barthel Index; DMFT: The Decayed, Missing and Filled Teeth; GI: Gingival Index; FIM: The Functional Independence Measure; IL-1α: interleukin 1alpha; IL-1β: interleukin 1beta; IL-1ra: interleukin 1 receptor antagonist; IL-2Rα/CD25: interleukin 2 receptor alpha/cluster of differentiation 25; IL-4: interleukin 4; IL-6: interleukin 6; IL-9: interleukin 9; IL-12 (p40): interleukin 12 homodimer; IL-16: interleukin 16; IL-17: interleukin 17; IL-18: interleukin 18; IFN-γ: interferon gamma; PI: Plaque Index; SBS: Sitting Balance Scale; TNF-α: tumor necrosis factor alpha; TNF-β: tumor necrosis factor beta; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand.
ROC Analysis
In our study, the salivary cytokine levels significantly distinguish ischemic stroke patients from healthy individuals. Among the analyzed markers, salivary TNF-α, TNF-β, IFN-γ, and IL-12 demonstrated exceptional diagnostic accuracy, attaining an AUC of 1 with 100% sensitivity and specificity (Table 1).
Discussion
In our previous study, we evaluated the diagnostic potential of unstimulated and stimulated saliva in assessing TNF-α, IL-6 and IL-10 in stroke patients [16]. Since unstimulated saliva proved more useful, this study focused on a detailed evaluation of its inflammatory profile. We found significantly higher levels of salivary pro-inflammatory cytokines (IL-1β, TNF-α, TNF-β), anti-inflammatory cytokines (IL-1ra, TRAIL), Th1 cytokines (IFN-γ and IL-12 (p40)), and Th2 cytokines (IL-6) in patients with ischemic stroke compared to healthy participants. However, the levels of IL-1α, IL-4, and IL-9 were significantly lower in the saliva of the study group. The obtained results do not indicate a dominance of any particular branch of the immune response.
Stroke is one of the leading causes of death worldwide. With the rising prevalence of stroke, developing simple and effective diagnostic methods to monitor disease progression is essential. Recently, saliva has gained increasing attention due to its non-invasive nature, safety, and the ability to collect multiple samples without requiring specialized personnel. The use of stress-free biofluid collection minimizes patient discomfort, which is especially important for the elderly. Whole saliva is a mixture of salivary gland filtrate, gingival fluid, nasal and pharyngeal secretions, cells lining the oral cavity, and microorganisms [37]. Moreover, due to the abundant vascularization of the salivary glands, a significant portion of the molecules that are present in blood can enter the saliva [38]. Cytokines can move from blood to saliva through active transport, ultrafiltration, or passive diffusion [39, 40]. Additionally, cytokines can enter the saliva through gingival fluid, transudation across the oral mucosa, and inflamed areas where "leaky patches" develop [41]. The dental examinations revealed that stroke patients had poor oral hygiene and periodontal health, which could make leaky patches a major source of cytokines in saliva. Moreover, the levels of salivary cytokines positively correlated with dental indices. We found a particularly strong relationship between pro-inflammatory cytokines (IL-1α, TNF-α, TNF-β, IL-6) and the Plaque Index (PI), and between IL-1α and TNF-β and the Gingival Index (GI). It is well known that dental caries and periodontal diseases significantly increase the risk of stroke [42]. The presence of even one tooth with caries correlates with an increased risk of stroke [43]. Dental infections starting within the tooth or in the periodontium can spread to nearby bones and soft tissues [44]. Periodontal dysbiosis not only triggers a local inflammatory response but also stimulates systemic inflammation through the dissemination of pathogens in the bloodstream [45]. Systemic inflammation, molecular mimicry, and bacteremia can lead to atherosclerosis and cardiovascular diseases [46-48]. Bacteria associated with periodontitis, such as Porphyromonas gingivalis and Prevotella intermedia, can disrupt the endothelial barrier and thus enter the atherosclerotic plaques directly from the bloodstream [49]. Therefore, moderate and severe periodontitis contribute to the progression of atherosclerosis, which in turn promotes the development of stroke [50]. Since oral inflammation is the primary source of salivary cytokines [51, 52], our study included a control group of individuals with comparable oral hygiene and periodontal health. This allowed for proper comparison of results between the study and control groups.
Receiver Operating Characteristic (ROC) analysis for salivary cytokines. IL-1alpha: interleukin 1alpha; IL-1beta: interleukin 1beta; IL-1ra: interleukin 1 receptor antagonist; IL-2Ralpha/CD25: interleukin 2 receptor alpha/cluster of differentiation 25; IL-4: interleukin 4; IL-6: interleukin 6; IL-9: interleukin 9; IL-12 (p40): interleukin 12 homodimer; IL-16: interleukin 16; IL-17: interleukin 17; IL-18: interleukin 18; IFN-gamma: interferon gamma; TNF-alpha: Tumor necrosis factor alpha; TNF-beta: Tumor necrosis factor beta; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand.
| Biomarker | Area | Cut off point | Sensitivity % | 95% CI | Specificity% | 95% CI | Likelihood ratio |
|---|---|---|---|---|---|---|---|
| pro-inflammatory cytokines | |||||||
| IL-1alpha | 0.77 | < 0.6638 | 71.43 | 50.04 - 86.19% | 61.9 | 40.88 - 79.25% | 1.88 |
| IL-1beta | 0.81 | > 152.9 | 81.82 | 61.48 - 92.69% | 81.82 | 61.48 - 92.69% | 4.5 |
| IL-16 | 0.5599 | < 0.7127 | 59.09 | 38.73 - 76.74% | 59.09 | 38.73 - 76.74% | 1.444 |
| IL-18 | 0.53 | < 0.2655 | 63.64 | 42.95 - 80.27% | 45.45 | 26.92 - 65.34% | 1.17 |
| TNF-alpha | 1 | > 21.18 | 100 | 83.18 - 100.0% | 95.45 | 78.20 - 99.77% | 22 |
| TNF-beta | 1 | > 21.18 | 100 | 83.18 - 100.0% | 95.45 | 78.20 - 99.77% | 22 |
| anti-inflammatory cytokines | |||||||
| IL-1ra | 0.94 | > 6.535 | 95.24 | 77.33 - 99.76% | 72.73 | 51.85 - 86.85% | 3.49 |
| TRAIL | 0.71 | > 0.5253 | 66.67 | 45.37 - 82.81% | 59.09 | 38.73 - 76.74% | 1.63 |
| Th1 cytokines | |||||||
| IFN-gamma | 1 | > 81.51 | 100 | 84.54 - 100.0% | 95.45 | 78.20 - 99.77% | 22 |
| IL-2Ralpha | 0.77 | > 51.58 | 77.27 | 56.56 - 89.88% | 77.27 | 56.56 - 89.88% | 3.4 |
| IL-12 (p40) | 1 | > 33.27 | 100 | 84.54 - 100.0% | 95.45 | 78.20 - 99.77% | 22 |
| Th2 cytokines | |||||||
| IL-4 | 0.74 | < 10.19 | 76.19 | 54.91 - 89.37% | 59.09 | 38.73 - 76.74% | 1.86 |
| IL-6 | 0.77 | > 64.56 | 85.71 | 65.36 - 95.02% | 68.18 | 47.32 - 83.64% | 2.69 |
| IL-9 | 0.75 | < 29.90 | 70 | 48.10 - 85.45% | 63.64 | 42.95 - 80.27% | 1.93 |
| Th17 cytokines | |||||||
| IL-17 | 0.57 | > 32.60 | 59.09 | 38.73 - 76.74% | 54.55 | 34.66 - 73.08% | 1.3 |
Inflammation resulting from a stroke is associated with secondary damage to brain tissues. During a stroke, the balance between pro-inflammatory and anti-inflammatory molecules is disturbed [29]. TNF-α, as one of the main pro-inflammatory cytokines, plays a significant role in enhancing the inflammatory response by regulating the expression of adhesion molecules and inducing the synthesis of free radicals [53]. In our study, the content of salivary TNF-α was significantly higher in stroke patients and correlated with cognitive impairment on the ACE-R scale. The ACE-R scale is used for initial differential diagnosis of dementia syndromes and for monitoring of disease progression. This scale assesses attention, orientation, memory, verbal fluency, language and visuospatial functions. It has been shown that ACE-R can be used for early assessment of patients with cerebrovascular changes [54]. We observed similar correlations in the ACE-R scale for TNF-β and IL-6. TNF-β activates signaling pathways associated with the transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and MAPK (mitogen-activated protein kinase), thereby stimulating inflammation and cell death leading to cognitive impairment [55, 56]. IL-6 increases prothrombotic activity and activates endothelial cells [57]. During the inflammatory process, IL-6 activates signaling pathways responsible for oxidative stress, proliferation, and lymphocyte differentiation [53]. However, IL-6 may have dual effects in brain tissue - in the early stage of stroke it shows pro-inflammatory activity, while in later stages, it has a potentially neurotrophic effect [4]. The protective effect of IL-6 may be indicated by the positive correlation of salivary IL-6 with the functional status of patients on the FIM and SBS scales.
The concentration of Th1 cytokines (IFN-γ and IL-12) and anti-inflammatory cytokines (IL-1ra, TRAIL) were also significantly higher in the saliva of stroke patients. IFN-γ synthesized by Th1 cells stimulates the polarization of microglia into the M1 phenotype, which exhibits pro-inflammatory activity, and activates the JAKs (Janus kinases) and STAT (signal transducer and activator of transcription proteins) signaling pathways [4]. It also exerts pro-inflammatory and pro-thrombotic effects by regulating adhesion molecule expression, activating the NADPH oxidase complex, and inducting adhesion molecule synthesis [58]. On the other hand, IL-12 stimulates the production of pro-inflammatory cytokines and chemotactic proteins, while also inducing the expression of adhesion molecules on endothelial cells to facilitate the influx of blood cells [59]. Anti-inflammatory cytokines modulate the immune response through various molecular mechanisms [60]. Interestingly, the concentration of Th2 cytokines in saliva (except for pro-inflammatory IL-6) was significantly lower in the study group compared to the control group. The differentiation of helper lymphocytes into Th2 is promoted by interleukins IL-4 and IL-9, and their reduced expression may indicate an impaired humoral response in stroke patients.
In our study, the salivary concentration of IL-2, IL-5, IL-7, IL-10, IL-12 (p70), IL-13, IL-15, and IFN-α2 was below the detection level in healthy controls (most commonly) and in stroke patients. Therefore, we cannot fully characterize the impact of stroke on the salivary cytokine profile. Indeed, the low concentration of certain biomarkers in saliva poses a limitation to its use in laboratory medicine [61]. The level of salivary biomarkers is also influenced by local factors (caries and periodontal inflammation [38]) and general factors (body hydration level, olfactory stimulation, time of day (during saliva collection), or time since the last meal [62]). However, saliva collection has numerous advantages. It is a non-invasive, non-infectious, simple, and inexpensive procedure [63] that is comfortable for patients of all ages [64]. Unlike blood, saliva can be safely collected from individuals with coagulation disorders without the involvement of medical personnel [65].
According to the Biomarkers Definition Working Group, a biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathological processes, or pharmacological responses to a therapeutic intervention [66]. Currently, there is no widely available and specific biomarker for stroke diagnosis. This prevents early diagnosis of the disease and above all, effective treatment of patients. An ideal stroke biomarker should have high sensitivity and specificity, be easily measurable in circulating biological fluids, and provide information on the effectiveness of treatment. More and more studies assess the biomarker potential of cytokines in stroke diagnosis. Christensen et al. assessed the levels of TNF-α, IL-1, IL-1ra, and IL-10 in the blood of 179 stroke patients. At the onset of stroke, the levels of these cytokines were significantly higher than three months after the incident [67]. In our study, we also noted higher levels of TNF-α, IL-1, and IL-1ra in the study group, however, IL-10 was undetectable in saliva. Beamer et al. showed that serum IL-Ra and IL-6 levels were significantly higher in stroke patients compared to healthy individuals [68]. Kim et al., in addition to elevated IL-6 levels, also showed a significant increase in IL-4 levels, and a considerable decrease in IL-2 levels, in the blood of stroke patients [69]. Tan et al. observed significantly higher levels of IL-4 and IL-9 in the blood of stroke patients, while IFN-γ levels did not differ between the groups [70]. ROC diagnostic utility analysis in our study indicates that salivary TNF-α, TNF-β, IFN-γ, and IL-12 levels significantly differentiate ischemic stroke patients from healthy individuals (AUC = 1, sensitivity = 100%, specificity = 100%). Although the number of patients in our study was statistically calculated, verification of results on a larger population of patients is necessary. Salivary cytokines may be a potential diagnostic biomarker for stroke. Saliva is particularly attractive as a diagnostic material for brain diseases [71, 72]. For many biomarkers, a stronger brain-saliva correlation has been demonstrated than a brain-blood correlation, which may be particularly important in the diagnosis of stroke [73-75]. Unfortunately, we did not assess the inflammatory profile of the blood of stroke patients, so we cannot compare inflammatory changes at the systemic (blood) and local (saliva) level. However, many studies indicate a strong saliva-blood correlation for cytokines [76-78].
Limitations
Our study also has numerous limitations. One of the biggest is that we only analyzed the inflammatory profile of unstimulated saliva. Saliva composition may differ depending on its type - unstimulated saliva is primarily produced by the submandibular glands (constituting plasma filtrate), while stimulated saliva mainly comes from the parotid glands and is less sensitive to systemic factors [79-82]. Furthermore, since we did not analyze the inflammatory profile in the blood and cerebrospinal fluid, we cannot determine whether the inflammation originates locally (from the salivary glands) or centrally (from the brain). Therefore, further analyses comparing different circulating fluids in a larger stroke patient population are necessary. Additionally, a comparison of the salivary inflammatory profiles between patients with ischemic and hemorrhagic stroke should also be explored.
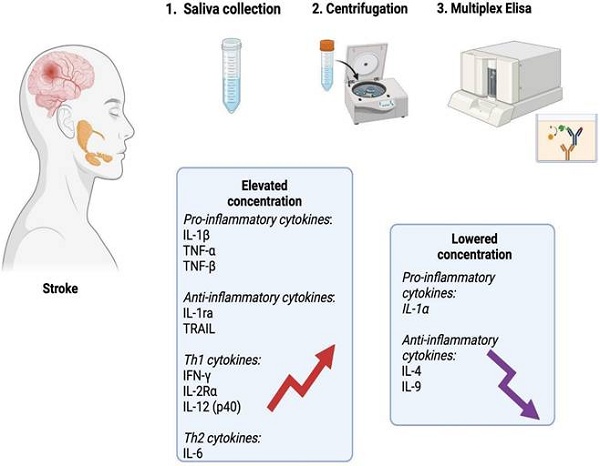
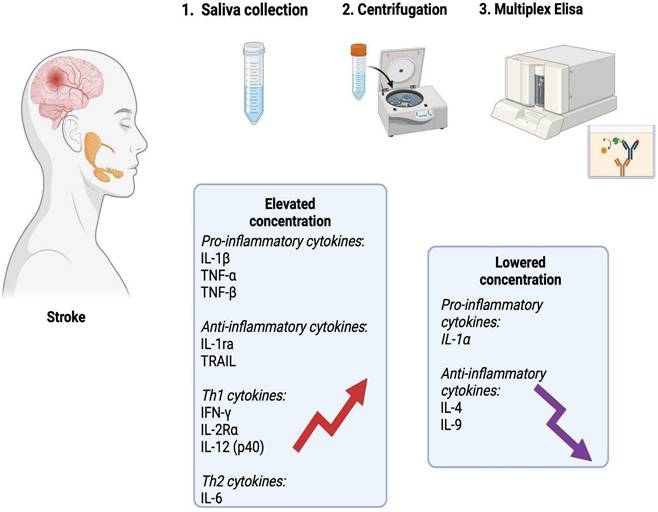
Graphical conclusions of the study (generated using biorender.com). In the unstimulated saliva of stroke patients, a significantly higher content of pro-inflammatory cytokines (IL-1β, TNF-α, TNF-β), anti-inflammatory cytokines (IL-1ra, TRAIL), Th1 cytokines (IFN-γ, IL-2Rα and IL-12 (p40)) and Th2 cytokines (IL-6) was found compared to healthy individuals. The content of IL-1α, IL-4 and IL-9 was significantly lower in the saliva of patients from the study group. IL-1α: interleukin 1alpha; IL-1β: interleukin 1beta; IL-1ra: interleukin 1 receptor antagonist; IL-2Rα: interleukin 2 receptor alpha; IL-4: interleukin 4; IL-6: interleukin 6; IL-9: interleukin 9; IL-12 (p40): interleukin 12 homodimer; IFN-γ: interferon gamma; TNF-α: tumor necrosis factor alpha; TNF-β: tumor necrosis factor beta; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand.
Conclusions
In summary, stroke patients exhibit changes in salivary composition including an increased secretion of inflammatory mediators. The obtained results confirm the inflammatory etiology of ischemic stroke, but do not indicate the dominance of any branch of the immune response. The levels of salivary TNF-α, TNF-β, IFN-γ, and IL-12 significantly differentiate ischemic stroke patients from healthy individuals, making salivary cytokines a potential biomarker for stroke (Figure 8). Further studies examining cytokine levels in the saliva and blood of a larger stroke patient population are necessary.
Key messages
Stroke patients exhibited modified salivary composition, with an increased release of inflammatory markers.
TNF-α, TNF-β, IFN-γ, and IL-12 are markers, which are useful to distinguish ischemic stroke survivors from healthy controls.
While it is essential to validate these results in a larger patient population, salivary cytokines could serve as virtual diagnostic markers.
Supplementary Material
Supplementary table S1.
Acknowledgements
The authors would like to express gratitude to the patients who participated in the research for their consent and cooperation, as well as to the head and staff of the health care center for inestimable help in the organization of examination of the patients.
Funding
Funding was provided by the Medical University of Białystok, Poland (Grant No. B.SUB.24.250).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions statement
D. Forszt: conception and design, saliva samples collection, stroke patients examination, analysis and interpretation of data, drafting of the paper.
K. Gerreth: conception and design, preparation of dental examination, saliva samples collection, stroke patients examination, study supervision, analysis and interpretation of data, critical review of the paper.
J. Kopczyński: analysis and interpretation of data.
A. Zalewska: conception and design, preparation of dental examination, healthy patients examination, saliva samples collection from control group, dentists calibration, study supervision, critical review of the paper.
K. Hojan: carrying out general examination, critical review of the paper.
R. Marchewka: carrying out physical - functional examination, critical review of the paper.
M. Bielas: translation and critical review of the paper.
M. Maciejczyk: conception and design, study supervision, analysis and interpretation of data, drafting of the paper, critical review of the paper.
All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ding S, Chen Q, Chen H. et al. The Neuroprotective Role of Neuroserpin in Ischemic and Hemorrhagic Stroke. Curr Neuropharmacol. 2021;19(8):1367-1378 doi: 10.2174/1570159X18666201008113052
2. Sudharsanan N, Deshmukh M, Kalkonde Y. Direct estimates of disability-adjusted life years lost due to stroke: a cross-sectional observational study in a demographic surveillance site in rural Gadchiroli, India. BMJ Open. 2019;9(11):e028695 doi: 10.1136/bmjopen-2018-028695
3. Farina M, Vieira LE, Buttari B. et al. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules. 2021 18;26(16):5001. doi: 10.3390/molecules26165001
4. Maida CD, Norrito RL, Daidone M. et al. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int J Mol Sci. 2020 4;21(18):6454. doi: 10.3390/ijms21186454
5. Kawabori M, Shichinohe H, Kuroda S. et al. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int J Mol Sci. 2020;21(19):7380 doi: 10.3390/ijms21197380
6. He J, Liu J, Huang Y. et al. Oxidative Stress, Inflammation, and Autophagy: Potential Targets of Mesenchymal Stem Cells-Based Therapies in Ischemic Stroke. Front Neurosci. 2021;15:641157 doi: 10.3389/fnins.2021.641157
7. Ren X, Hu H, Farooqi I. et al. Blood substitution therapy rescues the brain of mice from ischemic damage. Nat Commun. 2020;25;11(1):4078. doi: 10.1038/s41467-020-17930-x. Erratum in: Nat Commun. 2021;12(1):2957 doi: 10.1038/s41467-021-22615-0
8. Muhammad S, Barakat W, Stoyanov S. et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28(46):12023-12031 doi: 10.1523/JNEUROSCI.2435-08.2008
9. Fumagalli S, Perego C, Pischiutta F. et al. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81 doi: 10.3389/fneur.2015.00081
10. Liesz A, Dalpke A, Mracsko E. et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;14;35(2):583-598. doi: 10.1523/JNEUROSCI.2439-14.2015. Erratum in: J Neurosci. 2019;39(27):5419 doi: 10.1523/JNEUROSCI.0878-19.2019
11. Simats A, García-Berrocoso T, Montaner J. Neuroinflammatory biomarkers: From stroke diagnosis and prognosis to therapy. Biochim Biophys Acta. 2016;1862(3):411-424 doi: 10.1016/j.bbadis.2015.10.025
12. Okada T, Suzuki H, Travis ZD. et al. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr Neuropharmacol. 2020;18(12):1187-1212 doi: 10.2174/1570159X18666200528143301
13. Li W, Pan R, Qi Z. et al. Current progress in searching for clinically useful biomarkers of blood-brain barrier damage following cerebral ischemia. Brain Circ. 2018;4(4):145-152 doi: 10.4103/bc.bc_11_18
14. Kułak-Bejda A, Waszkiewicz N, Bejda G. et al. Diagnostic Value of Salivary Markers in Neuropsychiatric Disorders. Dis Markers. 2019 2;2019:4360612. doi: 10.1155/2019/4360612
15. Celec P, Tóthová Ľ, Šebeková K. et al. Salivary markers of kidney function - Potentials and limitations. Clin Chim Acta. 2016;30;453:28-37. doi: 10.1016/j. cca. 2015 11.028
16. Maciejczyk M, Mil KM, Gerreth P. et al. Salivary cytokine profile in patients with ischemic stroke. Sci Rep. 2021 25;11(1):17185. doi: 10.1038/s41598-021-96739-0
17. Maciejczyk M, Nesterowicz M, Zalewska A. et al. Salivary Xanthine Oxidase as a Potential Biomarker in Stroke Diagnostics. Front Immunol. 2022;13:897413 doi: 10.3389/fimmu.2022.897413
18. Easton JD, Saver JL, Albers GW. et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293
19. Sacco RL, Kasner SE, Broderick JP. et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064-2089
20. Albers GW, Caplan LR, Easton JD. et al. Transient ischemic attack - proposal for a new definition. N Engl J Med. 2002;347:1713-1716
21. Szulimowska J, Zalewska A, Taranta-Janusz K. et al. Association Between Salivary Cytokines, Chemokines and Growth Factors and Salivary Gland Function in Children with Chronic Kidney Disease. J Inflamm Res. 2023;16:1103-1120 doi: 10.2147/JIR.S399786
22. Klimiuk A, Zalewska A, Knapp M. et al. Could inflammation contribute to salivary gland dysfunction in patients with chronic heart failure? Front Immunol. 2022;13:1005981 doi: 10.3389/fimmu.2022.1005981
23. Silness J., Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964;22:121-135
24. Mioshi E, Dawson K, Mitchell J. et al. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21(11):1078-1085 doi: 10.1002/gps.1610
25. Prats-Sedano MA, Savulich G, Surendranathan A. et al. The revised Addenbrooke's Cognitive Examination can facilitate differentiation of dementia with Lewy bodies from Alzheimer's disease. Int J Geriatr Psychiatry. 2021;36(6):831-838 doi: 10.1002/gps.5483
26. Yi Y, Ding L, Wen H. et al. Is Barthel Index Suitable for Assessing Activities of Daily Living in Patients With Dementia? Front Psychiatry. 2020;11:282 doi: 10.3389/fpsyt.2020.00282
27. Kawamura K, Murayama K, Takamura J. et al. Effect of a weekly functional independence measure scale on the recovery of patient with acute stroke: A retrospective study. Medicine (Baltimore). 2022;101(11):e28974 doi: 10.1097/MD.0000000000028974
28. Medley A, Thompson M. Development, reliability, and validity of the Sitting Balance Scale. Physiother Theory Pract. 2011;27(7):471-481 doi: 10.3109/09593985.2010.531077
29. Nayak AR, Kashyap RS, Kabra D. et al. Time course of inflammatory cytokines in acute ischemic stroke patients and their relation to inter-alpha trypsin inhibitor heavy chain 4 and outcome. Ann Indian Acad Neurol. 2012;15(3):181-185 doi: 10.4103/0972-2327.99707
30. Dugue R, Nath M, Dugue A. et al. Roles of Pro- and Anti-inflammatory Cytokines in Traumatic Brain Injury and Acute Ischemic Stroke. 2017; InTech. doi: 10.5772/intechopen.70099.
31. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27-37 doi: 10.1097/AIA.0b013e318034194e
32. Zhao H, Li Y, Zhang Y. et al. Role of Immune and Inflammatory Mechanisms in Stroke: A Review of Current Advances. Neuroimmunomodulation. 2022;29(4):255-268 doi: 10.1159/000524951
33. Yu S, Cui W, Han J. et al. Longitudinal change of Th1, Th2, and Th17 cells and their relationship between cognitive impairment, stroke recurrence, and mortality among acute ischemic stroke patients. J Clin Lab Anal. 2022;36(7):e24542 doi: 10.1002/jcla.24542
34. Lappin MB, Campbell JD. The Th1-Th2 classification of cellular immune responses: concepts, current thinking and applications in haematological malignancy. Blood Rev. 2000;14(4):228-239 doi: 10.1054/blre.2000.0136
35. Jin R, Liu L, Zhang S. et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6(5):834-851 doi: 10.1007/s12265-013-9508-6
36. Chen X, Oppenheim JJ. Th17 cells and Tregs: unlikely allies. J Leukoc Biol. 2014;95(5):723-731 doi: 10.1189/jlb.1213633
37. Sculley DV, Langley-Evans SC. Salivary antioxidants and periodontal disease status. Proc Nutr Soc. 2002;61(1):137-143 doi: 10.1079/pns2001141
38. Williamson S, Munro C, Pickler R. et al. Comparison of biomarkers in blood and saliva in healthy adults. Nurs Res Pract. 2012;2012:246178 doi: 10.1155/2012/246178
39. Wang J, Liang Y, Wang Y. et al. Computational prediction of human salivary proteins from blood circulation and application to diagnostic biomarker identification. PLoS One. 2013 12;8(11):e80211. doi: 10.1371/journal.pone.0080211
40. Zięba S, Maciejczyk M, Antonowicz B. et al. Comparison of smoking traditional, heat not burn and electronic cigarettes on salivary cytokine, chemokine and growth factor profile in healthy young adults-pilot study. Front Physiol. 2024;15:1404944 doi: 10.3389/fphys.2024.1404944
41. Bosch JA. The use of saliva markers in psychobiology: mechanisms and methods. Monogr Oral Sci. 2014;24:99-108 doi: 10.1159/000358864
42. Gerreth P, Gerreth K, Maciejczyk M. et al. Is an Oral Health Status a Predictor of Functional Improvement in Ischemic Stroke Patients Undergoing Comprehensive Rehabilitation Treatment? Brain Sci. 2021;11(3):338 doi: 10.3390/brainsci11030338
43. Sen S, Logue L, Logue M. et al. Dental Caries, Race and Incident Ischemic Stroke, Coronary Heart Disease, and Death. Stroke. 2024;55(1):40-49 doi: 10.1161/STROKEAHA.123.042528
44. Shahi S, Farhoudi M, Dizaj SM. et al. The Link between Stroke Risk and Orodental Status-A Comprehensive Review. J Clin Med. 2022 2;11(19):5854. doi: 10.3390/jcm11195854
45. Shetty B, Fazal I, Khan SF. et al. Association between cardiovascular diseases and periodontal disease: more than what meets the eye. Drug Target Insights. 2023;17:31-38 doi: 10.33393/dti.2023.2510
46. Isola G, Polizzi A, Santonocito S. et al. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J Periodontol. 2022;93(1):135-145 doi: 10.1002/JPER.21-0049
47. Polizzi A, Nibali L, Tartaglia GM. et al. Impact of nonsurgical periodontal treatment on arterial stiffness outcomes related to endothelial dysfunction: A systematic review and meta-analysis. J Periodontol. 2024 doi: 10.1002/JPER.24-0422
48. LaValley EA, Sen S, Mason E. et al. Dental Caries a Risk Factor for Intracerebral Hemorrhage. Cerebrovasc Dis. 2024;53(1):98-104 doi: 10.1159/000530568
49. Chang Y, Woo HG, Lee JS. et al. Better oral hygiene is associated with lower risk of stroke. J Periodontol. 2021;92(1):87-94 doi: 10.1002/JPER.20-0053
50. Zheng X, Li X, Zhen J. et al. Periodontitis is associated with stroke. J Transl Med. 2023;21(1):697 doi: 10.1186/s12967-023-04545-1
51. Żukowski P, Maciejczyk M, Waszkiel D. Sources of free radicals and oxidative stress in the oral cavity. Arch Oral Biol. 2018;92:8-17 doi: 10.1016/j.archoralbio.2018.04.018
52. Zięba S, Maciejczyk M, Zalewska A. Ethanol- and Cigarette Smoke-Related Alternations in Oral Redox Homeostasis. Front Physiol. 2022;12:793028 doi: 10.3389/fphys.2021.793028
53. Tirandi A, Sgura C, Carbone F. et al. Inflammatory biomarkers of ischemic stroke. Intern Emerg Med. 2023;18(3):723-732 doi: 10.1007/s11739-023-03201-2
54. Siciliano M, Raimo S, Tufano D. et al. The Addenbrooke's Cognitive Examination Revised (ACE-R) and its sub-scores: normative values in an Italian population sample. Neurol Sci. 2016;37(3):385-392 doi: 10.1007/s10072-015-2410-z
55. Etemadi N, Holien JK, Chau D. et al. Lymphotoxin α induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J. 2013;280(21):5283-5297 doi: 10.1111/febs.12419
56. Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharmacol Ther. 2010;128(3):519-548 doi: 10.1016/j.pharmthera.2010.08.007
57. Liberale L, Ministrini S, Carbone F. et al. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic Res Cardiol. 2021;116(1):23 doi: 10.1007/s00395-021-00863-x
58. Yilmaz G, Arumugam TV, Stokes KY. et al. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113(17):2105-2112 doi: 10.1161/CIRCULATIONAHA.105.593046
59. Zhu H, Hu S, Li Y. et al. Interleukins and Ischemic Stroke. Front Immunol. 2022;13:828447 doi: 10.3389/fimmu.2022.828447
60. Peluzzo AM, Autieri MV. Challenging the Paradigm: Anti-Inflammatory Interleukins and Angiogenesis. Cells. 2022 8;11(3):587. doi: 10.3390/cells11030587
61. Tiwari M. Science behind human saliva. J Nat Sci Biol Med. 2011;2(1):53-58 doi: 10.4103/0976-9668.82322
62. Kaufman E, Lamster IB. The diagnostic applications of saliva-a review. Crit Rev Oral Biol Med. 2002;13(2):197-212 doi: 10.1177/154411130201300209
63. Xiao H, Wong DT. Proteomics and its applications for biomarker discovery in human saliva. Bioinformation. 2011 6;5(7):294-296. doi: 10.6026/97320630005294
64. Ghosh S, Dhobley A, Avula KK. et al. Role of Saliva as a Non-Invasive Diagnostic Method for Detection of COVID-19. Cureus. 2022;14(7):e27471 doi: 10.7759/cureus.27471
65. Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241-248
66. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89-95 doi: 10.1067/mcp.2001.113989
67. Christensen H, Boysen G, Christensen E. et al. Plasma cytokines in acute stroke. J Stroke Cerebrovasc Dis. 2002;11(2):72-79 doi: 10.1053/jscd.2002.126688
68. Beamer NB, Coull BM, Clark WM. et al. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37(6):800-805 doi: 10.1002/ana.410370614
69. Kim HM, Shin HY, Jeong HJ. et al. Reduced IL-2 but elevated IL-4, IL-6, and IgE serum levels in patients with cerebral infarction during the acute stage. J Mol Neurosci. 2000;14(3):191-196 doi: 10.1385/JMN:14:3:191
70. Tan S, Shan Y, Wang Y. et al. Exacerbation of oxygen-glucose deprivation-induced blood-brain barrier disruption: potential pathogenic role of interleukin-9 in ischemic stroke. Clinical Science. 2017;131(13):1499-1513 doi:10.1042/cs20170984
71. Gerreth P, Maciejczyk M, Zalewska A. et al. Comprehensive Evaluation of the Oral Health Status, Salivary Gland Function, and Oxidative Stress in the Saliva of Patients with Subacute Phase of Stroke: A Case-Control Study. J Clin Med. 2020;9(7):2252 doi: 10.3390/jcm9072252
72. Maciejczyk M, Gerreth P, Zalewska A. et al. Salivary Gland Dysfunction in Stroke Patients Is Associated with Increased Protein Glycoxidation and Nitrosative Stress. Oxid Med Cell Longev. 2020;10:6619439 doi: 10.1155/2020/6619439
73. Smith AK, Kilaru V, Klengel T. et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(1):36-44 doi: 10.1002/ajmg.b.32278
74. Martin J, Kagerbauer SM, Gempt J. et al. Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J Neuroendocrinol. 2018;3:e12596 doi: 10.1111/jne.12596
75. Thomas M, Knoblich N, Wallisch A. et al. Increased BDNF methylation in saliva, but not blood, of patients with borderline personality disorder. Clin Epigenetics. 2018;10(1):109 doi: 10.1186/s13148-018-0544-6
76. Browne RW, Kantarci A, LaMonte MJ. et al. Performance of multiplex cytokine assays in serum and saliva among community-dwelling postmenopausal women. PLoS One. 2013;8(4):e59498 doi: 10.1371/journal.pone.0059498
77. Byrne ML, O'Brien-Simpson NM, Reynolds EC. et al. Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain Behav Immun. 2013;34:164-175. doi: 10.1016/j.bbi.2013.08.010. Erratum in: Brain Behav Immun. 2020;89:696-697 doi: 10.1016/j.bbi.2020.07.003
78. Parkin GM, Kim S, Mikhail A. et al. Associations between saliva and plasma cytokines in cognitively normal, older adults. Aging Clin Exp Res. 2023;35(1):117-126 doi: 10.1007/s40520-022-02292-9
79. Dawes C. Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva. J Physiol. 1975;244(2):535-548 doi: 10.1113/jphysiol.1975.sp010811
80. Iorgulescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. 2009;2(3):303-307
81. Kado I, Kunimatsu R, Yoshimi Y. et al. Surveillance of salivary properties of pre-orthodontic patients in relation to age and sex. Sci Rep. 2021;11(1):6555 doi: 10.1038/s41598-021-85861-8
82. Kim J, Choi W, Kim KH. et al. Circadian Rhythms in Tongue Features. J Clin Med. 2024;13(12):3549 doi: 10.3390/jcm13123549
Author contact
![]() Corresponding author: Mateusz Maciejczyk, Department of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Mickiewicza 2C Street, 15-089 Bialystok, Poland; mat.maciejczykcom
Corresponding author: Mateusz Maciejczyk, Department of Hygiene, Epidemiology and Ergonomics, Medical University of Bialystok, Mickiewicza 2C Street, 15-089 Bialystok, Poland; mat.maciejczykcom

 Global reach, higher impact
Global reach, higher impact