3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(4):933-939. doi:10.7150/ijms.105995 This issue Cite
Research Paper
Pretreatment FDG PET in prognosis of locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiation therapy
1. Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.
2. Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China; Department of Radiation Oncology, Shanghai Clinical Research Center for Radiation Oncology, Shanghai, China; Shanghai Key Laboratory of Radiation Oncology, Shanghai, China.
*Xiaoshuang Niu and Fen Xue contributed equally to this article.
Received 2024-10-29; Accepted 2025-1-11; Published 2025-1-27
Abstract
Objectives: We aimed to investigate the long-term survival benefit of PET/CT compared with the routine examination (chest CT, abdominal enhanced CT and emission computed tomography (ECT)) for locally advanced nasopharyngeal carcinoma (NPC) before treatment.
Methods: From June 8th 2005 to August 10th 2017, 507 histologically diagnosed NPC patients with the 8th AJCC/UICC staging criteria III-IVA were enrolled in this study. Among them, patients underwent chest CT, abdominal enhanced CT and bone emission CT (control group), or replaced by positron emission tomography-CT (PET-CT group) to check for distant metastases.
Results: The numbers of patients in the control and PET-CT group were 344 (67.9%) and 163 (32.1%), respectively. With the median follow-up of 72 months, a total of 127 (25.0%) patients died. The 5-year and 8-year overall survival (OS) rates of the control and PET-CT group were 81.1% and 86.9%, 70.8% and 74.6% (P=0.087), respectively. Patients with T1-3, III stage and TPF showed improved 5-year and 8-year OS rates compared with T4, IVA stage and PF patients (P=0.001, P=0.000 and P=0.009). Patients with initially PET-CT-based staged showed improved 5-year and 8-year distant control (DC) compared with the control group (90.6% vs. 83.3% and 90.6% vs. 81.0%, P=0.013). There was no significant difference in local control (LC) and regional control (RC), between the control and PET-CT group.
Conclusions: Patients with initially PET-CT-based staged showed improved long-term DC compared with the control group. Initially PET-CT-based staged is recommended routinely in locoregionally advanced NPC.
Introduction
The proportion of distant metastasis in nasopharyngeal carcinoma (NPC) at initial diagnosis is high, ranging from 9.9% to 14.8% [1-3]. The common sites of distant metastasis are bone, liver and lung [4-5]. The treatment principles and prognosis differ greatly with/without distant metastasis in NPC. The 5-year overall survival (OS) rate for patients without distant metastasis was between 77.8% and 84.0% [6-9], while the 2-year OS was only 50.2%-76.4% for distant metastasis patients at initial diagnosis [10-13].
Traditionally, in order to rule out distant metastasis for newly diagnosed NPC, chest X-ray, abdominal ultrasound and ECT were performed [14-16]. PET-CT could detect potential distant metastasis, especially in medium-high risk patients [2, 16-20], while it might not be helpful in early-stage patients [21-22]. Yen TC's [17] study included 118 cases of newly diagnosed NPC (N0: 16, N1: 31, N2: 53, N3: 18 cases) and 22 cases of recurrent NPC. Among the M0 patients after routine examination (chest radiography, whole-body bone scanning, and liver sonography), PET-CT could detect metastatic lesions in 12.9% cases. Xiao BB's [21] retrospective analysis showed that there were no differences in OS, progression-free survival (PFS), relapse-free survival (RFS) or distant metastasis-free survival (DMFS) between conventional workup (CWU: chest radiograph, liver ultrasound, bone scintigraphy) and PET-CT+CWU group for stage I-II NPC. In another retrospective analysis, Yang PC [22] included stage I-IVA NPC with/without PET-CT before treatment. All patients in the control group (non-initially PET-CT-based staged) received abdominal ultrasound and chest X-ray for metastatic staging. Propensity score matching yielded 4366 patients in each group. The 5-year OS rates were 76.3% and 71.2% (P=0.0015). The 5-year OS rates for stage I were 91.9% and 88.9% (P=0.4431); for stage II, III, and IV, they were 88.7% and 84.1% (P=0.0260), 82.4% and 77.6% (P=0.0104), 61.9% and 56.8% (P=0.0299), respectively. In conclusion, most routine examination before treatment was based on chest X-ray, abdominal ultrasound and ECT, with relatively short observation time. It is uncertain that whether PET-CT plays an important role in the treatment and prognosis of NPC. Therefore, we reported the 8-year efficacy of locally advanced NPC performed with control group (chest CT, enhanced abdominal CT and ECT), compared to the PET-CT group.
Materials and Methods
Patients
From June 8th 2005 to August 10th 2017, 507 histologically diagnosed (WHO II/III) NPC patients with the 8th AJCC/UICC staging III-IVA were enrolled in this study. All patients had no history of a second malignant tumor with KPS≥70 and provided informed written consent before treatment. Initial assessment consisted of enhanced magnetic resonance imaging (MRI) of the nasopharynx and enhanced MRI/CT of the neck, chest CT, abdominal enhanced CT and bone emission CT (control group), or replaced by positron emission tomography-CT (PET-CT group). All patients were re-staged according to the 8th AJCC/UICC staging criteria.
Radiotherapy and chemotherapy
All patients were treated with IMRT. The prescribed dose given to primary tumor was 66 Gy in 30 fractions for T1 or T2 lesion and 70.4 Gy in 32 fractions for T3 or T4 disease (PTV-NX: GTV-NX +5 mm). A total dose of 66 Gy was given to the planned target volume of the lymph nodes (PTV-LN: GTV-LN +3 mm) in 30-32 fractions. The PTV-60 covering the high-risk CTV and a 5-mm margin was prescribed 60 Gy/30-32 F. The PTV-54 covering the low-risk CTV and a 5-mm margin was prescribed 54 Gy/30-32 F. Radiotherapy was given once daily, 5 fractions per week.
All patients received chemotherapy, including induction chemotherapy (IC) + concurrent chemotherapy (CCRT) or adjuvant chemotherapy (AC). Generally, the IC/AC regimens were delivered: TPF (docetaxel 60 mg/m2 d1, cisplatin 25 mg/m2 d1-3 and 5-FU 500 mg/m2 /d with 120-h infusion), or PF (cisplatin 25 mg/m2 d1-3 and 5-FU 500 mg/m2 /d with 120-h infusion). The regimens were repeated every 21 days for two cycles. CCRT was cisplatin 30 mg/m2 weekly or 80 mg/m2 d1-3 every 21 days during IMRT.
Follow-up and statistical analysis
After treatment completion, follow-ups occurred every 3 months for the first 2 years, every 6 months from the third through the fifth year and annually thereafter.
SPSS 23.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis in this study. The estimated OS, local control (LC), regional control (RC), and distant control (DC) were calculated by the Kaplan-Meier method. The duration of survival was measured from the time of treatment until death or the date of the last follow up visit for patients alive. LC was calculated from the date of initiation of treatment to the date of local failure or last follow-up. RC was calculated from the date of initiation of treatment to the date of regional failure or last follow-up. DC was calculated from the date of initiation of treatment to the date of metastasis or last follow-up. A 2-sided P<0.05 was considered statistically significant.
Results
Patient characteristics
From June 8th 2005 to August 10th 2017, 507 histologically diagnosed (WHO II/III) NPC patients with the 8th AJCC/UICC staging III-IVA were enrolled in this study. The numbers of patients in the control group and PET-CT group were 344 (67.9%) and 163 (32.1%), respectively. The median age was 48 years (range, 17-73 years). The study included 363 male (71.6%) and 144 female (28.4%). The treatment of patients with IC+RT+AC and IC+CCRT were 392 (77.3%) and 115 (22.7%), respectively. The characteristics of the two group patients were shown in Table 1.
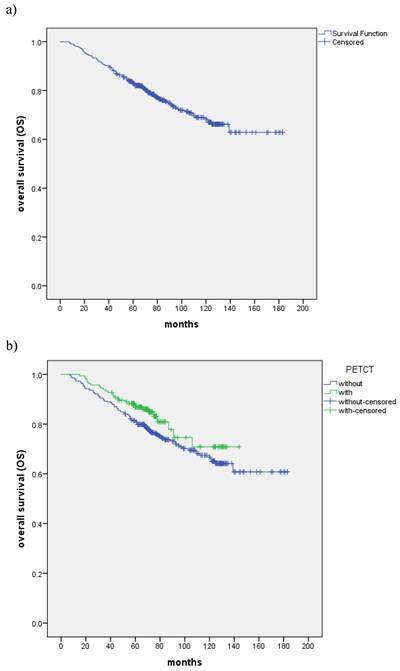
a) Kaplan-Meier estimate of OS curve for all the patients. b) The OS between patients with the control and PET-CT group, respectively.
Overall survival
With the median follow-up of 72 months, a total of 127 (25.0%) patients died: 32 patients died of recurrence in nasopharynx and/or regional lymph nodes, 7 of recurrence in regional lymph nodes, 46 patients died of distant metastasis, 8 of distant metastases accompanied by recurrence in nasopharynx and/or regional lymph nodes, 8 of distant metastases accompanied by recurrence in regional lymph nodes, 1 of massive hemorrhage, 6 of second tumor and 19 of other reasons. The all-cause death of the two group patients was shown in Table 2. The 5-year and 8-year OS rates were 82.9% and 72.4%, respectively (Fig. 1a). The 5-year and 8-year OS rates of the control and PET-CT group were 81.1% and 86.9%, 70.8% and 74.6% (P=0.087), respectively (Fig. 1b). Patients with T1-3, III stage and TPF showed improved 5-year and 8-year OS rates compared with T4, IVA stage and PF patients (P=0.001, P=0.000 and P=0.009) (Table 3).
Characteristics of patients
| Characteristic | No. of patients | ||
|---|---|---|---|
| Control group | PET-CT group | ||
| Age | ≤48 | 165 | 96 |
| >48 | 179 | 67 | |
| Gender | Female | 102 | 42 |
| Male | 242 | 121 | |
| KPS | 70-80 | 194 | 64 |
| 90-100 | 150 | 99 | |
| T stage | T1 | 24 | 15 |
| T2 | 72 | 48 | |
| T3 | 139 | 64 | |
| T4 | 109 | 36 | |
| N stage | N0 | 24 | 9 |
| N1 | 102 | 27 | |
| N2 | 129 | 75 | |
| N3 | 89 | 52 | |
| Clinical stage | III | 158 | 80 |
| IVa | 186 | 83 | |
| Treatment | IC+CCRT | 58 | 57 |
| IC+RT+AC | 286 | 106 | |
| Diagnosis year | 2005-2014 | 252 | 59 |
| 2015-2017 | 92 | 104 | |
| Chemotherapy | PF | 115 | 40 |
| TPF | 229 | 123 | |
All death causes of patients
| Whole group | Control group | PET-CT group | |
|---|---|---|---|
| nasopharyngeal ± neck lymph nodes | 32 | 22 | 10 |
| neck lymph nodes | 7 | 5 | 2 |
| distant metastasis | 46 | 37 | 9 |
| distant metastasis + nasopharyngeal ± neck lymph nodes | 8 | 7 | 1 |
| distant metastasis+neck lymph nodes | 8 | 6 | 2 |
| massive hemorrhage | 1 | 1 | 0 |
| second tumor | 6 | 4 | 2 |
| others | 19 | 17 | 2 |
Distant control
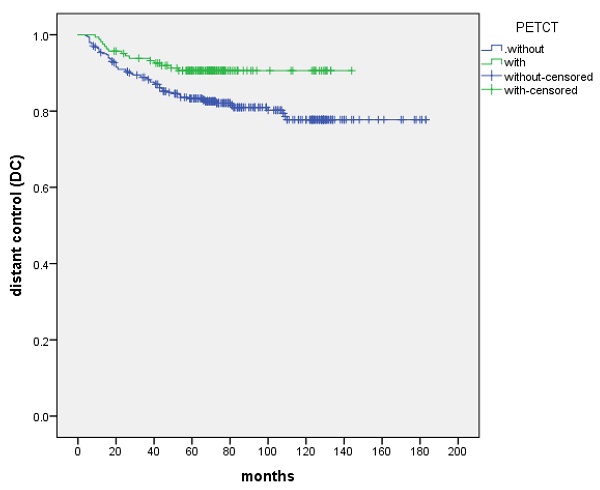
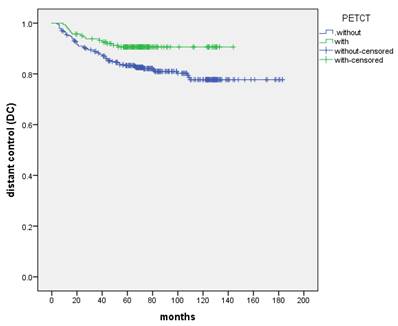
The 5-year and 8-year DC were 85.6% and 83.8%, respectively. DC was not significantly different in the T stage (P=0.115) and the treatment (IC+IMRT+AC or IC+CCRT) of chemotherapy (P=0.532). N stage appeared to be a prognostic factor for DC (P=0.001). Patients with initially PET-CT-based staged showed improved 5-year and 8-year DC compared with the control group (90.6% vs. 83.3% and 90.6% vs. 81.0%, P=0.013) (Figure 2, Table 4).
Local control and regional control
The 5-year and 8-year LC were 89.4% and 87.6%, respectively. T stage (P=0.000) was the factor that significantly influenced LC. Patients with T4 compared with those of T1-3 have worse LC. There was no significant difference in LC between the control and PET-CT group for T1, T2, T3, and T4 (P = 0.658, 0.725, 0.084, 0.207). The 5-year and 8-year RC were 91.5% and 89.9%, respectively. Patients with N2-3, compared with those of N0-1 have worse RC (5-year rates, 89.4% vs. 96.0%, 8-year rates, 86.9% vs. 96.0%, P=0.009). Initially PET-CT-based staged was not the factor that significantly influenced LC (P=0.494) and RC (P=0.618).
OS of different groups
| No. of patients | 5-year (%) | 8-year (%) | p | ||
|---|---|---|---|---|---|
| T stage | T1 | 6/39 | 92.3 | 86.2 | 0.001 |
| T2 | 24/120 | 85.8 | 78.1 | ||
| T3 | 44/203 | 84.7 | 75.1 | ||
| T4 | 53/145 | 75.6 | 59.9 | ||
| N stage | N0 | 7/33 | 81.6 | 74.2 | 0.412 |
| N1 | 33/129 | 87.5 | 71.6 | ||
| N2 | 45/204 | 83.2 | 77.4 | ||
| N3 | 42/141 | 78.7 | 67.5 | ||
| Stage | III | 43/238 | 88.2 | 79.8 | 0.000 |
| IV | 84/269 | 78.3 | 65.9 | ||
| Treatment | IC+IMRT+AC | 108/392 | 82.9 | 71.2 | 0.286 |
| IC+CCRT | 19/115 | 82.9 | 82.9 | ||
| Chemotherapy | PF | 49/155 | 75.9 | 63.8 | 0.009 |
| TPF | 78/352 | 86.0 | 76.0 | ||
| Pre-treatment examination | Control group | 99/344 | 81.1 | 70.8 | 0.087 |
| PET-CT group | 28/163 | 86.9 | 74.6 |
The DC between patients with the control and PET-CT group, respectively
Discussion
NPC is a malignant neoplasm of the epithelial tissue, which is sensitive to radiotherapy (RT) and chemotherapy and diagnosed as stage I-II for only 18.4%-25.4% at initial diagnosis [23-26]. More than 70% of newly diagnosed NPC cases are classified as locoregionally advanced disease and CCRT plays an essential role in the treatment of NPC. The 5-year OS rates of stage III, IVA and IVB (AJCC 7th) patients were 79.1-86.0%%, 65.1-74.3% and 48.6-63.1% [8, 26-27]. However, due to the high incidence of acute toxicity like oral mucositis, the tolerance of CCRT was unsatisfied and non-CCRT could also be considered as a treatment option for locoregionally advanced NPC [28-32]. Wang P [28] showed that compared with CCRT in other literature, IC-RT-AC achieved similar long-term survivals. The 5-year OS rates for patients with stage III or IVA were 86.5% and 56.5%. Yang Z [30] prospectively evaluated that there was no significant difference (P>0.05) in OS between IC+CCRT (102 cases) and CCRT (102 cases) for locoregionally advanced NPC. In this study, we retrospectively evaluated the 5-year and 8-year OS rates of stage III and IVA were 88.2% and 78.3%, 79.8% and 65.9% (P=0.000), respectively. OS was not significantly different between IC+RT+AC and IC+CCRT (P=0.286).
DC of different groups
| No. of patients | 5-year (%) | 8-year (%) | p | ||
|---|---|---|---|---|---|
| T stage | T1 | 6/39 | 84.6 | 84.6 | 0.115 |
| T2 | 14/120 | 89.0 | 87.9 | ||
| T3 | 29/203 | 88.5 | 84.5 | ||
| T4 | 31/145 | 78.8 | 78.8 | ||
| N stage | N0 | 1/33 | 96.6 | 96.6 | 0.001 |
| N1 | 16/129 | 89.6 | 88.1 | ||
| N2 | 27/204 | 89.4 | 85.9 | ||
| N3 | 36/141 | 74.0 | 74.0 | ||
| stage | III | 23/238 | 93.2 | 89.3 | 0.000 |
| IV | 57/269 | 78.9 | 78.9 | ||
| treatment | IC+IMRT+AC | 65/392 | 85.4 | 83.2 | 0.532 |
| IC+CCRT | 15/115 | 86.5 | 86.5 | ||
| Chemotherapy | PF | 25/155 | 83.9 | 83.0 | 0.730 |
| TPF | 55/352 | 86.4 | 84.2 | ||
| Pre-treatment examination | Control group | 65/344 | 83.3 | 81.0 | 0.013 |
| PET-CT group | 15/163 | 90.6 | 90.6 |
The proportion of distant metastasis in NPC at initial diagnosis is 9.9%-14.8% [1-3]. Initially PET-CT-based staged can detect potential distant metastasis and help with accurate staging and therapy compared with the routine examination, especially in N2-3 patients [33-35]. A meta-analysis [33] of NPC found that the pooled sensitivity and specificity were 85.7% and 98.1% for PET/CT (1474 patients), and 38.0% and 97.6% for CWUs (1329 patients). In a study [34] of 300 cases NPC, 61 cases (20.3%) were found to have distant metastases. In comparing CWUs (chest radiography, abdominal ultrasonography, and skeletal scintigraphy) with PET-CT, PET-CT was found to be more effective and higher sensitivity (P < 0.001). Further analysis showed that PET-CT was more effective in detecting chest and bone metastases (P < 0.001), and similar to abdominal ultrasound in detecting hepatic metastases (P=0.127). The study concluded that PET-CT could replace CWUs in primary M staging of NPC.
The main failure pattern in NPC is distant metastasis, followed by nasopharyngeal and regional lymph node recurrence [5-9, 23-27]. Distant metastases can occur in a single or multiple sites and be related to the N stage, with the bone, lung, and liver sorted by occurrence [36-37]. Al Tamimi AS [36] analyzed the distribution of 709 FDG avid lesions of NPC detected by PET/CT, of which 357/709 (50.35%) were locoregional nodal metastases (115 retropharyngeal, 226 cervical, and 16 supraclavicular). There were 352/709 (49.65%) distant metastases, including 104/352 (29.55%) in the chest, 45/352 (12.78%) in the abdomen, 11/352 (3.13%) in the pelvis, and 192/352 (54.55%) in the bones. Wu [23] analyzed the treatment outcomes of 614 NPC without distant metastasis over 10 years: 151 cases (24.6%) were stage I-II, and 463 cases (75.4%) were stage III-IV. After a median follow-up of 112.7 months, 123 cases (20.0%) had distant metastases and the 5-year and 10-year DMFS were 97.7% and 97.7% (N0), 85.9% and 83.8% (N1), 73.0% and 70.0% (N2), 54.4% and 54.4% (N3), respectively. In our study, the control group underwent ECT as reported in the literature, the different was that chest CT was used instead of chest X-ray, and abdominal enhanced CT instead of abdominal ultrasound. The long-term survival results in our research showed that the 5-year and 8-year DC were 83.3%, 81.0% and 90.6%, 90.6% in the control and PET-CT groups, respectively (P=0.013).
NPC is prone to invade out of the nasopharyngeal cavity and metastasize to regional lymph nodes. Clarifying the extent of tumor invasion of NPC is crucial for confirming stage, RT planning, and prognosis. Enhanced MRI is recommended as the first-line examination for NPC due to the high soft tissue resolution and multiparametric imaging [38-39]. Several studies have shown that nasopharynx enhanced MRI has advantages in detecting adjacent soft tissue involvement, skull base, intracranial invasion, retropharyngeal lymph nodes and so on [40-43]. However, owing to the low resolution, PET-CT alone may overestimate or underestimate retropharyngeal lymph nodes. Ng [16] analyzed 111 cases of newly diagnosed NPC to investigate with PET/CT and conventional imaging. PET/CT showed a discrepancy with head-and-neck MRI in 36 (32.4%) according to T stage. Among the discordant cases, MRI was superior in demonstrating tumor involvement in the retropharyngeal nodes, skull base, parapharyngeal space, intracranial area, and sphenoid sinus while PET/CT was superior sensitivity and specificity in demonstrating neck nodal metastasis [44-46]. Yang SS [44] analyzed the data of T3N1M0 NPC cases: Among the 269 cervical lymph nodes pathologically positive and 191 negative, 96.7% of positive and 75.9% of negative were correctly detected by PET/CT, while only 88.5% of positive and 70.7% of negative were correctly diagnosed by MRI (p<0.001). Patients who underwent both PET/CT and MRI had better OS, FFS, DMFS and LRRFS than those who underwent MRI alone (5-year OS, 95.7% vs. 90.4%, p<0.001; 5-year FFS, 85.7% vs. 71.7%, p<0.001; 5-year DMFS, 93.9% vs. 87.9%, p<0.001; 5-year LRRFS, 93.0% vs. 81.4%, p<0.001). In our study, all patients in both the control and PET-CT groups underwent nasopharynx enhanced MRI. The 5-year and 8-year LC rates of the control and PET-CT group were 88.6% and 91.1%, 87.0% and 88.8%, respectively (p=0.494). The 5-year and 8-year RC rates of the control and PET-CT group were 91.3% and 91.7%, 90.2% and 89.0%, respectively (p=0.618). The cost-effectiveness of PET-CT scans is a critical consideration. The primary benefit of PET-CT scans lies in their ability to detect diseases at an early stage, which can be crucial for effective treatment. This early detection can lead to more effective treatment plans and better patient prognoses. In addition, PET-CT is invaluable in the staging and monitoring of cancer treatments, as demonstrated by the ability to detect changes in metabolic activity that may not be apparent with other imaging techniques. Limitations of the study such as retrospective data, single center and lack of EBV-DNA should be considered in the future research.
Conclusion
Patients with initially PET-CT-based staged showed improved long-term DC compared with the control group (chest CT, enhanced abdominal CT and ECT) for locoregionally advanced NPC with nasopharynx enhanced MRI before therapy. Initially PET-CT-based staged and nasopharynx enhanced MRI are recommended routinely to confirm stage and improve efficacy in locoregionally advanced NPC.
Abbreviations
ECT: emission computed tomography
NPC: nasopharyngeal carcinoma
OS: overall survival
DC: distant control
LC: local control
RC: regional control
PFS: progression-free survival
RFS: relapse-free survival
DMFS: distant metastasis-free survival
IC: induction chemotherapy
CCRT: concurrent chemotherapy
AC: adjuvant chemotherapy
RT: radiotherapy
Ethical approval
The study was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center in accordance with the Declaration of Helsinki.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Xiao B B, Lin D F, Sun X S. et al. Nomogram for the prediction of primary distant metastasis of nasopharyngeal carcinoma to guide individualized application of FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(8):2586-2598
2. Tang L Q, Chen Q Y, Fan W. et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31(23):2861-2869
3. Chiesa F, De Paoli F. Distant metastases from nasopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63(4):214-216
4. Toumi N, Ennouri S, Charfeddine I. et al. Prognostic factors in metastatic nasopharyngeal carcinoma. Braz J Otorhinolaryngol. 2022;88(2):212-219
5. Qiu H Z, Zhang X, Liu S L. et al. M1 stage subdivisions based on (18)F-FDG PET-CT parameters to identify lo-coregional radiotherapy for metastatic nasopharyngeal carcinoma. Ther Adv Med Oncol. 2022;14:7436479
6. Ou X, Zhou X, Shi Q. et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget. 2015;6(35):38381-38397
7. Mao Y P, Tang L L, Chen L. et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer. 2016;35(1):103
8. Huang J, Yang Z Y, Wu B. et al. Long-term Therapeutic Outcome and Prognostic Factors of Patients with Nasopharyngeal Carcinoma Receiving Intensity-modulated Radiotherapy: An Analysis of 608 Patients from Low-endemic Regions of China. Curr Med Sci. 2021;41(4):737-745
9. Yang X, Ren H, Yu W. et al. Analysis of Clinical Target Volume Delineation in Local-regional Failure of Nasopharyngeal Carcinoma after Intensity-modulated Radiotherapy. J Cancer. 2020;11(7):1968-1975
10. You R, Liu Y P, Huang P Y. et al. Efficacy and Safety of Locoregional Radiotherapy With Chemotherapy vs Chemotherapy Alone in De Novo Metastatic Nasopharyngeal Carcinoma: A Multicenter Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(9):1345-1352
11. Du C, Ni M, Jiang J. et al. Taxane/gemcitabine-containing chemotherapy plus locoregional IMRT for patients with de novo metastatic nasopharyngeal carcinoma: the treatment outcomes and prognostic factors analysis. Eur Arch Otorhinolaryngol. 2022;279(8):3947-3956
12. Hu S X, He X H, Dong M. et al. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol. 2015;32(9):224
13. Li J X, Huang S M, Wen B X. et al. Prognostic factors on overall survival of newly diagnosed metastatic nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014;15(7):3169-3173
14. Chua M, Wee J, Hui E P. et al. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012-1024
15. Kumar M B, Lu J J, Loh K S. et al. Tailoring distant metastatic imaging for patients with clinically localized undifferentiated nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2004;58(3):688-693
16. Ng S H, Chan S C, Yen T C. et al. Staging of untreated nasopharyngeal carcinoma with PET/CT: comparison with conventional imaging work-up. Eur J Nucl Med Mol Imaging. 2009;36(1):12-22
17. Yen T C, Chang J T, Ng S H. et al. The value of 18F-FDG PET in the detection of stage M0 carcinoma of the nasopharynx. J Nucl Med. 2005;46(3):405-410
18. Chang M C, Chen J H, Liang J A. et al. Accuracy of whole-body FDG-PET and FDG-PET/CT in M staging of nasopharyngeal carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2013;82(2):366-373
19. Chen C, Xu T, Qiu X. et al. Selectively recommend (18)F-FDG PET/CT for patients with de novo nasopharyngeal carcinoma in endemic areas. Radiat Oncol. 2021;16(1):229
20. Ren Y Y, Li Y C, Wu H B. et al. Whole-body (18)F-FDG PET/CT for M staging in the patient with newly diagnosed nasopharyngeal carcinoma: Who needs? Eur J Radiol. 2017;89:200-207
21. Xiao B B, Chen Q Y, Sun X S. et al. Low value of whole-body dual-modality [18f]fluorodeoxyglucose positron emission tomography/computed tomography in primary staging of stage I-II nasopharyngeal carcinoma: a nest case-control study. Eur Radiol. 2021;31(7):5222-5233
22. Yang P C, Chen W M, Chen M. et al. Survival effect of pretreatment FDG-PET-CT on nasopharyngeal cancer. J Formos Med Assoc. 2023;122(1):36-46
23. Wu L R, Liu Y T, Jiang N. et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: An analysis of 614 patients from a single center. Oral Oncol. 2017;69:26-32
24. Zhao W, Lei H, Zhu X. et al. Investigation of long-term survival outcomes and failure patterns of patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: a retrospective analysis. Oncotarget. 2016;7(52):86914-86925
25. Xu M, Zang J, Luo S. et al. Long-term survival outcomes and adverse effects of nasopharyngeal carcinoma patients treated with IMRT in a non-endemic region: a population-based retrospective study. BMJ Open. 2021;11(8):e45417
26. Au K H, Ngan R, Ng A. et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2018;77:16-21
27. Tian Y M, Liu M Z, Zeng L. et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head Neck. 2019;41(5):1246-1252
28. Wang P, Dong F, Cai C. et al. Treatment outcomes of induction chemotherapy combined with intensity-modulated radiotherapy and adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma in Southeast China. Medicine (Baltimore). 2021;100(33):e27023
29. Lin S, Lu J J, Han L. et al. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10:39
30. Yang Z, Cai Z, Cai Q. et al. Sequential induction chemotherapy plus intensity-modulated radiotherapy versus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: the three-year report of a phase II, single center, randomized, non-inferiority trial. Cancer Med. 2021;10(12):3886-3895
31. Kong F, Zhai R, Huang J. et al. Long-Term Results of Intensity-Modulated Radiotherapy for T4 Nasopharyngeal Carcinoma: New Insight into the Value of Concurrent Chemotherapy. Cancer Invest. 2021;39(8):645-652
32. Sun X, Su S, Chen C. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398-403
33. Xu C, Zhang Y, Peng L. et al. Optimal Modality for Detecting Distant Metastasis in Primary Nasopharyngeal Carcinoma during Initial Staging: A Systemic Review and Meta-analysis of 1774 Patients. J Cancer. 2017;8(7):1238-1248
34. Liu F Y, Lin C Y, Chang J T. et al. 18F-FDG PET can replace conventional work-up in primary M staging of nonkeratinizing nasopharyngeal carcinoma. J Nucl Med. 2007;48(10):1614-1619
35. Liu F Y, Chang J T, Wang H M. et al. [18F]fluorodeoxyglucose positron emission tomography is more sensitive than skeletal scintigraphy for detecting bone metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2006;24(4):599-604
36. Al T A, Zaheer S, Ng D C. et al. The incidence and sites of Nasopharyngeal carcinoma (NPC) metastases on FDG PET/CT scans. Oral Oncol. 2015;51(11):1047-1050
37. Fangzheng W, Chuner J, Haiyan Q. et al. Survival without concurrent chemotherapy for locoregionally advanced nasopharyngeal carcinoma treated with induction chemotherapy plus intensity-modulated radiotherapy: Single-center experience from an endemic area. Medicine (Baltimore). 2019;98(51):e18484
38. Tang L L, Chen Y P, Chen C B. et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021;41(11):1195-1227
39. Bossi P, Chan A T, Licitra L. et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up(dagger). Ann Oncol. 2021;32(4):452-465
40. King A D. MR Imaging of Nasopharyngeal Carcinoma. Magn Reson Imaging Clin N Am. 2022;30(1):19-33
41. Vellayappan B A, Soon Y Y, Earnest A. et al. Accuracy of (18)F-flurodeoxyglucose-positron emission tomography/computed tomography in the staging of newly diagnosed nasopharyngeal carcinoma: a systematic review and meta-analysis. Radiol Oncol. 2014;48(4):331-338
42. King A D, Ma B B, Yau Y Y. et al. The impact of 18F-FDG PET/CT on assessment of nasopharyngeal carcinoma at diagnosis. Br J Radiol. 2008;81(964):291-298
43. Tang L L, Ma J, Chen Y. et al. The values of MRI, CT, and PET-CT in detecting retropharyngeal lymph node metastasis of nasopharyngeal carcinoma. Ai Zheng. 2007;26(7):737-741
44. Yang S S, Wu Y S, Chen W C. et al. Benefit of [18F]-FDG PET/CT for treatment-naive nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging. 2022;49(3):980-991
45. Peng H, Chen L, Tang L L. et al. Significant value of (18)F-FDG-PET/CT in diagnosing small cervical lymph node metastases in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Chin J Cancer. 2017;36(1):95
46. Chang J T, Chan S C, Yen T C. et al. Nasopharyngeal carcinoma staging by (18)F-fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2005;62(2):501-507
Author contact
![]() Corresponding authors: Chunying Shen and Xiayun He. Email address: shency2016com (C. Shen), hexiayun1962com (X. He).
Corresponding authors: Chunying Shen and Xiayun He. Email address: shency2016com (C. Shen), hexiayun1962com (X. He).

 Global reach, higher impact
Global reach, higher impact