Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(1):80-94. doi:10.7150/ijms.88508 This issue Cite
Review
Status of research on the development and regeneration of hair follicles
Department of Toxicology, School of Public Health, Jilin University, Changchun 130021, China.
Received 2023-7-26; Accepted 2023-10-17; Published 2024-1-1
Abstract
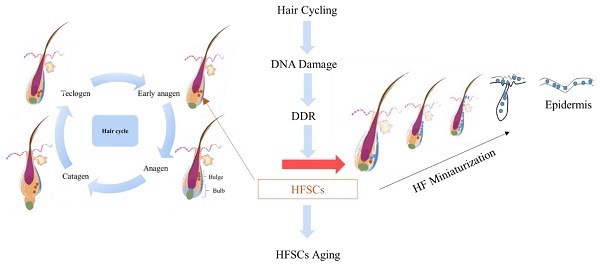
Hair loss, or alopecia, is a prevalent condition in modern society that imposes substantial mental and psychological burden on individuals. The types of hair loss, include androgenetic alopecia, alopecia areata, and telogen effluvium; of them, androgenetic alopecia is the most common condition. Traditional treatment modalities mainly involve medical options, such as minoxidil, finasteride and surgical interventions, such as hair transplantation. However, these treatments still have many limitations. Therefore, exploring the pathogenesis of hair loss, specifically focusing on the development and regeneration of hair follicles (HFs), and developing new strategies for promoting hair regrowth are essential. Some emerging therapies for hair loss have gained prominence; these therapies include low-level laser therapy, micro needling, fractional radio frequency, platelet-rich plasma, and stem cell therapy. The aforementioned therapeutic strategies appear promising for hair loss management. In this review, we investigated the mechanisms underlying HF development and regeneration. For this, we studied the structure, development, cycle, and cellular function of HFs. In addition, we analyzed the symptoms, types, and causes of hair loss as well as its current conventional treatments. Our study provides an overview of the most effective regenerative medicine-based therapies for hair loss.
Keywords: hair loss, hair follicle regeneration, hair follicle growth, stem cell therapy
1. Introduction
With the rapid increase in social demands and work-life pressure, hair loss has become a common and increasingly severe problem. Various factors, such as trauma, mental stimulation, genetics, endocrine imbalance, physical stress, and chemical exposure, can lead to severe hair loss, affecting patients' appearance and mental health [1]. Currently, the first-line treatment for hair loss involves the use of minoxidil and finasteride. However, these two drugs require long-term usage, which may result in drug tolerance and other side effects, Moreover, a hair loss often recurs upon discontinuation the drugs [2]. Although hair follicle (HF) transplantation has been gradually gaining acceptance, the low availability of HF donor sites for limits patient satisfaction. With the advancement of regenerative medicine, various treatment modalities have been introduced safely and effectively resolve the problem of hair loss. These modalities include low-level laser therapy (LLLT), micro needling, platelet-rich plasma (PRP) therapy, and stem cell therapy. The modern treatments have opened up new avenues for addressing challenges associated with traditional hair loss treatment [3]. In this review, we analyzed the types and causes of hair loss by exploring the molecular mechanisms underlying the development and regeneration of HFs, Herein, we discuss new treatment strategies for enhancing hair loss treatments. Our review provides a theoretical basis for the future application of stem cell therapy for hair loss.
2. Histomorphology of the hair follicle
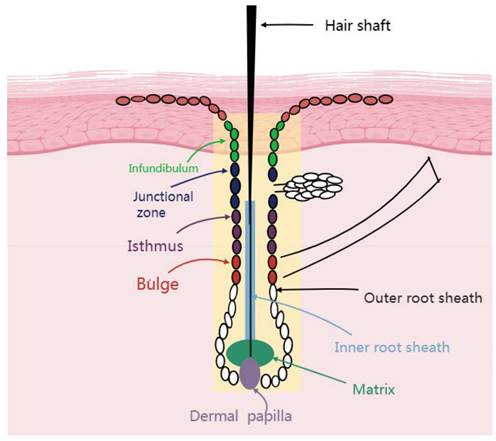
One distinctive traits of mammals are the presence of HFs, which are small skin organs. [4] Hair growth is driven by cellular activity within HFs which serve as the fundamental unit of hair [5]. Although different mammalian HF exhibit different morphological forms, they have similar structural features Figure 1. The HF is divided into two portions by a boundary known as the bulge. The upper portion of the HF include the infundibulum and isthmus. The lower portion of the HF consists of the bulb, including protrusions, hairballs, and papillae. Notably, the lower portion constitutes approximately one-third of the HF and participates in the hair growth hair loss cycle. The upper portion does not participate in the cycle, because it undergoes minimal apoptosis and regeneration in the follicular phase. Thus, the upper portion is known as the permanent component, whereas the lower portion is called the cycling component [6]. The layers of the HF, extending from the outside to the inside, include the outer hair root sheath (ORS), companion layer, inner hair root sheath (IRS) , and hair shaft (HS) [7, 8]. HF structures, such as the IRS and HS are formed by hair matrix cells which wrap around the HFs in the body to create the hair dermal papilla (DP). The infundibulum, isthmus, bulge, and bulb of the HF are part of the HF epidermis and originate from the ectoderm. The DP a raised structure formed by the mesoderm-derived dermal tissue connected to the hairball determines the hairball size, HS diameter and length, and hair growth duration. HS is the center of the HF epidermis the entire epidermis is surrounded by a connective tissue sheath of mesodermal origin.
Structure of a hair follicle.
3. HF morphogenesis and development
The morphogenesis and development of HFs are dependent on epidermis-interstitial interactions. This process is regulated by various signaling pathways, particularly the synergistic actions of the Wnt, bone morphogenetic protein (BMP), hedgehog, transforming growth factor (TGF)-β, fibroblast growth factor (FGF), and Notch signaling pathways [9, 10]. The Wnt/β-catenin signaling pathway is considered to be pivotal for HFs from the telogen phase to the anagen phase. This pathway is involved in all the stages of HF development and determines the differentiation fate of HF cells during development [11]. BMP is a crucial member of the TGF-β superfamily. The expression levels of BMP-2 and BMP-4 changes periodically during the HF cycle [12]. The Notch signaling pathway is essential for maintaining follicle structure and facilitating follicle formation and reepithelialization [13]. Ultimately, determining the morphology and development of the HFs [14, 15]. The morphogenesis and development of HFs proceed through three sequential stages: induction (production of hair placodes), organogenesis (downward growth of hair placodes), and cytodifferentiation (morphogenesis of HFs). The dermis provides the initiation signal for HF development [16]. In the embryonic stage, epidermal stem cells rapidly proliferate and differentiate to form hair placodes under the influence of signaling pathways such as the Wnt and BMP pathways. Hair placodes are a series of regular plate-like structures, and their formation indicates the commencement of HF development. After the formation of hair placodes, information is transmitted between two cellular compartments, epithelial and mesenchymal cells, which promote cell proliferation in both compartments by upregulating cyclins and facilitating hair germ formation. In these basal layer cells, upregulated Wnt-signaling induces the hedgehog signaling pathway, stimulating the proliferation of daughter cells with low levels of Wnt-signaling-related proteins. This process leads to the formation of an ORS and a bulge, which contain a pool of stem cells. Under signal stimulation, such as through the Wnt pathway, the hair embryo further proliferates into hair buds. Concurrently, hair placodes release signaling molecules, such as FGF20, into dermal fibroblasts, stimulating them to move directionally toward hair placodes, where they proliferate and aggregate to form the dermal condensate. The dermal condensate layer is then wrapped by HF epidermal cells, forming dermal papillae. Hair placodes grow toward the dermis. A group of highly proliferating cells at the bottom of the hair bud differentiates into matrix cells, forming the IRS [6, 17]. Melanocytes localized to the ridge and stromal sites secrete melanin to impart color to the HS [15]. Eventually, HF structures such as the IRS, ORS, and HS are formed.
4. HF cycle
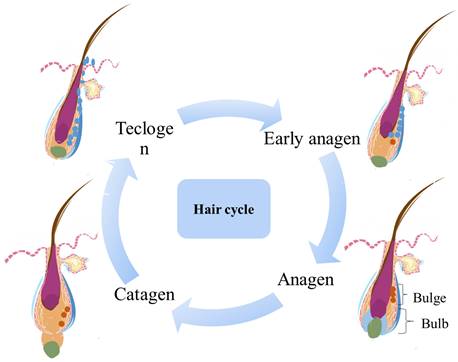
The morphology of mammalian HFs changes periodically, resulting in the division of HF growth into anagen, catagen, and telogen phases. Each phase is tightly regulated and is characterized by substantial changes in gene expression, cell proliferation, and cell differentiation. During the anagen phase, the HF produces a complete shaft from the top to the root. The anagen phase determines the length of hair and depends on the continued proliferation and differentiation of stromal cells at the base of the follicle. The portion below the follicle bulge grows and thickens, and the hair papilla enlarges and gradually moves away from the bulge, eventually becoming enveloped by hairballs extending into the adipose tissue [18]. The fat layer also thickens during the anagen phase, synchronized with the HF cycle. Adipocytes support the growth of HFs by producing various adipogenoids, such as leptin and adiponectin, and growth factors, such as macrophage colony-stimulating factor [18-20]. As the hairballs continue to expand and descend toward the muscle membrane in the subcutaneous tissue, the HFs stop descending and enter a phase of vigorous growth. The transition from the growth phase to the catagen phase is facilitated by several factors, including FGF5, epidermal growth factor, neurotrophic factors (e.g., brain-derived neurotrophic factor and p75 neurotrophic factor receptors), p53. and TGFβ family members (e.g., TGF- β1) [21]. After a period of continuous growth, HFs enter the apoptosis-mediated catagen phase. During the catagen phase, HFs stop growing, the lower portion of each HF is completely degraded [22], stromal cells and keratinocytes undergo apoptosis, and a circular structure called the bulb forms at the bottom of the dermis. This bulb ascends to the never-circulating HF to connect the hair DP with the bulge. When the DP reaches cells around the residual HS, it disappears completely. [21, 23]. After the catagen phase concludes and the telogen phase commences, HFs remain relatively quiescent, storing numerous pluripotent stem cells at the bulge site, which are ready to receive signals from the DP [24]. During the telogen phase, the DP is adjacent to the bulge, interacting with each other. When the stem cell activation signal reaches a threshold, the HFs begin to move into the next growth phase [25]. When the DP is missing, HF stem cells cannot receive a complete signal, leading to the failure of the HF cycle [26].
5. Mechanisms underlying HF regeneration
HF regeneration is based on complex signal interactions between the HF stem cell pool and the hair DP. Ectoderm stem cells serve as the primary cell source for the regeneration of HF. Mesenchymal hair papilla cells regulate this process by secreting signaling molecules. Taylor et al. [27] used bromodeoxyuridine and 3H-thymidine double labeling to demonstrate the presence of numerous stem cells at the site of the follicle bulge. Stem cells at the bulge site work synergistically with the DP to maintain the self-renewal and periodic growth of HF. HF stem cells typically remain quiescent and exhibit slow periodic activity. However, when stimulated by damage or growth signals, they can proliferate rapidly, producing numerous transit amplifying cells and postmitotic differentiating cells, which play essential roles in skin damage repair and HF reconstruction [28, 29]. A study in which HFs were cultured during the growth phase in four segments demonstrated that 95% of all clonal-forming cells originated from HF fragments in the bulge area [30], and a small number of cells were derived from the HF fragment of the hair bulb. HF stem cells mainly include ectoderm-derived hair keratinocyte stem cells, mesoderm-derived HF mesenchymal stem cells (MSCs), and neural crest-derived melanocyte stem cells [31]. Under certain conditions, these cells can be induced to differentiate into adipose tissues, bone, and various other tissues. Dermal papilla cells (DPCs), which belong to a group of mesenchymal cells in the HF structure, are characterized by marked heterogeneity and adult stem cell properties. They have been confirmed to be a type of MSCs. DPCs are dermis-derived cells located at the base of HF and play pivotal roles in HF morphogenesis, HF regeneration, HF reconstruction, hair growth signal transmissions, and HF cycle regulation [32, 33]. During the morphogenesis of HF, dermal fibroblasts receive signal stimuli from hair placode cells, which cause them to agglutinate and eventually develop into DPCs [34].
Hair follicle cycle.
Mechanisms underlying the generation of hair follicles.
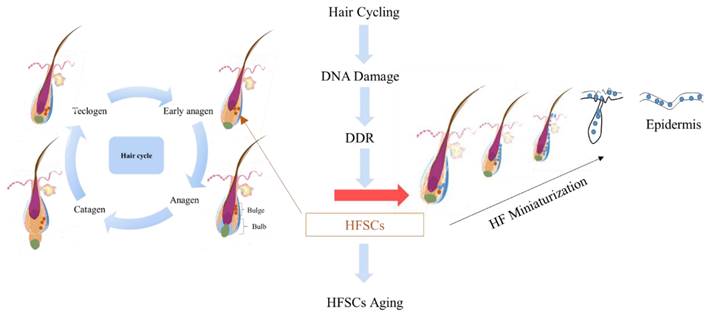
6. Current hair loss situation
Hair loss is a common and treatable condition that has evolved into a chronic problem, affecting a substantial portion of the population. An Israeli skin clinic reported that hair loss-related visits increased from 1.24% in 2010 to 9.44% in 2020. Furthermore, the highest increase was noted in the prevalence of androgenetic alopecia (AGA), from 17% in 2010 to 32% in 2020 [35]. Safavi et al. [36] indicated that the estimated incidence of AA was 20.2 per 100,000 person-years from 1975 to 1989. Furthermore, Mirzoyev et al. [37] reported that the estimated incidence of AA was 20.9 per 100,000 person-years from 1990 to 2009. Three epidemiological studies conducted in the United States have revealed a consistent increase, showing that the incidence of alopecia areata (AA) in the United States is increasing year by year over the years. Arash et al. [38] demonstrated that the estimated incidence of AA ranged from 91.46 per 100,000 person-years in 2016 to 92.90 per 100,000 person-years in 2019. In addition, hospital-based studies conducted in various countries, including India, Singapore, and Mexico [39-41], have reported that the incidence of AA ranges between 0.57% and 3.8% [42]. Paige et al. [43] found that the prevalence of hair loss among pediatric patients (mean age, 9 years) had doubled in the last decade, indicating a trend of hair loss in younger individuals. Hair loss not only affects aesthetics but also imposes a psychological burden on individuals. Moreover, it negatively affects personal image, mental health, quality of life, and even social competitiveness. In response to the challenges posed by baldness, researchers are actively seeking solutions for hair loss [44]. Recently, the demand for hair loss treatments has continually increased, reducing the incidence of mental and physical health disorders and the financial burden associated therewith [44]. However, very few drug regimens have been approved by regulatory agencies for clinical use; these drugs include mainly corticosteroids, minoxidil, and 5-alpha-reductase inhibitors (finasteride and dutasteride) [45, 46]. Although these medications have demonstrated effectiveness, their outcomes do not meet the needs of individuals with hair loss. Therefore, exploring new treatment approaches is imperative. The underlying causes of many types of hair loss involve the apoptosis and dysfunction of HF stem cells due to excessive hormone secretion, immune imbalances, and inflammatory response [25, 26, 47, 48]. Addressing hair loss mainly entails inhibiting the apoptosis of HF stem cells, maintaining the homeostasis of HF stem cells, and ensuring the normal functioning of the HF of the hair follicle cycle.
7. Causes of hair loss
Common types of hair loss include AGA, AA, and telogen effluvium (TE). Among these types, AGA—also known as premature baldness, male baldness, and seborrheic alopecia—is the most prevalent. Androgenic baldness is a polygenic genetic disorder caused by androgens [49]. HFs on the top of the head become more sensitive to androgens, leading to gradual HF atrophy, a shortened growth phase, a prolonged telogen phase, and eventual HF miniaturization, resulting in baldness [45]. Typical symptoms include hair thinning starting from the frontal corners on both sides of the forehead, reduced hair density, and a receding hairline that gradually extends to the top of the head [50].
AA is a psychogenic-dominated, autoimmune-related hair loss disease characterized by a sudden onset of localized patchy alopecia. It is a complex disease caused by the interaction between genetic and environmental factors and can occur in any region of the body. The affected skin appears smooth, with no inflammation, scaling, or scarring. AA can occur at any age but is more common in young and middle-aged individuals, demonstrating no substantial sex differences [51]. Typical symptoms include the sudden appearance of round or oval areas of hair loss with well-defined boundaries and varying numbers [52].
TE results from the disruption of the HF cycle, causing a large number of hairs in the anagen phase to simultaneously enter the telogen phase. This condition is characterized by a shortened anagen phase, a prolonged telogen phase, and the simultaneous shedding of the HS during the quiescent phase, resulting in the occurrence of telogen effluvium [53]. The amount of hair loss is proportional to the number of HFs simultaneously entering the telogen phase; this condition is known as acute telogen phase alopecia. Under normal circumstances, the human scalp contains approximately 100,000 HFs, with 90%-95% in the anagen phase and 5%-10% in the telogen phase, cycling continuously. However, because the cycle of each HF is not synchronized, the daily hair loss in normal individuals typically remains under 100 [54]. Factors such as malnutrition, micronutrient deficiencies, chronic illnesses, emergencies, and mental stress can affect the growth cycle of HFs. If the HF cycle is disrupted, it can result in increased hair loss.
8. Conventional treatments for hair loss
Hair loss treatment is a crucial aspect of clinical dermatology. In recent years, many treatments have been proven to promote hair regrowth. Traditional drug and surgical interventions continue to play crucial roles in addressing hair loss.
8.1 Drug therapy
To date, minoxidil and finasteride are the main drugs approved by the US Food and Drug Administration (FDA) for the treatment of AGA. Minoxidil, which was originally used to treat hypertensive disorders, was later discovered to effectively control the life cycle of HFs; thus, it was established as the primary treatment for hair loss in men and women [55]. Minoxidil is a potassium channel opener, exerting a vasodilating effect that extends the duration of the anagen phase and induces angiogenesis around the HFs [56]. In 1999, Vera et al. [57] assessed the efficacies of 5% and 2% topical minoxidil against AGA; they observed that topical minoxidil promoted hair growth and delayed hair loss within 96 weeks. Recent studies have also highlighted the effectiveness of minoxidil in combination with other drug treatments for hair loss [55, 58].
Finasteride is another preferred drug for the treatment of AGA. Androgens affect the development of HFs, and androgenic metabolic changes are closely related to the occurrence of AGA [59]. Testosterone is the main androgen in men; by contrast, the most crucial androgen in women is the weakly active form called androstenedione, which is secreted by the adrenal glands and ovaries [60, 61]. Testosterone and androstenedione are metabolized and converted by 5-alpha reductase. Finasteride is a specific inhibitor of type II 5-alpha reductase, which can irreversibly bind to 5-alpha reductase to treat AGA [62]. Piraccini et al. [59] evaluated the efficacy and safety of a topical finasteride spray in the treatment of AGA in men and reported that, compared with placebo, topical finasteride use significantly increased hair number.
Avodart is approximately thrice more potent than finasteride in inhibiting type II 5-alpha reductase [63]. Olsen et al. reported that dutasteride increased the number of hairs in the target area in a dose-dependent manner and was more effective than finasteride at 12 and 24 weeks [64]. Dutasteride is proposed as an effective therapy for frontal fibrosing alopecia [65] and is thus used as a new drug for AGA.
8.2 Surgical treatment
Hair transplantation involves the transplantation of hair extracted from a donor site into the bald area of the scalp (frontal lobe area/vertex). The preferred donor site is typically the occipital region of the scalp because of its resistance to androgens. When occipital hair is insufficient, hair can be harvested from areas such as the beard and chest [66]. Follicular unit transplantation is the gold standard for hair transplantation [67]. Two methods are used for graft harvesting: the strip method and follicular unit extraction. In the strip method, a section of the scalp is harvested from the occipital region, and individual follicles are dissected and then transplanted into a gap made in the recipient site. During the first few days to weeks after transplantation, some transplanted hair may fall out due to telogen shedding or transplant failure. Results typically become visible after at least 3 months, which is the time required for the transplanted hair to enter the anagen phase [67]. Hair transplantation can be combined with other treatments such as oral finasteride, topical minoxidil, and PRP to improve outcomes [68]. However, the essence of hair transplantation lies in the redistribution of self-dominant resources, and the low availability of donor sites limits the treatment satisfaction of hair transplant recipients [69].
9. New treatment strategies for hair loss
9.1 Physiotherapy
Scalp microneedle therapy, also known as micro needling or derma rolling for the scalp, is a non-surgical cosmetic procedure used to improve hair growth and overall scalp health. This procedure employs a device known as a microneedle roller, or derma roller. equipped with tiny needles on its surface. During treatment, a practitioner or an individual gently rolls the microneedle roller over the scalp. This action leads to the activation of genes associated with hair growth, the release of growth factors (e.g., platelet-derived growth factor), and the activation of stem cells with bulging hairs [70]. Scalp microneedling is frequently used in combination with PRP to enhance drug absorption by creating microchannels. Kang et al. [71] also pointed out that micro needle (MN) and nanoparticles (NP) transport systems are emerging treatments for hair loss. Kim et al. [72] demonstrated that repeated microneedling induces hair growth. Possible mechanisms underlying the efficacy of microneedling against AGA include inducing the overexpression of hair growth-related genes (e.g., vascular endothelial growth factor, β-catenin, Wnt3a, and Wnt10b), regulation the hair cycle, stimulating DP, and promoting hair growth [73]. The wound healing microenvironment created after microneedling treatment activates stem cells in the HF area, facilitating the delivery of drugs and increasing the absorption of various compounds in the skin [72]. This method has been observed to be particularly effective when used in combination with minoxidil therapy or topical steroids. In general, microneedling aids the penetration of drugs such as minoxidil, topical steroids, and PRP. The ease of use, cost-effectiveness, and safety of microneedling make it a potentially attractive therapy for the treatment of hair loss [74].
Fractional radiofrequency (FRF) therapy involves the use of specialized equipment and needles to accurately deliver ultra-high-frequency radio waves to targeted tissues for the treatment of diseases. Verner and Lotti evaluated the efficacy of FRF in stimulating hair growth in 25 patients who completed 31 treatment sessions (between-treatment interval: 2 weeks). The researchers reported that hair density and HS increased by 6.18% and 39%, respectively, and that the treatment outcomes were tolerated well [75]. Alsalhi et al. [76] demonstrated that FRF can enhance the delivery of topical minoxidil to the scalp. FRF not only reduces hair loss but also increases hair quantity and HS thickness. Another study indicated that FRF effectively reduced hair loss and stimulated hair growth in patients with AGA. A total of 25 patients completed 10 FRF sessions (administered every 2 weeks) and were followed up 2 months after the last session. The patients exhibited a 31.6% increase in hair counts and an 18% increase in HS thickness [77]. Both microneedling and FRF are physical methods that can be combined with drugs to treat hair loss by promoting drug absorption and delivery.
9.2 Light therapy
9.2.1 LLLT
LLLT, also known as photo biomodulation therapy, cold laser therapy, or red light therapy, is a non-invasive medical or cosmetic treatment that employs low-level lasers or light-emitting diodes to stimulate cellular function and promote tissue healing and regeneration. LLLT devices emit low-intensity laser or light-emitting diode light at specific wavelengths, typically in the red or near-infrared spectrum. These wavelengths range from 600 to 1000nm. LLLT operates through photo bioregulation, where red light triggers the release of nitric oxide from cytochrome C oxidase [78]. This event leads to the production of additional adenosine triphosphate, and reactive oxygen species are produced by the redox reaction of cytochrome C oxidase, which activates DNA transcription factors. These transcription factors direct protein synthesis that is integral to cell proliferation, migration, and adhesion. LLLT enhances levels of cytokines, growth factors, inflammatory mediators, and tissue oxygenation [79]. LLLT stimulates the generation of anti-inflammatory cytokines and antioxidants that accelerate the mitosis of keratinocytes and fibroblasts, ultimately promoting hair growth [80]. Gentile et al. [81] conducted a randomized controlled trial comparing the effectiveness of LLLT in patients with male pattern hair loss (MPHL) and those with female pattern hair loss (FPHL) against a control group and reported that LLLT is useful for hair loss treatment. In a case series involving 20 patients with AGA, after 16 weeks of follow-up, hair density increased by 12 ± 2 hairs/cm2 [82, 83].
9.2.2 Excimer lamp
Ultraviolet B light (290 to 320 nm) is widely used for treating AA [84], which is a T-cell autoimmune disorder. A 308-nm excimer laser has been reported to induce T-cell apoptosis in vitro [85]. Zakaria et al. [86] conducted a blinded controlled clinical trial by using a 308-nm excimer laser for AA treatment; their findings indicated that all patients experienced ≥16% improvements in plaque after direct beam photography treatment [86].
9.2.3 CO2 laser
The mechanism underlying CO2 fractional laser treatment for hair loss may involve the upregulation of Wnt β-catenin, which also enhances the delivery of traditional drugs, thereby promoting hair growth [87]. Studies have observed a significant increase in hair density and hair diameter after 4 months of a low-energy, high-density regimen administered every 2 weeks (for a total of six sessions), indicating that CO2 laser therapy leads to positive outcomes within a relatively short period of the time [88]. The efficacy of CO2 lasers is closely related to their energy parameters, which are often adjusted according to the patient's hair density or erythema area [89].
9.3 Regenerative medicine -based therapy
9.3.1 PRP therapy
PRP is an autologous blood preparation characterized by a considerably higher number of platelets than that noted in whole blood [90]. Upon activation, PRP releases a lysate rich in numerous growth factors, which play pivotal roles in promoting cell proliferation and tissue regeneration. The use of autologous PRP does not cause immune reactions, thereby broadening its applicability for various dermatological purposes, including wound healing, facial rejuvenation, scar repair, and hair growth [91, 92]. Although the FDA has not approved the use of PRP for hair loss treatment, several studies have demonstrated its efficacy against AGA [93-95]. Anitua et al. [96] evaluated the use of autologous PRP in 19 patients with AGA and observed significant increases in average hair density, hair diameter, and hair thickness at the 1-year follow-up. Alves and Grimalt [97] conducted a randomized, double-blind study involving 25 patients and noted that average hair count and hair density increased in PRP-treated areas 3 and 6 months after treatment. In another randomized, double-blind trial, Gentile et al. [98, 99] obtained the same results 3 months after PRP injection. These studies confirm the therapeutic advantages of PRP. Currently, PRP treatment for hair loss predominantly involves scalp injections, which can cause local infections, pain, bleeding, and uneven administration. Furthermore, multiple scalp injections can induce anxiety and fear in some patients [100-102].
9.3.2 Stem cell therapy
Stem cells exhibit self-renewal, migration, anti-inflammatory, and immunomodulatory functions, which are essential for the repair and recovery of damaged tissues or organs [103-105].
Human DPCs (hDPCs) are specialized dermal cells located at the base of HFs and are surrounded by dermal sheath cells (DSCs). Both hDPCs and DSCs are necessary for new hair development and hair renewal. Notably, hDPCs can activate HFSCs and substantially influence HF circulation, thereby enabling HF regeneration. In transplant surgery, the efficiency of HF formation is related to the ability of DPCs to induce HF regeneration [106]. In skin reconstruction and wound healing, DPCs not only retain the ability to form new DPCs but also promote the growth of DSCs and non-follicle-associated fibroblasts [107].Yamao et al. [108] co-transplanted highly passaged DPCs with cultured DSCs onto the dorsal side of nude mice. The researchers found that DSCs are involved in the induction of hair formation and that dermal sheath formation is key to the normal development of the HS. Ji et al. [5] investigated the HF regeneration mechanisms of DPCs and induced HF formation upon implantation in the hairless skin animals; their finding underscored that, when in contact with the epithelium, DPCs can generate new HFs. Tsuboi et al. [109] conducted a study involving 50 patients with MPHL who received a single injection of autologous DSC. The patient and 15 female pattern alopecia (FPHL)patients were randomized in a study, the results show, and the patient and 15 patients with female pattern alopecia (FPHL) who received a single injection of autologous DSCs. A substantial increase was noted in the number of DSC injection sites compared with the effects of a placebo intervention. Both men and women exhibited similar improvements. but neither sex had any severe adverse events [109]. In the beauty industry, the transplantation of dermal fibroblasts into the scalps of patients with hair loss has been approved for regenerative product implementation [110].
MSCs are pluripotent stem cells with differentiation potential. Because of their crucial roles in enhancing the regenerative potential of various tissues and their secretion of various cytokines, nerve growth factors, and glial neurotrophic factors, MSCs have become integral to regenerative medicine [111, 112]. Adult tissue sources of MSCs with self-renewal and multidirectional differentiation potential include HF-MSCs, human umbilical cord MSCs (hUC-MSCs), adipose MSCs (AD-MSCs), bone marrow MSCs (BM-MSCs), and mesenchymal-induced pluripotent stem cells (iPSC-MSCs). MSCs can regenerate HFs and other organs (e.g., sebaceous glands) in the skin through various mechanisms, including the reversal of pathological mechanisms and the formation of new HFs through organoid systems [113].
HF-MSCs possess several advantages over other adult tissue-derived stem cells. such as plentiful sources, easy accessibility, low immunogenicity, and no age restrictions, HF-MSCs play significant roles in HF development, the HF cycle, and HF regeneration. Kevin et al. [114] reported that HF-MSCs can promote chronic wound healing. Deng et al. [115] demonstrated that HF-MSCs reduced hair loss, alleviated inflammation around HFs, and increased follicle counts during the anagen phase in AA mice. Gentile et al. [116] [117] reported that an RCT study treated with HF-MSCs, which showed a significant increase in hair follicle density and number compared to the control group, demonstrating the long-term therapeutic advantages of HF-MSCs.
hUC-MSCs are useful for tissue repair and regeneration. Their ethical advantage, painless retrieval from discarded umbilical cords, and reduced risk of immune rejection make hUC-MSCs more beneficial than other MSCs [118-120]. Ko et al. [121] reported the crucial role of MSCs in wound healing. Exosomes from hUC-MSC can accelerate skin regeneration and wound healing [122]. Cord mesenchymal stromal cell-derived exosomes can also rescue the loss of outer hair cells [123]. Ahn et al. [124] successfully treated AA and generalized alopecia by using hUC-MSCs without administering immunosuppressive medications. These results indicate that hUC-MSC transplantation is safe and effective for the treatment of AA, with no hair loss during treatment or follow-up, no recurrence of hair loss or other side effects, and no immune rejection.
MSCs obtained from the adipose tissue include freshly derived primary pluripotent MSCs, known as adipogenic stromal vascular cells or adipogenic regenerative cells, as well as isolated and cultured pure MSCs (AD-MSCs). Saczonek et al. [113] indicated that AD-MSCs are necessary for the activation of epidermal stem cells in the skin and the normal cell growth of HFs. AD-MSCs secrete growth factors that play key roles in the activation of epidermal stem cells and hDPCs. These growth factors include vascular endothelial growth factor, which regulates hair growth and HF size by stimulating angiogenesis; hepatocyte growth factor, which is involved in determining the length of various stages of the hair cycle; platelet-derived growth factor, which induces and maintains the growth phase; and insulin-like growth factor-1, which controls the cycle of hair growth and hair cell differentiation [125-127]. Adipose tissue is essential for the extension of the anagen phase. During the transition of hair from the telogen to anagen phases, adipose progenitor cells are activated to proliferate and form mature adipocytes around regenerating HFs [128]. Zanzottera et al. [129] applied autologous AD-MSCs to the scalp of three patients and observed rapid wound healing, and improved hair growth, and a shortened telogen phase after 2 months of treatment. In their review, Yusuke et al. [75] indicated that AD-MSCs can improve the quality of aging skin and that the clinical use of these MSCs is allowed in dermatology and aesthetic surgery clinics in Japan, which highlights the safety and efficacy of adipose tissue-derived regenerative cells in the treatment of AA. According to Perez et al. [130], 19 out of 20 patients exhibited significant increases in hair diameter and hair density 3- 6 months after treatment [130]. A study including 50 patients with MPHL or FPHL investigated the effects of adipose-derived regenerative cells on hair growth by combining these cells with adipose tissue during transplantation. The results revealed a 7% increase in the average hair volume, compared with a 5% increase in patients receiving nonadjuvant adipose tissue treatment alone [131].
Elmaadawi et al. [132] examined the safety and efficacy of autologous BM-MSCs in combination with autologous follicular stem cells (FSCs) in 20 patients with AA and 20 patients with AGA. Each patient received an intradermal dose of BM-MSCs or FSCs, and the effects were examined after 6 months through immunostaining and digital dermoscopy. The results demonstrated that both autologous BM-MSCs and FSCs were safe, well-tolerated, and effective in the treatment of drug-resistant AA and AGA. No side effects were noted. A significant improvement in hair growth was observed in all patients, with no significant between-group differences.
Induced pluripotent stem cells (iPSCs) have been used to generate various cell types, including HF cells [133]. Subsequent studies have reported that HF-iPSCs can be used for hepatocyte regeneration in vitro [134]. Recently, Abaci et al. [135] used three-dimensional (3D) printing technology to create micropores with adjustable extensions in plastic molds, mimicking the shape of HF. DPCs were added along with keratinocytes to form a 3D structure resembling HFs. After 3 weeks of culture, HF-like structures of cells expressing hair-specific markers and hair fibers were observed, confirming that functional HFs can be generated using biomimetic developmental methods. In addition, human keratinocytes,when embedded in the Matrigel matrix and combined with nylon guidewires as scaffolds, can assemble human iPSC (hiPSC)-derived DPC aggregates into a 3D structure to reconstruct HFs [136]. Tsuji et al. [137] successfully bioengineered a 3D integumentary organ system comprising the skin, HF, sebaceous glands, and subcutaneous adipose tissue from mouse iPSCs (miPSCs). The resulting HF contained mature HF cells and structures such as bulge stem cells, melanocytes, DP, and DS. The hiPSCs have unlimited self-renewal capacity, which helps minimize the problem of cell shortages, and hiPSC-derived dermal and epidermal cells can induce hair genesis [138-140]. However, the disadvantages of hiPSC-induced hair regrowth cannot be ignored. Differentiation of hiPSCs into hair dermal cells or follicular epidermal cells is more time-consuming than the isolation and expansion of these cells from HFs. In addition, when using any hiPSC-derived product for cell-based therapy, the possibility of tumor formation in hiPSCs should be considered [141].
9.4 Non-medical approaches
Dietary supplementation with natural plant extracts, controlled shampoo use, and lifestyle changes are widely accepted nonmedical strategies for preventing hair loss. For a long time, the consumption of natural plants and their extracts has been recognized as a safe approach with minimal side effects for preventing hair loss. Major plants known for hair loss prevention include ginseng and Polygonum multiflorum [142]. Ginsenosides are among the main extracts of ginseng. Ginsenosides are classified into ternary diols (Rb1, Rb2, Rb3, Rg3, Rc, Rd, and Rh2), ternary alcohols (Re, Rf, Rg1, Rg2, and Rh1), and oleanolic acids. Ginsenoside Rb1 is the main bioactive component of ginseng and the most abundant ginsenoside in this plant [143]. P. multiflorum is an oriental traditional medicinal plant. It is the tuber root of P. multiflorum Thunb (Polygonum family). The main extracts of P. multiflorum are 2,3,5,40-tetrahydroxystilbene-2-O-D-glucoside and emodin [144]. In brief, the beneficial effects of herbs and their bioactive compounds and their potential mechanisms of action include the regulation of growth factors and cytokines, modulation of the Wnt/β-catenin pathway, inhibition of 5-alpha-reductase, involvement in sonic hedgehog signaling, and control of apoptosis and cell cycle progression. Park and Lee [142] comprehensively explored this subject in their review. Although phytochemicals have potential for treating alopecia, they have several limitations, including the limited availability of clinical evidence, varying levels of effectiveness, and slow appearance of results. In addition, phytochemical treatments are less effective for individuals with more severe hair loss [145]. Controlled shampoo use and lifestyle modifications are more direct approaches to the prevention and treatment of hair loss than phytochemical use. Shampoo effectively removes dirt, excess oils, sweat, and environmental pollutants from the hair and scalp, promoting cleanliness and scalp health. Regular use of shampoo contributes to maintaining good scalp hygiene and reducing the risk of scalp conditions such as dandruff and fungal infections. However, excessive shampooing can strip the scalp of its natural oils, potentially leading to dryness, itchiness, and oil overproduction ("rebound effect"). In addition, some shampoos contain sodium lauryl sulfate, parabens, formaldehyde, and alcohol; these chemicals have been widely reported to damage the scalp and HFs [146]. Even when using-silicone free shampoos, frequent washing over time may cause hair to become dependent on them because the scalp adjusts its oil production on the basis of the frequency of shampooing. This causes difficulty in reducing the frequency of shampooing. Therefore, selecting the right shampoo for your hair type and needs and monitoring usage frequency are essential. Finally, regardless of the nonmedical strategy used for treating hair loss, lifestyle changes are consistently advocated. A healthy lifestyle includes adequate hydration, a proper diet, and regular and adequate sleep. A balanced, nutrient-rich diet and proper hydration are essential for healthy hair growth. Hair is mainly made up of a protein called keratin. Thus, consuming adequate protein promotes hair growth. Lack of water can lead to dry hair. In addition, vitamin Bs, vitamin D, iron, zinc, and n-3 polyunsaturated fatty acids are essential for hair health. Foods such as lean meat, fish, eggs, nuts, fruits, vegetables, and whole grains should be included in the diet [147]. Furthermore, stress management techniques such as meditation, yoga, and deep breathing exercises can help prevent hair loss and improve overall physical health. In summary, nonmedical treatment strategies generally have fewer side effects than pharmacologic therapies or surgical interventions. Moreover, nonmedical approaches are cost-effective, which makes them accessible to a wider population. However, the long-term cost of the sustained use of these nonmedical approaches should be considered. Non-medical treatments may not lead to satisfactory outcomes in individuals with severe hair loss.
10. Conventional methods versus new strategies
Although conventional treatments for hair loss are highly effective, they are associated with considerable challenges that limit their routine clinical use. Pharmacological treatments for hair loss can lead to various side effects. For example, minoxidil mainly causes hirsutism and cardiovascular symptoms/signs in a dose-dependent manner, and oral finasteride and dutasteride are associated with sexual dysfunction and neuropsychiatric side effects [55]. Mouna et al. [148] reported cases of minoxidil-induced paresthesia, which may be due to irritant dermatitis caused by topical minoxidil. Studies have reported an increase in the number of hairs in women who continue to use minoxidil solution topically, but this effect disappears once the drug is discontinued [149]. Adverse events related to finasteride are primarily associated with the disruption of the balance between estrogen and androgens due to 5-alpha reductase inhibitors. This imbalance can increase the risk of breast cancer and result in other adverse effects, such as reduced sexual function, testicular pain, and breast tenderness [59]. The key surgical treatment for hair loss is hair transplantation, which involves the redistribution of self-dominant resources; however, the limited availability of donor sites reduces the treatment satisfaction of hair transplant recipients [69].
10.1 Minoxidil versus finasteride versus dutasteride
Minoxidil, finasteride, and dutasteride are the most common drugs used in hair loss therapy. Khandpur et al. [150] compared oral minoxidil, finasteride, and dutasteride in terms of their efficacy against AGA. The researchers revealed the following descending order of efficacy: avodart at 0.5 mg/day > finasteride at 5 mg/day > minoxidil at 5 mg/day > finasteride at 1 mg/day > minoxidil at 0.25 mg/day [150].
10.2 Microneedling versus minoxidil and finasteride
Lin et al. [151] examined the effects of 5% minoxidil and found that the mean improvement in total hair density from baseline to week 24 was 18.8/cm2 in patients who had received topical application alone and 38.3/cm2 in those who had received electrodynamic microneedling therapy plus topical 5% minoxidil.
10.3 LLLT versus minoxidil and finasteride
One study compared oral finasteride, topical minoxidil, and LLLT in terms of their efficacy against hair loss. The findings revealed that treatment with 1 mg of finasteride for 6 and 12 months resulted in 7.3% and 8.99% increases in the total hair count, respectively [152]. Treatment with 2% and 5% minoxidil for 48 weeks increased the total hair count by 8.84% and 12.3%, respectively [153]. After 26-week-long LLLT with 9- and 12-beam laser combs, the total hair count increased by 12.79% and 16.96%, respectively [80]. Thus, the effectiveness of LLLT may be comparable to that of conventional hair loss treatments [81].
Representative studies of traditional methods of hair loss treatment
| Treatment | Conventional methods | |||
|---|---|---|---|---|
| Minoxidil | Finasteride | Dutasteride | Hair transplantation | |
| Types | TE | AGA | FFA | AGA and FFA |
| Patients | 58 patients | 458 patients | 224 patients | Four cases |
| Dosage | 0.75-2.5 mg for 2.1 years | 0.25% solution once daily (volume of 50-200 µL) | From 1-7 capsules (0.5 mg) per week | Artificial hair implantation |
| Treatment results | Hair density↑ | Hair count↑ | Distance of the eyes from the hairline↓ | Hair count↑ |
| Adverse effects | Headaches, hypertrichosis, andirritation | Sexual dysfunction, altered libido, gynecomastia, and mood changes | Sexual dysfunction, altered libido, gynecomastia, and mood changes | Pain, bleeding, edema, and pruritis |
| References | 147 | 59 | 65 | 70 |
Representative studies of new strategies of hair loss treatment.
| Treatment | New strategies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microneedling | FRF | LLLT | Excimer lamp | CO2 laser | PRP | DPC | UC-MSCs | AD-MSCs | BM-MSCs | |
| Types | AGA and AA | AGA | AGA | AA | AGA | AGA | PHL | AA | AGA | AA and AGA |
| Patients | 50 patients | 25 patients | 110 patients | 105 patients | 45 patients | 25 patients | 65 patients | 3 patients | 3 patients | 40 patients |
| Dosage | Microneedling (needle length, 0.5 to 1.5 mm), 15 times a week | Ten treatment sessions at 2-week intervals | For 15 min, thrice a week for 26 weeks | 308-nm excimer lamp | 50-mm tip, 12-18 mJ/spot, 361 spots/cm2 | Once a month for 3 months | 7.1 × 107 autologous DSCs | 1 × 106 cells/mL, twice a month for 12 months | Cell suspension, injected subcutaneously | 1 × 104 cells/mL, injected intradermally |
| Treatment results | Hair shaft thickness↑ | Hair count↑ Hair shaft thickness↑ | Hair density↑ Hair loss↓ | Hair loss↓ | Hair count↑ Hair thickness↑ | Hair density↑ | Hair density↑ | Hair regrowth↑ | Wounds↓ Hair regrowth↑ | Hair regrowth↑ |
| Adverse effects | Pain, bruising, and discomfort at site | Scalp pain and slight burning sensation | Scalp tenderness, paresthesia, and mild urticaria | Scalp pain and slight burning sensation | Scalp tenderness and paresthesia | Scalp pain and pinpoint | Scalp pain and pinpoint | Scalp pain and pinpoint | Scalp pain and pinpoint | Scalp pain and pinpoint |
| References | 74 | 77 | 82 | 86 | 150 | 97 | 108 | 122 | 127 | 130 |
10.4 PRP versus finasteride
Gentile et al. [154] reported that posttreatment hair density was significantly higher in the PRP group than in the control group. After 12 weeks of treatment, the HF density increased by 31% ± 2% in the PRP group and by <1% in the control group. Nestle et al. [155] examined 212 patients with AGA who received 1 mg of finasteride daily. After 48 weeks of treatment, the HF density increased by 26% ± 3.1% in the finasteride group. The efficacy of PRP in promoting HF density was significantly stronger than that of finasteride.
10.5 PRP versus HFSCs
Gentile et al. [116] reported that after 23 weeks of treatment with HFSCs, HF density increased by 29% ± 5% hairs/cm2 compared with the density in the control group. After 23 weeks of PRP treatment, HF density increased by 28% ± 2% hairs/cm2 compared with that in the control group [154]. These studies demonstrate that PRP and HF-MSCs are equally effective in treating hair loss [156].
11. Conclusions and perspectives
Hair loss is a difficult-to-treat condition, and treatment with FDA-approved drugs alone is often associated with substantial side effects and recurrence after discontinuation. Various strategies for treating hair loss are currently being explored. New strategies, including regenerative medicine-based therapy, remain attractive emerging options. Although new strategies for hair loss treatment are effective, they still have some limitations and uncertainties, and more clinical treatment cases are needed for validation. Our research group has also been committed to studying HF development and regeneration. Some of our findings are as follows; Ggene therapy can promote HF proliferation and regulate their cycle processes [157-159]. Stem cell-derived exosomes or growth factors promote wound healing and HF proliferation [160, 161]. HF regeneration can be induced through tissue engineering [162]. HF-induced pluripotent stem cells can be induced through gene reprogramming [157]. The HF microenvironment regulates the HF cycle process [163, 164]. In the future,we will collaborate with other researchers to resolve the problem of hair loss and ensure sustainable clinical applications of effective therapeutic strategies.
Author Contributions
D. L. wrote the draft of the manuscript. Q. X. and X.M. were involved in discussing, drafting, and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation of China grant numbers 82073581 and 82273673.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kanti V, Messenger A, Dobos G, Reygagne P, Finner A, Blumeyer A. et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22
2. Anudeep TC, Jeyaraman M, Muthu S, Rajendran RL, Gangadaran P, Mishra PC. et al. Advancing Regenerative Cellular Therapies in Non-Scarring Alopecia. Pharmaceutics. 2022 14, 612
3. Owczarczyk-Saczonek A, Krajewska-Włodarczyk M, Kruszewska A, Banasiak Ł, Placek W, Maksymowicz W. et al. Therapeutic Potential of Stem Cells in Follicle Regeneration. Stem Cells Int. 2018;2018:1049641
4. Stone RC, Aviv A, Paus R. Telomere Dynamics and Telomerase in the Biology of Hair Follicles and their Stem Cells as a Model for Aging Research. J Invest Dermatol. 2021;141:1031-1040
5. Ji S, Zhu Z, Sun X, Fu X. Functional hair follicle regeneration: an updated review. Signal Transduct Target Ther. 2021;6:66
6. O'Sullivan JDB, Nicu C, Picard M, Chéret J, Bedogni B, Tobin DJ. et al. The biology of human hair greying. Biol Rev Camb Philos Soc. 2021;96:107-128
7. Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358:697-704
8. Tellechea O, Cardoso JC, Reis JP, Ramos L, Gameiro AR, Coutinho I. et al. Benign follicular tumors. An Bras Dermatol. 2015;90:780-796
9. Tripurani SK, Wang Y, Fan YX, Rahimi M, Wong L, Lee MH. et al. Suppression of Wnt/β-catenin signaling by EGF receptor is required for hair follicle development. Mol Biol Cell. 2018;29:2784-2799
10. Xing F, Yi WJ, Miao F, Su MY, Lei TC. Baicalin increases hair follicle development by increasing canonical Wnt/β-catenin signaling and activating dermal papillar cells in mice. Int J Mol Med. 2018;41:2079-2085
11. Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T. et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720-733
12. Hansdah K, Singh N, Bouzid A, Priyadarshi S, Ray CS, Desai A. et al. Evaluation of the Genetic Association and mRNA Expression of the COL1A1, BMP2, and BMP4 Genes in the Development of Otosclerosis. Genet Test Mol Biomarkers. 2020;24:343-351
13. Wang Z, Nan W, Si H, Wang S, Zhang H, Li G. Pantothenic acid promotes dermal papilla cell proliferation in hair follicles of American minks via inhibitor of DNA Binding 3/Notch signaling pathway. Life Sci. 2020;252:117667
14. Haslam IS, Paus R. The Hair Follicle as an Interdisciplinary Model for Biomedical Research: An Eclectic Literature Synthesis. Bioessays. 2020;42:e2000053
15. Hu X, Zhang X, Liu Z, Li S, Zheng X, Nie Y. et al. Exploration of key regulators driving primary feather follicle induction in goose skin. Gene. 2020;731:144338
16. Hwang SB, Park HJ, Lee BH. Hair-Growth-Promoting Effects of the Fish Collagen Peptide in Human Dermal Papilla Cells and C57BL/6 Mice Modulating Wnt/β-Catenin and BMP Signaling Pathways. Int J Mol Sci. 2022 23, 11904
17. Joost S, Annusver K, Jacob T, Sun X, Dalessandri T, Sivan U. et al. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell. 2020;26:441-457.e7
18. Chen CC, Plikus MV, Tang PC, Widelitz RB, Chuong CM. The Modulatable Stem Cell Niche: Tissue Interactions during Hair and Feather Follicle Regeneration. J Mol Biol. 2016;428:1423-1440
19. Oh JH, Jeong KH, Kim JE, Kang H. Synthesized Ceramide Induces Growth of Dermal Papilla Cells with Potential Contribution to Hair Growth. Ann Dermatol. 2019;31:164-174
20. Li W, Man XY, Li CM, Chen JQ, Zhou J, Cai SQ. et al. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp Cell Res. 2012;318:1633-1640
21. Alonso L. The hair cycle. Journal of Cell Science. 2006;119:391-393
22. Kloepper JE, Sugawara K, Al-Nuaimi Y, Gaspar E, van Beek N, Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. 2010;19:305-312
23. Panteleyev AA. Functional anatomy of the hair follicle: The Secondary Hair Germ. Exp Dermatol. 2018;27:701-720
24. Legrand JMD, Roy E, Ellis JJ, Francois M, Brooks AJ, Khosrotehrani K. STAT5 Activation in the Dermal Papilla Is Important for Hair Follicle Growth Phase Induction. J Invest Dermatol. 2016;136:1781-1791
25. Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13-25
26. Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4:a015180
27. Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451-461
28. Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713-724
29. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329-1337
30. Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473-13475
31. Rompolas P, Greco V. Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol. 2014;25-26:34-42
32. Jahoda CA, Whitehouse J, Reynolds AJ, Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12:849-859
33. Richardson GD, Arnott EC, Whitehouse CJ, Lawrence CM, Reynolds AJ, Hole N. et al. Plasticity of rodent and human hair follicle dermal cells: implications for cell therapy and tissue engineering. J Investig Dermatol Symp Proc. 2005;10:180-183
34. Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331
35. Lyakhovitsky A, Tzanani I, Gilboa S, Segal O, Galili E, Baum S. et al. Changing spectrum of hair and scalp disorders over the last decade in a tertiary medical centre. J Eur Acad Dermatol Venereol. 2023;37:184-193
36. Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633
37. Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142
38. Mostaghimi A, Gao W, Ray M, Bartolome L, Wang T, Carley C. et al. Trends in Prevalence and Incidence of Alopecia Areata, Alopecia Totalis, and Alopecia Universalis Among Adults and Children in a US Employer-Sponsored Insured Population. JAMA Dermatol. 2023;159:411-418
39. Rocha J, Ventura F, Vieira AP, Pinheiro AR, Fernandes S, Brito C. [Alopecia areata: a retrospective study of the paediatric dermatology department (2000-2008)]. Acta Med Port. 2011;24:207-214
40. Xiao FL, Yang S, Liu JB, He PP, Yang J, Cui Y. et al. The epidemiology of childhood alopecia areata in China: a study of 226 patients. Pediatr Dermatol. 2006;23:13-18
41. Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591
42. Juárez-Rendón KJ, Rivera Sánchez G, Reyes-López M, García-Ortiz JE, Bocanegra-García V, Guardiola-Avila I. et al. Alopecia Areata. Current situation and perspectives. Arch Argent Pediatr. 2017;115:e404-e411
43. Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403
44. Hosking AM, Juhasz M, Atanaskova Mesinkovska N. Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Skin Appendage Disord. 2019;5:72-89
45. Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-41.e5
46. Salim S, Kamalasanan K. Controlled drug delivery for alopecia: A review. J Control Release. 2020;325:84-99
47. Legrand JMD, Roy E, Ellis JJ, Francois M, Brooks AJ, Khosrotehrani K. STAT5 Activation in the Dermal Papilla Is Important for Hair Follicle Growth Phase Induction. J Invest Dermatol. 2016;136:1781-1791
48. Joulai Veijouye S, Yari A, Heidari F, Sajedi N, Ghoroghi Moghani F, Nobakht M. Bulge Region as a Putative Hair Follicle Stem Cells Niche: A Brief Review. Iran J Public Health. 2017;46:1167-1175
49. Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A. et al. Androgenetic alopecia: a review. Endocrine. 2017;57:9-17
50. Starace M, Orlando G, Alessandrini A, Piraccini BM. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am J Clin Dermatol. 2020;21:69-84
51. Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: A multifactorial autoimmune condition. J Autoimmun. 2019;98:74-85
52. Sterkens A, Lambert J, Bervoets A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021;21:215-230
53. Phillips TG, Slomiany WP, Allison R. Hair Loss: Common Causes and Treatment. Am Fam Physician. 2017;96:371-378
54. Wang ECE, Higgins CA. Immune cell regulation of the hair cycle. Exp Dermatol. 2020;29:322-333
55. Randolph M, Tosti A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746
56. Bertoli MJ, Sadoughifar R, Schwartz RA, Lotti TM, Janniger CK. Female pattern hair loss: A comprehensive review. Dermatol Ther. 2020;33:e14055
57. Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41:717-721
58. York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21:603-612
59. Piraccini BM, Blume-Peytavi U, Scarci F, Jansat JM, Falqués M, Otero R. et al. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol. 2022;36:286-294
60. Traish AM. Post-finasteride syndrome: a surmountable challenge for clinicians. Fertil Steril. 2020;113:21-50
61. Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS. Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J Clin Aesthet Dermatol. 2016;9:56-62
62. Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications. Endocr Rev. 2017;38:302-324
63. Bramson HN, Hermann D, Batchelor KW, Lee FW, James MK, Frye SV. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997;282:1496-1502
64. Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML. et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023
65. Pindado-Ortega C, Saceda-Corralo D, Moreno-Arrones Ó M, Rodrigues-Barata AR, Hermosa-Gelbard Á, Jaén-Olasolo P. et al. Effectiveness of dutasteride in a large series of patients with frontal fibrosing alopecia in real clinical practice. J Am Acad Dermatol. 2021;84:1285-1294
66. Rousso DE, Kim SW. A review of medical and surgical treatment options for androgenetic alopecia. JAMA Facial Plast Surg. 2014;16:444-450
67. Sovak M, Seligson AL, Kucerova R, Bienova M, Hajduch M, Bucek M. Fluridil, a rationally designed topical agent for androgenetic alopecia: first clinical experience. Dermatol Surg. 2002;28:678-685
68. Leavitt M, Perez-Meza D, Rao NA, Barusco M, Kaufman KD, Ziering C. Effects of finasteride (1 mg) on hair transplant. Dermatol Surg. 2005;31:1268-1276 discussion 76
69. Jimenez F, Alam M, Vogel JE, Avram M. Hair transplantation: Basic overview. J Am Acad Dermatol. 2021;85:803-814
70. Dhurat R, Mathapati S. Response to Microneedling Treatment in Men with Androgenetic Alopecia Who Failed to Respond to Conventional Therapy. Indian J Dermatol. 2015;60:260-263
71. Kang MS, Park TE, Jo HJ, Kang MS, Lee SB, Hong SW. et al. Recent Trends in Macromolecule-Based Approaches for Hair Loss Treatment. Macromol Biosci. 2023: e2300148.
72. Kim YS, Jeong KH, Kim JE, Woo YJ, Kim BJ, Kang H. Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model. Ann Dermatol. 2016;28:586-592
73. Ocampo-Garza SS, Fabbrocini G, Ocampo-Candiani J, Cinelli E, Villani A. Micro needling: A novel therapeutic approach for androgenetic alopecia, A Review of Literature. Dermatol Ther. 2020;33:e14267
74. Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32:564-569
75. Shimizu Y, Ntege EH, Sunami H. Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regen Ther. 2022;21:73-80
76. Alsalhi W, Alalola A, Randolph M, Gwillim E, Tosti A. Novel drug delivery approaches for the management of hair loss. Expert Opin Drug Deliv. 2020;17:287-295
77. Verner I, Lotti T. Clinical evaluation of a novel fractional radiofrequency device for hair growth: Fractional radiofrequency for hair growth stimulation. Dermatol Ther. 2018;31:e12590
78. Ivandic T. Low-Level Laser Therapy. Dtsch Arztebl Int. 2021;118:69
79. MF DEO, Johnson DS, Demchak T, Tomazoni SS, Leal-Junior EC. Low-intensity LASER and LED (photobiomodulation therapy) for pain control of the most common musculoskeletal conditions. Eur J Phys Rehabil Med. 2022;58:282-289
80. Jimenez JJ, Wikramanayake TC, Bergfeld W, Hordinsky M, Hickman JG, Hamblin MR. et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127
81. Gentile P, Garcovich S, Lee S-I, Han S. Regenerative Biotechnologies in Plastic Surgery: A Multicentric, Retrospective, Case-Series Study on the Use of Micro-Needling with Low-Level Light/Laser Therapy as a Hair Growth Boost in Patients Affected by Androgenetic Alopecia. Applied Sciences. 2022 12,217
82. Gentile P, Garcovich S. The Effectiveness of Low-Level Light/Laser Therapy on Hair Loss. Facial Plast Surg Aesthet Med. 2021 01,51
83. Galadari H, Shivakumar S, Lotti T, Wollina U, Goren A, Rokni GR. et al. Low-level laser therapy and narrative review of other treatment modalities in androgenetic alopecia. Lasers Med Sci. 2020;35:1239-1244
84. Novák Z, Bónis B, Baltás E, Ocsovszki I, Ignácz F, Dobozy A. et al. Xenon chloride ultraviolet B laser is more effective in treating psoriasis and in inducing T cell apoptosis than narrow-band ultraviolet B. J Photochem Photobiol B. 2002;67:32-38
85. Gupta AK, Carviel JL. Meta-analysis of 308-nm excimer laser therapy for alopecia areata. J Dermatolog Treat. 2021;32:526-529
86. Zakaria W, Passeron T, Ostovari N, Lacour JP, Ortonne JP. 308-nm excimer laser therapy in alopecia areata. J Am Acad Dermatol. 2004;51:837-838
87. Chen J, Wan Y, Lin Y, Jiang H. Fractional Carbon Dioxide Laser or Erbium:Yttrium-Aluminum-Garnet Laser Assisted by Topical Application/Intradermal Injection of Platelet-Rich Plasma for Postacne Scars. Plast Reconstr Surg. 2021;148:915e-927e
88. Salah M, Samy N, Fawzy MM, Farrag AR, Shehata H, Hany A. The Effect of the Fractional Carbon Dioxide Laser on Improving Minoxidil Delivery for the Treatment of Androgenetic Alopecia. J Lasers Med Sci. 2020;11:29-36
89. Huang Y, Zhuo F, Li L. Enhancing hair growth in male androgenetic alopecia by a combination of fractional CO(2) laser therapy and hair growth factors. Lasers Med Sci. 2017;32:1711-1718
90. Sclafani AP, Azzi J. Platelet Preparations for Use in Facial Rejuvenation and Wound Healing: A Critical Review of Current Literature. Aesthetic Plast Surg. 2015;39:495-505
91. Picard F, Hersant B, Bosc R, Meningaud JP. Should we use platelet-rich plasma as an adjunct therapy to treat "acute wounds," "burns," and "laser therapies": A review and a proposal of a quality criteria checklist for further studies. Wound Repair Regen. 2015;23:163-170
92. Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ. et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040-1046
93. Alves R, Grimalt R. Double-Blind, Placebo-Controlled Pilot Study on the Use of Platelet-Rich Plasma in Women With Female Androgenetic Alopecia. Dermatol Surg. 2018;44:132-133
94. Qu Q, He Y, Guo Z, Sun Y, Fan ZX, Yi YH. et al. Efficacy of Platelet-Rich Plasma plus Basic Fibroblast Growth Factor on the Treatment of Androgenic Alopecia. Plast Reconstr Surg. 2023;151:630e-640e
95. Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219
96. Anitua E, Pino A, Martinez N, Orive G, Berridi D. The Effect of Plasma Rich in Growth Factors on Pattern Hair Loss: A Pilot Study. Dermatol Surg. 2017;43:658-670
97. Alves R, Grimalt R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol Surg. 2016;42:491-497
98. Gentile P, Garcovich S, Scioli MG, Bielli A, Orlandi A, Cervelli V. Mechanical and Controlled PRP Injections in Patients Affected by Androgenetic Alopecia. J Vis Exp. 2018 27;56406
99. Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl Med. 2015;4:1317-1323
100. Singh B, Goldberg LJ. Autologous Platelet-Rich Plasma for the Treatment of Pattern Hair Loss. Am J Clin Dermatol. 2016;17:359-367
101. Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG. et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709
102. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020 21.7794
103. Gentile P, Garcovich S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells. 2019 8.466
104. Egger A, Tomic-Canic M, Tosti A. Advances in Stem Cell-Based Therapy for Hair Loss. CellR4 Repair Replace Regen Reprogram. 2020 8. e2894
105. Gentile P, Alves R, Cole JP, Andjelkov K, Van Helmelryck T, Fernandez J. et al. AIRMESS - Academy of International Regenerative Medicine & Surgery Societies: recommendations in the use of platelet-rich plasma (PRP), autologous stem cell-based therapy (ASC-BT) in androgenetic alopecia and wound healing. Expert Opin Biol Ther. 2021;21:1443-1449
106. Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ. 2007;49:185-195
107. Lu K, Han Q, Ma Z, Yan Q, Pei Y, Shi P. et al. Injectable platelet rich fibrin facilitates hair follicle regeneration by promoting human dermal papilla cell proliferation, migration, and trichogenic inductivity. Exp Cell Res. 2021;409:112888
108. Yamao M, Inamatsu M, Ogawa Y, Toki H, Okada T, Toyoshima KE. et al. Contact between dermal papilla cells and dermal sheath cells enhances the ability of DPCs to induce hair growth. J Invest Dermatol. 2010;130:2707-2718
109. Tsuboi R, Niiyama S, Irisawa R, Harada K, Nakazawa Y, Kishimoto J. Autologous cell-based therapy for male and female pattern hair loss using dermal sheath cup cells: A randomized placebo-controlled double-blinded dose-finding clinical study. J Am Acad Dermatol. 2020;83:109-116
110. Yuan X, Qin X, Wang D, Zhang Z, Tang X, Gao X. et al. Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nat Commun. 2019;10:2498
111. Assis-Ribas T, Forni MF, Winnischofer SMB, Sogayar MC, Trombetta-Lima M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev Biol. 2018;437:63-74
112. Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44:749-757
113. Owczarczyk-Saczonek A, Wociór A, Placek W, Maksymowicz W, Wojtkiewicz J. The Use of Adipose-Derived Stem Cells in Selected Skin Diseases (Vitiligo, Alopecia, and Nonhealing Wounds). Stem Cells Int. 2017;2017:4740709
114. Las Heras K, Royo F, Garcia-Vallicrosa C, Igartua M, Santos-Vizcaino E, Falcon-Perez JM. et al. Extracellular vesicles from hair follicle-derived mesenchymal stromal cells: isolation, characterization and therapeutic potential for chronic wound healing. Stem Cell Res Ther. 2022;13:147
115. Deng W, Zhang Y, Wang W, Song A, Mukama O, Huang J. et al. Hair follicle-derived mesenchymal stem cells decrease alopecia areata mouse hair loss and reduce inflammation around the hair follicle. Stem Cell Res Ther. 2021;12:548
116. Gentile P, Scioli MG, Cervelli V, Orlandi A, Garcovich S. Autologous Micrografts from Scalp Tissue: Trichoscopic and Long-Term Clinical Evaluation in Male and Female Androgenetic Alopecia. Biomed Res Int. 2020;2020:7397162
117. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58
118. Shareghi-Oskoue O, Aghebati-Maleki L, Yousefi M. Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem Cell Res Ther. 2021;12:454
119. Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9:226-40
120. Vangsness CT Jr, Sternberg H, Harris L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy. 2015;31:1836-1843
121. Ko SH, Nauta AC, Morrison SD, Hu MS, Zimmermann AS, Chung MT. et al. PHD-2 Suppression in Mesenchymal Stromal Cells Enhances Wound Healing. Plast Reconstr Surg. 2018;141:55e-67e
122. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int J Nanomedicine. 2020;15:5911-5926
123. Tsai SC, Yang KD, Chang KH, Lin FC, Chou RH, Li MC. et al. Umbilical Cord Mesenchymal Stromal Cell-Derived Exosomes Rescue the Loss of Outer Hair Cells and Repair Cochlear Damage in Cisplatin-Injected Mice. Int J Mol Sci. 2021 22.6664
124. Ahn H, Lee SY, Jung WJ, Lee KH. Alopecia treatment using minimally manipulated human umbilical cord-derived mesenchymal stem cells: Three case reports and review of literature. World J Clin Cases. 2021;9:3741-3751
125. Fukuoka H, Suga H. Hair Regeneration Treatment Using Adipose-Derived Stem Cell Conditioned Medium: Follow-up With Trichograms. Eplasty. 2015;15:e10
126. Huang CF, Chang YJ, Hsueh YY, Huang CW, Wang DH, Huang TC. et al. Assembling Composite Dermal Papilla Spheres with Adipose-derived Stem Cells to Enhance Hair Follicle Induction. Sci Rep. 2016;6:26436
127. Hwang I, Choi KA, Park HS, Jeong H, Kim JO, Seol KC. et al. Neural Stem Cells Restore Hair Growth Through Activation of the Hair Follicle Niche. Cell Transplant. 2016;25:1439-1451
128. Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M. et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761-771
129. Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose Derived Stem Cells and Growth Factors Applied on Hair Transplantation. Follow-Up of Clinical Outcome. Journal of Cosmetics, Dermatological Sciences and Applications. 2014;4:268-274
130. Perez-Meza D, Ziering C, Sforza M, Krishnan G, Ball E, Daniels E. Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning. 2017;10:1-10
131. Shimizu Y, Ntege EH, Sunami H, Inoue Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen Ther. 2022;21:527-539
132. Elmaadawi IH, Mohamed BM, Ibrahim ZAS, Abdou SM, El Attar YA, Youssef A. et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J Dermatolog Treat. 2018;29:431-440
133. Lim SJ, Ho SC, Mok PL, Tan KL, Ong AH, Gan SC. Induced pluripotent stem cells from human hair follicle keratinocytes as a potential source for in vitro hair follicle cloning. PeerJ. 2016;4:e2695
134. Shi X, Lv S, He X, Liu X, Sun M, Li M. et al. Differentiation of hepatocytes from induced pluripotent stem cells derived from human hair follicle mesenchymal stem cells. Cell Tissue Res. 2016;366:89-99
135. Abaci HE, Coffman A, Doucet Y, Chen J, Jacków J, Wang E. et al. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat Commun. 2018;9:5301
136. Fukuyama M, Tsukashima A, Kimishima M, Yamazaki Y, Okano H, Ohyama M. Human iPS Cell-Derived Cell Aggregates Exhibited Dermal Papilla Cell Properties in in vitro Three-Dimensional Assemblage Mimicking Hair Follicle Structures. Front Cell Dev Biol. 2021;9:590333
137. Takagi R, Ishimaru J, Sugawara A, Toyoshima K-e, Ishida K, Ogawa M. et al. Bioengineering a 3D integumentary organ system from iPS cells using an in vivo transplantation model. Science Advances. 2016;2:e1500887
138. Yang R, Zheng Y, Burrows M, Liu S, Wei Z, Nace A. et al. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nature Communications. 2014;5:3071
139. González R, Moffatt G, Hagner A, Sinha S, Shin W, Rahmani W. et al. Platelet-derived growth factor signaling modulates adult hair follicle dermal stem cell maintenance and self-renewal. npj Regenerative Medicine. 2017;2:11
140. Veraitch O, Mabuchi Y, Matsuzaki Y, Sasaki T, Okuno H, Tsukashima A. et al. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Scientific Reports. 2017;7:42777
141. Vatanashevanopakorn C, Sartyoungkul T. iPSC-based approach for human hair follicle regeneration. Front Cell Dev Biol. 2023;11:1149050
142. Park S, Lee J. Modulation of Hair Growth Promoting Effect by Natural Products. Pharmaceutics. 2021;13:2163
143. Jeong G, Shin SH, Kim SN, Na Y, Park BC, Cho JH. et al. Ginsenoside Re prevents 3-methyladenine-induced catagen phase acceleration by regulating Wnt/β-catenin signaling in human dermal papilla cells. J Ginseng Res. 2023;47:440-447
144. Shin JY, Choi YH, Kim J, Park SY, Nam YJ, Lee SY. et al. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement Med Ther. 2020;20:144
145. Kesika P, Sivamaruthi BS, Thangaleela S, Bharathi M, Chaiyasut C. Role and Mechanisms of Phytochemicals in Hair Growth and Health. Pharmaceuticals (Basel). 2023;16:206
146. Trüeb RM. Shampoos: ingredients, efficacy and adverse effects. J Dtsch Dermatol Ges. 2007;5:356-365
147. Almohanna HM, Ahmed AA, Tsatalis JP, Tosti A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol Ther (Heidelb). 2019;9:51-70
148. Korbi M, Said El Mabrouk R, Abdelaali M, Youssef M, Belhadjali H, Zili J. Topical minoxidil-induced paresthesia. Dermatol Ther. 2022;35:e15328
149. Burns LJ, De Souza B, Flynn E, Hagigeorges D, Senna MM. Spironolactone for treatment of female pattern hair loss. J Am Acad Dermatol. 2020;83:276-278
150. Gupta AK, Talukder M, Williams G. Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia. J Dermatolog Treat. 2022;33:2946-2962
151. Bao L, Gong L, Guo M, Liu T, Shi A, Zong H. et al. Randomized trial of electrodynamic microneedle combined with 5% minoxidil topical solution for the treatment of Chinese male Androgenetic alopecia. J Cosmet Laser Ther. 2020;22:1-7
152. Roberts JL, Fiedler V, Imperato-McGinley J, Whiting D, Olsen E, Shupack J. et al. Clinical dose ranging studies with finasteride, a type 2 5alpha-reductase inhibitor, in men with male pattern hair loss. J Am Acad Dermatol. 1999;41:555-563
153. Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, Tschen EH. et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47:377-385
154. Gentile P, Cole JP, Cole MA, Garcovich S, Bielli A, Scioli MG. et al. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int J Mol Sci. 2017;18:408
155. Van Neste D, Fuh V, Sanchez-Pedreno P, Lopez-Bran E, Wolff H, Whiting D. et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000;143:804-810
156. Gentile P, Garcovich S. Autologous activated platelet-rich plasma (AA-PRP) and non-activated (A-PRP) in hair growth: a retrospective, blinded, randomized evaluation in androgenetic alopecia. Expert Opin Biol Ther. 2020;20:327-337
157. Jiang Y, Liu F, Zou F, Zhang Y, Wang B, Zhang Y. et al. PBX homeobox 1 enhances hair follicle mesenchymal stem cell proliferation and reprogramming through activation of the AKT/glycogen synthase kinase signaling pathway and suppression of apoptosis. Stem Cell Res Ther. 2019;10:268
158. Wang Y, Sui Y, Lian A, Han X, Liu F, Zuo K. et al. PBX1 Attenuates Hair Follicle-Derived Mesenchymal Stem Cell Senescence and Apoptosis by Alleviating Reactive Oxygen Species-Mediated DNA Damage Instead of Enhancing DNA Damage Repair. Front Cell Dev Biol. 2021;9:739868
159. Wang B, Liu F, Liu Z, Han X, Lian A, Zhang Y. et al. Internalization of the TAT-PBX1 fusion protein significantly enhances the proliferation of human hair follicle-derived mesenchymal stem cells and delays their senescence. Biotechnol Lett. 2020;42:1877-1885
160. Bai T, Liu F, Zou F, Zhao G, Jiang Y, Liu L. et al. Epidermal Growth Factor Induces Proliferation of Hair Follicle-Derived Mesenchymal Stem Cells Through Epidermal Growth Factor Receptor-Mediated Activation of ERK and AKT Signaling Pathways Associated with Upregulation of Cyclin D1 and Downregulation of p16. Stem Cells Dev. 2017;26:113-122
161. Zhao G, Liu F, Lan S, Li P, Wang L, Kou J. et al. Large-scale expansion of Wharton's jelly-derived mesenchymal stem cells on gelatin microbeads, with retention of self-renewal and multipotency characteristics and the capacity for enhancing skin wound healing. Stem Cell Res Ther. 2015;6:38
162. Li P, Liu F, Wu C, Jiang W, Zhao G, Liu L. et al. Feasibility of human hair follicle-derived mesenchymal stem cells/CultiSpher(®)-G constructs in regenerative medicine. Cell Tissue Res. 2015;362:69-86
163. Lan S, Liu F, Zhao G, Zhou T, Wu C, Kou J. et al. Cyclosporine A increases hair follicle growth by suppressing apoptosis-inducing factor nuclear translocation: a new mechanism. Fundam Clin Pharmacol. 2015;29:191-203
164. Liu M, Liu X, Wang Y, Sui Y, Liu F, Liu Z. et al. Intrinsic ROS Drive Hair Follicle Cycle Progression by Modulating DNA Damage and Repair and Subsequently Hair Follicle Apoptosis and Macrophage Polarization. Oxid Med Cell Longev. 2022;2022:8279269
Author contact
![]() Corresponding authors: Xiaomei Liu; E-mail: liuxiaomedu.cn. Jinyu Liu, PhD; E-mail: jy_liuedu.cn.
Corresponding authors: Xiaomei Liu; E-mail: liuxiaomedu.cn. Jinyu Liu, PhD; E-mail: jy_liuedu.cn.

 Global reach, higher impact
Global reach, higher impact