3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(10):1363-1376. doi:10.7150/ijms.85746 This issue Cite
Review
Repeated Low-Level Red-Light Therapy for Controlling Onset and Progression of Myopia-a Review
1. Department of Pediatric Ophthalmology, Affiliated Hospital of Yunnan University, Kunming 650021, China.
2. Department of Ophthalmology, the First Affiliated Hospital of Kunming Medical University, Kunming 650031, China.
3. BioTissue (Tissue Tech, Inc.), Ocular Surface Center, and Ocular Surface Research & Education Foundation, Miami, FL, 33126 USA.
*These authors contributed equally to this article.
Received 2023-4-30; Accepted 2023-7-10; Published 2023-9-4
Abstract
Repeated low-level red-light (RLRL), characterized by increased energy supply and cellular metabolism, thus enhancing metabolic repair processes, has gained persistent worldwide attention in recent years as a new novel scientific approach for therapeutic application in myopia. This therapeutic revolution led by RLRL therapy is due to significant advances in bioenergetics and photobiology, for instance, enormous progresses in photobiomodulation regulated by cytochrome c oxidase, the primary photoreceptor of the light in the red to near infrared regions of the electromagnetic spectrum, as the primary mechanism of action in RLRL therapy. This oxidase is also a key mitochondrial enzyme for cellular bioenergetics, especially for the nerve cells in the retina and brain. In addition, dopamine (DA)-enhanced release of nitric oxide may also be involved in controlling myopia by activation of nitric oxide synthase, enhancing cGMP signaling. Recent evidence has also suggested that RLRL may inhibit myopia progression by inhibiting spherical equivalent refraction (SER) progression and axial elongation without adverse effects.
In this review, we provide scientific evidence for RLRL therapy as a unique paradigm to control myopia and support the theory that targeting neuronal energy metabolism may constitute a major target for the neurotherapeutics of myopia, with emphasis on its molecular, cellular, and nervous tissue levels, and the potential benefits of RLRL therapy for myopia.
Introduction
Myopia is caused by the parallel light penetrating via the eye refractive system and focusing the front retina as the eye is relaxed, which causes blurred images [1]. Myopia includes axial myopia due to the axial overgrowth and refractive myopia because of excessive curvature in the cornea and lens [2]. As the worldwide accepted, myopia is defined as spherical equivalent (SE) ≤ - 0.5 diopters (D), high myopia is defined as SE ≤ - 5.0 D (or - 6.0 D) or axial length (AL) greater than 26 mm [3-5].
Myopia has increasingly become a major public health problem, causing visual damage worldwide [6]. In Asia, for example, myopia is pandemic in China, due to constant increased prevalence in recent decades. In China, approximately 80% school age children suffer myopia when they complete schooling, of whom about 10-20% endure high myopia [7], which may cause serious myopic maculopathy, glaucomatous optic neuropathy and irreversible blindness [8]. A meta‑analysis indicates that the prevalence of myopia and high myopia in 2010 was 23% and 3% respectively in the world [8]. Unfortunately, the prevalence of myopia may increase to ~50% by 2050, thus 4.8 billion people worldwide may suffer from this disease [8]. Therefore, to prevent onset, to slow down progression of myopia have become the important strategies for myopia control in this world.
Myopia is linked closely with many complications, for instance, cataracts, macular degeneration and vision loss [9-12]. The causes related to myopia progression include but not limit to high educational pressure [13], little time outdoors [14] and more near-work activity [15] (for detailed reviews, see [1, 16]). Children born after 2010 are surrounded with a world through TV, smart phones, computers, which are now classified as causative factors [17, 18].
Previously, various interventions, including atropine, orthokeratology, spectacle and soft contact lenses, defocus incorporated multiple segment (DIMS) lenses and their combinations have been applied to slow down myopia progression with limited success [19-23]. For instance, Lam et al have demonstrated clear vision along with constant myopic defocus may reduce myopia progression by inhibiting axial elongation and myopia progression in myopic children [24]. Unfortunately, the efficiency for controlling myopia is between 30% to 60% with certain side effects using those methods [25]. Other measures to retard progression of myopia with similar efficacy include decrease of near work time [26], increase of time spent outdoors [27, 28] and improvement of scleral hypoxia [29]. Recently, more attention has been devoted to myopia prevention and control by red-light exposure, due to its high effectiveness for retardation of myopia with no side effects (reviewed in [30]).
Light and Red-Light
Light is defined as a form of electromagnetic radiation manifested by particles and wave properties. The waves of electromagnetic radiation possess unidirectional vectors, specified by wavelengths (λ, successive peak distance), frequencies (oscillations per second), and amplitudes (difference of trough and peak). The energy particles within electromagnetic radiation include photons, which travel at 3×108 m per second. Thus, mixture of various waves would elicit photons moving at different amplitudes and frequencies that are scattered and absorbed, reflected by various objects, for instance, biological materials.
By contrast, red-light is monochromatic, allowing high specificity as to molecular biomodulation. Red-light can be generated by lasers or light-emitting diodes (LEDs), appliable to photobiomodulation (PBM) in eyes. Lasers may generate coherent light energy efficiently to allow penetration to various tissues. The beam width of the lasers can be strengthened by coupling with fiber optic to permit energy distribution to larger areas. LEDs may generate efficient light without coherence in wavelengths of 4-10 nm. However, LEDs generate negligible heat, without any thermal injuries [31]. LEDs may also be coupled to the arrays with various functions, to deliver energy effectively to large areas, for instance, to the brain. Therefore, LEDs have been used in many human trials, and the use of LEDs has been approved by FDA [32]. Far red (FR) and near infrared (NIR) light generated by LEDs or a laser may promote cerebral blood flow (CBF) [33, 34], stimulate brain energy metabolism [35, 36] and increase the antioxidant capacity [37], mediate cell growth [38] and improve reparative ability in cells [32]. NIR and FR may also protect optical nerves, enhance their functional recoveries [39, 40].
Association of Light Wavelengths with Myopia
More recently, effects of different light wavelengths rather than those of different intensities on myopia onset and progression have drawn increasing attraction. Light waves are electromagnetic with different frequencies, including infrared rays, ultraviolet rays, radio waves, visible light (reviewed in [30]). Low frequency of electromagnetic fields (LF-EMFs) with a long wavelength may reduce expression of type I collagen in human fibroblasts and inhibit scleral remodeling, which may inhibit myopia onset and progression [41]. Interestingly, differences have been noted in the wavelength of light by different people and therefore visible light wavelengths are not consistent for retardation of myopia for different people [42].
Interestingly, chickens under blue-light may possess hyperopia, while those under red-light may possess myopia [43]. An experimental chick model has shown that blue light may retard myopia progression while red-light may enhance myopia progression [44]. Violet light at 360-400 nm may inhibit axial elongation in chicks and humans, by decreasing early growth response 1 (EGR1) gene expression known to inhibit myopia [45]. Violet light neuropsin (OPN5) in retina may also play a critical role to reduce myopia progression and mediate choroidal thickness in a mouse model [46]. Red-light may promote a hyperopic shift while blue light may not influence emmetropization in a mouse model [47]. Short wavelength light at 400 nm may delay eye growth, induce hyperopic shift, and prohibit myopia induced by lens [48]. In guinea pigs, blue light promotes hyperopic effects and inhibit axial growth while green light promotes myopia progression [49]. Positive lens promotes light to focus on the anterior part of retina, enhancing defocus-induced hyperopia while negative lens results in DIM Guinea pigs (reviewed in [30]). It is reported that blue light may inhibit axial myopia by negative lens and delay axial growth while red-light and positive lens do not induce hyperopia in a guinea pig model [50]. However, there is an inconsistency. For instance, researchers did not find that red-light promotes development of myopia but stimulates hyperopia instead [51]. Red-light may inhibit while blinking blue light may promote myopia progression in a tree shrew model [52]. Those results indicate that difference of wavelengths from different lights may cause different refractive and axial changes, which may be present independently of light intensity. The results from various species have added complexity in the association between light exposure and myopia [53-59].
Association of Light Color and Luminance with Myopia
Human color vision requires three types of color vision, short, middle and long wavelength sensitive cones, corresponding to their color perception channels. Precise information on color, light, dark and saturation is essential for two or more contrasts of cone and color vision channels [60]. Those signals may be affected by different pathways, such as brightness and 2 color vision alignment pathways [61-63].
Time-related changes from color contrast are defined as hyperopic defocus while time-related changes from luminance contrast are regarded as myopic defocus in absence of color changes [30]. The effect of the modulated ambient light on emmetropization have been studied extensively [64-75]. Nevertheless, due to species and experimental variabilities, the results reported are quite different. For instance, exposure to 0.5 and 5 Hz modulated illumination chronically has been reported to promote myopia progression in a guinea pig model [72]. Steady or flickering red-light may promote hyperopia, flickering blue light may promote myopia while steady blue light may not have any effects [52]. Gawne et al have also demonstrated that longitudinal chromatic aberration (LCA) is a critical visual cue of postnatal refractive development, and that short-wavelength temporal flickers are important for assessing and signaling defocus [76]. The visual system may use reactivity of longer-wavelength cones to blue light to adjust focus. As a result, blue light may promote emmetropization, and the amount of flicker detected by SWS channel may be more than that by long wavelength sensitive (LWS) channel [52]. Spatial contrast may also be an important factor for promoting myopia. In fact, eye may use a single retinal image for comparison of contrast from two different cone types to define state of hyperopia and myopia, without contrasting images in 2 different planes [61, 64]. In contrast of a single retinal image, eye may obtain information from comparing changes in color and luminance contrast along with changes from defocus of eye [77]. Those results suggest that LCA may reduce cone contrast from L- and M-cones, resembling when eye is in hyperopic or myopia defocus status.
In addition, an association between light luminance and myopia is present. An example is form-deprived myopia (FDM), referring to that induced myopia may be achieved by deprivation of form vision from eyes in a status of susceptibility, for example, lid-suture [78]. Low color temperature artificial lighting may retard form-deprived myopia progression in a juvenile monkey model, suggesting that low correlated color temperature (2700 K - 3000K) may delay ocular axial growth, when compared to high color temperature artificial lighting (4000 - 5000 K) [79]. One study has suggested that recovery from myopia in a chick model relies on the correlated color temperature of the light spectrum [80]. Another example is defocus-induced myopia (DIM) or lens-induced myopia (LIM), referring to myopia induced by concave or negative lenses, for instance, in chickens with lenses in front of eyes [81]. Light illuminance from outdoor is usually 10 to 1000 times higher than that from indoor [82, 83]. A DIM model has shown that chicks under high intensity light (15,000 lx) retarded development of myopia and slowed progression of myopia than those under low intensity light (500 lx) [84]. Similarly, an FDM model has demonstrated that chicks under high intensity light may delay myopia development [85-87]. Association of high light exposure and myopia control was later verified in FDM model using monkeys and mice [88, 89]. The association among near work, outdoor activity and myopia in school children was explored by Rose et al., the results of which have indicated that outdoor activity may decrease myopia even under high near-vision activity [27]. Zadnik et al have shown that outdoor activities may protect children against myopia [90], suggesting that intense light in outdoor environment may play a critical role in regulating onset and progression of myopia. Several studies have shown that the rate of myopia onset and progression are reduced in children with significant more time outdoors [91-93]. Unfortunately, the mechanism remains unclear.
Red-Light Targets and Possible Mechanism
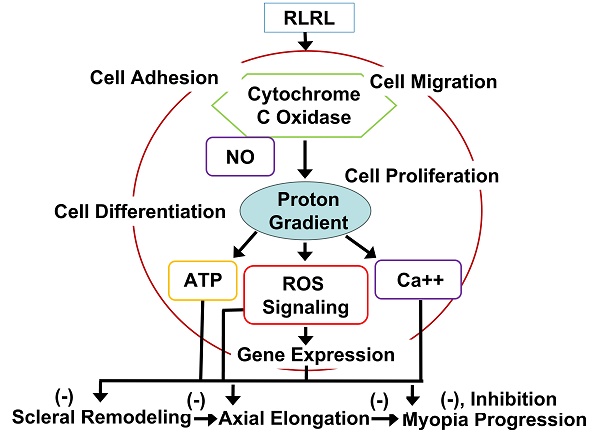
Red light may promote biological processes at a low dose but inhibit biological process at a high dose, in a U-shape response. Hormetic curves are better to predict biological responses within pharmacological threshold than linear curves [94]. It is very important since stimulatory responses from low-level red-light are mild, ~ 30% to 60% above controls. The hormetic dose-response is well known in various low-level red-light applications because photo--stimulatory or photo-inhibitory effects are shown in low (0.001 J/cm2) or high (0.10 J/cm2) energy densities respectively [95]. Positive responses may be obtained in that dose range for needed therapeutic effects [32, 96, 97]. Examples of low-level red-light effects and applications are listed for our understanding of usefulness of low-level red-light for potential medical applications. For example, wound healing may emerge in the rat skin under a low energy ruby laser [98] and under Gallium laser with 685 or 830 nm light [99]. Spinal cord injuries may be ameliorated by 810-nm light in a rat model [100]. Low-level red-light was beneficial for treating gingival incisions [101], oral mucositis [102], ulcers of skin [103], and wound healing [104], nerve repair [105-107], carpal tunnel syndrome [108, 109] and soft tissue injuries [110]. Inflammation could appear in light-stressed retina [111-113]. Microglial inhibitor naloxone may inhibit photoreceptor degeneration within retina [114, 115]. Pretreatment with LEDs can prevent neurons from apoptosis induced by cyanide due to inhibition of reactive oxygen synthesis and promotion of energy metabolism [116]. 670 nm red light has potential for medical applications reported by many researchers. For example, 670 nm light could protect neuronal cells under treatment of cyanide [32], protect photoreceptors in rat and promote wound healing in primate retina [117], increase of mitochondrial metabolism, decrease of retinal inflammation, and reduction of oxidative cell stress may be achieved probably by changes of respiratory chain complex I, II and IV to affect cytochrome c oxidase (CCO), resulting in better energy metabolism within mitochondria [118]. As reported, 670 nm red light may be used as an effective neuroprotectant against other light caused damage [119] and certain toxins [120]. Treatment with 670 nm red light decreases retinal inflammation by increasing mitochondria membrane potential [121], improves retinal healing [31], such as reducing raised intracellular pressure in rat retina [122], retards aging retinal functions [123]. 670 nm LED may regulate inflammation and immunity in the retina of a mouse with macular degeneration [124], likely through promoting CCO expression, along with reduced inflammation [125]. Respiration in aged retinal mitochondria may be enhanced by 670 nm light, showing better mitochondrial functions and inflammation reduction through activation of CCO. In fact, aged retina may possess a progressive oxidation increase by 670 nm light in 5 minutes [126]. 670 nm light can improve oxygen-mediated degeneration within retina in mouse, showing decreased oxidative stress marker expression and reduction in hyperoxia [127]. 670 nm light treatment may significantly retard lipid peroxidation, complement propagation in retina degeneration [128]. Low levels of 670 nm light may prevent retinopathy by oxygen induction and lung damage by excessive oxygen [129] and modulate expression of genes involved with inflammation, oxidative metabolism and apoptosis [130]. Evidence has indicated that CCO is the primary photo receptor [131], promoting oxidative metabolism [132] and increase ATP production [133], and is probably linked to increased CCO and reduced acrolein expression [134] through the reparative and/or the protective mechanism. In fact, abundant research results supports its potential benefits in retinal diseases [97], stroke [135, 136], neurodegeneration [137], neuromuscular disorders [138], hair regrowth [139], memory [140] and mood disorders [141].
Although many photoreceptors have been discovered, the mechanism of low-level red-light remains unclear since many molecules have photoreceptor capabilities. For instance, reactions from a molecular target induced by a wavelength can be complex. For example, NADH-dehydrogenase and flavoprotein have various photoreceptors from the violet to blue or from red to near-infrared spectral areas [133]. In addition, both terminal oxidase and superoxide dismutase have absorption peaks at high wavelengths of 670-680 nm, overlapping in the absorption spectrum of different photoreceptors [133, 142]. Those lights may enhance mitochondrial membrane potential, stimulate ATP exchange and production, promote synthesis of RNA, proteins, and usage of oxygen [143]. The effects from low-level red-light on mitochondria are wavelength-specific, and molecules absorbing low-level red-light in living cells are likely respiratory components [144]. For instance, RNA synthesis from HeLa cells may be enhanced by some wavelengths. The other example is cytochrome oxidase, especially cytochrome c oxidase, a primary light photoreceptor in the red and the near-infrared electromagnetic spectrum [145, 146], also a critical oxidase in cell bioenergetics in nerve cells from retina and brain [147]. Cytochrome oxidase exerts pump function by redox-coupled protons to stimulate transmembrane electrochemical gradient, synthesizing energy-generating ATP [148]. Cytochrome oxidase participates in free radical metabolism, glutamatergic regulation and apoptosis [149, 150]. Cytochrome oxidase is also involved in intraneuronal metabolic activity and neuronal functions [150]. In fact, cytochrome oxidase can be the only primary photoreceptor by sequential irradiation [151, 152]. Interestingly, absorption spectra from cytochrome oxidase is strongly linked to biological responses induced by low level red light [151].
Among the possible hypotheses for relationship of myopia and light intensity, the most possible hypothesis is that the light promotes synthesis and release of dopamine (DA) by retina [153]. DA is a critical neurotransmitter in retina, regulating multiple functions, such as refractive development, β receptor activation, visual signal transduction and myopia development, probably by activation of its receptor [154] (also reviewed in [30]). In fact, DA receptors are G-protein-coupled receptors present in retina, including D1, D2, D4, and D5 sub-receptors [155]. Among the sub-receptors, D2 receptors are more critical in mediation of myopia progression than D1 receptors in chicks (reviewed in [30]. Activation of D2 receptors may result in myopia, while activation of D1 receptors may cause hyperopia [155]. Light may promote DA release in a linear relationship with 4 log units of intensity [153]. DA release from retina may promote choroidal thickening with ocular growth retardation, probably by release of nitric oxide (NO) from retina and choroid to slow down myopia development [156-158]. Red, blue and UV light may also promote DA release from retina, with wavelength dependence [159]. For instance, blue and UV light may promote less deprivation myopia than red light (reviewed in [30]). Circadian rhythms could be another mechanism for light exposure to control myopia, overlapping with that of eye growth, in which DA is a critical mediator for the rhythms [160, 161]. Melatonin may also be critical in the association of the light and circadian functions [162]. AL and choroidal thickness of eyes may be opposite for the changes with circadian rhythms, which may be interfered by DIM modes, suggesting that optical defocus may play a critical role in regulation of AL and choroidal thickness [163-165]. In all, light intensity is negatively linked to onset and development of myopia.
DA may enhance synthesis and release of NO (nitric oxide) to mediate eye growth via activation of NOS (nitric oxide synthase). NO may decrease form deprived myopia dose dependently while NOS inhibitors may block myopia inhibition regulated by atropine [166, 167]. Activation of NOS may promote cGMP production to inhibit myopia in guinea pigs [168]. PBM may be caused by intracellular NO release [169, 170]. NO may inhibit oxidative agents, promote oxygen transport, and hemoglobin production. If combined with oxygen, NO may promote release of oxygen via NO competition [171]. Hypoxia and reoxygenation injury of cardiomyocytes may be relieved by 670 nm light, depending on NOS dependent or independent NO [172]. PBM may promote NO production via reduction of nitrite to NO mediated by CCO, a critical cytochrome oxidase, and myoglobin (Mb)/Hb [173]. CCO is a terminal complex for eukaryotic oxidative phosphorylation within mitochondria, which couples reduction of electron carriers in metabolism to reduction of molecular oxygen into water and proton translocation from internal mitochondrial matrix to inter-membrane [174]. A study has indicated that under 628 nm light for 3 days, the expression of the respiratory and antioxidant genes was promoted, as the expression of apoptotic genes is inhibited in human fibroblasts through CCO activation [175]. Photoreceptors of mammalian hemoglobin may be activated by certain light, manifesting that light absorption may be a differential sue to the redox state, allowing its quantification of oxygenation in clinical uses. CCO may be activated by light in FR to NIR range, causing cellular responses [32, 133]. Catalase, cryptochromes, cytochrome b, cytochrome c, guanylate cyclase, nitric oxide synthase and superoxide dismutase also possess photoreceptors [31].
Red light may induce oxidation in cytochrome c via activating CCO to increase use of oxygen, to elevate mitochondrial membrane sensitivity and pore permeability [133, 142]. The effects are mediated by the increase of electron flow in mitochondrial electron transportation chain. Red light may promote production of free radicals, via singlet oxygen due to photodynamic activation and generate superoxide ion through electron auto-oxidation. Red light can generate transient heat from chromophore due to electric or light oscillations [133], which can alter various molecules from the targets. RLRL may also promote energy transfer. Secondary effects from low-level red-light may appear as results of primary effects, for instance, biochemical and biological reactions which affects cellular homeostasis [176-178].
With treatment of monochromatic light, the symmetric myopic sign-dependency is manifested, resulting in the spherical defocus on the ocular acuity. The observed results suggest that the human visual system may integrate the chromatic differences in refraction to discern the signs of defocus [179]. Red light can activate signaling transduction from mitochondria to nucleus via activation of photoreceptors and translocation of signaling molecules to the nucleus, to alter gene expression. That is, red light may enhance beneficial NAD/NADH ratio changes in mitochondria to promote nitric oxide release via activation of cytochrome oxidase, and thus regulating ATP level. ATP may then activate ATP P2 receptors to cause inward calcium currents through calcium release from intracellular storages of calcium [180]. As a result of such ATP changes, which then mediate level of cAMP, inducing activation of several kinases to mediate gene expression, which can reduce inflammation and improve wound healing.
PBM may also activate TGFβ/Smad signaling in wound healing to mediate myopia progression [181, 182]. 670 nm light may promote production of collagen I and vascular endothelial growth factor (VEGF) in wound healing [183]. Decrease of collagen I alpha1 (COL1A1) and increase of α-SMA may be linked to sclera hypoxia [29]. Therefore, we deduce that FR and NIR treatment may promote choroid thickening and improve blood flow through release of NO acting as a vasodilator. NO may also have anti-hypoxia effect, inducing amelioration of scleral hypoxia. Activation of the TGFβ/Smad pathway may promote scleral remodeling via overproduction of COL1A1. Along with NO, trans-differentiation of sclera fibroblasts may be reversed, without any specific mechanism so far.
PBM and PBMT
PBM or low-level light therapy, via photon irradiation, has decades of clinical applications to soft tissue injuries due to promoting wound healing. Low-level red-light can penetrate tissues such as the brain, heart and spinal cord [184, 185], accelerate healing from ischemic heart injury [184], inhibit degeneration in injured optic nerve and retina [184, 186], and promote recovery in stroke [185, 187, 188]. These effects by low-level red-light are clearly associated with the absorption spectrum of cytochrome oxidase [31], by overexpression of cytoprotective factors [32, 116, 185, 186, 189-192], accumulation of antioxidants [120]. For example, gene profile analysis shows that low-level red-light promotes noncoding RNAs (ncRNA) to mediate certain gene expression [130]. During PBM, cytochrome oxidase, a primary photoreceptor of low-level red-light, plays a critical role in recoveries of injured eye and brain tissues because the enzyme in mitochondria is critical for oxidation. Low-level red-light has significant neuroprotective effects in regeneration of damaged retina via promoting mitochondrial function, improving blood flow to the damaged neurons, promoting expression and release of survival factors from cells [193].
PBMT (photobiomodulation therapy) has increasingly become popular in human medical therapies recently. Recent reports have suggested that laser therapy can be used for treatment of inflammation and promotion of wound healing [194]. Best of all, energy from PBMT is low without any side effect concern for tissue destruction due to overheating, however, good enough for medical purposes. For instance, PBMT may be applied as photodynamic therapy for skin diseases [195] and as thermal therapy of neuronal diseases [196].
Red-Light Therapy for Myopia Prevailing
Myopia pandemic affects a large number of population in the world [197-203] (reviewed in [204]). Higher Myopia may cause serious consequences such as cataract, choroidal degeneration, glaucoma, retinal tears and detachment [205-209]. The RLRL clinical trials are mainly conducted in China due to their recent invention of the new RLRL therapy machine. Xiong et al have shown that RLRL therapy may induce sustained choroidal thickening over the entire course of treatment [210]. Liu et al have shown that the choroid's thickening is not sufficient to explain the reasons for AL shortening, which may be linked to changes from the posterior segment [211]. Higher aberrations mean optical problems of eyes that alter quality of retinal image, causing visual progressive deterioration. Therefore, various myopia control methods have been developed such as use of orthokeratology, soft contact lenses (dual-focus or multi-focus) and near addition spectacle lenses, which have enormous effects for the world population [212]. Yam et al have also shown that 0.05%, 0.025%, and 0.01% atropine may delay myopia progression by concentration-dependent response, and of 3 concentrations used, 0.05% atropine was the best to controlling AL elongation and myopia progression in a 1-year trial [22]. Yam et al have later reported that the efficacy of 0.05% atropine is twice as good as that with 0.01% atropine in retarding myopia progression [213]. We recently have reported that 1% and 0.05% atropine respectively may delay moderate myopia progression in Chinese school children by a mega clinical investigation [214, 215] and long-term wear of orthokeratology lenses also retard myopia progression [216]. However, as our generation is aging, the prevalence of myopia is increasing continually [217] due to their limited effectiveness and obvious side effects. Therefore, we need to find more effective ways to control the development and progression of myopia.
Recently, several reports have indicated that RLRL may inhibit myopia progression by inhibiting SER progression and AL elongation [20, 218-220]. Hung et al have suggested that narrow-band with long-wavelength light may inhibit axial elongation typically produced by form deprivation and hyperopic defocus, due to eliciting direction signals related to myopic defocus [212]. However, not all the reports are comprehensive and many undiscovered characteristics remain to be explored, for instance, whether RLRL treatment may prevent myopia occurrence from preclinical status, whether such treatment is better than other conventional treatments for myopia, such as atropine, DIMS, multifocal contact lenses and outdoor exercises [91, 213, 221-226]. Wang et al have indicated that approximately a quarter school children have had inhibition of AL elongation by RLRL therapy, and the mean AL change is -0.142 mm per year [227]. Recently, there are new efficacy reports for inhibiting SER progression by a change of SER of 0.06 D by RLRL treatment in a 6-mouth trial reported by Dong et al [218], a change by 0.70 D in a 9-month trial from another report by Zhou et al [219] and a change by -0.59 D in a 12-month trial reported by Jiang et al [20]. In addition, time spent outdoors at school (40 more minutes a day for 3 years) can bring a change of SER 0.17 D presented by He et al [91], DIMS may bring a change of SER (-0.44 D) in a 2-year study reported by Lam et al [24], high add power contact lenses may bring a change of SER (0.46 D) in a 3-year study presented by Walline et al [228], spectacle lenses with aspherical lenselets may bring a change of SER (0.53 D) in a 1-year study reported by Bao et al [229], 0.01% atropine treatment for 1 year may bring a change of SER (0.26 D) presented by Wei et al [230] and 0.025% atropine for 2-year treatment can bring a change of SER change (-0.85 D) in LAMP (phase II) investigation reported by Yam et al [213]. Furthermore, the efficacy for inhibiting AL elongation may bring a change of axial growth by 0.11 mm in a 6-mouth RLRL trial by Dong et al [218], by 0.20 mm in a 9-mouth trial from another RLRL therapy by Zhou et al [219]. RLRL may also reduce AL elongation by 0.26 mm in a 12-mouth RLRL trial by Jiang et al [20]. Interestingly, time spent outdoors at school (40 more minutes a day for 3 years) may bring a change of axial growth by -0.03 mm presented by He et al [91], 0.01% atropine treatment for 1 year may bring axial growth change by 0.09 mm [230], and 0.025% atropine treatment for 1 year may lead to AL change by 0.29 mm in LAMP study by Yam et al [22] and 0.025% atropine treatment for 2-year may bring axial growth change by 0.50 mm (average 0.25 mm per year) in LAMP (phase II) study by Yam et al [213] and DIMS may bring a change of axial growth by 0.34 mm in a 2-year study (average 0.17 mm per year) by Lam et al [24] and high add power contact lenses may bring a change of axial growth by 0.42 mm in a 3-year study (average 0.14 mm per year) by Walline [228] and spectacle lenses with aspherical lenselets may bring a change of AL by 0.23 D in a 1-year study by Bao et al [229]. All evidence is clear that RLRL treatment may effectively retard myopia progression reported by Jiang et al, Zhang et al, Dong et al, Zhou et al and Xiong et al [20, 30, 218-220], without severe adverse events observed by Jiang et al [20]. As expected, compared to 0.01% atropine eye drops for myopia control, RLRL is more effective to retard AL elongation and myopia progression for 12 months observed by Chen et al [231]. Xiong et al have evaluated the long-term efficacy as well as safety of RLRL therapy over myopia control for 2 years and potential rebound effect after withdrawal of treatment and discovered that RLRL therapy has encouraging efficacy and safety for retardation of myopia progression without serious side effects over 2 years reported by Xiong et al [220]. Therefore, RLRL is an effective method to prevent and control myopia, probably with a slight myopic rebound after its withdrawal presented by Chen et al [232]. Dong et al have noted that the efficacy and safety of RLRL therapy for retarding myopia progression requires 100% original power to significantly reduce myopia progression over 6 months, compared to that from only 10% original power. Jiang et al have reported that RLRL therapy is a promising treatment for myopia control in children [20]. Further validation in long term randomized controlled trials are required for validation of the short-term trial results shown by Zhou et al [219]. The results for recent clinical trials of RLRL therapy to myopia are listed in Table 1.
Summary of Recent Clinical Trials for RLRL Therapy to Myopia.
| Authors | Year | Type of Studies | Study Duration (Months) | Sample Size | Efficacy and Study Results | Limitations |
|---|---|---|---|---|---|---|
| Zhou et al [219] | 2022 | Retrospective case series | 9 | 105 | At 9 months, the mean SER in RLRL group was -2.87 ± 1.89 D, significantly greater than that of the control (-3.57 ± 1.49 D). AL changes were -0.06 ± 0.19 mm and 0.26 ±0.15 mm in RLRL group and the control group. The subfoveal choroidal thickness changed by 45.32 ± 30.88 µm in RLRL group at the 9-month examination. Specifically, a substantial hyperopic shift (0.31 ± 0.24 D and 0.20 ± 0.14 D) was found in 8-14 years old, compared with 4-7 years old children. The decrease in AL in subjects with baseline AL >24 mm was -0.08 ± 0.19 mm, significantly greater than those with a baseline AL ≤24 mm (-0.04 ± 0.18 mm). The authors have concluded that repetitive exposure to RLRL therapy was associated with slower myopia progression and reduced axial growth after short durations of treatment. | Firstly, selection bias might be present because not all participants adhered strictly to treatment. Secondly, the sample size was small and the duration was short (9 months). The sample size of the control group and experimental groups was not the same or similar. Thirdly, the wavelength and power intensity used in this study was different from others, making it difficult to compare the results with previous studies. |
| Jiang et al [20] | 2022 | Randomized controlled trial | 12 | 264 | Adjusted 12-month axial elongation and SER progression were 0.13 mm (0.09-0.17mm) and -0.20 D (-0.29 to -0.11D) for RLRL treatment and 0.38 mm (0.34-0.42 mm) and -0.79 D (-0.88 to -0.69 D) for SVS treatment. The differences in axial elongation and SER progression were 0.26 mm (0.20-0.31 mm) and -0.59D (-0.72 to -0.46 D) between the RLRL and SVS groups. No severe adverse events (sudden vision loss ≥2 lines or scotoma), functional visual loss indicated by BCVA, or structural damage seen on OCT scans were observed. The authors have concluded that RLRL therapy is a promising alternative treatment for myopia control in children with good user acceptability and no documented functional or structural damage. | Firstly, because of pragmatic feasibility, the authors did not implement masking. Secondly, the level of compliance was not assigned randomly. Thirdly, because of coronavirus disease outbreak in 2019, approximately 50% of children were lost to follow-up at 6 months. Fourthly, the observed treatment efficacy in controlling myopic progression was generalizable only to the device used in the present study, not approved by studies using other wavelengths, power intensities, exposure durations, or frequencies of treatments. Fifthly, the duration of the trial was 1 year, which was not long enough. |
| Chen et al [231] | 2022 | Randomized controlled trial | 12 | 62 | The mean 1- year change in AL was 0.08 mm in RLRL group and 0.33 mm in low dose atropine (LDA) group, with a mean difference (MD) of -0.24mm. The 1-year change in SER was-0.03 D in the RLRL group and -0.60 D in LDA group. The progression of AL < 0.1 mm was 53.2% and 9.7% in the RLRL and LDA groups, respectively. For AL ≥ 0.36 mm, progression was 9.7% and in the RLRL and LDA groups, respectively. The authors have concluded that RLRL is more effective for controlling AL and myopia progression over 12 months of use compared with 0.01% atropine eye drops. | Firstly, the authors might not avoid the deliberate exclusion of false reports from parents. Secondly, this study was a localized but not a multicenter study, and the geographic representation was not great. Thirdly, only 0.01% atropine was used to compare the results from RLRL study, which is not the balanced dose for control of myopia by atropine. Fourthly, due to covid-19, online learning may bias the results in the study. Fifthly, the duration was 12 months, not long enough for a definite conclusion from this study. |
| Xiong et al [220] | 2022 | Post-trial follow up | 24 | 199 | In the second year, the mean changes in AL were 0.28 ± 0.14 mm, 0.05 ± 0.24 mm, 0.42 ± 0.20 mm and 0.12 ± 0.16 mm in SVS-SVS, SVS-RLRL, RLRL-SVS and RLRL-RLRL group, respectively (p < 0.001). The respective mean SER changes were -0.54 ± 0.39D, -0.09 ± 0.55D, -0.91 ± 0.48D, and -0.20 ± 0.56D respectively. Over the 2-year period, axial elongation and SER progression were smallest in RLRL-RLRL group (AL: 0.16 ± 0.37 mm; SER: -0.31 ± 0.79D), followed by SVS-RLRL (AL: 0.44 ± 0.37 mm; SER: -0.96 ± 0.70D), RLRL-SVS (AL: 0.50 ± 0.28 mm; SER: -1.07 ± 0.69D) and SVS-SVS group (AL: 0.64 ± 0.29 mm; SER: -1.24 ± 0.63D). No self-reported adverse events, functional or structural damage was noted. The authors have concluded that continued RLRL therapy sustained promising efficacy and safety in slowing myopia progression over 2 years. A modest rebound effect was noted after treatment cessation. | Firstly, the study was a post-trial follow-up research, and the participants were not randomly assigned to control and experimental groups. Secondly, the continuation or cessation of the treatment was not pre-determined, which might cause the bias in the results. Thirdly, the number of participants in the control and RLRL therapy groups was not balanced. Fourthly, the study was based on Chinese children, therefore, the generalizability of this study to other ethnicities needs further exploration. |
| Dong et al [218] | 2022 | Randomized controlled trial | 6 | 111 | The mean SER change over 6 months was 0.06 ± 0.30 D in the RLRL group and -0.11 ± 0.33 D in the sham device control group, with respective mean increases in AL of 0.02 ±0.11mm and 0.13 ± 0.10 mm. In the multivariate GEE models, children in the RLRL group showed less myopia progression and axial elongation than those in the sham device control group (SER: coefficient, 0.167 D; 0.050-0.283D; AL: coefficient, -0.101 mm; -0.139 to -0.062 mm). No treatment-related adverse events were reported. The authors have concluded that in myopic children, RLRL therapy with 100% power significantly reduced myopia progression over 6 months compared with those treated with a sham device of 10% original power. The RLRL treatment was well tolerated without treatment-related adverse effects. | Firstly, the duration of this trial was 6 months, not sufficient for the observations of the full myopia control effects. Secondly, the rebound effect of RLRL treatment was not present. Thirdly, online screen-based learning might have affected the results of myopia due to covid-19. Fourthly, in the control group, the authors used 10% of RLRL energy, which might not be appropriate since 10% of RLRL energy might also have certain effects. Fifthly, masking of participants was not strict. |
| Liu et al [211] | 2022 | Randomized controlled trial | 1 | 98 | A linear mixed-effects model showed that the AL of the subjects in RLRL decreased from 24.63 ±1.04 mm to 24.57 ± 1.04 mm, and the SChT thickened by 18.34 µm. CVI had a slight but significant increase in the 0-6 zone. However, all the anterior segment parameters did not change after RLRL treatment. The authors have concluded that choroid's thickening is insufficient to explain the AL shortening. The unchanged anterior segment and improved choroid blood flow suggest that AL shortening is related to changes in the posterior segment. | Firstly, the follow-up duration for this study is short. Secondly, the authors did not know how AL would rebound after discontinuation of their RLRL treatments in myopia. Thirdly, it is unclear how the short-term axial shortening links to long-term myopia control. Fourthly, it is unclear whether short-term axial shortening occurs in emmetropic subjects or only in myopic adults. |
| Tian et al [233] | 2022 | Randomized controlled trial | 6 | 224 | The median 6-month changes in AL of the LLRL and control groups were - 0.06 mm and 0.14 mm, respectively. The difference between groups was significant. The median 6-month changes in SER were 0.125 D and -0.25 D for the LLRL and control groups, respectively. The difference between groups was significant. Compared with the control, the proportion of children with hyperopic shift in the LLRL group was higher (51.65% vs. 3.41%), and the proportion of children with shortened AL in the LLRL group was higher (63.74% vs. 2.27%). No adverse event was observed. The authors have concluded that 650 nm LLRL significantly slowed down the myopia progression in children aged 6-12 years, and there was no observable side effect in the short term. | Firstly, the duration of this study was 1 year, and the cessation time was 3 months, not long enough for the controlling effect on myopia. Secondly, the sample size is not good enough to draw a scientific conclusion. Thirdly, not all examination results were objective, such as the examination for accommodation. Fourthly, LRL recorded was subjective. |
| Yang et al [234] | 2022 | Prospective study | 1 | 25 | The RFPD in LLRLT eyes significantly increased 5 min after LLRLT, and the increment was 1.70 ± 0.83%. The RFPD significantly decreased from 5 min to 1 h after LLRLT with a mean of -2.62 ± 0.86% decrement. The RFPD levels returned to baseline at 1 h after LLRLT. However, compared with insignificant RFPD changes in non-LLRLT eyes, there was no significant difference in RFPD changes at any sampling point. No significant changes in RFT, CFBF, and CFT were found in LLRLT eyes at each sampling point. Although 3 min of LLRLT has no effect on the choroid, it may cause a short-term transient increase in RFPD. The authors have concluded that their study provides theoretical support for the role of LLRLT in myopia control. | Firstly, the follow-up duration in this study was short, therefore, the long-term efficacy and safety of RLRL for myopia require further investigation. Secondly, the rebound effect was not determined. |
| Chen et al [232] | 2022 | Randomized controlled trial | 12 | 86 | AL elongation and myopic progression were 0.01 mm (- 0.05 to 0.07 mm) and 0.05 D (- 0.08 to 0.19 D) in the LRL group, which were less than 0.39 mm (0.33 to 0.45 mm) and - 0.64 D (- 0.78 to - 0.51 D) in the SFS group. The change of SFCT in the LRL group was greater than that in the SFS group. Accommodative response and positive relative accommodation in the LRL group were more negative than those in the SFS group. Forty-two subjects completed the observation of LRL cessation, AL and SER increased by 0.16 mm (0.11 to 0.22 mm) and - 0.20 D (95%- 0.26 to - 0.14 D) during the cessation, and SFCT returned to baseline. The authors have concluded that LRL is an effective measure for preventing and controlling myopia, and it may also have the ability to improve the accommodative function. There may be a slight myopic rebound after its cessation. | Firstly, only 25 cases were included in this study, which might cause the bias in their conclusions. Secondly, the data of RLRL therapy was from only one device, which needs confirmation from data obtained from other machines because different devices for RLRL therapy might have other power intensities and wavelengths. Thirdly, the appropriate exposure durations were not determined. |
| Wang et al [235] | 2023 | Retrospective study | 12 | 434 | The mean age of participants was 9.7 (2.6) years with SER of -3.74 (2.60) diopters. There were 115 (26.50%), 76 (17.51%), and 20 (4.61%) children with AL shortening based on cutoffs of 0.05 mm/year, 0.10 mm/year, and 0.20 mm/year, respectively. In the multivariable model, AL shortening was significantly associated with older baseline age, female gender, and longer baseline AL or greater SER. Among AL shortened eyes, the mean AL difference was -0.142 (0.094) mm/year. Greater AL shortening was observed among children who were younger and had longer baseline AL. The authors have concluded that more than a quarter of children have had AL shortening [0.05 mm following RLRL therapy, and the overall mean AL change was -0.142 mm/year. | Firstly, the children were Chinese ethnicity, which might not be representative in other geographic regions of the world. Secondly, this study duration is not long enough for follow-up study of RLRL therapy on the control of myopia. Secondly, AL measurements might be affected by the changes in choroidal thickness. Thirdly, no data on outdoor activities on myopia was available in the retrospective study, which could cause significant bias. Fourthly, the study duration is 1 year for follow-up information, not sufficient for an estimate of myopia reduction that should take a few years to complete. |
Controversies
Effects of various lights on myopia may be quite different. For instance, red-light, a long wavelength light should lead to hyperopia defocus [50, 159, 236] and induce myopia [30]. However, it is not always the case. The effect of red-light on progression of myopia is quite different. For instance, some researchers have reported that the predominant effect of red-light is to induce hyperopia but not myopia [51, 52]. Little or no difference has been found in refractive error in a rhesus monkey model with or without quasi monochromatic red-light [75] while rhesus monkeys with long red wavelength filters show significant hyperopia [51]. These results suggest that chromatic signaling is not required in certain cases [75]. Reduction of the luminance contrast may lead to chromatic contrast mainly in emmetropization [61, 237], causing imbalance in strengths of short and long wavelength signals [51]. Other reports have suggested that spectral wavelength and specific wavelength ranges may significantly influence myopia progression. For example, 630 nm light and 624 nm light may significantly affect myopia progression in rhesus monkey and tree shrew models [212, 238]. RLRL at 650 nm and 1600 lx may significantly inhibit progression of myopia in school children [20]. Tian et al have also concluded that 650 nm RLRL therapy may significantly retard myopia progression in children of 6-12 years old, without serious side effects in a 6-month trial [233]. Yang et al have demonstrated that 650 nm RLRL therapy for 3 min has had no effect on the choroid but had effect with a transient increase of retinal fovea perfusion density [234]. Low intensity and long wavelength red-light (635 nm) may also inhibit myopia progression in school children in Eastern China [219].
Prospectives
Myopia has been increasing steadily, especially in East Asia. Red-light may improve choroidal blood perfusion and may be a critical tool for control of myopia via cytochrome and nitric oxide signaling. In all, application of red-light may retard myopia effectively, possessing high potential for prevention of myopia onset and control of myopia progression. However, the safety and the implementation of RLRL therapy are two biggest issues for extensive investigations. In addition, whether such a therapy is effective in high myopia requires further scientific studies. Furthermore, whether RLRL therapy can prevent onset of myopia remains unknown. Finally, whether RLRL therapy can be used to replace atropine therapy for myopia control needs comparative studies.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith Iii EL, Zhou X. et al. Myopia. Nat Rev Dis Primers. 2020;6:99
2. Hashemi H, Heydarian S, Hooshmand E, Saatchi M, Yekta A, Aghamirsalim M. et al. The Prevalence and Risk Factors for Keratoconus: A Systematic Review and Meta-Analysis. Cornea. 2020;39:263-70
3. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet (London, England). 2012;379:1739-48
4. Bremond-Gignac D. [Myopia in children]. Med Sci (Paris). 2020;36:763-8
5. Bressler NM. Reducing the Progression of Myopia. JAMA. 2020;324:558-9
6. Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H. et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1:e339-49
7. Morgan IG, Jan CL. China Turns to School Reform to Control the Myopia Epidemic: A Narrative Review. Asia Pac J Ophthalmol (Phila). 2022;11:27-35
8. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P. et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036-42
9. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157:9-25 e12
10. Lakawicz JM, Bottega WJ, Fine HF, Prenner JL. On the mechanics of myopia and its influence on retinal detachment. Biomech Model Mechanobiol. 2020;19:603-20
11. Pan CW, Cheng CY, Saw SM, Wang JJ, Wong TY. Myopia and age-related cataract: a systematic review and meta-analysis. Am J Ophthalmol. 2013;156:1021-33 e1
12. Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The Complications of Myopia: A Review and Meta-Analysis. Investigative ophthalmology & visual science. 2020;61:49
13. Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1-38
14. Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080-5
15. Saw SM, Zhang MZ, Hong RZ, Fu ZF, Pang MH, Tan DT. Near-work activity, night-lights, and myopia in the Singapore-China study. Arch Ophthalmol. 2002;120:620-7
16. Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M. et al. IMI Prevention of Myopia and Its Progression. Investigative ophthalmology & visual science. 2021;62:6
17. Alvarez-Peregrina C, Sanchez-Tena MA, Martinez-Perez C, Villa-Collar C. The Relationship Between Screen and Outdoor Time With Rates of Myopia in Spanish Children. Front Public Health. 2020;8:560378
18. Khezri R, Rezaei F, Darvish Motevalli F. Covid-19 pandemic and risk of Myopia. Ann Med Surg (Lond). 2022;79:103944
19. Singh H, Singh H, Latief U, Tung GK, Shahtaghi NR, Sahajpal NS. et al. Myopia, its prevalence, current therapeutic strategy and recent developments: A Review. Indian J Ophthalmol. 2022;70:2788-99
20. Jiang Y, Zhu Z, Tan X, Kong X, Zhong H, Zhang J. et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology. 2022;129:509-19
21. Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:341-5
22. Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E. et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. 2019;126:113-24
23. Lee YC, Wang JH, Chiu CJ. Effect of Orthokeratology on myopia progression: twelve-year results of a retrospective cohort study. BMC ophthalmology. 2017;17:243
24. Lam CSY, Tang WC, Tse DY, Lee RPK, Chun RKM, Hasegawa K. et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020;104:363-8
25. Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H. et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. 2016;123:697-708
26. Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of Myopia. Asia Pac J Ophthalmol (Phila). 2016;5:386-93
27. Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W. et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279-85
28. Zhang J, Deng G. Protective effects of increased outdoor time against myopia: a review. J Int Med Res. 2020;48:300060519893866
29. Wu H, Chen W, Zhao F, Zhou Q, Reinach PS, Deng L. et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. 2018;115:E7091-E100
30. Zhang P, Zhu H. Light Signaling and Myopia Development: A Review. Ophthalmol Ther. 2022;11:939-57
31. Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP. et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559-67
32. Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E. et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-71
33. Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med. 2016;48:343-9
34. Nawashiro H, Wada K, Nakai K, Sato S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed Laser Surg. 2012;30:231-3
35. Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME. et al. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017;49:165-82
36. Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer's disease-related pathology in cerebral cortex - evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6:2
37. Leung MC, Lo SC, Siu FK, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med. 2002;31:283-8
38. Whelan HT, Smits RL Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA. et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305-14
39. Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain. 2011;3:49-67
40. Assia E, Rosner M, Belkin M, Solomon A, Schwartz M. Temporal parameters of low energy laser irradiation for optimal delay of post-traumatic degeneration of rat optic nerve. Brain Res. 1989;476:205-12
41. Wang J, Cui J, Zhu H. Suppression of type I collagen in human scleral fibroblasts treated with extremely low-frequency electromagnetic fields. Mol Vis. 2013;19:885-93
42. Sliney DH. What is light? The visible spectrum and beyond. Eye (Lond). 2016;30:222-9
43. Seidemann A, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002;42:2409-17
44. Rucker FJ, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009;49:1775-83
45. Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T. et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine. 2017;15:210-9
46. Jiang X, Pardue MT, Mori K, Ikeda SI, Torii H, D'Souza S. et al. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc Natl Acad Sci U S A. 2021 118
47. Yang J, Yang L, Chen R, Zhu Y, Wang S, Hou X. et al. A role of color vision in emmetropization in C57BL/6J mice. Sci Rep. 2020;10:14895
48. Strickland R, Landis EG, Pardue MT. Short-Wavelength (Violet) Light Protects Mice From Myopia Through Cone Signaling. Investigative ophthalmology & visual science. 2020;61:13
49. Liu R, Qian YF, He JC, Hu M, Zhou XT, Dai JH. et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Experimental eye research. 2011;92:447-53
50. Jiang L, Zhang S, Schaeffel F, Xiong S, Zheng Y, Zhou X. et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Res. 2014;94:24-32
51. Smith EL 3rd, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of Long-Wavelength Lighting on Refractive Development in Infant Rhesus Monkeys. Investigative ophthalmology & visual science. 2015;56:6490-500
52. Gawne TJ, Siegwart JT Jr, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Experimental eye research. 2017;155:75-84
53. Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407-13
54. Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625-8
55. Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK. et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Investigative ophthalmology & visual science. 2005;46:3074-80
56. Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Res. 1992;32:833-42
57. Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Investigative ophthalmology & visual science. 1999;40:214-29
58. Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vision Res. 1993;33:1311-24
59. Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28:323-8
60. Swanson WH, Cohen JM. Color vision. Ophthalmol Clin North Am. 2003;16:179-203
61. Rucker FJ, Kruger PB. Accommodation responses to stimuli in cone contrast space. Vision Res. 2004;44:2931-44
62. Kelly DH, van Norren D. Two-band model of heterochromatic flicker. J Opt Soc Am. 1977;67:1081-91
63. Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127-53
64. Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists). 2013;33:196-214
65. Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686:169-81
66. Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. Neuroreport. 2002;13:1029-32
67. Crewther SG, Barutchu A, Murphy MJ, Crewther DP. Low frequency temporal modulation of light promotes a myopic shift in refractive compensation to all spectacle lenses. Experimental eye research. 2006;83:322-8
68. Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J. et al. Temporal constraints on experimental emmetropization in infant monkeys. Investigative ophthalmology & visual science. 2007;48:957-62
69. Rucker F, Britton S, Spatcher M, Hanowsky S. Blue Light Protects Against Temporal Frequency Sensitive Refractive Changes. Investigative ophthalmology & visual science. 2015;56:6121-31
70. Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res. 1997;37:2661-73
71. Di Y, Liu R, Chu RY, Zhou XT, Zhou XD. Myopia induced by flickering light in guinea pigs: a detailed assessment on susceptibility of different frequencies. Int J Ophthalmol. 2013;6:115-9
72. Di Y, Lu N, Li B, Liu R, Chu RY, Zhou XT. et al. Effects of chronic exposure to 0.5 Hz and 5 Hz flickering illumination on the eye growth of guinea pigs. Current eye research. 2013;38:1182-90
73. Yu Y, Chen H, Tuo J, Zhu Y. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic research. 2011;46:80-7
74. Luo X, Li B, Li T, Di Y, Zheng C, Ji S. et al. Myopia induced by flickering light in guinea pig eyes is associated with increased rather than decreased dopamine release. Mol Vis. 2017;23:666-79
75. Liu R, Hu M, He JC, Zhou XT, Dai JH, Qu XM. et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Investigative ophthalmology & visual science. 2014;55:1901-9
76. Gawne TJ, Ward AH, Norton TT. Juvenile Tree Shrews Do Not Maintain Emmetropia in Narrow-band Blue Light. Optom Vis Sci. 2018;95:911-20
77. Rucker FJ, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012;12:23
78. Yinon U. Myopia induction in animals following alteration of the visual input during development: a review. Current eye research. 1984;3:677-90
79. Hu YZ, Yang H, Li H, Lv LB, Wu J, Zhu Z. et al. Low color temperature artificial lighting can slow myopia development: Long-term study using juvenile monkeys. Zool Res. 2022;43:229-33
80. Muralidharan AR, Low SWY, Lee YC, Barathi VA, Saw SM, Milea D. et al. Recovery From Form-Deprivation Myopia in Chicks Is Dependent Upon the Fullness and Correlated Color Temperature of the Light Spectrum. Investigative ophthalmology & visual science. 2022;63:16
81. Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639-57
82. Dharani R, Lee CF, Theng ZX, Drury VB, Ngo C, Sandar M. et al. Comparison of measurements of time outdoors and light levels as risk factors for myopia in young Singapore children. Eye (Lond). 2012;26:911-8
83. Lingham G, Mackey DA, Lucas R, Yazar S. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. 2020;104:593-9
84. Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Investigative ophthalmology & visual science. 2010;51:5247-53
85. Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative ophthalmology & visual science. 2009;50:5348-54
86. Stone RA, Cohen Y, McGlinn AM, Davison S, Casavant S, Shaffer J. et al. Development of Experimental Myopia in Chicks in a Natural Environment. Investigative ophthalmology & visual science. 2016;57:4779-89
87. Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Investigative ophthalmology & visual science. 2014;56:299-309
88. Smith EL 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investigative ophthalmology & visual science. 2012;53:421-8
89. Chen S, Zhi Z, Ruan Q, Liu Q, Li F, Wan F. et al. Bright Light Suppresses Form-Deprivation Myopia Development With Activation of Dopamine D1 Receptor Signaling in the ON Pathway in Retina. Investigative ophthalmology & visual science. 2017;58:2306-16
90. Zadnik K, Mutti DO. Outdoor Activity Protects Against Childhood Myopia-Let the Sun Shine In. JAMA Pediatr. 2019;173:415-6
91. He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J. et al. Effect of Time Spent Outdoors at School on the Development of Myopia Among Children in China: A Randomized Clinical Trial. JAMA. 2015;314:1142-8
92. French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013;120:2100-8
93. Li M, Lanca C, Tan CS, Foo LL, Sun CH, Yap F. et al. Association of time outdoors and patterns of light exposure with myopia in children. Br J Ophthalmol. 2023;107:133-9
94. Calabrese EJ, Stanek EJ 3rd, Nascarella MA, Hoffmann GR. Hormesis predicts low-dose responses better than threshold models. International journal of toxicology. 2008;27:369-78
95. Mussttaf RA, Jenkins DFL, Jha AN. Assessing the impact of low level laser therapy (LLLT) on biological systems: a review. Int J Radiat Biol. 2019;95:120-43
96. Yu W, Naim JO, Lanzafame RJ. The effect of laser irradiation on the release of bFGF from 3T3 fibroblasts. Photochem Photobiol. 1994;59:167-70
97. Eells JT, Gopalakrishnan S, Valter K. Near-Infrared Photobiomodulation in Retinal Injury and Disease. Adv Exp Med Biol. 2016;854:437-41
98. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. American journal of surgery. 1971;122:532-5
99. Viegas VN, Abreu ME, Viezzer C, Machado DC, Filho MS, Silva DN. et al. Effect of low-level laser therapy on inflammatory reactions during wound healing: comparison with meloxicam. Photomed Laser Surg. 2007;25:467-73
100. Wu X, Dmitriev AE, Cardoso MJ, Viers-Costello AG, Borke RC, Streeter J. et al. 810 nm Wavelength light: an effective therapy for transected or contused rat spinal cord. Lasers Surg Med. 2009;41:36-41
101. Neiburger EJ. Rapid healing of gingival incisions by the helium-neon diode laser. J Mass Dent Soc. 1999;48:8-13 40
102. Whelan HT, Connelly JF, Hodgson BD, Barbeau L, Post AC, Bullard G. et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg. 2002;20:319-24
103. Schindl A, Schindl M, Pernerstorfer-Schon H, Mossbacher U, Schindl L. Low intensity laser irradiation in the treatment of recalcitrant radiation ulcers in patients with breast cancer-long-term results of 3 cases. Photodermatol Photoimmunol Photomed. 2000;16:34-7
104. Medrado AR, Pugliese LS, Reis SR, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med. 2003;32:239-44
105. Gigo-Benato D, Geuna S, Rochkind S. Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve. 2005;31:694-701
106. Anders JJ, Geuna S, Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res. 2004;26:233-9
107. Anders JJ, Borke RC, Woolery SK, Van de Merwe WP. Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med. 1993;13:72-82
108. Branco K, Naeser MA. Carpal tunnel syndrome: clinical outcome after low-level laser acupuncture, microamps transcutaneous electrical nerve stimulation, and other alternative therapies-an open protocol study. J Altern Complement Med. 1999;5:5-26
109. Irvine J, Chong SL, Amirjani N, Chan KM. Double-blind randomized controlled trial of low-level laser therapy in carpal tunnel syndrome. Muscle Nerve. 2004;30:182-7
110. Simunovic Z, Ivankovich AD, Depolo A. Wound healing of animal and human body sport and traffic accident injuries using low-level laser therapy treatment: a randomized clinical study of seventy-four patients with control group. J Clin Laser Med Surg. 2000;18:67-73
111. Ng TF, Streilein JW. Light-induced migration of retinal microglia into the subretinal space. Investigative ophthalmology & visual science. 2001;42:3301-10
112. Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Investigative ophthalmology & visual science. 2002;43:3511-21
113. Rutar M, Provis JM, Valter K. Brief exposure to damaging light causes focal recruitment of macrophages, and long-term destabilization of photoreceptors in the albino rat retina. Current eye research. 2010;35:631-43
114. Ni YQ, Xu GZ, Hu WZ, Shi L, Qin YW, Da CD. Neuroprotective effects of naloxone against light-induced photoreceptor degeneration through inhibiting retinal microglial activation. Investigative ophthalmology & visual science. 2008;49:2589-98
115. Yang L, Kim JH, Kovacs KD, Arroyo JG, Chen DF. Minocycline inhibition of photoreceptor degeneration. Arch Ophthalmol. 2009;127:1475-80
116. Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl A. et al. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639-49
117. Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M. et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439-44
118. Heinig N, Schumann U, Calzia D, Panfoli I, Ader M, Schmidt MHH. et al. Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration. Int J Mol Sci. 2020 21
119. Albarracin R, Eells J, Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Investigative ophthalmology & visual science. 2011;52:3582-92
120. Ying R, Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain Res. 2008;1243:167-73
121. Kokkinopoulos I, Colman A, Hogg C, Heckenlively J, Jeffery G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol Aging. 2013;34:602-9
122. Del Olmo-Aguado S, Nunez-Alvarez C, Osborne NN. Red light of the visual spectrum attenuates cell death in culture and retinal ganglion cell death in situ. Acta Ophthalmol. 2016;94:e481-91
123. Sivapathasuntharam C, Sivaprasad S, Hogg C, Jeffery G. Aging retinal function is improved by near infrared light (670 nm) that is associated with corrected mitochondrial decline. Neurobiol Aging. 2017;52:66-70
124. Kokkinopoulos I. 670 nm LED ameliorates inflammation in the CFH(-/-) mouse neural retina. J Photochem Photobiol B. 2013;122:24-31
125. Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PloS one. 2013;8:e57828
126. Kaynezhad P, Tachtsidis I, Jeffery G. Optical monitoring of retinal respiration in real time: 670 nm light increases the redox state of mitochondria. Experimental eye research. 2016;152:88-93
127. Albarracin R, Natoli R, Rutar M, Valter K, Provis J. 670 nm light mitigates oxygen-induced degeneration in C57BL/6J mouse retina. BMC Neurosci. 2013;14:125
128. Rutar M, Natoli R, Albarracin R, Valter K, Provis J. 670-nm light treatment reduces complement propagation following retinal degeneration. Journal of neuroinflammation. 2012;9:257
129. Natoli R, Valter K, Barbosa M, Dahlstrom J, Rutar M, Kent A. et al. 670nm photobiomodulation as a novel protection against retinopathy of prematurity: evidence from oxygen induced retinopathy models. PloS one. 2013;8:e72135
130. Natoli R, Zhu Y, Valter K, Bisti S, Eells J, Stone J. Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol Vis. 2010;16:1801-22
131. Karu TI, Afanas'eva NI. [Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light]. Dokl Akad Nauk. 1995;342:693-5
132. Silveira PC, Streck EL, Pinho RA. Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol B. 2007;86:279-82
133. Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1-17
134. Gkotsi D, Begum R, Salt T, Lascaratos G, Hogg C, Chau KY. et al. Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Experimental eye research. 2014;122:50-3
135. Iglesias-Rey R, Castillo J. New strategies for ischemic stroke: internal photobiomodulation therapy. Neural Regen Res. 2020;15:1658-9
136. Casalechi HL, Dumont AJL, Ferreira LAB, de Paiva PRV, Machado C, de Carvalho PTC. et al. Acute effects of photobiomodulation therapy and magnetic field on functional mobility in stroke survivors: a randomized, sham-controlled, triple-blind, crossover, clinical trial. Lasers Med Sci. 2020;35:1253-1262
137. Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516-33
138. Finsterer J, Zarrouk-Mahjoub S. Treatment of muscle weakness in neuromuscular disorders. Expert Rev Neurother. 2016;16:1383-95
139. Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-92
140. Zamani ARN, Saberianpour S, Geranmayeh MH, Bani F, Haghighi L, Rahbarghazi R. Modulatory effect of photobiomodulation on stem cell epigenetic memory: a highlight on differentiation capacity. Lasers Med Sci. 2020;35:299-306
141. Maruani J, Geoffroy PA. Bright Light as a Personalized Precision Treatment of Mood Disorders. Front Psychiatry. 2019;10:85
142. Kubota S, Yang JT. Bis[cyclo(histidylhistidine)]copper(II) complex that mimicks the active center of superoxide dismutase has its catalytic activity. Proc Natl Acad Sci U S A. 1984;81:3283-6
143. Greco M, Guida G, Perlino E, Marra E, Quagliariello E. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-34
144. Karu T. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol B. 1989;3:638-40
145. Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol. 2000;76:863-70
146. Yamanaka T, Fukumori Y, Numata M, Yamazaki T. The variety of molecular properties of bacterial cytochromes containing heme a. Ann N Y Acad Sci. 1988;550:39-46
147. Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99-116
148. Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015-69
149. Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res Brain Res Rev. 2005;48:1-15
150. Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends in neurosciences. 1989;12:94-101
151. Karu TI, Pyatibrat LV, Kalendo GS, Esenaliev RO. Effects of monochromatic low-intensity light and laser irradiation on adhesion of HeLa cells in vitro. Lasers Surg Med. 1996;18:171-7
152. Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of cell monolayers relevant to mechanisms of laser phototherapy: reduction or oxidation of cytochrome c oxidase under laser radiation at 632.8 nm. Photomed Laser Surg. 2008;26:593-9
153. Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Experimental eye research. 2012;103:33-40
154. Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Experimental eye research. 2013;114:106-19
155. Zhou X, Pardue MT, Iuvone PM, Qu J. Dopamine signaling and myopia development: What are the key challenges. Prog Retin Eye Res. 2017;61:60-71
156. Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Experimental eye research. 2009;88:1092-9
157. Nickla DL, Wildsoet CF. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci. 2004;81:111-8
158. Sekaran S, Cunningham J, Neal MJ, Hartell NA, Djamgoz MB. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. The European journal of neuroscience. 2005;21:2199-208
159. Wang M, Schaeffel F, Jiang B, Feldkaemper M. Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Investigative ophthalmology & visual science. 2018;59:4413-24
160. Burfield HJ, Patel NB, Ostrin LA. Ocular Biometric Diurnal Rhythms in Emmetropic and Myopic Adults. Investigative ophthalmology & visual science. 2018;59:5176-87
161. Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Experimental eye research. 2013;114:35-47
162. Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom. 2019;102:99-108
163. Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Investigative ophthalmology & visual science. 2011;52:5121-9
164. Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:399-407
165. Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Experimental eye research. 2012;103:47-54
166. Carr BJ, Stell WK. Nitric Oxide (NO) Mediates the Inhibition of Form-Deprivation Myopia by Atropine in Chicks. Sci Rep. 2016;6:9
167. Mathis U, Feldkaemper M, Wang M, Schaeffel F. Studies on retinal mechanisms possibly related to myopia inhibition by atropine in the chicken. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020;258:319-33
168. Wu J, Liu Q, Yang X, Yang H, Wang XM, Zeng JW. Time-course of changes to nitric oxide signaling pathways in form-deprivation myopia in guinea pigs. Brain Res. 2007;1186:155-63
169. Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol. 2009;47:256-63
170. Shiva S, Gladwin MT. Shining a light on tissue NO stores: near infrared release of NO from nitrite and nitrosylated hemes. J Mol Cell Cardiol. 2009;46:1-3
171. Kosaka H. Nitric oxide and hemoglobin interactions in the vasculature. Biochim Biophys Acta. 1999;1411:370-7
172. Zhang R, Mio Y, Pratt PF, Lohr N, Warltier DC, Whelan HT. et al. Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J Mol Cell Cardiol. 2009;46:4-14
173. Quirk BJ, Whelan HT. What Lies at the Heart of Photobiomodulation: Light, Cytochrome C Oxidase, and Nitric Oxide-Review of the Evidence. Photobiomodul Photomed Laser Surg. 2020;38:527-30
174. Watson SA, McStay GP. Functions of Cytochrome c oxidase Assembly Factors. Int J Mol Sci. 2020 21
175. Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 2003;120:849-57
176. Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84:1091-9
177. Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358-83
178. Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602-18
179. Leube A, Kostial S, Alex Ochakovski G, Ohlendorf A, Wahl S. Symmetric visual response to positive and negative induced spherical defocus under monochromatic light conditions. Vision Res. 2018;143:52-7
180. Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg. 2010;28:159-60
181. Mokoena D, Dhilip Kumar SS, Houreld NN, Abrahamse H. Role of photobiomodulation on the activation of the Smad pathway via TGF-beta in wound healing. J Photochem Photobiol B. 2018;189:138-44
182. Mokoena DR, Houreld NN, Dhilip Kumar SS, Abrahamse H. Photobiomodulation at 660 nm Stimulates Fibroblast Differentiation. Lasers Surg Med. 2020;52:671-81
183. Otterco AN, Andrade AL, Brassolatti P, Pinto KNZ, Araujo HSS, Parizotto NA. Photobiomodulation mechanisms in the kinetics of the wound healing process in rats. J Photochem Photobiol B. 2018;183:22-9
184. Clarke G, Lumsden CJ, McInnes RR. Inherited neurodegenerative diseases: the one-hit model of neurodegeneration. Hum Mol Genet. 2001;10:2269-75
185. Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B. et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke. 2007;38:1843-9
186. Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg. 2001;19:29-33
187. Garbuzova-Davis S, Willing AE, Saporta S, Bickford PC, Gemma C, Chen N. et al. Novel cell therapy approaches for brain repair. Prog Brain Res. 2006;157:207-22
188. Ilic S, Leichliter S, Streeter J, Oron A, DeTaboada L, Oron U. Effects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brain. Photomed Laser Surg. 2006;24:458-66
189. Oron U, Yaakobi T, Oron A, Mordechovitz D, Shofti R, Hayam G. et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103:296-301
190. Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O. et al. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg Med. 2001;28:204-11
191. Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT. et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21:67-74
192. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-6
193. Beirne K, Rozanowska M, Votruba M. Photostimulation of mitochondria as a treatment for retinal neurodegeneration. Mitochondrion. 2017;36:85-95
194. Peat FJ, Colbath AC, Bentsen LM, Goodrich LR, King MR. In Vitro Effects of High-Intensity Laser Photobiomodulation on Equine Bone Marrow-Derived Mesenchymal Stem Cell Viability and Cytokine Expression. Photomed Laser Surg. 2018;36:83-91
195. Morton CA, McKenna KE, Rhodes LE, British Association of Dermatologists Therapy G, Audit S, the British Photodermatology G. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-66
196. Fork RL. Laser stimulation of nerve cells in Aplysia. Science. 1971;171:907-8
197. Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC ophthalmology. 2020;20:27
198. Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983;101:405-7
199. Lin LL, Chen CJ, Hung PT, Ko LS. Nation-wide survey of myopia among schoolchildren in Taiwan, 1986. Acta Ophthalmol Suppl. 1988;185:29-33
200. Quek TP, Chua CG, Chong CS, Chong JH, Hey HW, Lee J. et al. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists). 2004;24:47-55
201. Vitale S, Sperduto RD, Ferris FL 3rd. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127:1632-9
202. Benavente-Perez A, Nour A, Troilo D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Investigative ophthalmology & visual science. 2012;53:6479-87
203. Dolgin E. The myopia boom. Nature. 2015;519:276-8
204. Tran HDM, Tran YH, Tran TD, Jong M, Coroneo M, Sankaridurg P. A Review of Myopia Control with Atropine. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2018;34:374-9
205. Burton TC. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc. 1989;87:143-55 discussion 55-7
206. Zadnik K. It's the retina, stupid. Optom Vis Sci. 2001;78:179-80
207. Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109:704-11
208. Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists). 2005;25:381-91
209. Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Investigative ophthalmology & visual science. 2007;48:3524-32
210. Xiong R, Zhu Z, Jiang Y, Wang W, Zhang J, Chen Y. et al. Longitudinal Changes and Predictive Value of Choroidal Thickness for Myopia Control after Repeated Low-Level Red-Light Therapy. Ophthalmology. 2023;130:286-96
211. Liu G, Li B, Rong H, Du B, Wang B, Hu J. et al. Axial Length Shortening and Choroid Thickening in Myopic Adults Treated with Repeated Low-Level Red Light. J Clin Med. 2022 11
212. Hung LF, Arumugam B, She Z, Ostrin L, Smith EL 3rd. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental eye research. 2018;176:147-60
213. Yam JC, Li FF, Zhang X, Tang SM, Yip BHK, Kam KW. et al. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology. 2020;127:910-9
214. Zhu Q, Tang Y, Guo L, Tighe S, Zhou Y, Zhang X. et al. Efficacy and Safety of 1% Atropine on Retardation of Moderate Myopia Progression in Chinese School Children. Int J Med Sci. 2020;17:176-81
215. Zhu Q, Tang GY, Hua ZJ, Xue LP, Zhou Y, Zhang JY. et al. 0.05% atropine on control of myopia progression in Chinese school children: a randomized 3-year clinical trial. Int J Ophthalmol. 2023;16:939-46
216. Zhu Q, Yin J, Li X, Hu M, Xue L, Zhang J. et al. Effects of Long-Term Wear and Discontinuation of Orthokeratology Lenses on the Eyeball Parameters in Children with Myopia. Int J Med Sci. 2023;20:50-6
217. Holden BA, Jong M, Davis S, Wilson D, Fricke T, Resnikoff S. Nearly 1 billion myopes at risk of myopia-related sight-threatening conditions by 2050 - time to act now. Clin Exp Optom. 2015;98:491-3
218. Dong J, Zhu Z, Xu H, He M. Myopia Control Effect of Repeated Low-Level Red-Light Therapy in Chinese Children: A Randomized, Double-Blind, Controlled Clinical Trial. Ophthalmology. 2023;130:198-204
219. Zhou L, Xing C, Qiang W, Hua C, Tong L. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists). 2022;42:335-44
220. Xiong R, Zhu Z, Jiang Y, Kong X, Zhang J, Wang W. et al. Sustained and rebound effect of repeated low-level red-light therapy on myopia control: A 2-year post-trial follow-up study. Clinical & experimental ophthalmology. 2022;50:1013-1024
221. Lam CS, Tang WC, Lee PH, Zhang HY, Qi H, Hasegawa K. et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. Br J Ophthalmol. 2022;106:1110-4
222. Kang P, McAlinden C, Wildsoet CF. Effects of multifocal soft contact lenses used to slow myopia progression on quality of vision in young adults. Acta Ophthalmol. 2017;95:e43-e53
223. Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Investigative ophthalmology & visual science. 2012;53:7077-85
224. Sun Y, Xu F, Zhang T, Liu M, Wang D, Chen Y. et al. Orthokeratology to control myopia progression: a meta-analysis. PloS one. 2015;10:e0124535
225. Chan DK, Fung YK, Xing S, Hagger MS. Myopia prevention, near work, and visual acuity of college students: integrating the theory of planned behavior and self-determination theory. J Behav Med. 2014;37:369-80
226. Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J. et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95:551-66
227. Wang A, Yang C, Shen L, Wang J, Zhang Z, Yang W. Axial length shortening after orthokeratology and its relationship with myopic control. BMC ophthalmology. 2022;22:243
228. Walline JJ, Walker MK, Mutti DO, Jones-Jordan LA, Sinnott LT, Giannoni AG. et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA. 2020;324:571-80
229. Bao J, Yang A, Huang Y, Li X, Pan Y, Ding C. et al. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. Br J Ophthalmol. 2022;106:1171-1176
230. Wei S, Li SM, An W, Du J, Liang X, Sun Y. et al. Safety and Efficacy of Low-Dose Atropine Eyedrops for the Treatment of Myopia Progression in Chinese Children: A Randomized Clinical Trial. JAMA Ophthalmol. 2020;138:1178-84
231. Chen Y, Xiong R, Chen X, Zhang J, Bulloch G, Lin X. et al. Efficacy Comparison of Repeated Low-Level Red Light and Low-Dose Atropine for Myopia Control: A Randomized Controlled Trial. Translational vision science & technology. 2022;11:33
232. Chen H, Wang W, Liao Y, Zhou W, Li Q, Wang J. et al. Low-intensity red-light therapy in slowing myopic progression and the rebound effect after its cessation in Chinese children: a randomized controlled trial. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2023;261:575-84
233. Tian L, Cao K, Ma DL, Zhao SQ, Lu LX, Li A. et al. Investigation of the Efficacy and Safety of 650 nm Low-Level Red Light for Myopia Control in Children: A Randomized Controlled Trial. Ophthalmol Ther. 2022;11:2259-70
234. Yang W, Lin F, Li M, Wei R, Zhou J, Zhou X. Immediate effect in retina and choroid after 650 nm low-level red light therapy in children. Ophthalmic research. 2023;66:312-318
235. Wang W, Jiang Y, Zhu Z, Zhang S, Xuan M, Chen Y. et al. Clinically Significant Axial Shortening in Myopic Children After Repeated Low-Level Red Light Therapy: A Retrospective Multicenter Analysis. Ophthalmol Ther. 2023;12:999-1011
236. Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Investigative ophthalmology & visual science. 2013;54:8004-12
237. Wildsoet CF, Howland HC, Falconer S, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Res. 1993;33:1593-603
238. Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55-65
Author contact
![]() Corresponding authors: Liping Xue, Department of Pediatric Ophthalmology, the Affiliated Hospital of Yunnan University, Kunming 650021, China. Telephone: 0118613708499237; Fax: 011860871-65156650; E-mail: xueliping001com or Ying-Ting Zhu, Research and Development Department, BioTissue (TissueTech, Inc.), 7230 Corporate Center Drive, Suite B, Miami, FL 33126, USA. Telephone: (786) 456-7632; Fax: (305) 274-1297; E-mail: yzhucom
Corresponding authors: Liping Xue, Department of Pediatric Ophthalmology, the Affiliated Hospital of Yunnan University, Kunming 650021, China. Telephone: 0118613708499237; Fax: 011860871-65156650; E-mail: xueliping001com or Ying-Ting Zhu, Research and Development Department, BioTissue (TissueTech, Inc.), 7230 Corporate Center Drive, Suite B, Miami, FL 33126, USA. Telephone: (786) 456-7632; Fax: (305) 274-1297; E-mail: yzhucom

 Global reach, higher impact
Global reach, higher impact