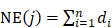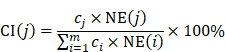3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(13):1824-1834. doi:10.7150/ijms.77451 This issue Cite
Research Paper
A study of the therapeutic mechanism of Jakyakgamcho-Tang about functional dyspepsia through network pharmacology research
1. Division of Longevity and Biofunctional Medicine, School of Korean Medicine, Pusan National University, Yangsan 50612, Republic of Korea.
2. Department of Food and Nutrition, College of BioNano Technology, Gachon University, Seongnam 13120, Republic of Korea.
*These authors contributed equally to this work.
Received 2022-7-25; Accepted 2022-10-5; Published 2022-10-17
Abstract
Herbal medicines have traditionally been used as an effective digestive medicine. However, compared to the effectiveness of Herbal medicines, the treatment mechanism has not been fully identified. To solve this problem, a system-level treatment mechanism of Jakyakgamcho-Tang (JGT), which is used for the treatment of functional dyspepsia (FD), was identified through a network pharmacology study. The two components, paeoniae radix alba and licorice constituting JGT were analyzed based on broad information on chemical and pharmacological properties, confirming 84 active chemical compounds and 84 FD-related targets. The JGT target confirmed the relationship with the regulation of various biological movements as follows: cellular behaviors of muscle and cytokine, calcium ion concentration and homeostasis, calcium- and cytokine-mediated signalings, drug, inflammatory response, neuronal cells, oxidative stress and response to chemical. And the target is enriched in variety FD-related signaling as follows: MAPK, Toll-like receptor, NOD-like receptor, PI3K-Akt, Apoptosis and TNF signaling pathway. These data give a new approach to identifying the molecular mechanisms underlying the digestive effect of JGT.
Keywords: Jakyakgamcho-Tang, Functional dyspepsia, Network pharmacology, Traditional medicine
Introduction
The characteristic of functional dyspepsia (FD) is clinical syndrome accompanied by long-term or repetitive upper abdominal pain and discomfort even without a fundamental organic disease that can be pointed out as the cause of symptoms [1]. Pharmacological treatment for functional dyspepsia is still insufficient [2]. Several trials to address this were generally disappointing, with small improvements in Helicobacter pylori eradication [3], proton-pump inhibitors [4], and histamine H2-receptor antagonists [5] compared to placebo. Furthermore, despite the poor effects, pharmacological preparations also pose a danger of side effects (e.g. cisapride).
Nevertheless, despite the perception that herbal medicines have few side effects and excellent effects on symptoms, the number of systematic clinical studies available has been limited due to the lack of standardization of herbal medicine ingredients.
Jakyakgamcho-Tang (JGT) is a widely prescribed spasmolytic in traditional chinese medicine, consisting of Radix Paeoniae and Radix Glycyrrhizae and, and of indicator substances such as albiflorin, benzoylpaeoniflorin, glycyrrhizin, isoliquiritin, liquiritigenin, and paeoniflorin. And it has the effect of skeletal muscle cramps caused by hemodialysis and nervous stimulation [6,7]. It is also reported that JGT is effective in relieving myodynia caused by chemotherapy or peripheral nerve damage, and can alleviate intestinal cramps through smooth muscle relaxation [8,9,10]. In particular, Radix Glycyrrhizae in JGT contains isoliquiritin and glycyrrhizin, which has a remarkable convulsive effect [11]. The muscle-relaxing effect of JGT through the interaction of complex constituents has not yet been clearly identified, but it has fewer side effects than the muscle relaxation effect of the muscle relaxants that directly acts on the central nervous system [11]. However, despite many of these clinical effects, understanding of mechanisms is still insufficient, requiring investigation of the pharmacological characteristics of JGT at the systemic level.
Because of the complex pharmacological properties of multi-compound multi-target multi-pathway preparations of traditional herb drugs, investigating a comprehensive mechanism of action with the existing biological experimental methodological approach has fundamental limitations [12-19]. With the rapid development of bioinformatics and systems biology, a new research method called network pharmacology has been proposed [20,21]. Network pharmacology collects information about biological systems that are discovered through systematic disturbances of biological systems into big data and classifies them into monitoring of gene, protein, and information pathway response. Then apply mathematical models to describe the system structure and its response to individual perturbations [22]. Unlike existing pharmacology research strategies based on 'one target, single drug', network pharmacology describes the complexity between among biological systems, drugs and diseases from a network perspective, which shares a holistic philosophy with traditional chinese Medicine [23]. Through this, the mechanism of disease and the mechanism of medicine activity are identified through interaction between the constituents of drug and genes, and between genes and disease-related factors [12-19]. Until now, these network pharmacology characteristics have wonderfully investigated the poly-pharmacological characteristics of traditional medicines by suggesting that herbal medicines have pharmacological mechanisms and effects (e.g., therapeutic adjustment of biological procedure such as angiogenesis, cell cycle regulation, apoptosis, inflammation, insulin metabolism, reduction, oxidation and proliferation) through interactions between compounds and targets for medical cure of variety diseases, including arthritis, diabetes, ischemic stroke and cancer [12-19,24-34]. In addition, the components of JGT were analyzed through a network pharmacology study, and the therapeutic effects for antidepressants, respiratory diseases, and chronic gastritis was confirmed [35-37]. Through this network pharmacology study, we try for identify from a system perspective the therapeutic mechanisms underlying the characteristics of digestive properties of JGT.
Materials and methods
Sorting of active chemical compounds in Jakyakgamcho-Tang
In order to know the herbal components constituting JGT, information on chemical compounds were searched using the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database [38]. And then, TCMSP was used to screen compounds that met previously proposed criteria [70,87,88] based on ADME properties (absorption, distribution, metabolism and excretion) (i.e., oral bioavailability (OB), Caco-2 permeability, and drug-likeness (DL)) [38]: OB ≥ 30%, Caco-2 permeability ≥ -0.4, and DL ≥ 0.18. Among these, OB, one of the most important considerations in the design and development of drugs, refers to the ratio of oral administered drug compounds to general circulation [38,39]. In general, OB is 30% or more as a standard for effectively absorbing compounds into the living body [38,39]. Caco-2 permeability is an essential indicator of intestinal permeability and medicine efflux and is based on the assessment of rate of the absorption and diffusion of compounds through Caco-2 human intestinal cells [38,40-42]. The Caco-2 permeability criterion is -0.4, and if it is lower than that, the compound is regarded to be non-permeable in the intestinal epithelium [43,44]. DL is a criterion for qualitatively evaluating which specific compounds are structurally and physicochemically suitable for use as a medicine [38,45]. As an indicator for active compounds, DL is based on 0.18 or higher, which is usually used as the limitation for determining the pharmacological potential of the compound because the average DL of all medicine is 0.18 [38,45].
Target identification
Target information of the compound was found by searching for TCMSP [38]. The target proteins were linked to the official gene name through the UniProtKB database [46].
Functional enrichment analysis
For perform gene ontology (GO) enrichment analysis, the target gene list was entered into query of g:Profiler and analyzed [47]. Pathway enrichment analysis was achieved using Kyoto Encyclopedia of Genes and Genomes (KEGG) database [48]. Functional association analysis of the target gene list was performed through GeneMANIA [49].
Construction of network
First, herbal medicine-active chemical compound (H-C) network was created from the herbal medicines with their active chemical compounds, and the active chemical compound-target (C-T) network was created from the compounds with their targets, finally the target-pathway (T-P) network by connecting the targets with the signaling pathways in which they are enriched. In addition, the Protein-Protein Interaction (PPI) network was created by applying the target gene to the STRING database (interaction confidence score ≥ 0.9) [50]. A network consists of nodes and edges that describe the interactions between nodes [51]. And the degree is defined as the number of edges on the node [51]. The analysis and visualization of the created network was carried out using Cytoscape software [52].
Contribution index evaluation
In order to evaluate the network- and efficacy-based contribution index (CI) about active chemical compounds of JGT, it was calculated as follows according to the previous procedure [53]:
where, m represents the number of compounds, n represents the number of targets of chemical compound j, di represents the number of links of target i of chemical compound j, and ci (or cj) represents the number of previous studies in which “functional dyspepsia” and compound i (or j) are included in the title or abstract from the PubMed database. (https://pubmed.ncbi.nlm.nih.gov/). The chemical compounds with the highest CIs were considered to take on more important role in the pharmacological activity of the herbal medicine [53].
Results
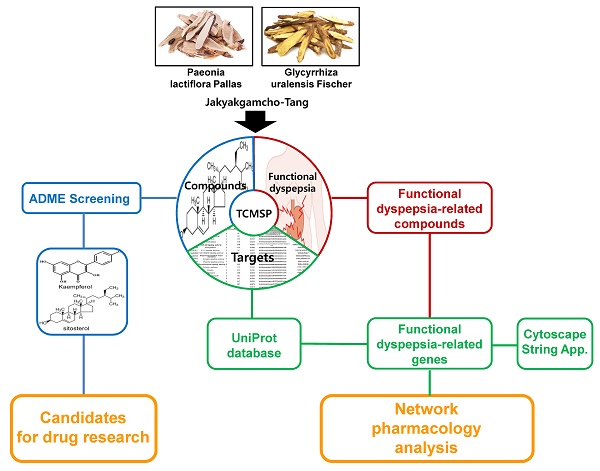
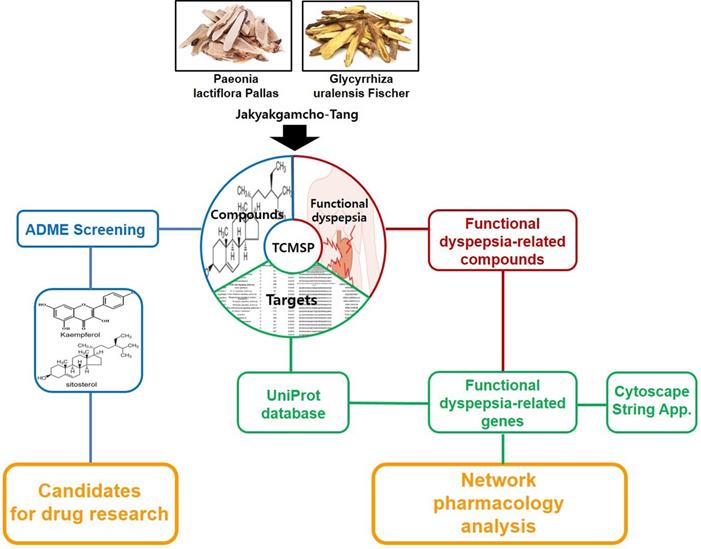
The network pharmacology study about mechanism of JGT was carried out as follows (Figure 1). Using TCMSP, the chemical components about the two main herbs of JGT were analyzed to investigate bioactive compounds according to the ADME characteristics and identify targets of active compounds. Then, extensive herbal medicine-related data was integrated into the network and network pharmacology analysis was performed (Figure 1).
The Schematic diagram of pharmacological network of the digestive mechanisms of Jakyakgamcho-Tang.
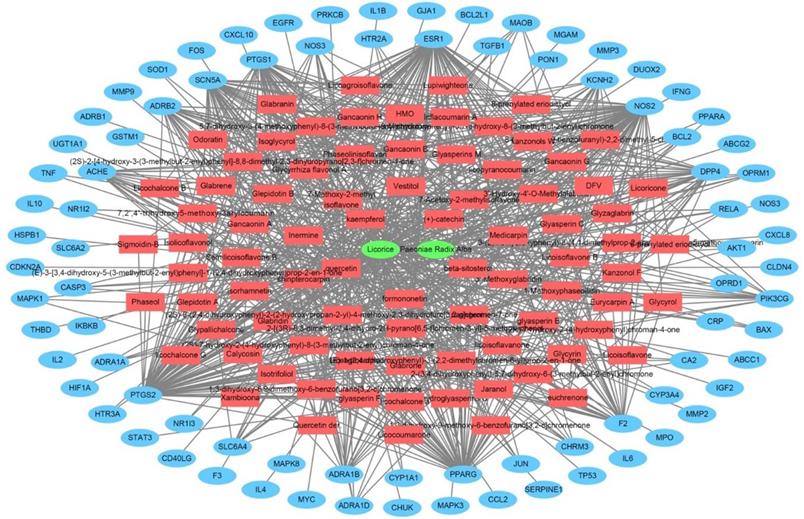
The herbal medicine-active chemical compound-target network of Jakyakgamcho-Tang. Green nodes are herbal medicines; red nodes are active chemical compounds; blue nodes are FD-related targets.
Total chemical compounds and active chemical compounds of Jakyakgamcho-Tang
For comprehensive information of compounds constituting JGT, was acquired through TCMSP [38] (Supplementary Table S1), of which compounds corresponding to with OB ≥ 30%, Caco-2 permeability ≥ -0.4, and DL ≥ 0.18 were defined as active compounds [13,38,53]. As a result, it was confirmed that 94 active compounds are present in JGT (Supplementary Table S2).
Targets of Jakyakgamcho-Tang
We used the TCMSP database to analyze the targets about the active chemical compounds of JGT [38], confirming that there were 73 human FD-associated and 146 non FD-associated targets for JGT (Supplementary Table S3).
Network pharmacology-based analysis of Jakyakgamcho-Tang
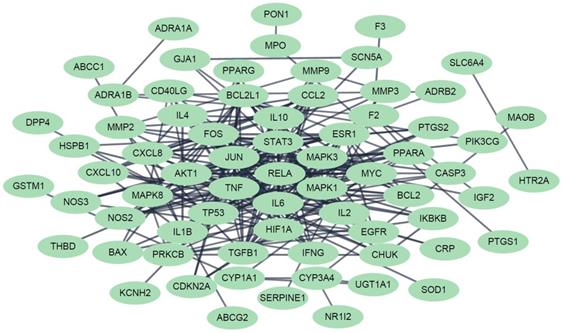
In order to perform a network pharmacological analysis about the pharmacological characteristics of JGT, an herbal medicine-active chemical compound-target (H-C-T) network consisting of 170 nodes (herbal medicine = 2, active chemical compound = 84, FD-associated target = 84) and 779 edges (Figure 2 and Supplementary Table S3) was constructed using detailed information on herbs. Among the active compounds, quercetin (target number = 63) and kaempferol (target number = 26) were found as compounds with comparatively many targets (Figure 2 and Supplementary Table S3), indicating that they are active compounds more important for the therapeutic effect of JGT. And it found that there are 42 genes/proteins targeted by two or more active chemical compounds out of all related genes/proteins (Figure 2), indicating that the poly-pharmacological mechanism works. JGT has many targets and the interactions between them play an important role in biological function and therapeutic potential, so to investigate this, we created a PPI network (70 nodes and 265 links) composed of targets of JGT (Figure 3) [54,55]. And the degree as a hub was confirmed in the revealed network, we found a specific node with a high degree that has more than twice the average degree as a hub that was found based on the criteria presented in existing network pharmacology studies [56,57]. Through the analysis results, it was showed that RELA (degree = 26), JUN (degree = 24), STAT3 (degree = 24), MAPK3 (degree = 22), TNF (degree = 21), MAPK1 (degree = 21), IL6 (degree = 21), TP53 (degree = 19) and AKT1 (degree = 19) act as a hubs (Figure 3), and it was found that they were the main targets related to the digestive activity of JGT. These herbs can be used as potent targets to induce digestive effects by engaging in the regulation of FD-associated processes. NF-kappa-B is a protein formed by binding Rel-like domain-containing proteins NFKB1/p50, NFKB1/p105, NFKB2/p52, REL, RELB and RELA/p65, and is divided into homo- or heterodimeric complex according to binding combinations, of which heterodimeric p65-p50 complex is most abundant. NF-kappa-B exists in almost all cell types and is a pleiotropic transcription factor, initiated by a wide range of stimuli associated to many biological processes such as apoptosis, cell growth, differentiation, immunity, inflammation and tumorigenesis, and corresponds to the endpoint of sign transduction events. This wide range of effects is also expected to affect FD [58]. STAT3 acts as a ground-state transcription factor and plays a role about cell proliferation in many types of cells. Cytokines or growth factors such as interleukin (IL)-6 increased by the activation of STAT3 promote stem cell self-regeneration, cell proliferation, and Th17 cell differentiation. As a result of GO analysis (Biological Process), it was confirmed that STAT3 was also related to eating behavior [59,60]. MAPK1 and MAPK3 are members of the MAP kinase family. MAP kinases receive variety extracellular signals to regulate variety cell processes such as cell cycle progression, differentiation and proliferation. In particular, MAPK1 and MAPK3 play an essential role in the MAPK signal pathway. By suppressing the MAPK pathway, it can increase intestinal moisture and promote intestinal peristalsis [61]. IL-6 is a powerful inducer for acute reactions and is involved in monocyte and lymphocyte differentiation, and in particular, plays an important role about the final differentiation of B-cells into IL-secreting cells [62]. Studies on experimental animals have shown that IL-6 plays a partial role in inhibiting food intake and weight due to glucagon-like peptide-1 (GLP-1) receptor stimulation in Central nervous system [63]. TNF is an adipokine and a cytokine. TNF is secreted from macrophages and induces cell death of certain cancer cell lines. It is a powerful pyrogen of heat that causes fever via stimulation of interleukin-1 secretion or direct action by itself, and also plays a role in inducing cachexia. Certain conditions may induce cell proliferation or cell differentiation [64]. It is also polyhedral as another feature of TNF and plays an essential role in controlling immunity, metabolism, and food intake [65,66,67]. AKT1 is one of the three components (AKT1, 2, 3) that make up AKT kinase and is involved in many processes, including cell survival, angiogenesis, growth, metabolism and proliferation [68]. AKT controls glucose uptake by intervening the insulin-induced translocation of SLC2A4/GLUT4 glucose transporter in the cell surface [69].
For a more reliable evaluation, we evaluated the pharmacological contribution to the digestive effect of herbal medicines by measuring the CI of active compounds of JGT [53,70]. As a result of the evaluation, it was confirmed that naringenin has the highest CI at 98.94%. Naringenin is a compound contained in licorice and is associated with ABCC1, AKT1, BCL2, CASP3, ESR1, MAPK1, MAPK3, PIK3CG, PPARA, PPARG, PTGS1, PTGS2, RELA, SOD1 and UGT1A1 (Figure 2). It was found that the compound contributed significantly to the digestive activity of JGT.
Together these results, we were able to confirm the system-level pharmacological Characteristics of the digestive effect of JGT.
The protein-protein interaction network for the FD-related targets of Jakyakgamcho-Tang. Green nodes are FD-related targets.
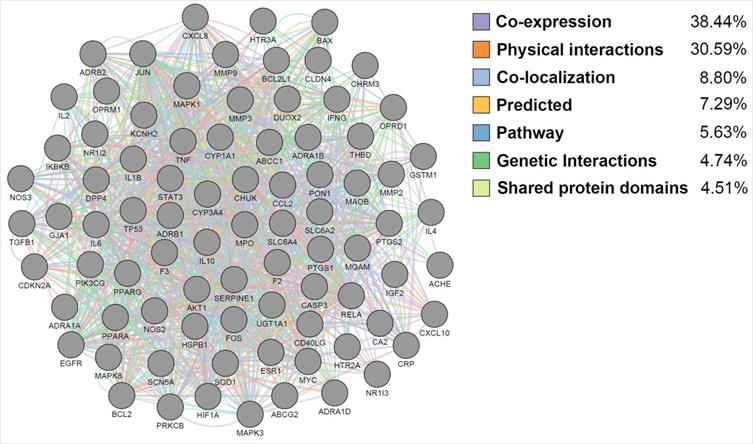
Functional interaction analysis of the FD-related targets of Jakyakgamcho-Tang. Gray nodes are FD-associated targets; colored edges are mechanisms of the function interactions between the targets.
Functional enrichment investigation about networks of Jakyakgamcho-Tang
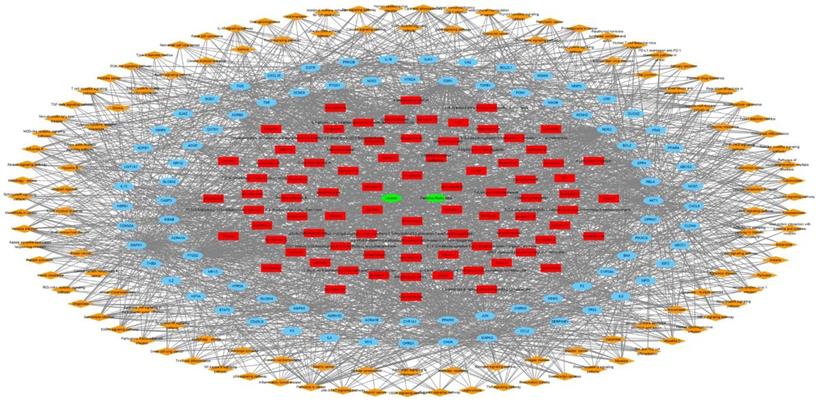
The targets of JTG were analyzed using GO enrichment analysis, a method that has been recently spotlighted in investigating molecular mechanisms. The results were concentrated in GO terms related to regulation of various biological activities, including cellular behaviors of muscle and cytokine, calcium ion concentration and homeostasis, calcium- and cytokine-mediated signalings, drug, inflammatory response, neuronal cells, oxidative stress and response to chemical (Supplementary Table S4). And the results are matched with previously reported therapeutic mechanisms of herbal medicines [71,72,8,73,74,75,76,77-80]. Furthermore, GeneMANIA analysis showed that JGT targets interact with each other and function through various mechanisms (Figure 4), suggesting the similarity in pharmacological roles. Since it was known that various signaling pathways were related to FD [81-86], we performed pathway enrichment analysis and found following signalings rich in targets of JGT (Figure 5 and Supplementary Table S4). These signalings, which contain well-known digestion-related pathways, can be used as therapeutic targets for alleviating FD. Human T-cell leukemia virus 1 infection, Shigellosis, Yersinia infection, Epstein-Barr virus infection, Salmonella infection, Pathogenic Escherichia coli infection, Human immunodeficiency virus 1 infection, Pancreatic cancer, Colorectal cancer, Hepatocellular carcinoma, Amoebiasis, Inflammatory bowel disease, Gastric cancer, Malaria, Chronic myeloid leukemia, Graft-versus-host disease, Renal cell carcinoma and Type I diabetes mellitus are diseases accompanied by dyspepsia [87-104]. Apoptosis, TNF signaling pathway, MAPK signaling pathway, PI3K-Akt signaling pathway, NOD-like receptor signaling pathway, Chemokine signaling pathway, Calcium signaling pathway, NF-kappa B signaling pathway, cAMP signaling pathway, Toll-like receptor signaling pathway, Neurotrophin signaling pathway, Thyroid hormone signaling pathway, Autophagy, Growth hormone synthesis, secretion and action, Gap junction, Salivary secretion and p53 signaling pathway are associated with functions related to digestion, which are involved in the occurrence of FD, and functional regulation can alleviate FD [105-117]. Totally, this result confirmed the pathway- and molecular-level mechanisms supporting the therapeutic activity of JGT.
Discussion
Traditionally used herbal medicine has been used as an effective digestive medicine with excellent therapeutic activity as an alternative to existing medicines under the general recognition that they have fewer side effects. JGT is also a well-known herbal medicine prescribed to relieve stomach cramps and indigestion [8-10]. Therefore, network pharmacology research was conducted to identify the therapeutic mechanisms underlying the digestive effect of JGT. First, 84 active chemical compounds of JGT and 84 FD-associated human molecular targets were identified through network pharmacology investigation and ADME evaluation. And enrichment analysis confirmed that the target of JGT was related with the regulation of biological activity, including involving cellular behaviors of muscle and cytokine, calcium ion concentration and homeostasis, calcium- and cytokine-mediated signalings, drug, inflammatory response, neuronal cells, oxidative stress and response to chemical, coincidental with previously reported therapeutic mechanisms of herbal medicines [71,72,8,73,74,75,76,77-80]. We further revealed that JGT might target variety digestion signalings to exert its dyspepsia-relieving activity, which involve Apoptosis, MAPK, TNF, PI3K-Akt, Toll-like receptor signaling pathway and NOD-like receptor.
The herbal medicine - active chemical compound - target - pathway network of Jakyakgamcho-Tang. Green nodes are herbal medicines; red nodes are active chemical compounds; blue nodes are FD-related targets; orange nodes are signaling pathways.
Contribution index analysis for the digestive effect of active chemical compounds of Jakyakgamcho-Tang. A graph depicting the analysis result of contribution index (CI) for the digestive effect of active chemical compounds of JGT. The CI of naringenin was found to be higher than 98%. Note that the CIs of compounds that are not present in the graph is '0'.
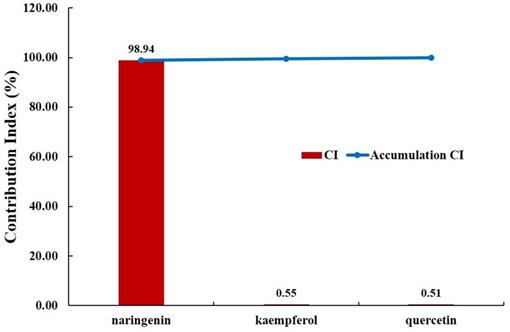
There have been reports that chemical components of JGT contribute to digestive activity. quercetin promotes protein absorption through the internalization of digested oligopeptides in the intestinal epithelium Rather than increasing intracellular permeability [118]. Kaempferol has been studied to have a positive effect on anti-peptic ulcer activity [119] Medicarpin act on DRD1 receptor. DRD1 is a kind of dopamine receptor and is widely distributed in the enteric nervous system such as colon, gastroesophageal junction, pylorus, small intestine and stomach [120]. Dopamine, the substrate of DRD1, weakens human gastric motility and pressure. Thus, DA antagonists such as domperidone can improve GI mobility. Actually, DA receptors regulate gastric smooth muscle cell response through two actions: relaxation of the longitudinal smooth muscle layer and/or contraction of the circular smooth muscle layer [121]. Naringenin increase the factors related to gastrointestinal movement, ICC markers (c-Kit and SCF) and AQP3 [122]. It alleviates dyspeptic symptoms through increasing gastrointestinal motility [123]. Especially, since naringenin has the highest CI (98.94%), JGT suggested that naringenin induces an increase in gastrointestinal movement factors, ICC markers (c-kit and SCF) and AQP3 to increase gastrointestinal motility in relieving FD. It is thought to play a key role in alleviating FD (Figure 6).
This study through network pharmacological analysis would provide to the following improvements in herbal medicine treatments: (i) Evaluation of the therapeutic efficacy of JGT digestive activity against FD; and (ii) Comprehensive exploration of various pharmacological perspectives on the system-level mechanisms of herbal medicine digestive characteristics.
In conclusion, we researched the pharmacological characteristics of JGT from a system perspective, a widely prescribed herbal medicine for alleviate FD. JGT was analyzed based on a network pharmacological approach to find 84 active chemical compounds and their 84 FD-related targets related to the digestion effect of JGT. Targets of JGT were related with the regulation of biological functions such as cellular behaviors of muscle and cytokine, calcium ion concentration and homeostasis, calcium- and cytokine-mediated signalings, drug, inflammatory response, neuronal cells, oxidative stress and response to chemical, indicating system-level treatment mechanism of JGT therapies. Furthermore, through enrichment analysis, we found that the targets of JGT play a role in various pathways related to the pathophysiology of digestion, such as Apoptosis, MAPK, TNF, PI3K-Akt, Toll-like receptor signaling pathway and NOD-like receptor. Totally, a novel systematic approach to the poly-pharmacological properties of JGT was provided, and the mechanistic basis for the clinical significance of JGT in the treatment of FD was confirmed.
Supplementary Material
Supplementary tables.
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A3042479).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Clouse RE, Mayer EA, Aziz Q. et al. Functional abdominal pain syndrome. Gastroenterology. 2006;130(5):1492-1497
2. Holtmann G, Talley NJ. Functional dyspepsia. Current treatment recommendations. Drugs. 1993;45(6):918-930
3. Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with nonulcer dyspepsia. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;134(5):361-369
4. Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127(5):1329-1337
5. Talley NJ, McNeil D, Hayden A, Piper DW. Randomized, double-blind, placebo-controlled crossover trial of cimetidine and pirenzepine in nonulcer dyspepsia. Gastroenterology. 1986;91(1):149-156
6. Dezaki K, Kimura I, Miyahara K, Kimura M. Complementary effects of paeoniflorin and glycyrrhizin on intracellular Ca2+ mobilization in the nerve-stimulated skeletal muscle of mice. Jpn J Pharmacol. 1995;69(3):281-284
7. Hyodo T, Taira T, Takemura T. et al. Immediate effect of Shakuyaku-kanzo-to on muscle cramp in hemodialysis patients. Nephron Clin Pract. 2006;104(1):c28-c32
8. Tsuji S, Yasuda K, Sumi G. et al. Shakuyaku-kanzo-to inhibits smooth muscle contractions of human pregnant uterine tissue in vitro. J Obstet Gynaecol Res. 2012;38(7):1004-1010
9. Yamamoto K, Hoshiai H, Noda K. Effects of shakuyaku-kanzo-to on muscle pain from combination chemotherapy with paclitaxel and carboplatin. Gynecol Oncol. 2001;81(2):333-334
10. Yoshida T, Sawa T, Ishiguro T, Horiba A, Minatoguchi S, Fujiwara H. The efficacy of prophylactic Shakuyaku-Kanzo-to for myalgia and arthralgia following carboplatin and paclitaxel combination chemotherapy for non-small cell lung cancer. Support Care Cancer. 2009;17(3):315-320
11. Lee KK, Omiya Y, Yuzurihara M, Kase Y, Kobayashi H. Antispasmodic effect of shakuyakukanzoto extract on experimental muscle cramps in vivo: role of the active constituents of Glycyrrhizae radix [published correction appears in J Ethnopharmacol. 2013 Mar 7;146(1):431]. J Ethnopharmacol. 2013;145(1):286-293
12. Wang Y, Dong B, Xue W. et al. Anticancer Effect of Radix Astragali on Cholangiocarcinoma In vitro and Its Mechanism via Network Pharmacology. Med Sci Monit. 2020;26:e921162. Published 2020 Apr 4
13. Lee HS, Lee IH, Park SI, Lee DY. Network Pharmacology-Based Investigation of the System-Level Molecular Mechanisms of the Hematopoietic Activity of Samul-Tang, a Traditional Korean Herbal Formula. Evid Based Complement Alternat Med. 2020;2020:9048089. Published 2020 Feb 13
14. Lee WY, Lee CY, Kim YS, Kim CE. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules. 2019;9(8):362. Published 2019 Aug 13
15. Xu T, Wang Q, Liu M. A Network Pharmacology Approach to Explore the Potential Mechanisms of Huangqin-Baishao Herb Pair in Treatment of Cancer. Med Sci Monit. 2020;26:e923199. Published 2020 Jul 1
16. Zhang SQ, Xu HB, Zhang SJ, Li XY. Identification of the Active Compounds and Significant Pathways of Artemisia Annua in the Treatment of Non-Small Cell Lung Carcinoma based on Network Pharmacology. Med Sci Monit. 2020;26:e923624. Published 2020 May 31
17. Poornima P, Kumar JD, Zhao Q, Blunder M, Efferth T. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol Res. 2016;111:290-302
18. Mi JL, Liu C, Xu M, Wang RS. Network Pharmacology to Uncover the Molecular Mechanisms of Action of LeiGongTeng for the Treatment of Nasopharyngeal Carcinoma. Med Sci Monit Basic Res. 2020;26:e923431. Published 2020 May 25
19. He R, Ou S, Chen S, Ding S. Network Pharmacology-Based Study on the Molecular Biological Mechanism of Action for Compound Kushen Injection in Anti-Cancer Effect. Med Sci Monit. 2020;26:e918520. Published 2020 Jan 1
20. Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110-1111 Published 2007 Oct 25
21. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682-690 Published 2008 Nov 4
22. Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annu Rev Genomics Hum Genet. 2001;2:343-372 Published 2001 Feb 1
23. Zhang R, Zhu X, Bai H, Ning K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front Pharmacol. 2019;10:123. Published 2019 Feb 21
24. Hu Z, Yang M, Yang L. et al. Network Pharmacology-Based Identification of the Mechanisms of Shen-Qi Compound Formula in Treating Diabetes Mellitus. Evid Based Complement Alternat Med. 2020;2020:5798764. Published 2020 Jun 4
25. Jiang Y, Zhong M, Long F, Yang R. Deciphering the Active Ingredients and Molecular Mechanisms of Tripterygium hypoglaucum (Levl.) Hutch against Rheumatoid Arthritis Based on Network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:2361865. Published 2020 Jan 13
26. Li DH, Su YF, Sun CX, Fan HF, Gao WJ. A Network Pharmacology-Based Identification Study on the Mechanism of Xiao-Xu-Ming Decoction for Cerebral Ischemic Stroke. Evid Based Complement Alternat Med. 2020;2020:2507074. Published 2020 Oct 19
27. Liu W, Fan Y, Tian C. et al. Deciphering the Molecular Targets and Mechanisms of HGWD in the Treatment of Rheumatoid Arthritis via Network Pharmacology and Molecular Docking. Evid Based Complement Alternat Med. 2020;2020:7151634. Published 2020 Aug 26
28. Qian H, Jin Q, Liu Y. et al. Study on the Multitarget Mechanism of Sanmiao Pill on Gouty Arthritis Based on Network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:9873739. Published 2020 Aug 4
29. Ren B, Tan L, Xiong Y. et al. Integrated Analysis of the Mechanisms of Da-Chai-Hu Decoction in Type 2 Diabetes Mellitus by a Network Pharmacology Approach. Evid Based Complement Alternat Med. 2020;2020:9768414. Published 2020 Apr 28
30. Wang W, Zhang Y, Luo J, Wang R, Tang C, Zhang Y. Virtual Screening Technique Used to Estimate the Mechanism of Adhatoda vasica Nees for the Treatment of Rheumatoid Arthritis Based on Network Pharmacology and Molecular Docking. Evid Based Complement Alternat Med. 2020;2020:5872980. Published 2020 Sep 29
31. Xiao K, Li K, Long S, Kong C, Zhu S. Potential Molecular Mechanisms of Chaihu-Shugan-San in Treatment of Breast Cancer Based on Network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:3670309. Published 2020 Sep 25
32. Yang K, Zeng L, Ge J. Exploring the Pharmacological Mechanism of Danzhi Xiaoyao Powder on ER-Positive Breast Cancer by a Network Pharmacology Approach. Evid Based Complement Alternat Med. 2018;2018:5059743. Published 2018 Mar 5
33. Zhang C, Liao Y, Liu L. et al. A Network Pharmacology Approach to Investigate the Active Compounds and Mechanisms of Musk for Ischemic Stroke. Evid Based Complement Alternat Med. 2020;2020:4063180. Published 2020 Jul 3
34. Zhou J, Wang Q, Xiang Z. et al. Network Pharmacology Analysis of Traditional Chinese Medicine Formula Xiao Ke Yin Shui Treating Type 2 Diabetes Mellitus. Evid Based Complement Alternat Med. 2019;2019:4202563. Published 2019 Sep 8
35. Zhang H, Zhang S, Hu M. et al. An integrative metabolomics and network pharmacology method for exploring the effect and mechanism of Radix Bupleuri and Radix Paeoniae Alba on anti-depression. J Pharm Biomed Anal. 2020;189:113435. Published 2020 Sep 10
36. Ijaz M, Huang X, Buabeid M. et al. Mechanistic Investigation of Glycyrrhiza uralensis Effects against Respiratory Ailments: Application of Network Pharmacology and Molecular Docking Approaches. Letters in Drug Design & Discovery 2022;5(19);397-412(16). Published. 2022 May 1
37. Wang T, Feng Y, Wang H. et al. The Mechanisms of Sijunzi Decoction in the Treatment of Chronic Gastritis Revealed by Network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:8850259. Published 2020 Oct 23
38. Ru J, Li P, Wang J. et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. Published 2014 Apr 16
39. Wang CK, Craik DJ. Cyclic peptide oral bioavailability: Lessons from the past. Biopolymers. 2016;106(6):901-909
40. Kono Y, Iwasaki A, Matsuoka K, Fujita T. Effect of Mechanical Agitation on Cationic Liposome Transport across an Unstirred Water Layer in Caco-2 Cells. Biol Pharm Bull. 2016;39(8):1293-1299
41. Volpe DA. Variability in Caco-2 and MDCK cell-based intestinal permeability assays. J Pharm Sci. 2008;97(2):712-725
42. Garcia MN, Flowers C, Cook JD. The Caco-2 cell culture system can be used as a model to study food iron availability. J Nutr. 1996;126(1):251-258
43. Li Y, Zhang J, Zhang L. et al. Systems pharmacology to decipher the combinational anti-migraine effects of Tianshu formula. J Ethnopharmacol. 2015;174:45-56
44. Zhang J, Li Y, Chen X, Pan Y, Zhang S, Wang Y. Systems pharmacology dissection of multi-scale mechanisms of action for herbal medicines in stroke treatment and prevention. PLoS One. 2014;9(8):e102506. Published 2014 Aug 5
45. Lee AY, Park W, Kang TW, Cha MH, Chun JM. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J Ethnopharmacol. 2018;221:151-159
46. UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506-D515
47. Raudvere U, Kolberg L, Kuzmin I. et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47(W1):W191-W198
48. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27-30
49. Montojo J, Zuberi K, Rodriguez H, Bader GD, Morris Q. GeneMANIA: Fast gene network construction and function prediction for Cytoscape. F1000Res. 2014;3:153. Published 2014 Jul 1
50. Szklarczyk D, Gable AL, Lyon D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607-D613
51. Barabási AL, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet. 2004;5(2):101-113
52. Shannon P, Markiel A, Ozier O. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498-2504
53. Yue SJ, Xin LT, Fan YC. et al. Herb pair Danggui-Honghua: mechanisms underlying blood stasis syndrome by system pharmacology approach. Sci Rep. 2017;7:40318. Published 2017 Jan 11
54. Cho DY, Kim YA, Przytycka TM. Chapter 5: Network biology approach to complex diseases. PLoS Comput Biol. 2012;8(12):e1002820
55. Jeong H, Mason SP, Barabási AL, Oltvai ZN. Lethality and centrality in protein networks. Nature. 2001;411(6833):41-42
56. Zhu J, Yi X, Zhang Y, Pan Z, Zhong L, Huang P. Systems Pharmacology-Based Approach to Comparatively Study the Independent and Synergistic Mechanisms of Danhong Injection and Naoxintong Capsule in Ischemic Stroke Treatment. Evid Based Complement Alternat Med. 2019;2019:1056708. Published 2019 Feb 4
57. Zhong J, Liu Z, Zhou X, Xu J. Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair. Molecules. 2017;22(9):1465. Published 2017 Sep 4
58. Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A. 2005;102(14):5138-5143
59. Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30(9):1005-1014
60. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798-809
61. Xu L, Hu G, Xing P, Zhou M, Wang D. Corrigendum to "Paclitaxel alleviates the sepsis-induced acute kidney injury via lnc-MALAT1/miR-370-3p/HMGB1 axis" [Life Sci. 2020 Dec 1; 262:118505. doi:10.1016/j.lfs.2020.118505. Epub 2020 Sep 28]. Life Sci. 2021;272:119159
62. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin-6 Signaling in Clinic. Immunity. 2019;50(4):1007-1023
63. Shirazi R, Palsdottir V, Collander J. et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc Natl Acad Sci U S A. 2013;110(40):16199-16204
64. Nie H, Zheng Y, Li R. et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19(3):322-328
65. Plata-Salamán CR. Cytokines and Feeding. News Physiol Sci. 1998;13:298-304
66. Cannon JG. Inflammatory Cytokines in Nonpathological States. News Physiol Sci. 2000;15:298-303
67. Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222-234
68. Rönnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61(19-20):2535-2548
69. Beg M, Abdullah N, Thowfeik FS, Altorki NK, McGraw TE. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. Elife. 2017;6:e26896. Published 2017 Jun 7
70. Yue SJ, Liu J, Feng WW. et al. System Pharmacology-Based Dissection of the Synergistic Mechanism of Huangqi and Huanglian for Diabetes Mellitus. Front Pharmacol. 2017;8:694. Published 2017 Oct 5
71. Hinoshita F, Ogura Y, Suzuki Y. et al. Effect of orally administered shao-yao-gan-cao-tang (Shakuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: a preliminary study. Am J Chin Med. 2003;31(3):445-453
72. Lee KK, Omiya Y, Yuzurihara M, Kase Y, Kobayashi H. Antispasmodic effect of shakuyakukanzoto extract on experimental muscle cramps in vivo: role of the active constituents of Glycyrrhizae radix [published correction appears in J Ethnopharmacol. 2013 Mar 7;146(1):431]. J Ethnopharmacol. 2013;145(1):286-293
73. Chen IC, Lin TH, Hsieh YH. et al. Formulated Chinese Medicine Shaoyao Gancao Tang Reduces Tau Aggregation and Exerts Neuroprotection through Anti-Oxidation and Anti-Inflammation. Oxid Med Cell Longev. 2018;2018:9595741. Published 2018 Oct 28
74. Zhang Y, Jia X, Yang J. et al. Effects of Shaoyao-Gancao Decoction on Infarcted Cerebral Cortical Neurons: Suppression of the Inflammatory Response following Cerebral Ischemia-Reperfusion in a Rat Model. Biomed Res Int. 2016;2016:1859254
75. Omiya Y, Suzuki Y, Yuzurihara M. et al. Antinociceptive effect of shakuyakukanzoto, a Kampo medicine, in diabetic mice. J Pharmacol Sci. 2005;99(4):373-380
76. Zhang J, Lv C, Wang HN, Cao Y. Synergistic interaction between total glucosides and total flavonoids on chronic constriction injury induced neuropathic pain in rats. Pharm Biol. 2013;51(4):455-462
77. Chen CM, Chen WL, Hung CT. et al. Shaoyao Gancao Tang (SG-Tang), a formulated Chinese medicine, reduces aggregation and exerts neuroprotection in spinocerebellar ataxia type 17 (SCA17) cell and mouse models. Aging (Albany NY). 2019;11(3):986-1007
78. Jeong SJ, Lim HS, Seo CS. et al. Traditional herbal formula Jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-κB in HaCaT cells. Phytomedicine. 2015;22(2):326-332
79. Kang TH, Baek HY, Kim YC. Protective effect of jakyak-gamcho-tang extract and its constituents against t-BHP-induced oxidative damage in HT22 cells. Am J Chin Med. 2005;33(2):181-189
80. Maeda T, Shinozuka K, Baba K, Hayashi M, Hayashi E. Effect of shakuyaku-kanzoh-toh, a prescription composed of shakuyaku (Paeoniae Radix) and kanzoh (Glycyrrhizae Radix) on guinea pig ileum. J Pharmacobiodyn. 1983;6(3):153-160
81. Wauters L, Talley NJ, Walker MM, Tack J, Vanuytsel T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 2020;69(3):591-600
82. Page AJ, Li H. Meal-Sensing Signaling Pathways in Functional Dyspepsia. Front Syst Neurosci. 2018;12:10. Published 2018 Apr 5
83. Van Oudenhove L, Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):158-167
84. O'Mahony S, Dinan TG, Keeling PW, Chua AS. Central serotonergic and noradrenergic receptors in functional dyspepsia. World J Gastroenterol. 2006;12(17):2681-2687
85. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397-414
86. Talley NJ. Functional Dyspepsia: Advances in Diagnosis and Therapy. Gut Liver. 2017;11(3):349-357
87. Kaymak Cihan M, Karabulut HG, Yürür Kutlay N, Ilgın Ruhi H, Tükün A, Olcay L. Association Between N363S and BclI Polymorphisms of the Glucocorticoid Receptor Gene (NR3C1) and Glucocorticoid Side Effects During Childhood Acute Lymphoblastic Leukemia Treatment. Glukokortikoid Reseptör Geninin (NR3C1) N363S ve BclI Polimorfizmleri ile Çocukluk Çağı Akut Lenfoblastik Lösemi Tedavisi Sırasında Görülen Glukokortikoid Yan Etkileri Arasındaki İlişki. Turk J Haematol. 2017;34(2):151-158
88. Saps M, Pensabene L, Di Martino L. et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152(6):812-816.e1
89. Porter CK, Choi D, Cash B. et al. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol. 2013;13:46. Published 2013 Mar 8
90. Morales-Sánchez A, Torres J, Cardenas-Mondragón MG. et al. Detection of Epstein-Barr Virus DNA in Gastric Biopsies of Pediatric Patients with Dyspepsia. Pathogens. 2020;9(8):623. Published 2020 Jul 30
91. Mearin F, Pérez-Oliveras M, Perelló A. et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129(1):98-104
92. Petrović Lj, Milosević V, Zujović J, Josipović Z. Dispepije izazvane patogenim sojevima Escherichia coli kod odojacadi i male dece. Klinicka zapazanja [Dyspepsia caused by pathogenic strains of Escherichia coli in infants and small children. Clinical observation]. Srp Arh Celok Lek. 1969;97(4):419-424
93. Santos AS, Silveira EA, Falco MO. Gastrointestinal Symptoms in HIV-Infected Patients: Female Sex and Smoking as Risk Factors in an Outpatient Cohort in Brazil. PLoS One. 2016;11(10):e0164774. Published 2016 Oct 17
94. Bazaldua OV, Schneider FD. Evaluation and management of dyspepsia. Am Fam Physician. 1999;60(6):1773-1788
95. Ray D, Morimoto M. Malrotation of the Intestine in Adult and Colorectal Cancer. Indian J Surg. 2015;77(6):525-531
96. Kim NM, Doh YS, Jang JW. et al. Discrepancy between the Actual Clinical Status of Patients with Hepatocellular Carcinoma and Expectations from Hepatocellular Carcinoma Surveillance: a Single-Center Study. J Liver Cancer. 2019 19(1);30-37
97. CHENE P, SIMON A. Dyspepsie gastrique et amibiase [Gastric dyspepsia and amebiasis]. Arch Mal Appar Dig Mal Nutr. 1949;38(5-6):338-345
98. Barros LL, Farias AQ, Rezaie A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: Prevalence, diagnosis and treatment. World J Gastroenterol. 2019;25(31):4414-4426
99. Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol. 2008;14(8):1149-1155
100. Wilairatana P, Riganti M, Looareesuwan S, Punpoowong B, Srisopark P, Charoenlarp P. Dyspepsia in acute falciparum malaria: a clinico-pathological correlation. Southeast Asian J Trop Med Public Health. 1992;23(4):788-794
101. Karaoglu AO, Kadikoylu G, Yukselen V, Yasa MH, Bolaman Z. Gastrointestinal lesions and Helicobacter pylori in patients with myeloproliferative disorders. Saudi Med J. 2004;25(12):1913-1916
102. Weisdorf DJ, Snover DC, Haake R. et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76(3):624-629
103. Al Juboori A, Kaur S, Reddy A. Metastatic Renal Cell Carcinoma Presenting as Gastric Ulcer: Case Report and Literature Review. Case Rep Gastrointest Med. 2017;2017:2509294
104. Sfarti C, Trifan A, Hutanasu C, Cojocariu C, Singeap AM, Stanciu C. Prevalence of gastroparesis in type 1 diabetes mellitus and its relationship to dyspeptic symptoms. J Gastrointestin Liver Dis. 2010;19(3):279-284
105. Hagiwara SI, Kaushal E, Paruthiyil S, Pasricha PJ, Hasdemir B, Bhargava A. Gastric corticotropin-releasing factor influences mast cell infiltration in a rat model of functional dyspepsia. PLoS One. 2018;13(9):e0203704. Published 2018 Sep 7
106. Yao X, Mei Y, Mao W. Quercetin Improves Mitochondrial Function and Inflammation in H2O2-Induced Oxidative Stress Damage in the Gastric Mucosal Epithelial Cell by Regulating the PI3K/AKT Signaling Pathway. Evid Based Complement Alternat Med. 2021;2021:1386078. Published 2021 Nov 27
107. Castaño-Rodríguez N, Kaakoush NO, Goh KL, Fock KM, Mitchell HM. The NOD-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer: a case-control study and gene expression analyses [published correction appears in PLoS One. 2015;10(1):e0117870]. PLoS One. 2014;9(6):e98899. Published 2014 Jun 5
108. Wu YY, Zhong ZS, Ye ZH. et al. D-galacturonic acid ameliorates the intestinal mucosal permeability and inflammation of functional dyspepsia in rats. Ann Palliat Med. 2021;10(1):538-548
109. Zhao M, Chen Y, Wang C. et al. Systems Pharmacology Dissection of Multi-Scale Mechanisms of Action of Huo-Xiang-Zheng-Qi Formula for the Treatment of Gastrointestinal Diseases. Front Pharmacol. 2019;9:1448. Published 2019 Jan 11
110. Pak ME, Oh YC, Park YJ. et al. Banhasasim-Tang Ameliorates Spatial Memory by Suppressing Oxidative Stress through Regulation of ERK/p38 Signaling in Hippocampus of Mice. Oxid Med Cell Longev. 2021;2021:6970578. Published 2021 Dec 2
111. Li Q, Winston JH, Sarna SK. Noninflammatory upregulation of nerve growth factor underlies gastric hypersensitivity induced by neonatal colon inflammation. Am J Physiol Regul Integr Comp Physiol. 2016;310(3):R235-R242
112. Pei H, Wu S, Zheng L, Wang H, Zhang X. Identification of the active compounds and their mechanisms of medicinal and edible Shanzha based on network pharmacology and molecular docking. J Food Biochem. 2022;46(1):e14020
113. Thein W, Po WW, Choi WS, Sohn UD. Autophagy and Digestive Disorders: Advances in Understanding and Therapeutic Approaches. Biomol Ther (Seoul). 2021;29(4):353-364
114. Zhang G, Xie S, Hu W. et al. Effects of Electroacupuncture on Interstitial Cells of Cajal (ICC) Ultrastructure and Connexin 43 Protein Expression in the Gastrointestinal Tract of Functional Dyspepsia (FD) Rats. Med Sci Monit. 2016;22:2021-2027 Published 2016 Jun 14
115. Ueno H, Shiiya T, Nakazato M. Translational research of ghrelin. Ann N Y Acad Sci. 2010;1200:120-127
116. Yamawaki H, Futagami S, Wakabayashi M. et al. Management of functional dyspepsia: state of the art and emerging therapies. Ther Adv Chronic Dis. 2018;9(1):23-32
117. Aksit-Bicak D, Emekli-Alturfan E, Ustundag UV, Akyuz S. Assessment of dental caries and salivary nitric oxide levels in children with dyspepsia. BMC Oral Health. 2019;19(1):11. Published 2019 Jan 11
118. Cheng Y, Liu Y, Chen D. et al. Dual effects of quercetin on protein digestion and absorption in the digestive tract. Food Chem. 2021;358:129891
119. Kumadoh D, Archer MA, Yeboah GN. et al. A review on anti-peptic ulcer activities of medicinal plants used in the formulation of Enterica, Dyspepsia and NPK 500 capsules. Heliyon. 2021;7(12):e08465. Published 2021 Nov 29
120. Feng XY, Li Y, Li LS. et al. Dopamine D1 receptors mediate dopamine-induced duodenal epithelial ion transport in rats. Transl Res. 2013;161(6):486-494
121. Zhao M, Chen Y, Wang C. et al. Systems Pharmacology Dissection of Multi-Scale Mechanisms of Action of Huo-Xiang-Zheng-Qi Formula for the Treatment of Gastrointestinal Diseases. Front Pharmacol. 2019;9:1448. Published 2019 Jan 11
122. Yin J, Liang Y, Wang D. et al. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 2018;41(2):649-658
123. Ho L, Zhong CCW, Wong CHL. et al. Chinese herbal medicine for functional dyspepsia: a network meta-analysis of prokinetic-controlled randomised trials. Chin Med. 2021;16(1):140. Published 2021 Dec 20
Author contact
![]() Corresponding authors: Byung Joo Kim, Division of Longevity and Biofunctional Medicine, Pusan National University School of Korean Medicine, 49 Busandaehakro, Mulgeum-eup, Yangsan 50612, Republic of Korea. Telephone: +82-51-510-8469; Fax: +82-51-510-8420; E-mail: visionac.kr. Eun-Jung Park, Department of Food and Nutrition, College of BioNano Technology, Gachon University, Seongnam 13120, Republic of Korea. Telephone: +82-31-724-4408; Fax: +82-31-724-4411; E-mail: ejparkac.kr.
Corresponding authors: Byung Joo Kim, Division of Longevity and Biofunctional Medicine, Pusan National University School of Korean Medicine, 49 Busandaehakro, Mulgeum-eup, Yangsan 50612, Republic of Korea. Telephone: +82-51-510-8469; Fax: +82-51-510-8420; E-mail: visionac.kr. Eun-Jung Park, Department of Food and Nutrition, College of BioNano Technology, Gachon University, Seongnam 13120, Republic of Korea. Telephone: +82-31-724-4408; Fax: +82-31-724-4411; E-mail: ejparkac.kr.

 Global reach, higher impact
Global reach, higher impact