3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(12):1816-1823. doi:10.7150/ijms.76139 This issue Cite
Research Paper
Possibility of Multiple Drug-Drug Interactions in Patients Treated with Statins: Analysis of Data from the Japanese Adverse Drug Event Report (JADER) Database and Verification by Animal Experiments
1. Laboratory of Analytical Pharmaceutics and Informatics, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan.
2. Laboratory of Pharmacy Management, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan.
3. Josai University Pharmacy, Saitama, Japan.
4. Student Learning Assistance Center, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan.
Abstract
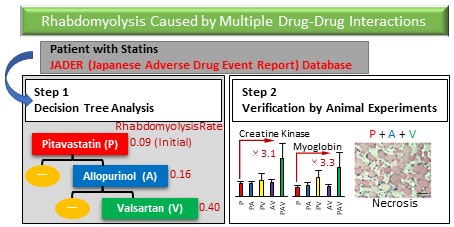
Adverse drug events due to drug-drug interactions can be prevented by avoiding concomitant use of causative drugs; therefore, it is important to understand drug combinations that cause drug-drug interactions. Although many attempts to identify drug-drug interactions from real-world databases such as spontaneous reporting systems have been performed, little is known about drug-drug interactions caused by three or more drugs in polypharmacy, i.e., multiple drug-drug interactions. Therefore, we attempted to detect multiple drug-drug interactions using decision tree analysis using the Japanese Adverse Drug Event Report (JADER) database, a Japanese spontaneous reporting system. First, we used decision tree analysis to detect drug combinations that increase the risk of rhabdomyolysis in cases registered in the JADER database that used six statins. Next, the risk of three or more drug combinations that significantly increased the risk of rhabdomyolysis was validated with in vivo experiments in rats. The analysis identified a multiple drug-drug interaction signal only for pitavastatin. The reporting rate of rhabdomyolysis for pitavastatin in the JADER database was 0.09, and it increased to 0.16 in combination with allopurinol. Furthermore, the rate was even higher (0.40) in combination with valsartan. Additionally, necrosis of leg muscles was observed in some rats simultaneously treated with these three drugs, and their creatine kinase and myoglobin levels were elevated. The combination of pitavastatin, allopurinol, and valsartan should be treated with caution as a multiple drug-drug interaction. Since multiple drug-drug interactions were detected with decision tree analysis and the increased risk was verified in animal experiments, decision tree analysis is considered to be an effective method for detecting multiple drug-drug interactions.
Keywords: multiple drug-drug interaction, decision tree analysis, spontaneous reporting system, statin, rhabdomyolysis, polypharmacy

 Global reach, higher impact
Global reach, higher impact