Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(12):1816-1823. doi:10.7150/ijms.76139 This issue Cite
Research Paper
Possibility of Multiple Drug-Drug Interactions in Patients Treated with Statins: Analysis of Data from the Japanese Adverse Drug Event Report (JADER) Database and Verification by Animal Experiments
1. Laboratory of Analytical Pharmaceutics and Informatics, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan.
2. Laboratory of Pharmacy Management, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan.
3. Josai University Pharmacy, Saitama, Japan.
4. Student Learning Assistance Center, Faculty of Pharmacy and Pharmaceutical Sciences, Josai University, Saitama, Japan.
Received 2022-6-14; Accepted 2022-9-13; Published 2022-10-9
Abstract
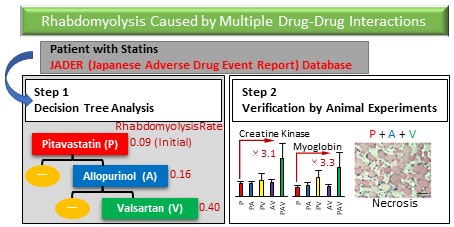
Adverse drug events due to drug-drug interactions can be prevented by avoiding concomitant use of causative drugs; therefore, it is important to understand drug combinations that cause drug-drug interactions. Although many attempts to identify drug-drug interactions from real-world databases such as spontaneous reporting systems have been performed, little is known about drug-drug interactions caused by three or more drugs in polypharmacy, i.e., multiple drug-drug interactions. Therefore, we attempted to detect multiple drug-drug interactions using decision tree analysis using the Japanese Adverse Drug Event Report (JADER) database, a Japanese spontaneous reporting system. First, we used decision tree analysis to detect drug combinations that increase the risk of rhabdomyolysis in cases registered in the JADER database that used six statins. Next, the risk of three or more drug combinations that significantly increased the risk of rhabdomyolysis was validated with in vivo experiments in rats. The analysis identified a multiple drug-drug interaction signal only for pitavastatin. The reporting rate of rhabdomyolysis for pitavastatin in the JADER database was 0.09, and it increased to 0.16 in combination with allopurinol. Furthermore, the rate was even higher (0.40) in combination with valsartan. Additionally, necrosis of leg muscles was observed in some rats simultaneously treated with these three drugs, and their creatine kinase and myoglobin levels were elevated. The combination of pitavastatin, allopurinol, and valsartan should be treated with caution as a multiple drug-drug interaction. Since multiple drug-drug interactions were detected with decision tree analysis and the increased risk was verified in animal experiments, decision tree analysis is considered to be an effective method for detecting multiple drug-drug interactions.
Keywords: multiple drug-drug interaction, decision tree analysis, spontaneous reporting system, statin, rhabdomyolysis, polypharmacy
Introduction
Adverse drug events (ADEs) due to drug-drug interactions (DDIs) can be prevented by avoiding concomitant use of causative drugs, unlike the side effects of single drugs [1-4]. Therefore, to prevent ADEs, it is important to identify drug combinations that lead to DDIs. However, it is not possible to detect all DDIs during the clinical trials of drug development due to the limited target patients and concomitant drugs [5]. In fact, many drugs such as solivudine, cerivastatin, mibefradil, cisapride, and terfenadine have been withdrawn from the market owing to the occurrence of ADEs due to DDIs post-marketing [6-8]. Therefore, it is important to detect DDIs using real-world databases such as the spontaneous reporting system (SRS) to identify ADEs in the early stage of post-marketing and to get feedback for clinical practice [9-14].
On the other hand, the number of patients with polypharmacy is increasing in developed nations owing to the rising number of comorbidities per patient [15]. As patients with polypharmacy who use more than 5-6 drugs have higher rates of emergency hospitalization, re-hospitalization, death, and onset of ADEs [16-18], polypharmacy is recognized as a problem owing to the number of drugs. Therefore, studies have focused on reducing the number of prescribed drugs, i.e., deprescribing [19]. However, although DDIs caused by three or more specific drugs (i.e., multiple DDIs) may potentially underlie ADEs due to polypharmacy, the existence of multiple DDIs is poorly known. In Japan, which has been facing a serious problem of polypharmacy due to the aging population, the Japanese Adverse Drug Event Report (JADER) database was released in 2012 as an SRS, and its use for the detection of DDIs has been discussed. From the early release of this database, the detection of multiple DDIs has been a challenging issue [20].
To the best of our knowledge, the only previous study that detected multiple DDIs using the SRS database was by Yao et al. They used the SRS database to search for combinations of multiple DDIs that cause myopathy and detected a significant signal from a combination of seven drugs [21]. However, since this method comprehensively investigates all drug combinations, many small signals are detected, thus, making it difficult to interpret results.
Multiple logistic regression analysis is often used as a method to evaluate pairwise DDIs from the SRS [22]. However, to evaluate three or more DDIs with a generalized linear model, such as logistic regression analysis, a large number of interaction terms must be included in the regression equation. This results in the detection of many small signals because of which it is difficult to interpret the results. On the contrary, decision tree analysis, which is one of the nonlinear data mining methods, enables the division of the population with optimal variables without using predictive equations, and indicates complex interactions easily and clearly [23,24].
In the medical field, decision tree analysis is used to predict patient outcomes [25], and attempts have been made to including the drug as a predictor [22]. We considered that multiple DDIs could be detected using drug as a predictor of rhabdomyolysis. Although a sufficient number of samples are required for decision tree analysis, a large database, such as JADER, is a suitable resource for the analysis [26].
In this study, we applied decision tree analysis to the JADER database to identify multiple DDIs that increase the risk of occurrence of rhabdomyolysis, a characteristic ADE of the six statins prescribed in Japan. Additionally, according to the World Health Organization, signals detected from the analysis of SRS database require further validation [27]. Therefore, some recent reports on signal detection have attempted to increase the verifiability of signals by combining in vivo or in vitro studies [10,12]. In this study, we conducted in vivo experiments in rats to verify whether the detected drug combinations increase the risk of occurrence of rhabdomyolysis.
Methods
Database information
The JADER database was downloaded from the website of the Pharmaceuticals and Medical Devices Agency. Cases reported from April 2004 to July 2017 were studied. JADER database consists of the following four tables linked by a common identification number: “patient demographic information (DEMO)”, “drug information (DRUG)”, “adverse events (REAC)”, and “primary disease information (HIST)”. The DEMO table contains basic patient information (e.g., age, sex), DRUG table contains drug information (e.g., drug name, start date, end date, route of administration), REAC table contains ADE information (e.g., ADEs, date of onset), and HIST table contains primary disease information. In this study, the DEMO, DRUG, and REAC tables were used. All ADEs in the JADER database are registered by preferred terms listed in the Japanese version of the International Conference on Harmonization's Medical Dictionary for Regulatory Activities (version 20.0) [17].
Extraction of cases
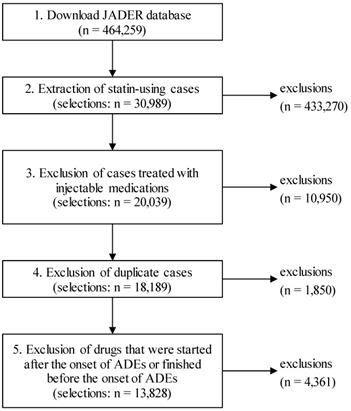
The procedure for extracting cases from the JADER database is shown in Fig. 1. First, cases treated with the six statins prescribed in Japan, atorvastatin, simvastatin, rosuvastatin, fluvastatin, pravastatin, and pitavastatin, were extracted from the downloaded data. Next, cases treated with injectable medications were excluded from the study because injectable medications are more likely to be used for treatment of ADEs during hospitalization. Futhermore, cases with matching “sex,” “age,” and “registered drug names” were considered duplicate cases, and only the first registered case was included in the analysis.
The JADER database also registers the drugs that were started after the onset of ADEs or finished before the onset of ADEs [19]. In this study, the drugs that were used at the time of ADE onset were included in the analysis in principle; thus, all drugs started after the onset of ADEs were excluded. However, the discontinued drugs were included in the analysis only if they were used within one week of the onset of ADEs because of the possibility of causing DDIs for a certain period. On the other hand, drugs started before the onset of ADEs without the date of end of administration were included in the analysis only if they were started within 1 year of the onset of ADEs because they might have been used at the time of onset of ADEs. For cases not registered with the onset dates of ADEs, all registered drugs were included in the study.
Flowchart of data cleaning.
Data analysis
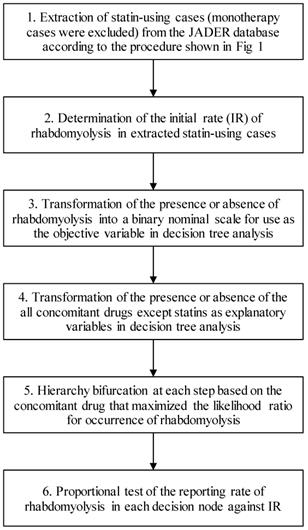
The analysis procedure is shown in Fig. 2. The analysis was performed for each statin. First, we determined the initial rate (IR) of rhabdomyolysis (PT 10039020) in each statin-using case (monotherapy cases were excluded) selected for the study. Thereafter, for each case, the presence or absence of rhabdomyolysis was transformed into a binary nominal scale and used as the objective variable in decision tree analysis. Further, all concomitant drugs except statins were transformed into a binary nominal scale for each case and used as an explanatory variable in decision tree analysis. The number of used drugs was also included as an explanatory variable. The number of cases in each hierarchy (decision nodes) was set to a minimum of 10 cases, and at each step, the hierarchy was branched by the concomitant drug that maximized the likelihood ratio of occurrence of rhabdomyolysis. Finally, a proportional test was used to compare the reporting rate of rhabdomyolysis to the IR for each decision node. Benjamini-Hochberg adjustment was used to correct for multiple comparisons.
Flowchart for data analysis.
Materials
Pitavastatin calcium, allopurinol, and valsartan were purchased from TAKATA Pharmaceutical Co., Ltd. (Saitama, Japan). Rat myoglobin (MYO-2) enzyme-linked immunosorbent assay was obtained from Life Diagnostics, Inc. (West Chester, PA, USA). Multirotor II VLA was acquired from Central Scientific Commerce Inc. (Tokyo, Japan). Tissue-Tek® Mayer's hematoxylin solution and Tissue-Tek® eosin solution were purchased from Sakura Finetek Japan Co., Ltd. (Tokyo, Japan). Heparin sodium was obtained from Mochida Pharmaceutical Co., Ltd. (Tokyo, Japan). Formalin neutral buffer solution (10%), xylene, and anhydrous ethanol were acquired from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Otsuka normal saline was purchased from Otsuka Pharmaceutical Factory, Inc. (Tokushima, Japan).
Animals
Female Wistar rats were purchased from Japan SLC, Inc. (Shizuoka, Japan). Rats were reared under normal environmental conditions (temperature: 25 °C ± 2°C, humidity: 55% ± 5%, and lights on: 7:00 to 19:00 h). Furthermore, the rats were provided tap water and solid feed (Labo MR Stock, Nosan Corporation, Kanagawa, Japan), and they were acclimated for at least 1 week before the experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of Josai University (Approval No.: JU19011-2019/04/18).
Drug administration and verification of rhabdomyolysis
Fifty 6-week-old female Wistar rats were divided into the following groups based on the drug administered (n = 10 per group): pitavastatin (P) group; pitavastatin and allopurinol (PA) group; pitavastatin and valsartan (PV) group; allopurinol and valsartan (AV) group; and pitavastatin, allopurinol, and valsartan (PAV) group. Although the development of rhabdomyolysis with high-dose statins usually occurs within 14 days [28-30], no in vivo animal study has reported the development of rhabdomyolysis with pitavastatin. The only study we found reported some skeletal muscle involvement (atrophy, vacuolation, and necrosis) in some female rats treated with 50 mg/kg/day pitavastatin in repeated toxicity studies for 28 days (31). Therefore, we determined the dose of pitavastatin as 50 mg/kg/day in this study. On the other hand, allopurinol and valsartan were administered at 24 and 60 mg/kg/day, respectively, as the no observed adverse effect level [32, 33]. The drugs were suspended in pure water (Elix® UV, Merck Millipore, Tokyo, Japan) and administered orally once daily for 14 days.
On days 7 and 14 of drug administration, blood was collected from the tail vein, and blood creatine kinase (CK) was measured using a VetScan (Daiichi Chemical Co., Ltd., Tokyo, Japan). After 4 h from the final dose administration on day 14, blood was collected from the jugular vein of rats under anesthesia with pentobarbital, and plasma was separated by centrifuging the blood samples at 13,000 rpm at 4 °C for 5 min. Drainage was performed immediately after blood collection via cardiac perfusion with 200 mL of saline containing heparin (5 units/mL) for 10 min. Subsequently, tissue fixation was performed via cardiac perfusion using 200 mL of 10% formalin neutral buffer solution for 10 min, and the lower leg muscles were excised. Myoglobin level in the collected plasma was measured using a rat myoglobin enzyme-linked immunosorbent assay kit (Life Diagnostics, West Chester, PA, USA). The excised lower leg muscles were embedded in paraffin, and they were sliced into 3-µm sections with a microtome. Next, the slides were immersed in xylene thrice and then in a series of ethanol concentrations (100%, 100%, 95%, and 95%). After rinsing with water, slides were stained with hematoxylin and eosin. Slides were dehydrated in a series of ethanol concentrations (95%, 95%, 100%, 100%), permeated three times with xylene, and sealed with glass slides.
Analysis software
JMP® 5.1.2 (SAS Institute Japan, Tokyo, Japan) was used for decision tree analysis whereas R software (R 3.2.2, Project for statistical computing) was used for other data analyses.
Results
Analysis of data from the JADER database
Table 1 summarizes statistically significant drug combinations that increased the risk of rhabdomyolysis detected via decision tree analysis. Of the six investigated statins, pitavastatin was the only statin for which three or more drug combinations, i.e., multiple DDIs, were detected (Fig. S1).
Drug combinations that significantly increased the risk of rhabdomyolysis
| Statin | Concomitant drugs | Cases | Cases (+) | Rate |
|---|---|---|---|---|
| Simvastatin | - | 353 | 44 | 0.12 (IR) |
| - | - | - | ||
| Pitavastatin calcium | - | 739 | 69 | 0.09 (IR) |
| Benzbromarone | 13 | 5 | 0.38 | |
| Allopurinol + Valsartan | 10 | 4 | 0.40 | |
| Fluvastatin sodium | - | 354 | 25 | 0.07 (IR) |
| - | - | - | ||
| Pravastatin sodium | - | 1201 | 93 | 0.08 (IR) |
| Bezafibrate | 11 | 4 | 0.36 | |
| Benzbromarone | 21 | 5 | 0.24 | |
| Carbocisteine | 23 | 5 | 0.22 | |
| Flunitrazepam | 12 | 3 | 0.25 | |
| Rosuvastatin calcium | - | 1185 | 91 | 0.08 (IR) |
| Sitagliptin phosphate hydrate | 13 | 5 | 0.38 | |
| Allopurinol | 15 | 5 | 0.33 | |
| Amlodipine besilate | 22 | 6 | 0.27 | |
| Atorvastatin calcium hydrate | - | 1905 | 144 | 0.08 (IR) |
| Metformin hydrochloride | 16 | 7 | 0.44 | |
| Loxoprofen sodium hydrate | 11 | 5 | 0.45 | |
| Doxazosin mesilate | 12 | 4 | 0.33 | |
| Pioglitazone hydrochloride | 14 | 4 | 0.29 | |
| Allopurinol | 28 | 7 | 0.25 | |
| Flunitrazepam | 19 | 4 | 0.21 | |
| Isosorbide mononitrate | 10 | 3 | 0.30 | |
| Benidipine hydrochloride | 12 | 3 | 0.25 |
IR: Initial rate; Decision tree analysis revealed drug combinations that increased the reporting rate of rhabdomyolysis. The ratios indicated that the drug combinations indicated in this table significantly increased the risk of rhabdomyolysis (false discovery rate was set at 0.05 after adjustment using Benjamini-Hochberg correction). “Cases” represent the number of cases stratified based on concomitant drugs. “Cases (+)” represent the number of rhabdomyolysis events in “Cases” identified after stratification. “Rate” represents the reporting rate of rhabdomyolysis cases identified after stratification. The first row of each table shows the IR of rhabdomyolysis in each statin-use case. It also shows the ratio of the number of patients with rhabdomyolysis to the total number of patients included in the analysis.
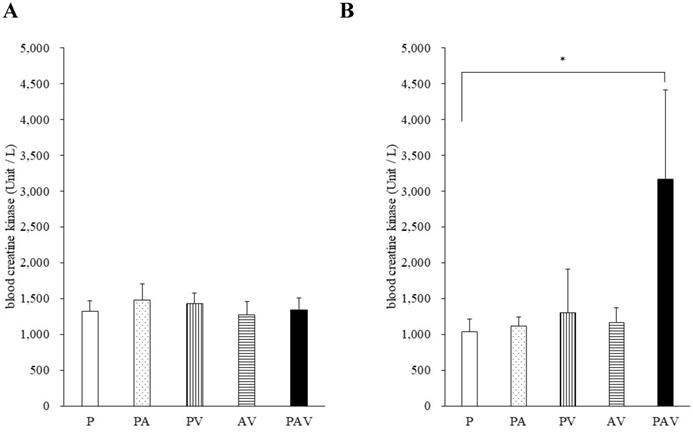
Blood levels of creatine kinase following administration of pitavastatin. Data are expressed as mean ± standard error of the mean (SEM) on (A) day 7 and (B) day 14 of drug administration. P, pitavastatin; PA, pitavastatin + allopurinol. PV, pitavastatin + valsartan; AV, allopurinol + valsartan; PAV, pitavastatin + allopurinol + valsartan. Dunnett's multiple comparison test was used to identify statistically significant differences vs. P group (* p < 0.05).
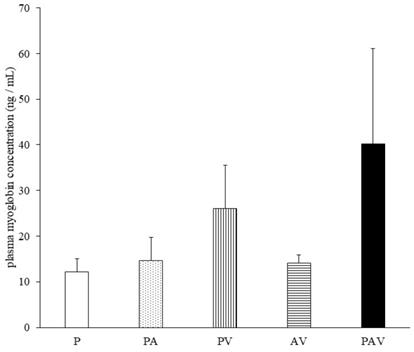
Plasma myoglobin levels following administration of pitavastatin. Data are expressed as mean ± standard error of the mean (SEM). P, pitavastatin; PA, pitavastatin + allopurinol; PV, pitavastatin + valsartan; AV, allopurinol + valsartan; PAV, pitavastatin + allopurinol + valsartan. Dunnett's multiple comparison test was used to identify statistically significant differences vs. P group. Significance was set at P < 0.05 (No significant difference).
Among the drug combinations listed in Table 1, eight drugs, metformin, loxoprofen, doxazosin, pioglitazone, allopurinol, flunitrazepam, isosorbide nitrate, and benidipine, significantly increased atorvastatin's risk of rhabdomyolysis. Similarly, three drugs, sitagliptin, allopurinol, and amlodipine, increased the risk of rhabdomyolysis for rosuvastatin; four drugs, bezafibrate, benzbromarone, carbocisteine, and flunitrazepam, increased the risk of rhabdomyolysis for pravastatin, and only benzbromarone increased the risk of rhabdomyolysis for pitavastatin except multiple DDIs. No combination with fluvastatin and simvastatin increased the risk of developing rhabdomyolysis.
Verification by animal experiments
There was one death in the PV group on day 13 of drug administration and one death in the PAV group on day 14 of drug administration. No deaths occurred in the remaining groups (Table 2). Therefore, pathological evaluation of CK, myoglobin levels, and lower leg muscles was performed on day 14 in rats that survived until the timepoint immediately before cardiac perfusion.
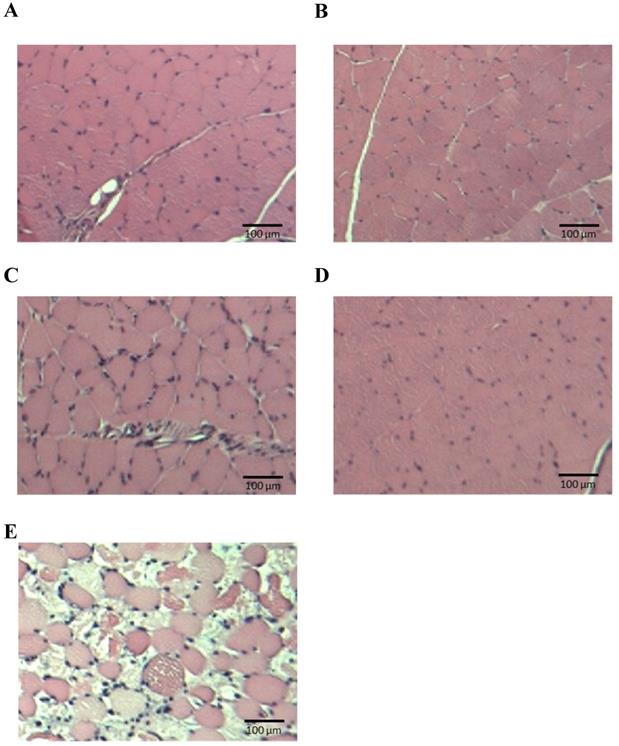
On day 7 of drug administration, the CK level in all groups was similar to that in P group. However, on day 14, the CK level in PAV group was significantly higher than that in P group (Fig. 3, Table S1). Moreover, no significant difference was observed in the myoglobin concentration on day 14, but it tended to increase only in the PAV group (Fig. 4, Table S1). Pathological evaluation of lower leg muscles verified necrosis of the lower leg muscles in two of the nine surviving rats in PAV group only (Table 2, Fig. 5).
Pathological specimens of lower leg muscles. (A) P, pitavastatin; (B) PA, pitavastatin + allopurinol; (C) PV, pitavastatin + valsartan; (D) AV, allopurinol + valsartan; (E) PAV, pitavastatin + allopurinol + valsartan.
Pathological evaluation of necrosis in lower leg muscles
| Group | Necrosis/survived |
|---|---|
| P | 0/10 |
| PA | 0/10 |
| PV | 0/9 |
| AV | 0/10 |
| PAV | 2/9 |
P, pitavastatin; PA, pitavastatin + allopurinol; PV, pitavastatin + valsartan; AV, allopurinol + valsartan; and PAV, pitavastatin + allopurinol + valsartan.
Discussion
In this study, multiple DDIs were identified with pitavastatin, allopurinol, and valsartan. Although cytochromes P450 (CYPs) are involved in the metabolism of many statins, pitavastatin is rarely metabolized by CYPs and it is considered a statin with low risk of CYP-related DDIs [34]. Therefore, pitavastatin is the preferred drug for polypharmacy patients at high risk of DDIs. However, in the present study, only pitavastatin was found to have multiple DDIs.
Musculoskeletal ADEs have been reported for allopurinol [35], and rhabdomyolysis is distributed as a severe ADE in the Japanese package insert [14]. However, no study has reported ADEs due to DDIs associated with the concomitant use of pitavastatin and allopurinol. Both pitavastatin and valsartan are taken up in the liver by OATP1B1 and are excreted into the bile [36,37]. Our study findings suggested that the mechanism of multiple DDIs of “pitavastatin + allopurinol + valsartan” identified in the decision tree analysis of JADER database is an additive action between the combination of pitavastatin and allopurinol on rhabdomyolysis and a decrease in the excretion of pitavastatin and valsartan due to competition for uptake into the liver. Further studies are required to clarify the mechanism of multiple DDIs to improve the level of evidence for the detected signals.
In this study, we also identified pairwise DDIs. Several drugs listed in Table 1 such as bezafibrate, pioglitazone, sitagliptin, which significantly increased the risk of statin-induced rhabdomyolysis, have been reported to be associated with rhabdomyolysis [38-40]. Furthermore, the combination of statins with amlodipine or sitagliptin has been reported to increase the risk of rhabdomyolysis [41-43]. Therefore, the results of this study using decision tree analysis reflect previous findings and may be useful for detecting not only multiple DDIs but also pairwise DDIs.
We have previously reported that the number of days of statin administration in patients developing rhabdomyolysis is reduced by concomitant drugs [44]. We believe that the number of days of administration is an important factor in DDIs, for example, in determining whether multiple DDIs further reduce or do not change the number of days of administration before the onset of rhabdomyolysis. Therefore, we considered it necessary to include the number of days of administration in the analysis of multiple DDIs in this study, and attempted to analyze them. However, in the PAV combination group, the number of days of administration could be calculated in only one case, making analysis difficult.
JADER is an SRS that can perform time-to-onset analysis because it registers the start date of administration of each drug and the onset date of each ADE [17]. However, the deficiency rate of JADER is approximately 30% [45], and the accumulation of cases with no deficiency is necessary to increase the number of available cases for decision tree analysis.
Limitations
Statins rarely cause rhabdomyolysis; however, they pose a risk of developing rhabdomyolysis in a dose-dependent manner [46]. Therefore, the statin dose used in animal experiments to evaluate the risk of rhabdomyolysis is usually high [28-30]. As a result, although the animal experiments in this study supported the results of decision tree analysis, they do not reflect the actual clinical doses.
Moreover, as the JADER database population consists of patients with ADEs, the incidence of rhabdomyolysis cannot be calculated using data from the database alone. The reporting rate of rhabdomyolysis obtained in this study should be used only for the relative comparison of concomitant risk for the cases registered in JADER, and it should not be extrapolated to the incidence in daily clinical practice.
Conclusions
A decision tree analysis of cases registered in the JADER database detected multiple DDIs with the combination of pitavastatin, allopurinol, and valsartan. Furthermore, animal studies supported the DDI signals obtained from decision tree analysis of JADER database, indicating that the risk of rhabdomyolysis increased with the combination of these three drugs. Our results suggested that decision tree analysis using the JADER database provided a method for detecting multiple DDIs, which has been expected to be utilized in the SRS database and will contribute useful findings to the advancement of theoretical deprescribing.
Abbreviations
ADEs: Adverse drug events; DDIs: drug-drug interactions; IR: initial rate; SRS: spontaneous reporting system; JADER: Japanese Adverse Drug Event Report; CK: creatine kinase.
Supplementary Material
Supplementary figure and table.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ahn EK, Cho SY, Shin D, Jang C, Park RW. Differences of reasons for alert overrides on contraindicated co-prescriptions by admitting department. Healthc Inform Res. 2014;20(4):280-7
2. Fokter N, Mozina M, Brvar M. Potential drug-drug interactions and admissions due to drug-drug interactions in patients treated in medical departments. Wien Klin Wochenschr. 2010;122(3-4):81-8
3. Missiakos O, Baysari MT, Day RO. Identifying effective computerized strategies to prevent drug-drug interactions in hospital: A user-centered approach. Int J Med Inform. 2015;84(8):595-600
4. Yeh ML, Chang YJ, Wang PY, Li YC, Hsu CY. Physicians' responses to computerized drug-drug interaction alerts for outpatients. Comput Methods Programs Biomed. 2013;111(1):17-25
5. Rogers AS. Adverse drug events: Identification and attribution. Drug Intell Clin Pharm. 1987;21(11):915-20
6. Diasio RB. Sorivudine and 5-fluorouracil; a clinically significant drug-drug interaction due to inhibition of dihydropyrimidine dehydrogenase. Br J Clin Pharmacol. 1998;46(1):1-4
7. Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72(6):685-91
8. Klotz U, Beil W, Gleiter C, Drewelow B, Garbe E, Gillessen A, Mutschler E. Drug interactions. Mechanisms and clinical relevance. Internist (Berl). 2003;44(11):1444-9
9. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112-5
10. Ikemura K, Hiramatsu SI, Shinogi Y, Nakatani Y, Tawara I, Iwamoto T, Katayama N, Okuda M. Concomitant febuxostat enhances methotrexate-induced hepatotoxicity by inhibiting breast cancer resistance protein. Sci Rep. 2019;9(1):20359
11. Yue Z, Shi J, Li H, Li H. Association between concomitant use of acyclovir or valacyclovir with NSAIDs and an increased risk of acute kidney injury: Data mining of FDA Adverse Event Reporting System. Biol Pharm Bull. 2018;41(2):158-62
12. Floyd JS, Kaspera R, Marciante KD, Weiss NS, Heckbert SR, Lumley T, Wiggins KL, Tamraz B, Kwok PY, Totah RA, Psaty BM. A screening study of drug-drug interactions in cerivastatin users: An adverse effect of clopidogrel. Clin Pharmacol Ther. 2012;91(5):896-904
13. Inaba I, Kondo Y, Iwasaki S, Tsuruhashi S, Akaishi A, Morita K, Oniki K, Saruwatari J, Ishitsuka Y, Irie T. Risk evaluation for acute kidney injury induced by the concomitant use of valacyclovir, analgesics, and renin-angiotensin system inhibitors: The detection of signals of drug-drug interactions. Front Pharmacol. 2019;10:874
14. Gosho M. Rhabdomyolysis risk from the use of two-drug combination of antidyslipidemic drugs with antihypertensive and antidiabetic medications: A signal detection analysis. Fundam Clin Pharmacol. 2019;33(3):339-46
15. Hovstadius B, Petersson G. The impact of increasing polypharmacy on prescribed drug expenditure-a register-based study in Sweden 2005-2009. Health Policy. 2013;109(2):166-74
16. Kim HA, Shin JY, Kim MH, Park BJ. Prevalence and predictors of polypharmacy among Korean elderly. PLoS One. 2014;9(6):e98043
17. Abe J, Umetsu R, Uranishi H, Suzuki H, Nishibata Y, Kato Y, Ueda N, Sasaoka S, Hatahira H, Motooka Y, Masuta M, Nakamura M. Analysis of polypharmacy effects in older patients using Japanese Adverse Drug Event Report database. PLoS One. 2017;12(12):e0190102
18. Mortazavi SS, Shati M, Keshtkar A, Malakouti SK, Bazargan M, Assari S. Defining polypharmacy in the elderly: A systematic review protocol. BMJ Open. 2016;6(3):e010989
19. Reeve E, Thompson W, Farrell B. Deprescribing: A narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med. 2017;38:3-11
20. Susuta Y, Takahashi Y. Safety risk evaluation methodology in detecting the medicine concomitant use risk which might cause critical drug rash. 2014; 19(1): 39-49.
21. Yao X, Tsang T, Sun Q, Quinney S, Zhang P, Ning X, Li L, Shen L. Mining and visualizing high-order directional drug interaction effects using the FAERS database. BMC Med Inform Decis Mak. 2020;20(Suppl 2):50
22. Imai S, Yamada T, Kasashi K, Kobayashi M, Iseki K. Usefulness of a decision tree model for the analysis of adverse drug reactions: Evaluation of a risk prediction model of vancomycin-associated nephrotoxicity constructed using a data mining procedure. J Eval Clin Pract. 2017;23(6):1240-6
23. Gao L, Smielewski P, Li P, Czosnyka M, Ercole A. Signal information prediction of mortality identifies unique patient subsets after severe traumatic brain injury: a decision-tree analysis approach. J Neurotrauma. 2020;37(7):1011-9
24. Birjandi SM, Khasteh SH. A survey on data mining techniques used in medicine. J Diabetes Metab Disord. 2021;20(2):2055-71
25. Nakayama N, Oketani M, Kawamura Y, Inao M, Nagoshi S, Fujiwara K, Tsubouchi H, Mochida S. Algorithm to determine the outcome of patients with acute liver failure: A data-mining analysis using decision trees. J Gastroenterol. 2012;47(6):664-77
26. Song YY, Lu Y. Decision tree methods: Applications for classification and prediction. Shanghai Arch Psychiatry. 2015;27(2):130-5
27. World Health Organization. Quality Assurance and Safety of Medicines Team. Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. Geneva, Switzerland: World Health Organization. 2002
28. Dorajoo Rs, Pereira BP, Yu Z, Gopalakrishnakone P, Leong CC, Wee A, Lee E. Role of multi-drug resistance-associated protein-1 transporter in statin-induced myopathy. Life Sci. 2008;82(15-16):823-30
29. Naba H, Kakinuma C, Ohnishi S, Ogihara T. Improving effect of ethyl eicosapentanoate on statin-induced rhabdomyolysis in Eisai hyperbilirubinemic rats. Biochem Biophys Res Commun. 2006;340(1):215-20
30. Schaefer WH, Lawrence JW, Loughlin AF, Stoffregen DA, Mixson LA, Dean DC, Raab CE, Yu NX, Lankas GR, Frederick CB. Evaluation of ubiquinone concentration and mitochondrial function relative to cerivastatin-induced skeletal myopathy in rats. Toxicol Appl Pharmacol. 2004;194(1):10-23
31. Akiba T, Shibuta T, Amano Y, Okubo M, Asanuma A, Koga T, Tanaka M, Takimoto M. 28-day repeated oral toxicity study of a hypolipidemic agent, NK-104 in rats. J Toxicol Sci. 1998;23(Suppl 5):701-11
32. Suzuki Y, Sudo J, Tanabe T. Allopurinol toxicity: Its toxic organ-specificity between the liver and the kidney in the rat. J Toxicol Sci. 1984;9(4):343-51
33. Goyal S, Bharti S, Sahoo KC, Sharma AK, Arya DS. Valsartan, an angiotensin II receptor blocker, attenuates cardiac dysfunction and oxidative stress in isoproterenol-induced cardiotoxicity. Cardiovasc Toxicol. 2011;11(2):148-56
34. Mukhtar RYA, Reid J, Reckless JPD. Pitavastatin. Int J Clin Pract. 2005;59(2):239-52
35. Ryu HJ, Song R, Kim HW, Kim JH, Lee EY, Lee YJ, Song YW, Lee EB. Clinical risk factors for adverse events in allopurinol users. J Clin Pharmacol. 2013;53(2):211-6
36. Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013;65(2):809-48
37. Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311(1):139-46
38. Kanterewicz E, Sanmartí R, Riba J, Trias I, Autonell J, Brugués J. Bezafibrate induced rhabdomyolysis. Ann Rheum Dis. 1992;51(4):536-8
39. Slim R, Ben Salem C, Zamy M, Biour M. Pioglitazone-induced acute rhabdomyolysis. Diabetes Care. 2009;32(7):e84
40. Labat V, Arnaud M, Miremont-Salamé G, Salvo F, Bégaud B, Pariente A. Risk of myopathy associated with DPP-4 inhibitors in combination with statins: A disproportionality analysis using data from the WHO and French spontaneous reporting databases. Diabetes Care. 2017;40(3):e27-9
41. Bhome R, Penn H. Rhabdomyolysis precipitated by a sitagliptin-atorvastatin drug interaction. Diabet Med. 2012;29(5):693-4
42. Khan S, Khan I, Novak M, Regmi A, Difilippo W. The concomitant use of atorvastatin and amlodipine leading to rhabdomyolysis. Cureus. 2018;10(1):e2020
43. Skovbølling SL, Lindelof M. Myopathy and rhabdomyolysis after treatment with simvastatin, amlodipine, and roxithromycin. Ugeskr Laeger. 2014;176(41):V04140212
44. Akimoto H, Negishi A, Oshima S, Okita M, Numajiri S, Inoue N, Ohshima S, Kobayashi D. Onset timing of statin-induced musculoskeletal adverse events and concomitant drug-associated shift in onset timing of MAEs. Pharmacol Res Perspect. 2018;6(6):e00439
45. Hasegawa S, Ikesue H, Satake R, Inoue M, Yoshida Y, Tanaka M, Matsumoto K, Wakabayashi W, Oura K, Muroi N, Hashida T, Iguchi K, Nakamura M. Osteonecrosis of the jaw caused by denosumab in treatment-naïve and pre-treatment with zoledronic cid groups: A time-to-onset study using the Japanese Adverse Drug Event Report (JADER) database. Drugs Real World Outcomes. 2022
46. Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: A case series of 354 patients. Pharmacotherapy. 2010;30(6):541-53
Author contact
![]() Corresponding author: Dr. Shinji Oshima; Tel: +81-49-271-8018; E-mail: soshimaac.jp.
Corresponding author: Dr. Shinji Oshima; Tel: +81-49-271-8018; E-mail: soshimaac.jp.

 Global reach, higher impact
Global reach, higher impact