3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(14):3106-3111. doi:10.7150/ijms.60200 This issue Cite
Research Paper
Microbial growth and importance of flushing inside closed-type infusion devices during administration of lipid emulsion in vitro setting
Faculty of Pharmacy, Osaka Ohtani University.
Abstract
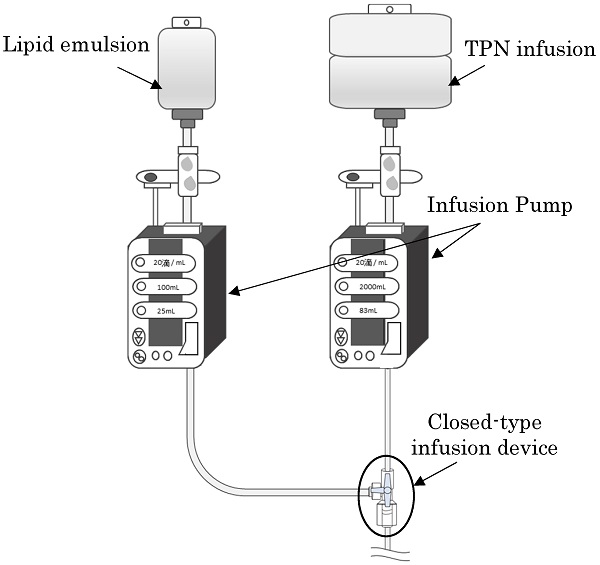
Background: We investigated the extent of growth of microorganisms with simultaneous administration of lipid emulsions with infusions for Total Parenteral Nutrition (TPN), assuming that the lipid emulsions contaminated with microorganisms are stagnant in a closed-type infusion device. We also investigated if bacterial growth can be prevented in the infusion device by flushing the inside of the infusion device with saline solution after the administration of lipid emulsion from the side tube in vitro setting.
Methods: We made a preparation by adding Escherichia coli to the lipid emulsion and started the infusion simultaneously with the infusion solution for TPN and lipid emulsion with the piggyback method. Immediately after the completion of lipid emulsion infusion, we conducted flushing with saline solution. The volume of saline solution was none, 5, 10, or 20 mL at a flow rate of 1 mL/s. Infusion solution that was stagnant in the infusion device was collected immediately before completing the lipid emulsion infusion and 20 h after flushing, i.e., 24 h after starting the infusion for TPN, and the number of viable bacteria was determined.
Results: The number of viable E. coli increased in the infusion device of all three species used in this experiment 24 h after starting the lipid emulsion infusion without flushing. We found that bacterial growth could be prevented through flushing with saline solution after the completion of lipid emulsion infusion and flushing out the stagnant infusion solution in the closed-type infusion device.
Conclusions: We found that if E. coli was present in the closed-type infusion device, it would multiply. We also found that the number of viable bacteria varied according to the variety and internal structure of the closed-type infusion device as well as the liquid volume used for flushing, although flushing can prevent the growth of microorganisms. Proper management and manipulation of infusion is required to prevent infection.
Keywords: Total Parenteral Nutrition (TPN), lipid emulsion, closed-type infusion device, flushing, catheter-related blood stream infections (CRBSI)

 Global reach, higher impact
Global reach, higher impact