Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(5):1198-1206. doi:10.7150/ijms.50039 This issue Cite
Research Paper
Risk factors for mortality of critically ill patients with COVID-19 receiving invasive ventilation
1. Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
2. Department of Clinical Laboratory, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
3. Department of Epidemiology and Biostatistics, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Received 2020-6-28; Accepted 2020-12-22; Published 2021-1-11
Abstract
Rationale: Early invasive ventilation may improve outcomes for critically ill patients with COVID-19. The objective of this study is to explore risk factors for 28-day mortality of COVID-19 patients receiving invasive ventilation.
Methods: 74 consecutive adult invasively ventilated COVID-19 patients were included in this retrospective study. The demographic and clinical data were compared between survivors and non-survivors, and Cox regression analysis was used to explore risk factors for 28-day mortality. The primary outcome was 28-day mortality after initiation of invasive ventilation. Secondary outcome was the time from admission to intubation.
Results: Of 74 patients with COVID-19, the median age was 68.0 years, 53 (71.6%) were male, 47 (63.5%) had comorbidities with hypertension, and diabetes commonly presented. The most frequent symptoms were fever and dyspnea. The median time from hospital admission to intubation was similar in survivors and non-survivors (6.5 days vs. 5.0 days). The 28-day mortality was 81.1%. High Sequential Organ Failure Assessment (SOFA) score (hazard ratio [HR], 1.54; 95% confidence interval [CI], 1.23-1.92; p < 0.001) and longer time from hospital admission to intubation (HR, 2.41; 95% CI, 1.15-5.07; p = 0.020) were associated with 28-day mortality in invasively ventilated COVID-19 patients.
Conclusions: The mortality of invasively ventilated COVID-19 patients was particularly striking. Patients with high SOFA score and receiving delayed invasive ventilation were at high risk of mortality.
Keywords: COVID-19, critically ill, mortality, risk factor, invasive ventilation
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) first occurred in Wuhan, China. As of November 26, 2020, 60074174 SARS-CoV-2 infections had been reported worldwide. The clinical spectrum of COVID-19 is heterogeneous, ranging from asymptomatic infection to acute hypoxic respiratory failure [1]. Among hospitalized patients, 2.3% to 19% required supportive invasive mechanical ventilation as part of their treatments for days to weeks [2-6]. Although it was reported that dexamethasone could reduce mortality in invasively ventilated COVID-19 patients [7], owing to the lack of effective agents to date, the timely intubation and invasive mechanical ventilation play a crucial role in countering a progressively oxygen debt and saving life in critically ill COVID-19 patients [8]. Determining whether and when to intubate and mechanically ventilate a COVID-19 case with hypoxemic respiratory failure is an intricate decision based on both patient conditions and clinician evaluation, and may have vital implications for individual prognosis. For example, delayed mechanical ventilation is associated with worsened clinical outcomes in ARDS [9]. High respiratory drive leading to self-induced lung injury has been suggested as a potential mechanism underlying these observations [10]. Correspondingly, utilization of high-flow nasal cannula, which can produce 30-60 L/min of supplemental oxygen and reduce dead space, may cover up the clinical deterioration, therefore delay the time to endotracheal intubation and exacerbate respiratory failure [9]. COVID-19 patients presenting with acute respiratory failure were intubated empirically in particular with those manifesting no improvement with non-invasive ventilation, persistent respiratory distress and poor oxygenation (PaO2 to FiO2 ratio < 150 mmHg) after 2-hours high-flow oxygen therapy or noninvasive ventilation. It may be rational to intubate and mechanically ventilate following the early indications of non-invasive ventilation failure. To date, information about clinical characteristics of patients who required invasive ventilation remains scarce, and risk factors associated with poor outcome of these patients have not been identified, which are of great importance to reduce mortality of critically ill COVID-19 patients.
To address these questions, we carried out a cohort study of invasively ventilated patients with COVID-19. Our primary hypothesis was that longer time from hospital admission to intubation would be associated with increased 28-day mortality. In addition, we explored risk factors associated with 28-day mortality in invasively ventilated COVID-19 patients.
Methods
Study design and participants
This single-centered, retrospective, observational study included consecutive patients from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, which was a designated hospital for severe and critically ill patients with COVID-19 in Wuhan, China. From January 10 to February 29, 2020, Adult patients admitted to intensive care unit (ICU) and receiving invasive ventilation were enrolled in this study. COVID-19 was diagnosed according to the World Health Organization (WHO) interim guidance. The study was approved by the Institutional Ethics Board of Tongji Hospital (TJ-IRB20200347). Written informed consent was waived due to the rapid emergence of this infectious disease.
Data collection
Epidemiological, demographic, clinical, laboratory, radiological, and treatment data were extracted from electronic medical records. All data were checked and reviewed by three physicians (QL, PY, YT). Information recorded included demographic data, comorbidities, symptoms from illness onset to hospital admission, chest computed tomographic (CT) scans, treatments, living status, etc.
Throat swab samples were obtained for SARS-CoV-2 detection using real-time RT-PCR. 200 µL of sample was used for laboratory confirmation of SARS-CoV-2 RNA with RNA isolation kit (Yuesui equipment No.20170583 and 20150302, Da'an gene Co., Ltd, Sun Yat-sen University, China) according to the manufacturer's instructions. A cycle threshold value less than 40 was defined as positive response.
Laboratory values on the day of hospital admission and within 24 hours before endotracheal intubation were recorded, including complete blood count, erythrocyte sedimentation rate, blood biochemical examinations (i.e., liver and renal function, blood glucose, lactate dehydrogenase [LDH], and electrolytes), myocardial enzymes, coagulation profile, cytokine profile, N-terminal pro-B-type natriuretic peptide (NT-proBNP), D-dimer, high sensitivity C-reactive protein (hsCRP), procalcitonin, and ferritin.
Study outcomes
The primary outcome was 28-day mortality after receipt of invasive mechanical ventilation invasive ventilation. Secondary outcome was the time from hospital admission to intubation.
Definitions
The disease severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (Trial Version 7), in which patients with respiratory failure who require mechanical ventilation, or shock, or other organ failure who require intensive care treatment, are classified as critical illness [11]. Acute cardiac injury was defined as serum level of high-sensitivity cardiac troponin I (hsTnI) above 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography [12]. ARDS was defined according to the Berlin definition [13]. Septic shock and sepsis were defined according to the Third International Consensus Definitions for Sepsis and Septic Shock [14]. Acute heart failure was defined according to the European Society of Cardiology criteria [15]. Acute kidney injury was defined according to the KDIGO clinical practice guidelines [16]. Disseminated intravascular coagulation (DIC) was defined according to the JAAM DIC diagnostic criteria [17].
Statistical analysis
Continuous variables were described as medians and inter-quartile ranges (IQR), and categorical variables as numbers and percentages. The differences between survivors and non-survivors were compared using Mann-Whitney U test, χ² test, or Fisher's exact test, as appropriate. The differences in laboratory findings between hospital admission and before invasive ventilation were compared using Wilcoxon signed-rank test or McNemar's test, as appropriate. For survival analysis, Kaplan-Meier plots were made to visualize the effects of age, cardiac injury and the time from hospital admission to intubation (≥120 h) on 28-day mortality with log-rank test. Univariable and multivariable Cox proportional hazards regression models were used to explore the risk factors associated with 28-day mortality in COVID-19 patients receiving invasive ventilation. Models were adjusted for age, sex and comorbidities. We excluded variables from univariable analysis if their between-group differences were not significant, if the numbers of events were too small to calculate hazard ratios, and if they had collinearity with Sequential Organ Failure Assessment (SOFA) score. The analyses regarding different factors were based on non-missing data, and missing data were not imputed. All statistical analyses were conducted using SPSS version 19.0 and MedCalc software with the level of significance set to α=0.05 (two-tailed).
Results
Patient characteristics
109 critically ill patients receiving invasive ventilation were enrolled. After excluding 32 patients without confirmed COVID-19, and 3 patients with incomplete core information in their electronic medical records, 74 patients were included in the final analysis, and 60 (81.1%) of them died at 28 days. As of April 14, 2020, 63 (85.1%) of 74 patients died, 11 (14.9%) extubated and discharged.
Of the 74 patients, the median age was 68.0 years (inter-quartile range [IQR], 61.5-74.0; range, 44.0-87.0 years), and 53 (71.6%) were male. The proportion of non-survivors aged 70 years and older was significantly higher than survivors (37/60 [61.6%] vs. 4/14 [28.6%]; p = 0.036). 47 (63.5%) of 74 patients had one or more comorbidities, with hypertension, cardiovascular disease and diabetes commonly presented. The most frequent symptom was fever (68 [91.9%]), followed by dyspnea (44 [59.5%]), dry cough (43 [58.1%]), fatigue (27 [36.5%]), and diarrhea (24 [32.4%]). Less common symptoms were sputum production, myalgia, headache, nausea/vomiting, and abdominal pain (Table 1). Abnormalities in thoracic CT scans were observed among all of the 74 patients, and typical CT findings included bilateral pulmonary parenchymal ground-glass and consolidative opacities. Of the 74 patients, the median time from symptom onset to hospital admission, the time from hospital admission to intubation, and the time from symptom onset to intubation were 10.5 days (IQR, 7.0-15.3), 6.0 days (IQR, 4.0-10.0), and 18.4 days (IQR, 15.0-24.0) respectively. The time from endotracheal intubation to death was 5.0 (IQR, 3.0-9.0) days in non-survivors. Demographic, clinical, and radiological features did not show significant differences between survivors and non-survivors (Table 1).
Demographics and clinical characteristics in patients with COVID-19
| Total (n = 74) | Non-survivors (n = 60) | Survivors (n = 14) | p-value | ||
|---|---|---|---|---|---|
| Age, years | 68.0 (61.5-74.0) | 69.0 (63.0-74.0) | 66.5 (57.8-72.8) | 0.365 | |
| 18-69 years | 33/74 (44.6%) | 23/60 (38.3%) | 10/14 (71.4%) | 0.036 | |
| ≥ 70 years | 41/74 (55.4%) | 37/60 (61.6%) | 4/14 (28.6%) | .. | |
| Gender, male | 53/74 (71.6%) | 43/60 (71.7%) | 10/14 (71.4%) | 1.000 | |
| Comorbidities | |||||
| Hypertension | 29/74 (39.2%) | 25/74 (33.8%) | 4/14 (28.6%) | 0.545 | |
| Diabetes | 14/74 (18.9%) | 12/60 (20.0%) | 2/14 (14.3%) | 1.000 | |
| Cardiovascular disease | 13/74 (27.7%) | 12/60 (20.0%) | 1/14 (7.1%) | 0.440 | |
| Cerebrovascular disease | 5/74 (6.8%) | 4/60 (6.7%) | 1/14 (7.1%) | 1.000 | |
| Chronic obstructive pulmonary disease | 7/74 (9.5%) | 6/60 | 1/14 (7.1%) | 1.000 | |
| Chronic kidney disease | 1/74 (1.4%) | 1/60 | 0/14 (0.0%) | 1.000 | |
| Chronic liver disease | 1/74 (1.4%) | 1/60 | 0/14 (0.0%) | 1.000 | |
| Symptoms | |||||
| Fever | 68/74 (91.9%) | 55/60 (91.7%) | 13/14 (92.9%) | 1.000 | |
| Dry cough | 43/74 (58.1%) | 34/60 (56.7%) | 9/14 (64.3%) | 0.271 | |
| Sputum production | 18/74 (24.3%) | 17/60 (28.3%) | 1/14 (7.1%) | 0.165 | |
| Dyspnea | 44/74 (59.5%) | 35/60 (58.3%) | 9/14 (64.3%) | 0.167 | |
| Fatigue | 27/74 (36.5%) | 24/60 (40.0%) | 3/14 (21.2%) | 0.233 | |
| Diarrhea | 24/74 (32.4%) | 22/60 (36.7%) | 2/14 (14.3%) | 0.127 | |
| Myalgia | 10/74 (13.5%) | 9/60 (15.0%) | 1/14 (7.1%) | 0.676 | |
| Nausea/vomiting | 5/74 (6.8%) | 4/60 (6.7%) | 1/14 (7.1%) | 1.000 | |
| Headache | 8/74 (10.8%) | 7/60 (11.75) | 1/14 (7.1%) | 1.000 | |
| Abdominal pain | 2/74 (2.7%) | 2/60 (3.3%) | 0/14 (0.0%) | 1.000 | |
| Vital signs | |||||
| Heart rate, beats per min | 90.0 (80.0-102.0) | 89.5 (79.3-102.0) | 92.0 (82.0-102.0) | 0.729 | |
| Respiratory rate, breaths per min | 22.5 (20.0-30.0) | 23.0 (20.0-30.0) | 22.0 (20.0-30.0) | 0.801 | |
| Mean arterial pressure, mmHg | 99.0 (89.7-107.3) | 99.3 (91.3-107.3) | 94.7 (86.7-105.7) | 0.404 | |
| Time from symptom onset to hospital admission, days | 10.5 (7.0-15.3) | 10.5 (7.0-14.8) | 12.5 (8.3-18.5) | 0.283 | |
| Time from hospital admission to invasive ventilation, days | 6.0 (4.0-10.0) | 6.5 (4.3-10.0) | 5.0 (1.5-10.3) | 0.225 | |
| Time from symptom onset to invasive ventilation, days | 18.4 (15.0-24.0) | 18.4 (15.0-23.7) | 18.9 (14.0-26.5) | 0.836 | |
Data are median (inter-quartile range) or n (%). COVID-19, coronavirus disease 2019.
Laboratory findings
On hospital admission, neutrophilia (neutrophil count ≥ 6.3 × 109/L), lymphopenia (lymphocyte count < 0.8 × 109/L), eosinopenia (eosinophil count < 0.02 × 109/L) and hypoalbuminemia (level of albumin < 30 g/L) were detected in 41 (55.4%), 52 (70.3%), 62 (83.8%) and 26 (35.6%) patients, respectively. Increased levels of LDH, D-dimer, hsTnI, and NT-proBNP beyond the normal values were detected in 68 (91.9%), 63 (91.3%), 19 (30.6%), and 49 (84.5%) patients, respectively. Levels of hsCRP, procalcitonin, ferritin, interleukin-2 receptor (IL-2R), and interleukin-6 (IL-6) in most patients were far beyond the normal values. Non-survivors had higher creatine kinase isoenzyme-MB and NT-proBNP than survivors (Table 2).
Before intubation, white blood cell (WBC) and neutrophil counts were increased, and leukocytosis (WBC count ≥ 10×109/L) and increased neutrophils (≥ 6.3×109/L) were more frequent than hospital admission in the 74 patients (Table 3). Compared with survivors, non-survivors had more frequent cardiac injury with increased hsTnI and NT-proBNP, coagulation dysfunction with prolonged prothrombin time and increased international normalized ratio, and remarkably higher Acute Physiology and Chronic Health Evaluation (APACHE) II and SOFA scores before invasive ventilation (Table 3).
Laboratory findings in patients with COVID-19 on hospital admission
| Total (n = 74) | Non-survivors (n = 60) | Survivors (n = 14) | p-value | |
|---|---|---|---|---|
| White blood cell count, ×109/L | 8.0 (5.7-11.6) | 8.1 (5.9-11.6) | 7.5 (3.9-16.9) | 0.535 |
| < 4 | 6/74 (8.1%) | 3/60 (5.0%) | 3/14 (21.4%) | 0.015 |
| 4-10 | 46/74 (62.2%) | 42/60 (67.7%) | 4/14 (28.6%) | .. |
| ≥ 10 | 22/74 (29.7%) | 15/62 (24.2%) | 7/14 (50.0%) | .. |
| Neutrophil count, ×109/L | 7.2 (4.3-11.2) | 7.2 (4.7-9.9) | 6.1 (2.8-12.3) | 0.408 |
| < 1.8 | 1/74 (1.4%) | 0/60 (0.0%) | 1/14 (7.1%) | < 0.001 |
| 1.8-6.3 | 32/74 (43.2%) | 25/60 (41.7%) | 7/14 (50.0%) | .. |
| ≥ 6.3 | 41/74 (55.4%) | 35/60 (58.3%) | 6/14 (42.9%) | .. |
| Lymphocyte count, ×109/L | 0.64 (0.44-0.94) | 0.64 (0.42-0.95) | 0.73 (0.49-0.96) | 0.359 |
| < 0.8 | 52/74 (70.3%) | 44/52 (84.6%) | 10/14 (71.4%) | 0.264 |
| Monocyte count, × 109/L | 0.41 (0.29-0.60) | 0.39 (0.28-0.60) | 0.53 (0.33-0.62) | 0.444 |
| Eosinophil count, × 109/L | 0.00 (0.00-0.01) | 0.00 (0.00-0.01) | 0.00 (0.00-0.02) | 0.712 |
| < 0.02 | 62/74 (83.8%) | 49/60 (81.7%) | 13/14 (92.9%) | 0.432 |
| Hemoglobin, g/dL | 135.0 (124.0-144.0) | 135.0 (124.3-144.0) | 137.0 (117.8-146.3) | 0.907 |
| Platelet count, × 109/L | 162.0 (133.5-222.0) | 160.5 (131.0-222.0) | 169.5 (134.5-235.3) | 0.760 |
| < 100 | 10/74 (13.5%) | 9/60 (15.0%) | 1/14 (7.1%) | 0.676 |
| Alanine transaminase, U/L | 29.5 (19.0-44.5) | 33.0 (19.0-45.5) | 22.0 (155-38.8) | 0.197 |
| Aspartate transaminase, U/L | 42.0 (28.0-59.5) | 42.5 (30.0-61.5) | 34.5 (23.8-58.0) | 0.214 |
| > 40 | 40/74 (54.1%) | 35/60 (58.3%) | 5/14 (35.7%) | 0.126 |
| Albumin, g/L | 31.6 (29.1-34.6) | 31.8 (28.4-34.7) | 31.1 (29.7-32.7) | 0.801 |
| < 30 | 26/73 (35.6%) | 22/60 (36.7%) | 4/13 (30.8%) | 0.760 |
| Total bilirubin, μmol/L | 13.4 (10.0-19.3) | 13.4 (9.7-19.2) | 12.9 (10.5-20.2) | 0.735 |
| Direct bilirubin, μmol/L | 6.2 (4.4-10.5) | 5.8 (4.4-10.2) | 8.5 (4.9-11.5) | 0.266 |
| Indirect bilirubin, μmol/L | 6.4 (4.7-9.0) | 6.6 (4.9-9.1) | 5.4 (3.8-8.7) | 0.204 |
| Lactate dehydrogenase, U/L | 495.5 (416.5-691.8) | 530.5 (427.8-721.0) | 462.5 (245.8-653.0) | 0.086 |
| > 225 | 68/72 (91.9%) | 58/58 (100.0%) | 10/14 (71.4%) | 0.001 |
| γ-glutamyl transpeptidase, U/L | 81.0 (61.5-98.0) | 40.5 (26.8-80.0) | 50.5 (34.3-80.3) | 0.584 |
| Blood urea nitrogen, mmol/L | 7.2 (5.3-10.4) | 7.5 (5.3-10.4) | 6.4 (4.5-10.6) | 0.330 |
| Creatinine, μmol/L | 81.0 (61.5-98.0) | 82.0 (59.0-97.0) | 74.0 (62.5-107.3) | 0.823 |
| > 104 | 14/73 (19.2%) | 11/59 (18.6%) | 3/14 (21.4%) | 1.000 |
| Potassium, mmol/L | 4.33 (3.77-4.74) | 4.32 (3.75-4.74) | 4.64 (3.79-4.78) | 0.474 |
| Sodium, mmol/L | 138.4 (134.6-142.6) | 139.0 (136.2-143.2) | 133.3 (131.8-137.0) | 0.001 |
| Calcium, mmol/L | 2.01 (1.92-2.09) | 2.06 (1.99-2.15) | 1.98 (1.95-2.04) | 0.006 |
| Blood glucose, mmol/L | 7.29 (6.36-10.42) | 7.29 (6.39-10.38) | 7.48 (5.89-13.37) | 0.784 |
| > 7.00 | 32/49 (65.3%) | 25/38 (65.8%) | 8/14 (57.1%) | 0.566 |
| Prothrombin time, seconds | 14.8 (13.7-16.3) | 14.9 (13.7-16.7) | 14.0 (13.2-15.7) | 0.181 |
| Activated partial thromboplastin time, seconds | 38.4 (34.7-43.3) | 38.2 (34.7-43.7) | 39.7 (33.7-43.4) | 0.887 |
| International normalized ratio | 1.15 (1.04-1.30) | 1.17 (1.06-1.33) | 1.09 (1.01-1.24) | 0.190 |
| D-dimer, μg/mL | 6.8 (1.3-21.0) | 7.9 (1.6-21.0) | 2.3 (0.5-18.8) | 0.130 |
| < 0.5 | 6/69 (8.7%) | 3/56 (5.4%) | 3/14 (21.4%) | 0.672 |
| 0.5-21 | 37/69 (53.6%) | 29/56 (51.8%) | 8/14 (57.1%) | .. |
| > 21 | 26/69 (37.7%) | 23/56 (41.1%) | 3/14 (21.4%) | .. |
| High-sensitivity cardiac troponin I, ng/L | 19.2 (8.1-46.2) | 22.0 (8.8-53.2) | 9.2 (6.1-19.0) | 0.058 |
| ≥ 34.2 | 19/62 (30.6%) | 18/49 (36.7%) | 1/13 (7.7%) | 0.050 |
| Myoglobin, ng/mL | 133.1 (59.5-199.7) | 134.0 (77.9-198.2) | 71.0 (41.4-244.1) | 0.412 |
| Creatine kinase isoenzyme-MB, ng/ML | 1.3 (0.8-2.7) | 1.7 (0.9-3.2) | 1.0 (0.6-1.4) | 0.025 |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 636.0 (193.0-1373.5) | 751.0 (336.5-1661.0) | 249.0 (69.3-555.8) | 0.004 |
| > 161 | 49/58 (84.5%) | 32/46 (69.6%) | 7/12 (58.3%) | 0.460 |
| High sensitivity C-reactive protein, pg/mL | 75.5 (45.5-146.6) | 75.8 (46.0-155.2) | 65.8 (22.7-119.9) | 0.441 |
| Erythrocyte sedimentation rate, mm/h | 32.0 (19.0-63.0) | 30.5 (18.3-61.5) | 75.0 (32.0-84.0) | 0.073 |
| Procalcitonin, ng/mL | 0.16 (0.10-0.33) | 0.16 (0.11-0.33) | 0.22 (0.10-0.36) | 0.612 |
| < 0.05 | 3/60 (5.0%) | 3/50 (6.0%) | 0/10 (0.0%) | 0.098 |
| 0.05-0.49 | 48/60 (80.0%) | 39/50 (78.0%) | 9/10 (90.0%) | .. |
| 0.5-1.99 | 5/60 (8.3%) | 5/50 (10.0%) | 0/10 (0.0%) | .. |
| ≥ 2 | 4/60 (6.7%) | 3/50 (6.0%) | 1/10 (10.0%) | .. |
| Serum ferritin, μg/L | 1598.5 (1064.5-2547.1) | 1587.3 (1066.5-2517.6) | 1598.5 (225.0-2014.5) | 0.860 |
| ≥ 400 | 33/35 (94.4%) | 31/32 (96.9%) | 2/3 (66.7%) | 0.166 |
| Interleukin-1β, pg/mL | 5.0 (5.0-5.0) | 5.0 (5.0-5.0) | 5.0 (5.0-7.4) | 0.280 |
| ≥ 5 pg/mL | 6/45 (13.3.0%) | 4/37 (10.8%) | 2/8 (25.0%) | 0.290 |
| Interleukin-2 receptor, U/L | 1072.0 (829.5-1409.5) | 1063.0 (829.5-1409.5) | 1087.5 (823.0-1452.8) | 0.812 |
| ≥ 710 | 37/45 (82.2%) | 30/37 (81.1%) | 7/8 (87.5%) | 1.000 |
| Interleukin-6, pg/mL | 40.3 (19.1-123.1) | 58.2 (23.5-134.5) | 25.8 (10.2-37.1) | 0.093 |
| ≥ 7 | 45/45 (100.0%) | 37/37 (100.0%) | 8/8 (100.0%) | 1.000 |
| Interleukin-8, pg/mL | 37.2 (15.7-63.4) | 37.4 (17.2-63.4) | 24.9 (10.5-119.5) | 0.635 |
| ≥ 62 pg/mL | 11/45 (24.4%) | 9/37 (24.3%) | 2/8 (25.0%) | 1.000 |
| Interleukin-10, pg/mL | 11.3 (5.2-17.8) | 9.8 (5.4-15.7) | 23.8 (7.5-49.1) | 0.063 |
| ≥ 9.2 pg/mL | 25/45 (55.6%) | 19/37 (51.4%) | 6/8 (75.0%) | 0.629 |
| Tumor necrosis factor-α, pg/mL | 11.2 (7.2-15.3) | 9.9 (7.5-13.0) | 10.7 (5.8-14.2) | 0.850 |
| > 8.1 | 30/45 (66.7%) | 25/37 (67.6%) | 5/8 (62.5%) | 1.000 |
Data are median (inter-quartile range) or n (%). COVID-19, coronavirus disease 2019.
Laboratory findings in patients with COVID-19 before invasive ventilation
| Total (n = 74) | Non-survivors (n = 60) | Survivors (n = 14) | p-value | |
|---|---|---|---|---|
| White blood cell count, × 109/L | 14.9 (10.6-17.9) | 15.1 (11.3-18.3) | 10.5 (7.8-17.9) | 0.103 |
| < 4 | 0/66 (0.0%) | 0/52 (0.0%) | 0/14 (0.0%) | 0.015 |
| 4-10 | 13/66 (19.7%) | 7/52 (13.4%) | 6/14 (42.9%) | .. |
| ≥ 10 | 53/66 (80.3%) | 46/52 (88.5%) | 7/14 (50.0%) | .. |
| Neutrophil count, × 109/L | 13.8 (9.7-16.9) | 14.1 (10.1-17.0) | 9.4 (5.8-16.8) | 0.083 |
| < 1.8 | 1/66 (1.5%) | 0/52 (0.0%) | 1/14 (7.1%) | < 0.001 |
| 1.8-6.3 | 3/66 (4.5%) | 0/52 (0.0%) | 3/14 (21.4%) | .. |
| ≥ 6.3 | 62/66 (94.0%) | 52/52 (100.0%) | 10/14 (71.4%) | .. |
| Lymphocyte count, × 109/L | 0.50 (0.37-0.72) | 0.49 (0.36-0.69) | 0.68 (0.37-0.79) | 0.265 |
| < 0.8 | 55/66 (83.3%) | 44/52 (84.6%) | 11/14 (78.6%) | 0.688 |
| Monocyte count, × 109/L | 0.46 (0.34-0.63) | 0.46 (0.34-0.64) | 0.46 (0.28-0.62) | 0.748 |
| Eosinophil count, × 109/L | 0.00 (0.00-0.05) | 0.00 (0.00-0.07) | 0.00 (0.00-0.08) | 1 |
| < 0.02 | 46/66 (69.7%) | 36/52 (69.2%) | 10/14 (71.4%) | 1.000 |
| Hemoglobin, g/dL | 127.5 (118.8-135.0) | 127.0 (119.5-134.8) | 130.5 (115.0-142.0) | 0.632 |
| Platelet count, × 109/L | 160.0 (98.0.0-222.3) | 160.0 (81.0-221.8) | 169.5 (128.7-258.8) | 0.308 |
| < 100 | 17/66 (25.8%) | 15/52 (28.8%) | 2/14 (14.3%) | 0.327 |
| Alanine transaminase, U/L | 27.0 (17.0-45.0) | 27.0 (17.0-47.0) | 27.0 (18.3-45.3) | 0.969 |
| Aspartate transaminase, U/L | 32.5 (21.0-44.8) | 26.0 (19.0-37.0) | 28.0 (21.5-53.8) | 0.962 |
| > 40 | 22/66 (33.3%) | 18/52 (34.6%) | 4/14 (28.6%) | 0.529 |
| Albumin, g/L | 29.5 (25.3-33.1) | 29.3 (25.2-33.1) | 30.5 (27.9-33.2) | 0.458 |
| < 30 | 33/65 (50.8%) | 27/52 (51.9%) | 6/13 (46.2%) | 0.764 |
| Total bilirubin, μmol/L | 16.7 (12.0-22.7) | 17.1 (12.6-22.7) | 14.2 (9.2-20.7) | 0.234 |
| Direct bilirubin, μmol/L | 7.9 (6.2-11.3) | 8.2 (6.2-11.2) | 7.2 (4.7-12.1) | 0.43 |
| Indirect bilirubin, μmol/L | 7.7 (5.4-10.5) | 7.7 (5.8-10.8) | 6.3 (3.3-9.5) | 0.165 |
| Lactate dehydrogenase, U/L | 595.5 (431.8-863.3) | 608.0 (426.8-926.8) | 477.0 (396.8-688.0) | 0.132 |
| > 225 | 64/66 (97.0%) | 51/52 (98.1%) | 13/14 (92.9%) | 0.382 |
| γ-glutamyl transpeptidase, U/L | 50.0 (35.0-81.0) | 49.0 (31.5-75.5) | 65.5 (38.3-93.0) | 0.342 |
| Blood urea nitrogen, mmol/L | 8.0 (5.4-11.4) | 8.7 (5.6-12.3) | 5.4 (4.8-10.5) | 0.051 |
| Creatinine, μmol/L | 67.0 (55.0-87.0) | 67.0 (57.5-87.5) | 69.0 (50.3-87.3) | 0.671 |
| > 104 | 9/67 (13.4%) | 7/53 (13.2%) | 2/14 (14.3%) | 1.000 |
| Potassium, mmol/L | 4.45 (3.85-4.80) | 4.46 (3.88-4.81) | 4.19 (3.62-4.89) | 0.54 |
| Sodium, mmol/L | 139.9 (134.4-144.0) | 141.3 (136.1-144.6) | 133.0 (129.8-137.3) | < 0.001 |
| Calcium, mmol/L | 2.01 (1.92-2.09) | 2.01 (1.93-2.09) | 2.00 (1.91-2.09) | 0.787 |
| Blood glucose, mmol/L | 9.07 (6.54-11.81) | 7.86 (6.36-11.30) | 9.95 (6.80-14.34) | 0.458 |
| > 7.00 | 32/49 (65.3%) | 25/38 (67.8%) | 7/11 (63.6%) | 1.000 |
| Prothrombin time, seconds | 15.7 (14.9-17.3) | 16.0 (15.0-18.1) | 15.0 (13.6-16.2) | 0.016 |
| Activated partial thromboplastin time, seconds | 37.8 (34.4-43.2) | 37.9 (35.0-42.9) | 37.1 (32.2-44.2) | 0.641 |
| International normalized ratio | 1.24 (1.14-1.38) | 1.25 (1.16-1.45) | 1.18 (1.05-1.31) | 0.046 |
| D-dimer, μg/mL | 14.3 (4.0-21.0) | 15.6 (6.0-21.0) | 8.2 (1.8-21.0) | 0.192 |
| < 0.5 | 1/55 (38.2%) | 1/42 (2.4%) | 0/13 (0.0%) | 0.672 |
| 0.5-21 | 33/55 (60.0%) | 24/42 (57.1%) | 9/13 (69.2%) | .. |
| ≥ 21 | 21/55 (1.8%) | 17/42 (40.4%) | 4/13 (30.8%) | .. |
| High-sensitivity cardiac troponin I, ng/L | 32.3 (13.7-232.6) | 43.8 (15.1-303.1) | 16.5 (8.7-36.0) | 0.044 |
| ≥ 34.2 | 26/51 (51.0%) | 22/41 (53.7%) | 4/10 (40.0%) | 0.499 |
| Myoglobin, ng/mL | 86.9 (57.3-186.1) | 89.1 (59.4-189.4) | 73.8 (49.1-164.6) | 0.409 |
| Creatine kinase isoenzyme-MB, ng/ML | 2.0 (1.2-4.0) | 2.2 (1.2-4.6) | 1.9 (1.1-2.1) | 0.264 |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 590.0 (306.0-2032.5) | 793.0 (399.3-2608.0) | 427.0 (195.0-771.0) | 0.044 |
| > 161 | 46/49 (93.9%) | 37/38 (97.4%) | 9/11 (81.8%) | 0.629 |
| High sensitivity C-reactive protein, pg/mL | 68.2 (37.4-152.1) | 76.3 (32.9-152.1) | 66.6 (43.3-167.3) | 0.736 |
| Erythrocyte sedimentation rate, mm/h | 50.0 (16.0-65.5) | 48.0 (15.5-66.3) | 50.0 (20.5-65.5) | 0.916 |
| Procalcitonin, ng/mL | 0.23 (0.11-0.59) | 0.24 (0.13-0.71) | 0.18 (0.09-0.33) | 0.145 |
| < 0.05 | 0/33 (0.0%) | 0/24 (0.0%) | 0/9 (0.0%) | 0.098 |
| 0.05-0.49 | 24/33 (21.2%) | 15/24 (62.5%) | 9/9 (100.0%) | .. |
| 0.5-1.99 | 7/33 (72.7%) | 7/24 (29.2%) | 0/9 (10.8%) | .. |
| ≥ 2 | 2/33 (6.1%) | 2/24 (8.3%) | 0/9 (0.0%) | .. |
| Serum ferritin, μg/L | 1328.5 (1022.7-2399.0) | 1304.0 (1135.3-1866.9) | 2360.9 (386.1-3197.7) | 0.730 |
| ≥ 400 | 17/18 (94.4%) | 13/13 (100.0%) | 4/5 (80.0%) | 0.278 |
| Interleukin-1β, pg/mL | 5.0 (5.0-7.1) | 5.0 (5.0-7.7) | 5.7 (5.0-6.8) | 0.800 |
| ≥ 5 pg/mL | 16/33 (48.5%) | 10/24 (41.7%) | 6/9 (66.7%) | 0.259 |
| Interleukin-2 receptor, U/L | 1049.0 (822.5-1484.0) | 1049.0 (716.0-1550.0) | 1048.5 (886.3-1381.8) | 0.710 |
| ≥ 710 | 27/33 (81.8%) | 18/23 (78.3%) | 9/10 (90.0%) | 0.640 |
| Interleukin-6, pg/mL | 58.9 (27.2-172.3) | 68.3 (37.2-173.4) | 26.8(14.1-89.2) | 0.021 |
| ≥ 7 | 33/33 (100.0%) | 23/23 (100.0%) | 10/10 (100.0%) | 1.000 |
| Interleukin-8, pg/mL | 40.3 (25.0-74.5) | 54.2 (25.9-101.0) | 28.3 (13.5-34.0) | 0.050 |
| ≥ 62 | 16/33 (30.3%) | 14/23 (39.1%) | 2/10 (20.0%) | 0.453 |
| Interleukin-10, pg/mL | 11.3 (5.2-17.8) | 11.3 (5.3-16.8) | 10.8 (5.0-14.0) | 0.984 |
| ≥ 9.2 | 18/33 (54.5%) | 13/23 (56.5%) | 5/10 (50.0%) | 0.730 |
| Tumor necrosis factor-α, pg/mL | 11.2 (7.2-15.3) | 11.2 (7.7-15.5) | 11.3 (7.1-14.0) | 0.754 |
| > 8.1 | 23/33 (69.7%) | 17/23 (73.9%) | 6/10 (60.4%) | 0.444 |
| APACHE II score | 17.0 (15.0-20.0) | 19.0 (16.0-21.0) | 14.0 (12.0-18.0) | < 0.001 |
| SOFA score | 6.0 (5.0-7.0) | 6.0 (5.0-8.0) | 5.0 (4.0-6.0) | 0.001 |
Data are median (inter-quartile range) or n (%). COVID-19, coronavirus disease 2019; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment.
Interventions and complications
Of the 74 patients, 68 (91.9%) patients received antivirals (abidol, 55 [74.3%]; oseltamivir, 19 [25.7%]; lopinavir/ritonavir, 15 [20.3%]; remdesivir/placebo, 2 [2.7%]), and all patients were prescribed with empirical antibiotics (moxifloxacin, 62 [83.8%]; cefoperazone sulbactam, 36 [48.6%]; carbapenem, 12 [16.2%]; levofloxacin, 7 [9.5%]). Additionally, 60 (81.1%) patients received systematic corticosteroids, and albumin therapy was administered to 23 (31.1%) patients. For oxygen therapy, 66 (89.2%) patients received HFNC and 63 (85.1%) received NIV before invasive ventilation. Of the 14 patients who survived, 1 had received extracorporeal membrane oxygenation support. There were no significant differences in pharmacological treatments, prone position ventilation, and continuous renal replacement therapy between survivors and non-survivors.
In terms of complications, all the survivors and non-survivors had ARDS and sepsis. Compared with survivors, more non-survivors experienced acute heart failure (85.0% vs. 14.3%; p < 0.001), acute kidney injury (58.3% vs. 0.0%; p < 0.001), DIC (78.3% vs. 28.5%; p = 0.001), and septic shock (100% vs. 57.1%; p < 0.001).
Survival analysis and predictors of 28-day mortality
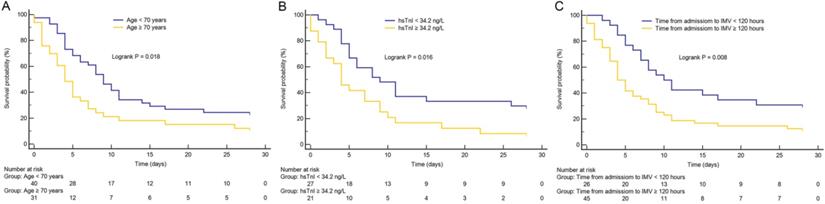
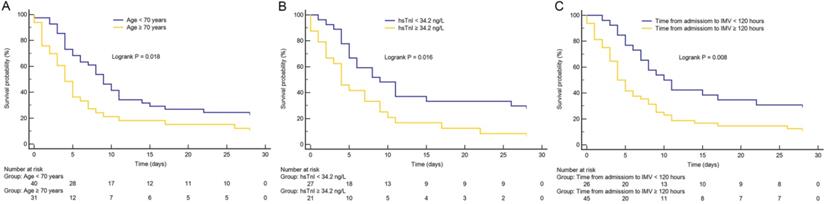
As shown in Figure 1, Kaplan-Meier survival curves showed that patients with advanced age (≥ 70 years), longer time from hospital admission to intubation (≥ 120 hours), and cardiac injury had higher 28-day mortality than patients with younger age, shorter time from hospital admission to intubation (< 120 hours), and non-cardiac injury (all p < 0.05), respectively.
In univariable Cox proportional hazard regression analysis, advanced age, high SOFA score, longer time from hospital admission to intubation, high hsTnI and LDH levels were associated with death. After adjusting for age, gender and comorbidities, the multivariable model showed that high SOFA score (hazard ratio [HR], 1.54; 95% confidence interval [CI], 1.23-1.92; p < 0.001) and longer time from hospital admission to intubation (≥ 120 hours) (HR, 2.41; 95% CI, 1.15-5.07; p = 0.020) were independent predictors of 28-day mortality in COVID-19 patients receiving invasive ventilation (Table 4).
Discussion
We reported 74 critically ill cases with COVID-19 receiving invasive ventilation tended to be older, and characterized by cytokine storm and multiple organ dysfunction. 60 (81.1%) of 74 patients died within 28 days after initiation of invasive ventilation. High SOFA score and delayed intubation were associated with 28-day mortality.
Kaplan-Meier survival curves. (A) The impact of age on mortality in critically ill patients with coronavirus disease 2019 (COVID-19). (B) The impact of high-sensitivity cardiac troponin I (hsTnI) level on mortality in critically ill patients with COVID-19. (C) The impact of time from hospital admission to invasive mechanical ventilation (IMV) on mortality in critically ill patients with COVID-19. Groups were compared by using log-rank test. All log-rank p value < 0.05. Numbers at risk are indicated.
Univariate and multivariate Cox regression analysis
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age ≥ 70 years vs. < 70 years | 1.80 (1.08-2.99) | 0.025 | |||
| Gender, male | 0.99 (0.57-1.73) | 0.982 | |||
| Hypertension | 1.38 (0.83-2.32) | 0.219 | |||
| Diabetes | 1.22 (0.65-2.31) | 0.537 | |||
| Time from hospital admission to invasive ventilation ≥ 120 hours vs. < 120 hours | 2.03 (1.16-3.53) | 0.013 | 2.41 (1.15-5.07) | 0.020 | |
| Comorbidities | .. | 0.332 | |||
| 1 | 1.28 (0.70-2.33) | 0.422 | |||
| 2 | 1.26 (0.59-2.68) | 0.555 | |||
| 3 | 2.26 (0.95-5.37) | 0.066 | |||
| White blood cell × 109/L | 1.02 (0.99-1.05) | 0.217 | |||
| Lymphocyte × 109/L | 0.93 (0.38-2.28) | 0.868 | |||
| Lactate dehydrogenase, U/L | 1.00 (1.00-1.00) | 0.048 | |||
| Prothrombin time, Seconds | 1.03 (0.98-1.09) | 0.273 | |||
| High-sensitivity cardiac troponin I, ≥ 34.2 ng/L vs. < 34.2 ng/L | 2.04 (1.10-3.80) | 0.024 | |||
| N-terminal pro-B-type natriuretic peptide, pg/mL | 1.00 (1.00-1.00) | 0.392 | |||
| D-dimer | 1.02 (0.98-1.06) | 0.326 | |||
| Interleukin-6 | 1.00 (1.00-1.00) | 0.063 | |||
| SOFA score | 1.34 (1.16-1.56) | <0.001 | 1.54 (1.23-1.92) | <0.001 | |
HR, hazard ratio; CI, confidence interval; SOFA, Sequential Organ Failure Assessment.
Recent studies have demonstrated that survivals among COVID-19 patients with respiratory failure are similar to those in other viral pneumonias and ARDS [18, 19]. However, these studies did not analyze the outcomes on the basis of timing of intubation. Consideration for substantial fluctuates in transpulmonary pressures and self-induced lung injury have been proposed as reasons for early intubation in COVID-19 [20, 21]. In this case, a study would be warranted to address if the timing of intubation influences the clinical outcomes. Our results provide evidence that delayed intubation is substantially associated with 28-day mortality in this vulnerable population of critically ill patients. Noteworthy, these data suggest that strategy for initiating intubation need be adjusted, and early intubation for invasive mechanical ventilation should be applied in critically ill COVID-19 patients. The primary finding of our study is not in accord with the study of Alfonso et al., which reported that timing of intubation was not associated with the risk of death [22]. It is requisite to consider that the characteristics, comorbidities, and baseline severity of illness between studies are different. The average SOFA score in the early intubation subgroup in previous study was 10.5, but the median value was 6.0 in present study. These differences in the cohorts reflect the tendency to admit preemptively to ICU for ventilation when necessary, even the condition of COVID-19 patients is slightly deteriorated due to the clinical course. This indicates that our patients were intubated and received invasive ventilation when in a less critical status or in the earlier stage of disease than those in previous study [22]. Additionally, the possible explanation is that the definition of early intubation was different between two studies (time from ICU admission to intubation < 8 hours vs. time from hospital admission to intubation ≥120 hours).
In our cohort, 28-day mortality was 81.1%, which was particularly high. Advanced age has been reported to be associated with the severity of SARS-CoV-2 infection, and also an independent predictor of mortality in COVID-19 patients [23-26]. In this cohort, invasively ventilated cases were middle-aged and old, with a median age of 68.0 years, confirming that older patients are likely to develop more serious SARS-CoV-2 infection with high mortality. Patients aged 70 and older had shorter time (11.5 days) from illness onset to death than younger patients (20.0 days) [27], indicting a rapid progression of COVID-19 in the elderly. The most fatal complication during SARS-CoV-2 infection was ARDS with an incidence of 100% in this cohort, which was higher than that of 71% in previous study [28]. The elderly have been proved to be more likely to develop ARDS than younger patients [29]. Only COVID-19 patients had respiratory failure presenting with serious hypoxemia or multiple organ failure are adequately treated with invasive ventilation. This can also explain why the mortality in this cohort was remarkably higher than other studies in which most patients did not require invasive ventilation. Additionally, other possible reasons of high mortality included shortage of medical resources and delayed hospital admission that exist at the early stage of COVID-19 outbreak in Wuhan. We assume that monitoring the progression of SARS-CoV-2 infection in older patients might help physicians make medical decision to reduce the risk of mortality.
Recently, several studies have explored the risk factors for adverse outcomes of COVID-19 [23, 30]. The SOFA score has been used to evaluate organ dysfunction, and showed significant association with in-hospital mortality in patients with ARDS [31]. We found that non-survivors had higher SOFA scores than survivors before invasive ventilation, which was associated with mortality. Hence, high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Previous study has reported that high SOFA score at hospital admission was a risk factor for death in COVID-19 patients [23]. Of the 74 patients in this cohort, leucocytosis, increased neutrophil count and procalcitonin were more common before invasive ventilation, suggesting that secondary bacterial infection might has been developed in a large proportion of patients. More frequent lymphopenia and eosinopenia, as well as increased LDH, hsCRP, NT-proBNP, ferritin, IL-2R, IL-6 and tumor necrosis factor-α, were detected before invasive ventilation, which were similar to previous study [32]. These abnormalities of laboratory data indicated invasively ventilated COVID-19 patients commonly experienced severe systemic inflammation and life-threatening conditions, manifested by clinical symptoms and cytokine storm. In this cohort, non-survivors had more fatal complications than survivors after ICU admission. Besides ARDS, most non-survivors eventually developed acute heart failure, acute cardiac injury, acute kidney injury, DIC, and septic shock.
In this cohort, survivors and non-survivors were comparable for clinical features and laboratory findings on hospital admission. The time from symptom onset to hospital admission in survivors and non-survivors were comparable, indicating that patients with different outcomes in our cohort had similar courses of SARS-CoV-2 infection on hospital admission.
Our study has several limitations. Firstly, limited by a small sample size, especially the number of non-survivors, variables with statistical non-significance may not be ruled out. Risk factors for mortality identified in this cohort should be verified in large-sample, multi-center, prospective trails. Secondly, the average time from symptom onset to hospital admission in this cohort was 10.5 days, which indicated delayed hospital admission of patients. Further studies are needed to investigate the optimal timing of endotracheal intubation, which may be decided comprehensively according to the actual conditions of patients.
Conclusion
The mortality of invasively ventilated COVID-19 patients was rather high. High SOFA score and delayed invasive ventilation were independent predictors of 28-day mortality. Critically ill patients with COVID-19, who had severe hypoxemia and respiratory failure, may benefit from earlier invasive ventilation.
Abbreviations
COVID-19: Coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; HFNC: high flow nasal cannula; NIV: non-invasive ventilation; SOFA: High Sequential Organ Failure Assessment; ARDS: acute respiratory distress syndrome; ICU: intensive care unit; CT: Computerized tomography; WBC: white blood cell; LDH: lactate dehydrogenase; NT-proBNP: N-terminal pro-B-type natriuretic peptide; hsTnI: high-sensitivity cardiac troponin I.
Acknowledgements
We acknowledge all health-care workers involved in the diagnosis and treatment of patients in Wuhan, China.
Author Contributions
TY, LA, TX, and GF conceived of and designed the study. TY, YP, ZY, WX, LQ, WJ, HJ, HN collected data, TY, WK, WCL and GF conceived of the analysis. TY, ZY, ZJ and GF conducted the analysis and prepared manuscript. All authors interpreted the findings, contributed to writing the manuscript, and approved the final version for publication.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81974168, 81771191, 81601481).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782-793
2. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell C-RC, Barnaby DP, Becker LB, Chelico JD. et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052-2059
3. Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, Chang BP, Chau KH, Choi JJ, Gavin N. et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996
4. Vena A, Giacobbe DR, Di Biagio A, Mikulska M, Taramasso L, De Maria A, Ball L, Brunetti I, Loconte M, Patroniti NA. et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26(11):1537-1544
5. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R. et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581
6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC. et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720
7. Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A. et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020
8. Alhazzani W, Moller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A. et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease. 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887
9. Kangelaris KN, Ware LB, Wang CY, Janz DR, Zhuo H, Matthay MA, Calfee CS. Timing of Intubation and Clinical Outcomes in Adults with Acute Respiratory Distress Syndrome. Crit Care Med. 2016;44(1):120-129
10. Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195(4):438-442
11. National Health Commission of the People's Republic of China. Chinese management guideline for COVID-19 (version 7.0). March 3, 2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf
12. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q. et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020
13. Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533
14. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810
15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200
16. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4c):179-184
17. Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, Ikeda T, Ishikura H, Iba T, Ueyama M, Eguchi Y. et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145-150
18. Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, Jabaley CS, Carpenter D, Kaplow R, Hernandez-Romieu AC. et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med. 2020;48(9):e799-e804
19. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770
20. Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323(22):2329-2330
21. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099-1102
22. Hernandez-Romieu AC, Adelman MW, Hockstein MA, Robichaux CJ, Edwards JA, Fazio JC, Blum JM, Jabaley CS, Caridi-Scheible M, Martin GS. et al. Timing of Intubation and Mortality Among Critically Ill Coronavirus Disease 2019 Patients: A Single-Center Cohort Study. Crit Care Med. 2020;48(11e):1045-1053
23. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062
24. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629
25. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F. et al. COVID-19 with Different Severity: A Multi-center Study of Clinical Features. Am J Respir Crit Care Med. 2020
26. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q. et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur Respir J. 2020
27. Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441-447
28. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020
29. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534
30. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C. et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020
31. Kao KC, Hsieh MJ, Lin SW, Chuang LP, Chang CH, Hu HC, Wang CH, Li LF, Huang CC, Wu HP. Survival predictors in elderly patients with acute respiratory distress syndrome: a prospective observational cohort study. Sci Rep. 2018;8(1):13459
32. Wang L, He W, Yu X, Hu D, Bao M, Liu H, Zhou J, Jiang H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639-645
Author contact
![]() Corresponding author: Pro. Feng Gao, Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Dadao, Wuhan 430030, China. Phone: 008613971587381; e-mail: fgaotjmu.edu.cn.
Corresponding author: Pro. Feng Gao, Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Dadao, Wuhan 430030, China. Phone: 008613971587381; e-mail: fgaotjmu.edu.cn.

 Global reach, higher impact
Global reach, higher impact