3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2020; 17(11):1652-1664. doi:10.7150/ijms.46034 This issue Cite
Research Paper
Cancer Cell enters reversible quiescence through Intracellular Acidification to resist Paclitaxel Cytotoxicity
1. Institute of Biotherapy, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, Guangdong, China.
2. Department of Vascular Surgery, The Third Affiliated Hospital, Southern Medical University, Guangzhou, Guangdong, China.
3. Department of Urology, The First Affiliated Hospital, Guangdong Pharmaceutical University, Guangzhou, Guangdong, China.
4. Clinical Laboratory, The First Affiliated Hospital, Guangdong Pharmaceutical University, Guangzhou, Guangdong, China.
#These authors contributed equally to this work.
Abstract
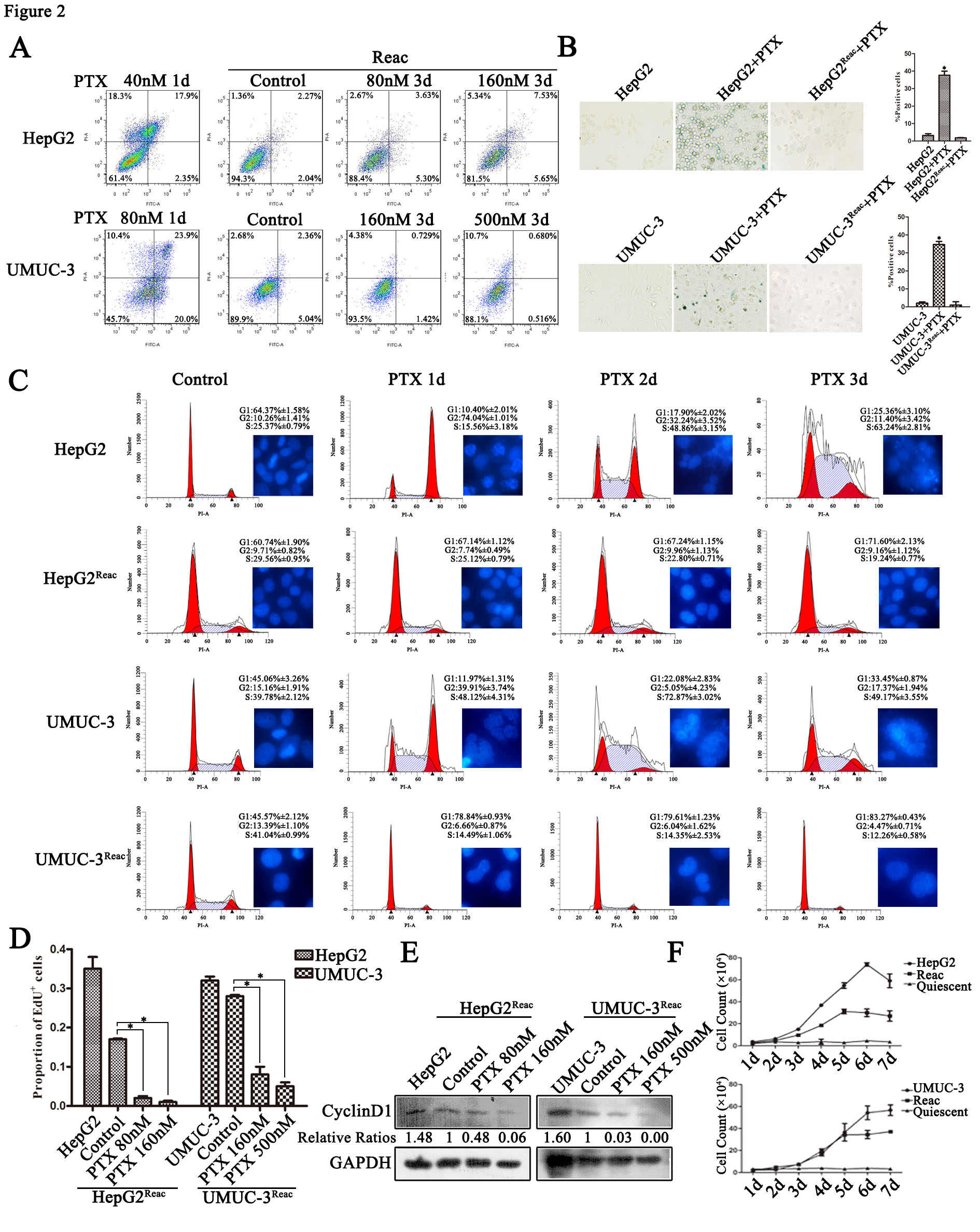
Cancer cells can enter quiescent or dormant state to resist anticancer agents while maintaining the potential of reactivation. However, the molecular mechanism underlying quiescence entry and reactivation remains largely unknown. In this paper, cancer cells eventually entered a reversible quiescent state to resist long-term paclitaxel (PTX) stress. The quiescent cells were characterized with Na+/H+ exchanger 1 (NHE1) downregulation and showed acidic intracellular pH (pHi). Accordingly, decreasing pHi by NHE1 inhibitor could induce cell enter quiescence. Further, acidic pHi could activate the ubiquitin-proteasome system and inhibiting proteasome activity by MG132 prevented cells entering quiescence. In addition, we show that after partial release, the key G1-S transcription factor E2F1 protein level was not recovered, while MCM7 protein returned to normal level in the reactivated cells. More importantly, MCM7 knockdown inhibited G1/S genes transcription and inhibited the reactivated proliferation. Taken together, this study demonstrates a regulatory function of intracellular acidification and subsequent protein ubiquitination on quiescence entry, and reveals a supportive effect of MCM7 on the quiescence-reactivated proliferation.
Keywords: chemo-resistance, quiescence, intracellular acidification, NHE1, MCM7

 Global reach, higher impact
Global reach, higher impact