Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(11):1462-1470. doi:10.7150/ijms.6632 This issue Cite
Research Paper
Totally Laparoscopic Distal Gastrectomy with D2 Lymphadenectomy and Billroth II Gastrojejunostomy for Gastric Cancer: Short- and Medium-term Results of 139 Consecutive Cases from a Single Institution
Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Institute of Micro-invasive Surgery, Zhejiang University, Hangzhou, China
* Ke Chen and Xiaowu Xu contributed equally to this work.
Received 2013-5-7; Accepted 2013-8-16; Published 2013-8-28
Abstract
Objective: The goal of this study was to investigate the feasibility, safety, and associated 3-year survival outcomes of the totally laparoscopic distal gastrectomy (TLDG) for the treatment of gastric cancer.
Methods: Herein, we analyzed the clinical data from 139 consecutive patients with gastric cancer who received TLDG at our institution from March of 2007 to March of 2013.
Results: TLDG was successfully carried out in 139 patients; no cases were converted to open surgery. The mean operation time was 228.6 ± 51.0 minutes, mean blood loss was 131.2 ± 85.2 mL, and mean number of dissected lymph nodes was 31.1 ± 9.0. The average time to flatus, time to fluid diet, and length of hospital stay were 3.6 ± 1.1 days, 4.8 ± 1.6 days, and 9.8 ± 4.0 days, respectively. The postoperative morbidity was 10.1%. A total of 135 patients were followed for a subsequent 1-73 months (median, 24.0 months). The 3-year disease-free survival (DFS) and overall survival (OS) rates were 82.3% and 82.9%, respectively. When divided by stage, the 3-year DFS for stage I, II, and III were 100%, 86.2%, and 48.8%, respectively; and the 3-year OS for stage I, II, and III were 98.0%, 92.3%, and 51.6%, respectively.
Conclusions: In this preliminary report, TLDG was found to be a safe, feasible, and efficacious procedure for the treatment of gastric cancer with encouraging 3-year overall and stage-by-stage survival rates.
Keywords: laparoscopy, gastric cancer, clinical study, survival
Introduction
Over a span of about 20 years, the excision range of laparoscopic radical gastrectomy has been extended from the distal gastrectomy to the more complicated total gastrectomy [1,2] and, at the same time, the lymph node dissection range has been extended from a D1 to a standard D2 for more radical treatments [3,4]. Although laparoscopy is fairly common for the treatment of early gastric cancer (EGC), some countries and regions have also explored its use and potential for the treatment of advanced gastric cancer (AGC) [4-6]. Laparoscopic radical gastrectomy has been reported to reduce intra-operative blood loss and to shorten hospital stay relative to conventional open gastrectomy [7-9]. Some studies reported that patients who receive laparoscopic gastrectomy have similar clinical benefits in the long term as those who receive laparotomy [3,4,10].
The most popular version of laparoscopic distal gastrectomy is laparoscopic-assisted distal gastrectomy (LADG), wherein the lymph node dissection is completed under the laparoscope. An epigastrium auxiliary incision is then made to facilitate the excision of the specimen and the reconstruction of the digestive tract. Another version is the totally laparoscopic distal gastrectomy (TLDG), which is characterized by an intracorporeal anastomosis without auxiliary incision and no touching of the tumor; it is considered “incisionless”, with the exception of the trocar wounds [11]. However, given the safety concerns associated with laparoscopic reconstruction of the gastrointestinal tract, many surgeons choose to continue performing LADG, while the TLDG operation remains less well developed.
It should be noted that the inclusion of the auxiliary incision in LADG makes it divergent from the minimally invasive treatment concept pursued in laparoscopic surgery. Furthermore, reconstruction through the small incision also has disadvantages, such as a potentially challenging specimen extrusion, contamination via the incision, and excessive pulling on the residual stomach [12]. Hence, there is a need to develop a standardized methodology got reconstructing the digestive tract by the laparoscopic approach that is as simple and safe as possible. With the aim of fulfilling this need and on the basis of our extensive laparoscopic experience gained from LADG, laparoscopic distal pancreatectomy, and other laparoscopic operations [13-15], we were encouraged to develop TLDG for the treatment of gastric cancer. Here, we report the short- and medium -term outcomes of 139 patients who received TLDG from March of 2007 to March of 2013 at our institution.
Materials and methods
Patients
Consecutive patients who received TLDG for gastric cancer from March of 2007 to March of 2013 were identified from a prospective, institutional review board-approved database. Patients with any of the following conditions were excluded: 1) laparoscopic gastric cancer palliative resection; 2) distant metastasis (e.g. peritoneal metastasis or peritoneal lavage cytology positive for carcinoma cells, hepatic metastasis); 3) tumors invading adjacent structures; or 4) tumors that could not be confirmed pathologically as being malignant. All TLDG procedures were performed by the same surgical team. Clinical and pathological staging were determined according to the American Joint Committee on Cancer (seventh edition), the tumor-node-metastasis (TNM) classification scheme. The trial received approval from the local research ethics committee, and written informed consent was obtained from all patients before the investigation.
Surgical procedure
Position and trocar location
The patient was placed in a supine position under general anesthesia. The surgeon had two assistants: one assistant stood on the right side of the patient and held the laparoscope, and another stood on the left side of the patient. Carbon dioxide pneumoperitoneum (15 mmHg) was instituted through a Veress needle. One initial 10-mm trocar was inserted for laparoscopy below the umbilicus. Another four trocars (one 12-mm trocar and three 5-mm trocars) were inserted into the left upper flank, left flank, right upper flank, and right flank quadrants. The five trocars were inserted in a V-shape arrangement.
Lymphadenectomy and specimen resection
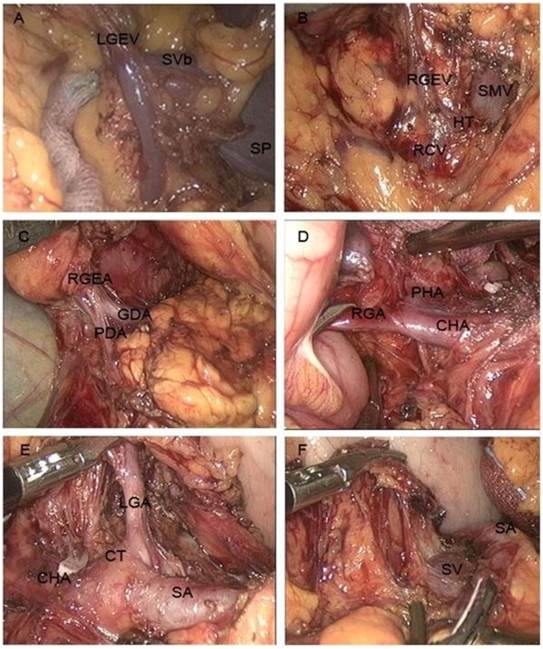
In principle, lymph node dissection was performed in almost the same manner as conventional laparotomy, defined according to the Japanese classification and treatment guidelines for gastric carcinoma [16,17]. First, the greater omentum was dissected along the border of the transverse colon with ultrasonic coagulating shears (Harmonic Ace Scalpel, Ethicon Endo-Surgery, Cincinnati, OH). The dissection was continued towards the left side of the patient until the splenic hilus and tail of the pancreas were visualized. The left gastroepiploic vessels and lymph nodes (No. 4d) were divided along the greater curvature (Fig. 1A).
Next, the superior leaf of the mesocolon and the anterior leaf of the pancreas were resected rightward towards the pylorus. Dissection was continued upward along the right colic vein and to Henle's trunk. The superior mesenteric vein was visualized near the pancreas neck, and the lymph nodes in front of it were dissected (No. 14v; Fig. 1B). The dissection was continued upward, as close as possible to the pancreas head. The bifurcation of the gastroduodenal artery and right gastroepiploic artery were divided (Fig. 1C). Then, the right gastroepiploic vessel was clamped at its origin and cut. The dissection was continued towards the right until the duodenum was visualized and the infrapyloric lymph nodes were dissected (No. 6). The right gastric artery was divided and cut at its origin, and the dissection was continued upward along the hepatoduodenal ligament. The proper hepatic artery was visualized completely, and the surrounding lymph nodes (No. 12a) were dissected (Fig. 1D). To expose the gastropancreatic fold, the stomach was turned upwards towards the patient's head, with the greater omentum folded up onto the anterior aspect of the stomach. The plica between the pancreas and stomach was opened near the superior margin of the pancreas. The lymph nodes near the common hepatic artery (No. 8a), the left gastric artery (No. 7), and the splenic artery (No. 11p; Fig. 1E) were dissected. The left gastric artery was cut away from the celiac trunk, removing the lymph nodes surrounding celiac artery (No. 9). The dissection was continued along the rear of the stomach, and then the crus dextrum diaphragmatis was opened up to the right side of the cardia, and the right paracardial lymph nodes (No. 1) were dissected (Fig. 1F).
After the stomach and the greater omentum were returned to their normal positions, the left lobe of the liver was retracted upward, while the stomach was stretched downward to expose the lesser omentum. The hepatogastric ligament was explored over the pylorus, and the suprapyloric lymph nodes (No. 5) were dissected. The lesser omentum was resected along the edge of the liver to the esophagogastric junction, and then the lymph nodes around the junction were dissected away from it (No. 1,3). With endoscopic linear staplers, the duodenum was divided at a point 1 cm distal to the pylorus (Endocutter 60 staple, Blue Cartridge; Ethicon, Endo-Surgery, Cincinnati, OH), and the stomach was divided at a point 6 cm from the superior margin of the mass (Endocutter 60 staple, Green Cartridge; Ethicon, Endo-Surgery, Cincinnati, OH). The proximal and distal margins of the resected specimen were examined. After the sample was placed into the sample bag, the incision below the umbilicus was extended to 3 cm, and the bag was externalized through the incision.
Lymph node dissection of TLDG. Shown are the dissections of the lymph nodes: A) at the root of the left gastroepiploic vein, B) at the root of the right gastroepiploic vein, C) at the root of the right gastroepiploic artery, D) at the root of the right gastric artery, E) at the root of the left gastric artery, and F) above the splenic artery. Abbreviations: LGEV, left gastroepiploic vein; SVb, splenic vein branch; SP, spleen; RGEV, right gastroepiploic vein; HT, Henle's trunk; RCV, right colic vein; SMV, superior mesenteric vein; RGEA, right gastroepiploic artery; GDA, gastroduodenal artery; PDA, pancreaticoduodenal artery; RGA, right gastric artery; PHA, proper hepatic artery; CHA, common hepatic artery; CT, coeliac trunk; LGA, left gastric artery; SA, splenic artery; SV, splenic vein.
Gastrointestinal reconstruction
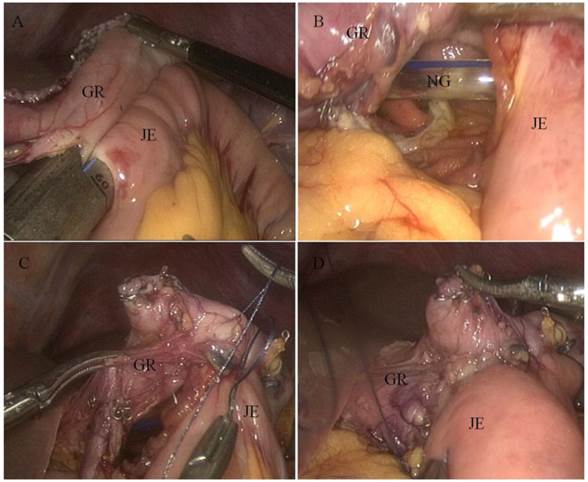
The incision was sutured, and the pneumoperitoneum was reestablished. Two access openings were created: one on the antimesenteric side of the efferent jejunal (15-cm distal to the ligament of Treitz), and the other on the posterior wall of the gastric stump 2 cm towards the cutting margin. One of the endoscopic linear stapler (Endocutter 60 staple, Blue Cartridge; Ethicon, Endo-surgery, Cincinnati, OH) legs was inserted into the jejunum opening to draw the jejunum to the rear of the gastric stump. Then, the second leg was inserted into the stomach opening. After stapling, an antecolic Billroth II side-to-side gastrojejunostomy was constructed. The common opening was closed with a continuous 3-0 Vicryl suture (Fig. 2). A single drain was placed in the abdominal cavity through a 5-mm port on the patient's right side, and other port sites were closed (Fig. 3).
Postoperative management
Patients were supported by total parenteral nutrition (TPN) until they could consume a liquid diet. After the patients could tolerate the liquid diet, they were transferred gradually to a semiliquid diet. To be discharged from the hospital, patients had to be able to tolerate a semiliquid diet and have a normal blood work panel and temperature, with no obvious discomfort. Adjuvant chemotherapy with 5-fluorouracil (5-FU)-based regimens (mostly 5-FU with cisplatin) was recommended to all eligible patients, except those with stage I cancer.
Billroth II gastrojejunostomy as viewed through a laparoscope. A) Endoscopic linear stapler completing the anastomosis. B) Internal view of the anastomosis. C) Laparoscopically closed common opening sewn by hand. D) Completed gastrojejunostomy. Abbreviations: GR, gastric remnant; JE, jejunum; NG, nasogastric tube
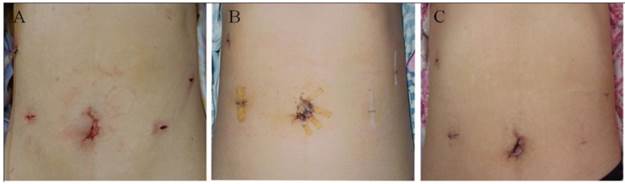
TLDG surgical incision. Shown are images of the incision at: A) the end of the operation and B) 7 days and C) 30 days after the operation.
Patient data and follow-up evaluation
Data related to patient demographics, the surgical procedure, and postoperative outcomes were collected. Outcome parameters included the total operative time, estimated blood loss, need for blood transfusion, time to passing the first flatus, time to oral intake, length of postoperative hospital stay, pathological findings, and nodal status. Follow-up data were collected for at least 3 years, including alternating semiannual abdominopelvic CT scans or ultrasound examinations. An endoscopic surveillance was performed annually or earlier if the patient had symptoms or there was any suspicion of recurrence.
Statistical analysis
Quantitative data are given as the means ± standard deviations (SD). Local and distant recurrence, disease-free survival (DFS), and overall survival (OS) rates were evaluated with the Kaplan-Meier method. 3-year follow-up data were reported for oncologic outcomes because less than one-third of the data were available for the 5-year follow-up time point. All statistical analyses were performed with SPSS software, version 18.0 (SPSS Inc, Chicago, United States).
Results
Clinical characteristics and pathological features
Table 1 shows the clinical characteristics and pathologic features of the patients. The mean age of the patients was 59.2 years (range, 34-81 years) and the male: female ratio was 2.4:1 (98 males). Their mean body mass index (BMI) was 22.7 kg/m2 (range, 15.4-32.9 kg/m2). Slightly more than a third (51/139; 36.7%) of the patients had comorbidities, the most common being hypertension. The mean neoplasm size was 3.8 cm (range, 0.5-11.5 cm) and the vast majority of neoplasms were located in the stomach antrum (90.0%). About half of the patients had lesions that were staged as T1 (41.7%), N0 (48.9%), stage I (50.4%) neoplasm. Approximately 60% of the patients had advanced gastric cancer, defined as tumor invasion into the proper muscular layer.
Operative findings and postoperative clinical course
The operative findings and subsequent postoperative clinical course data are shown in Table 2. The operation was completed successfully in all cases with no conversions to laparoscopic-assisted or open operations. The mean operation time was 228.6 minutes (range, 150-360 minutes), with a mean blood loss of 131.2 mL (range, 20-400 mL). Only four patients (2.9%) required transfusion. The mean number of retrieved lymph nodes per patient was 31.1 (range, 18-66). The mean proximal and distal resection margins were 5.3 cm (range, 2-10 cm) and 5.2 cm (range, 2-11 cm), respectively. The proximal and distal margins were examined in frozen sections; an R0 resection was achieved in all cases. The mean times to first flatus were 3.6 days (range, 2-7 days). The mean times to starting liquid and soft diets were 4.8 days (range, 3-17 days) and 6.7 days (range, 4-20 days), respectively. Finally, the mean postoperative hospital stay was 9.8 days (range, 6-42 days).
Clinical characteristics and pathologic features of the cohort of 139 patients who underwent TLDG.
| Variable | Value (%) |
|---|---|
| Gender (male/female) | 98 (70.5)/41 (29.5) |
| Age (years) | 59.2 ± 10.9 |
| BMI (kg/m2) | 22.7 ± 3.0 |
| ASA classification (I/II/Ⅲ) | 72 (51.8)/59 (42.4) /8 (5.8) |
| Comorbidities (yes)* | 51 (36.7) |
| Hypertension | 30 (21.6) |
| Diabetes mellitus | 12 (8.6) |
| Cardiovascular | 9 (6.5) |
| Pulmonary | 5 (3.6) |
| Liver | 5 (3.6) |
| Others | 5 (3.6) |
| Tumor size (cm) | 3.8 ± 2.1 |
| Tumor location (body/antrum) | 14 (10.0)/125 (90.0) |
| Histology (differentiated/undifferentiated) | 72 (51.8)/67 (48.2) |
| T stage (T1/T2/T3/T4) | 58(41.7)/18(12.9)/22(15.8)/41(29.5) |
| N stage (N0/N1/N2/N3) | 68(48.9)/35(25.2)/21(15.1)/15(10.8) |
| TNM stage (I/II/III/IV) | 70(50.4)/25(18.0)/44(31.7)/0(0.0) |
*Nine (7.4%) of 122 patients had more than two comorbidities. Abbreviation: BMI, body mass index.
Operative findings postoperative clinical course.
| Variable | Value (%) |
|---|---|
| Operation time (min) | 228.6 ± 51.0 |
| Blood loss (mL) | 131.2 ± 85.2 |
| Transfusion (patients) | 4 (2.9) |
| Number of retrieved lymph nodes | 31.1 ± 9.0 |
| Proximal resection margin (cm) | 5.3 ± 1.5 |
| Distal resection margin (cm) | 5.2 ± 1.4 |
| Time to first flatus (days) | 3.6 ± 1.1 |
| Time to starting liquid diet (days) | 4.8 ± 1.6 |
| Time to starting soft diet (days) | 6.7 ± 2.2 |
| Postoperative hospital stay (days) | 9.8 ± 4.0 |
Postoperative morbidity and mortality
The postoperative complications are listed in Table 3. The rate of postoperative morbidity was 10.1% (14/139 patients), and there was no perioperative mortality. Incidences of morbidity included one case of anastomotic leakage at the gastrojejunostomy site (requiring an operative correction) and two cases of hemorrhage (one from the gastroduodenal artery and one from the branch of splenic artery), which required a second operation to stop the bleeding. Other complications included abdominal abscess (n = 2), pulmonary infection (n = 3), delayed gastric emptying (n = 4), ileus (n = 1), and lymphorrhea (n = 2). These complications were controlled with conservative treatment.
Follow-up results
Among the 139 patients, survival data were available for 135 patients. Four patients were lost to follow-up but were known to be free of disease at 10, 10, 12, and 33 months, respectively. The median follow-up duration was 24 months (range = 1-73 months). A total of 18 patients were dead at the time of analysis. The causes of death included 16 postoperative recurrences, 1 primary hepatocellular carcinoma, and 1 old age. Another 3 patients experienced recurrences, but were still alive at the end of the study period. The causes of recurrence included 3 local recurrences (average interval of 29.7 months), 5 hepatic metastases (average interval of 14 months), 4 peritoneal metastases (average interval of 13.8 months), 5 lymphatic metastases (average interval of 20.8 months), 1 ovarian metastasis (4 months), and 1 pulmonary metastasis (44 months).
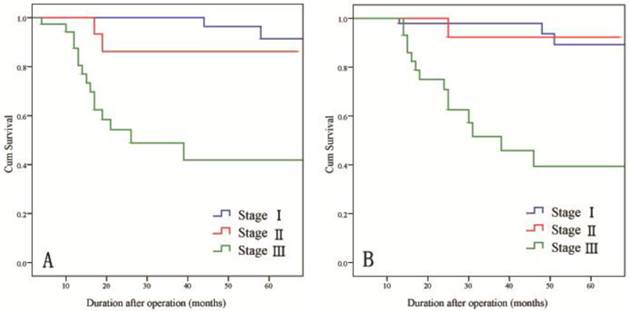
For all patients, the 3-year DFS and OS rates were 82.3% and 82.9%, respectively. When subdivided by stage, the 3-year DFS rates were 100% for stage I disease, 86.2% for stage II disease, and 48.8% for stage III disease and the 3-year OS rates were 98.0% for stage I disease, 92.3% for stage II disease, and 51.6% for stage III disease (Fig. 4).
Postoperative complications.
| Variable | Value (%) |
|---|---|
| Present/absent | 14 (10.1)/125 (89.9) |
| Anastomotic leakage | 1 |
| Postoperative hemorrhage | 2 |
| Abdominal abscess | 2 |
| Pulmonary infection | 3 |
| Delayed gastric emptying | 4 |
| Ileus | 1 |
| Lymphorrhea | 2 |
| Reoperation | 3 (2.2) |
| Mortality | 0 (0.0) |
*One patient experienced hemorrhage and pulmonary infection, simultaneously.
Long-term survival rates of patients by stage of gastric cancer. A) DFS rates and B) OS rates.
Discussion
The totally laparoscopic gastrectomy method was first conceptualized in 1992 by the Singapore scholar, Dr. Goh, who reported two TLDGs for the treatment of peptic ulcers [18]. In 1996, Dr. Ballesta-Lopez first applied this surgical method to treat gastric cancer and documented its feasibility and efficacy [19]. Although TLDG has been in use for over 20 years, its development has been limited because successful reconstruction of the digestive tract laparoscopically has been difficult to achieve. However, with recent advancements of laparoscopic surgical instruments and the accumulation of operative experience, laparoscopic gastrointestinal anastomosis has gradually become mature. Today, the Billroth I, Billroth II, and Roux-en-Y anastomosis can be completed laparoscopically[20-22]. TLDG has become attractive to laparoscopic surgeons and may become the favored method of laparoscopic radical gastrectomy [23].
The methods of gastrointestinal anastomosis after laparoscopic distal gastrectomy are the same as standard laparotomy which include the Billroth I, Billroth II, and Roux-en-Y methods. The choice between these methods depends on the patient's condition and economic situation, and on the surgeon's operating habits. Laparoscopic Roux-en-Y reconstruction has been the preferred method to prevent reflux gastritis and esophagitis and to decrease the probability of gastric cancer recurrence. However, the procedure is complex and time-consuming, and the extensive use of endoscopic linear staplers can result in higher costs. The Billroth I reconstruction method has the advantage of technical simplicity, involving only one anastomotic site and maintaining physiological intestinal continuity [24]. However, gastroesophageal and duodenogastric reflux are common sequalae [25]. Additionally, this technique may have limitations in its use in that it may not be feasible in obese patients or in patients with large tumors in the low- to mid-stomach. For large tumors or tumor located toward the middle section of the stomach, the recommended treatment consists of a radical resection of the distal four-fifths of the stomach with a 5-cm free margin, which makes the Billroth I anastomosis unlikely. At present, Japanese and Korean operators prefer to perform Billroth I anastomosis because approximately 50% of their patients are diagnosed at an early stage (meaning a lower range of excision). However, the situation is less favorable in China, where most gastric cancer cases are AGC, and the economic resources of the Chinese patients are limited. Given these circumstances, totally laparoscopic Billroth II gastrectomy is generally the standard treatment in China, as it can be used more liberally in gastric cancer resection. Therefore, it was the treatment of choice for all 139 patients in our cohort.
Regarding anastomosis methodology, although preferred for its low cost, manual suturing is relatively difficult and time consuming to perform laparoscopically. Therefore, it is rarely used in TLDG, in which an apparatus is often favored to perform the anastomosis [22]. The most common tool used in side-to-side anastomosis within the digestive tract is the endoscopic linear stapler. Some surgeons suggested that the position of the jejunum and gastric stump should be fixed by using one or two stitches before endoscopic linear staples are applied [26]. However, we have found that the suture suspends and angulates the jejunum, sacrificing the mobility of jejunum, making placement of the endoscopic linear stapler difficult. According to our experience, we prefer placing one arm of the endoscopic linear stapler into the jejunum opening and clamping the two arms without stapling, and then, with the help of the stapler, drawing the jejunum up to the rear of the gastric stump and then releasing the two arms to place the second arm into the gastric stump opening, so as to complete the anastomosis after the apposition has been confirmed to be ideal.
Endoscopic linear staplers can also be used to close the common opening. However, if the closure is not accurate, it will make the anastomotic stoma too narrow or permit leakage. For this reason, the common opening was closed with a manual continuous suture in all 139 patients in this study. Although initially more time consuming, with experience, the overall time for the anastomosis becomes shorter.
Because the reconstruction step of TLDG is tricky, operating safety is an continuing worry for surgeons. In our study, only 14 patients (10.1%) developed postoperative complications. Only one of these 14 patients developed complications directly related to the anastomosis (e.g., anastomotic leakage); thus, the rate of anastomosis relevant complications was only 0.72%. Similarly, a Japanese study (N = 1,185) and a Korean study (N = 1,237) followed patients who received LADG and reported postoperational complication rates of 12.7% and 13.1%, respectively, of which the anastomosis related complications were involved in 5.1% and 1.9% of cases, respectively [23,27]. Thus, it is our view that laparoscopic surgeons with ample experience could be able to achieve a safe and effective digestive tract reconstruction using the TLDG method with a complication rate comparable to that observed with LADG, which is a relatively more mature operation for laparoscopic radical gastrectomy. In addition, it is important to note that there were two patients in our cohort who developed abdominal abscesses, whereas we observed no such complications in our earlier LADG study. Both abdominal abscesses were located at the upper edge of the pancreas near the anastomotic stoma. Because the gastric cavity needs to be opened temporarily during TLDG due to the nature of the gastrointestinal reconstruction in the procedure, we believe that the main reason for abdominal abscesses occurring near the anastomotic stoma may be leakage of some gastric content into the abdominal cavity during the operation. In our experience, sufficient gastrointestinal decompression before opening the stomach and continuous local peritoneal irrigation after opening should be done to avoid abscess development.
Some studies have shown that TLDG is advantageous over LADG because the patient experiences less blood loss during the operation and has a faster recovery after the operation [28-31]. In practice [15], we have found that TLDG is preferable to LADG for three additional reasons. First, it is an in situ operation that avoids excessive pulling on the internal organs. When conducting LADG, the physician must pull the gastric stump outside of the body to operate on it. This pulling puts tremendous stress on the gastric stump and may even lead to tearing of the spleen envelope, causing bleeding from the spleen envelope. Also, when conducting LADG (especially for patients with a high tumor location), the short gastric blood vessels must be divided which may result in more intra-operative blood loss. Conversely, when conducting TLDG, the entire gastrointestinal anastomosis procedure is performed in situ, which reduces stress on the gastric stump and retains its blood supply and function. Second, TLDG is more suitable for a “no touch tumor” operation. When conducting LADG, the physician is limited to working through a small incision, which leads to the inevitable squeezing of the tumor. Also, there is a higher possibility that the tumor will directly contact the incision. When conducting TLDG, the physician can achieve a “zero extrusion,” wherein the tumor does not come into direct contact with the incision because it is enclosed within a sample bag. Finally, TLDG requires only a small incision and imparts more selectivity to the surgeon than LADG. When conducting LADG, an auxiliary 6-cm incision is generally made below the xiphoid. For overweight patients, however, the incision may need to be extended to 8-10 cm. When conducting TLDG, because the hypogastrium wall has more ductility, the surgeon can simply expand the incision for the 10-mm trocar below the umbilicus to a 3-4-cm semicircle incision around the navel to enable the sample to be taken out properly.
Although adjuvant chemotherapy was associated with a survival benefit for gastric cancer [32,33], radical gastrectomy with regional lymph node dissection still remains the first choice of treatment [34,35]. The curative potential of surgical treatment should be evaluated based on the extent of lymph node dissection involved as well as the proximal and distal resection margins. The quantity of laparoscopic lymph nodes dissected is closely related to the surgical technique level of the operator. In our study, the mean number of retrieved lymph nodes per patient was 31.1, which is consistent with the requirements of the Japanese gastric cancer treatment guidelines. As the technique has matured in recent years, some researchers have reported that not only was the overall number of lymph nodes that could be retrieved laparoscopically similar to that of laparotomy, but also that the specific lymph nodes such as No. 7, 8a, 9, 11p, 12a and 14v retrieved were also similar to that of laparotomy, which were once considered difficult to dissect under laparoscope [36,37].
Cancer recurrence and long-term survival rate are two critical outcomes for evaluating surgical interventions in oncological therapy. A convergence of research indicates that the long-term survival rates of patients after laparoscopic and open radical surgery for gastric cancers are not significantly different from each other, even in AGC [3-6]. In this study, the 3-year DFS and OS rates of patients with stage I, II, and III cancers (DFS: 100%, 86.2%, and 48.8%; OS: 98.0%, 92.3%, and 51.6%, respectively) are consistent with previous reports in the literature [10, 38]. Recently, Park et al. [39] reported an analysis of the follow-up results of 239 cases of AGC in which the patients received laparoscopic radical gastrectomies; 130 of the cases were T2 stage, 63 were T3, and 46 were T4. The 5-year survival rates for the T2, T3, and T4 patients were 86.6%, 77.4%, and 58.7% respectively. These outcomes are similar to those observed with concurrent laparotomy, which is encouraging. With continuous improvement of the laparoscopic technique, so long as surgeons apply the radical cure principle of malignant tumor surgery resolutely and choose appropriate cases accurately, we expect that, in addition to minimizing invasiveness, TLDG can yield long-term efficacy that is on par with laparotomy.
In conclusion, despite a relatively small number of patients, this study indicates that TLDG with D2 lymphadenectomy and Billroth II gastrojejunostomy is safe, feasible, and yields acceptable medium-term oncologic outcomes. A major benefit of the procedure for the patient is the avoidance of a major incision. Additionally, the procedure offers some benefits of convenience during operating compared with LADG. In our opinion, future research should be directed at long-term oncologic outcome, survival, and quality of life in addition to the outcomes reported in this study.
Abbreviations
TLDG: totally laparoscopic distal gastrectomy; LADG: laparoscopic-assisted distal gastrectomy; DFS: disease-free survival; OS: overall survival; TNM: tumor node metastasis; TPN: total parenteral nutrition; 5-FU: 5-fluorouracil; SD: standard deviation; BMI: body mass index; EGC: early gastric cancer; AGC: advanced gastric cancer.
Acknowledgements
This work was supported by a Key Project Grant from the Science and Technology Department of Zhejiang Province, China (No. 2011C13036-2) and the Department of Health of Zhejiang Province, China (No. 2010KYA112, No. 2012ZDA024).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jeong GA, Cho GS, Kim HH. et al. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146:469-474
2. Okabe H, Obama K, Kan T. et al. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg. 2010;211:e1-6
3. Sato H, Shimada M, Kurita N. et al. Comparison of long-term prognosis of laparoscopy-assisted gastrectomy and conventional open gastrectomy with special reference to D2 lymph node dissection. Surg Endosc. 2012;26:2240-2246
4. Hamabe A, Omori T, Tanaka K. et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2012;26:1702-1709
5. Park do J, Han SU, Hyung WJ. et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548-1553
6. Chen K, Xu XW, Mou YP. et al. Systematic review and meta-analysis of laparoscopic and open gastrectomy for advanced gastric cancer. World J Surg Oncol. 2013;11:182
7. Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173
8. Chen K, Xu XW, Zhang RC. et al. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365-5376
9. Viñuela EF, Gonen M, Brennan MF. et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446-456
10. Pak KH, Hyung WJ, Son T. et al. Long-term oncologic outcomes of 714 consecutive laparoscopic gastrectomies for gastric cancer: results from the 7-year experience of a single institute. Surg Endosc. 2012;26:130-136
11. Shawki S, Bashankaev B, Denoya P. et al. What is the definition of "conversion" in laparoscopic colorectal surgery? Surg Endosc. 2009;23:2321-2326
12. Oki E, Sakaguchi Y, Ohgaki K. et al. The impact of obesity on the use of a totally laparoscopic distal gastrectomy in patients with gastric cancer. J Gastric Cancer. 2012;12:108-112
13. Yan JF, Mou YP, Xu XW. et al. Laparoscopic distal pancreatectomy: the experience of 68 cases in a single centre. Chin J Surg. 2012;50:802-805
14. Xu X, Chen K, Zhou W. et al. Laparoscopic transgastric resection of gastric submucosal tumors located near the esophagogastric junction. J Gastrointest Surg. 2013 [Epub ahead of print]
15. Wang W, Chen K, Xu XW. et al. Case-matched comparison of laparoscopy-assisted and open distal gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:3672-3677
16. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112
17. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123
18. Goh P, Tekant Y, Isaac J. et al. The technique of laparoscopic Billroth II gastrectomy. Surg Laparosc Endosc. 1992;2:258-260
19. Ballesta-Lopez C, Bastida-Vila X, Catarci M. et al. Laparoscopic Billroth II distal subtotal gastrectomy with gastric stump suspension for gastric malignancies. Am J Surg. 1996;171:289-292
20. Lee WJ, Wang W, Chen TC. et al. Totally laparoscopic radical BII gastrectomy for the treatment of gastric cancer: a comparison with open surgery. Surg Laparosc Endosc Percutan Tech. 2008;18:369-374
21. Kanaya S, Gomi T, Momoi H. et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287
22. Kim JJ, Song KY, Chin HM. et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008;22:436-442
23. Kitano S, Shiraishi N, Uyama I. et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72
24. Weil PH, Buchberger R. From Billroth to PCV: a century of gastric surgery. World J Surg. 1999;23:736-742
25. Pääkkönen M, Aukee S, Syrjänen K. et al. Gastritis, duodenogastric reflux and bacteriology of the gastric remnant in patients operated for peptic ulcer by Billroth I operation. Ann Clin Res. 1985;17:32-36
26. Bouras G, Lee SW, Nomura E. et al. Surgical outcomes from laparoscopic distal gastrectomy and Roux-en-Y reconstruction: evolution in a totally intracorporeal technique. Surg Laparosc Endosc Percutan Tech. 2011;21:37-41
27. Kim W, Song KY, Lee HJ. et al. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg. 2008;248:793-799
28. Kinoshita T, Shibasaki H, Oshiro T. et al. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. 2011;25:1395-1401
29. Song KY, Park CH, Kang HC. et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg. 2008;12:1015-1021
30. Lee WJ, Wang W, Chen TC. et al. Totally laparoscopic radical BII gastrectomy for the treatment of gastric cancer: a comparison with open surgery. Surg Laparosc Endosc Percutan Tech. 2008;18:369-374
31. Ikeda O, Sakaguchi Y, Aoki Y. et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2374-2379
32. Sakuramoto S, Sasako M, Yamaguchi T. et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820
33. Bang YJ, Kim YW, Yang HK. et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321
34. Catalano V, Labianca R, Beretta GD. et al. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127-164
35. Songun I, Putter H, Kranenbarg EM. et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449
36. Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008;22:655-659
37. Chen QY, Huang CM, Lin JX. et al. Laparoscopy-assisted versus open D2 radical gastrectomy for advanced gastric cancer without serosal invasion: a case control study. World J Surg Oncol. 2012;10:248
38. Hwang SH, Park do J, Jee YS. et al. Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg. 2009;144:559-564 discussion 565
39. Park do J, Han SU, Hyung WJ. et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548-1553
Author contact
![]() Corresponding author: Yiping Mou, Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Institute of Micro-invasive Surgery, Zhejiang University, 3 East Qingchun Road, Hangzhou 310016, China. Tel: +86-571-86006952, Fax: +86-571-86044817, E-mail: mouyiping2002com
Corresponding author: Yiping Mou, Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Institute of Micro-invasive Surgery, Zhejiang University, 3 East Qingchun Road, Hangzhou 310016, China. Tel: +86-571-86006952, Fax: +86-571-86044817, E-mail: mouyiping2002com

 Global reach, higher impact
Global reach, higher impact