3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(6):462-466. doi:10.7150/ijms.4242 This issue Cite
Research Paper
Rap System of Stress Stimulation Can Promote Bone Union after Lower Tibial Bone Fracture: A Clinical Research
1. Department of Orthopaedic Surgery, the Affiliated Dongnan Hospital of Xiamen University, Orthopaedic Trauma Center of the 175th Hospital of PLA, Zhangzhou, Fujian, 363000, PR China.
2. The Orthopaedic Department of the 4th Hospital of Taizhou, Jiangsu, 225300, PR China.
Received 2012-2-16; Accepted 2012-7-16; Published 2012-7-31
Abstract
Background Lower tibial bone fracture may easily cause bone delayed union or nonunion because of lacking of dynamic mechanical load.
Objective Research Group would design a new instrument as Rap System of Stress Stimulation (RSSS) to provide dynamic mechanical load which would promote lower tibial bone union postoperatively.
Methods This clinical research was conducted from January 2008 to December 2010, 92 patients(male 61/female 31, age 16-70years, mean 36.3years) who suffered lower tibial bone closed fracture were given intramedullary nail fixation and randomly averagely separated into experimental group and control group(according to the successively order when patients went for the admission procedure). Then researchers analysed the clinical healing time, full weight bearing time, VAS (Visual Analogue Scales) score and callus growth score of Lane-Sandhu in 3,6,12 months postoperatively. The delayed union and nonunion rates were compared at 6 and 12 months separately.
Results All the 92 patients had been followed up (mean 14 months). Clinical bone healing time in experimental group was 88.78±8.80 days but control group was 107.91±9.03days. Full weight bearing time in experimental group was 94.07±9.81 days but control group was 113.24±13.37 days respectively (P<0.05). The delayed union rate in 6 months was 4.3% in experimental group but 10.9% in control group(P<0.05). The nonunion rate in 12 months was 6.5% in experimental group but 19.6% in control group(P<0.05). In 3, 6, 12 months postoperatively, VAS score and Lane-Sandhu score in experimental group had more significantly difference than them in control group.
Conclusions RSSS can intermittently provide dynamic mechanical load and stimulate callus formation, promote lower tibial bone union, reduce bone delayed union or nonunion rate. It is an adjuvant therapy for promoting bone union after lower tibial bone fracture.
Keywords: Rap, Stress, Mechanical Load, Bone Fracture, Bone Union, Nonunion.
Introduction
In orthopedic department, plenty of patients suffered lower tibial bone fractures because of traffic accident injury, weight crushed injury, falling injury or other high energy injures, which may cause lower tibial bone delayed union or nonunion. Researchers have studied out some methods to stimulate bone union, such as electromagnetic stimulation[1], ultrasonicoscillation stimulation[2], autologous bone graft[3], allogous bone graft[4, 5], bone tissue engineering[5, 6], mechanical engineering[7], but many of them had somewhat disadvantages.
After injury patients must lie in bed for a long time, the fractured lower tibial bone is somewhat lack of physiological stress stimulation that may cause bone delayed union or nonunion. While bone secondary healing is an extremely complicated biological reconstituted procedure and needs mechanical load to stimulate bone callus formation and mineralization[8], which can promote bone union rate and shorten bone union time[9-15]. So the aim of this research is to design the RSSS instrument, which can imitate humans working dynamic mechanical load and provide physiological stress stimulation, to promote bone union.
Methods
Instrument Design
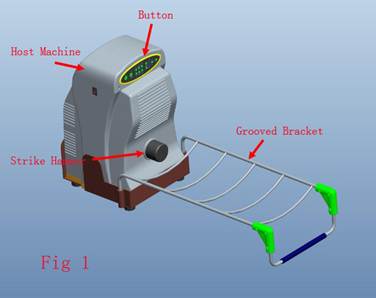
In collaboration with Xiamen Double Engine Medical Material Co, Ltd, we designed RSSS and obtained a patent (patent number: CN1803117). RSSS consists of four parts as host machine, button, grooved bracket and remote controller (Fig 1 is the RSSS introduction and Fig 2 is a case that RSSS instrument treating the right leg of a patient). The host machine contains stepping motor, integrated circuit plate and strike hammer. The stress record of the high-low (between 50 to 350 Newton), frequency (0.5-3Hz), strike time (5-35 second) and time interval (3-19 second) could be regulated both by the control panel or remote controller. All these parameter could be revealed on its liquid crystal display. The strike hammer can strike heel, imitate humans upright walking and produce an intermittent dynamic mechanical load, which can stimulate bone callus formation and mineralization.
is the RSSS instrument introduction, the arrows show parts of the instrument, host machine is the main construction member contains engine and electric circuits, button controls the engine and electric circuits, strike hammer strikes patients heel, grooved bracket supports patients injured lower limb.
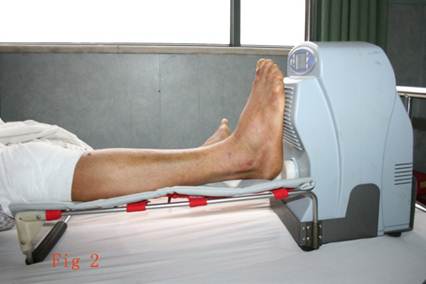
shows the RSSS instrument treating the right leg of a patient, male, 38 years old, closed fracture(fracture type Tscherne & Oestern[16] Type1) because of traffic accident, after intramedullary nail fixation the patient has given RSSS treatment.
Clinical Data
The research was approved by the Medical Ethical Committee of 175th Hospital. From January 2008 to December 2010, 92 patients(male 61/female 31, age 16-70years, mean 36.3years) who suffered lower tibial bone closed fractures were selected in local orthopaedic trauma center and were given intramedullary nail fixation( All the intramedullary nails were provided by Xiamen Double Engine Medical Material Co, Ltd) and randomly averagely separated into experimental group and control group(each n=46, according to the successively order when patients went for the admission procedure). The inclusion criteria is that patients have been diagnosed high-energy injuries to lower tibial bone, closed fractured bone Tscherne & Oestern[16] type 0&1(type 0 is simple fracture, type1 is low-grade fracture) and need internal fixation surgeries; the exclusion criteria is that fracture line spreads to ankle, intra-articular fracture, age less than 16 years or older than 70 years, fracture gaps exceeds 5mm, pathological fracture, active infection, osteomyelitis or tumor, heart cerebrovascular diseases and other serious diseases, pregnancy and lactation women, mental illness or dementia patients, coagulant function obstacle, diabetes, taking immune inhibitors or along term of smoking. Main information such as gender, age, closed Tscherne & Oestern[16] type and fixed pattern was recorded (Table 1). In control group patients stayed in bed only giving psychological comfort but not giving RSSS treatment, while in experimental group patients received RSSS treatment at 1 week postoperatively. At the beginning of RSSS treatment doctors informed total treatment programme to patients that stress was set 2-2.5N/Per Kilogram Body Weight in the first week and increased to 2.5-3N/ Per Kilogram Body Weight in the following weeks. Parameters setting: strike time 20s and 10s interval; frequency 3 Hz; 30 mins a time with 8 hours interval, 2 times a day, continue 1 to 3 months.
Assessment
In this research, we used Lane-Sandhu score[17] (Table 2) to evaluate callus formation rate and visible level of fracture line, evaluated clinical bone healing time, the full weight bearing time(when the fracture line was vanished and 100% callus formation), VAS(visual analogue scales) score of these patients in 3,6,12 months postoperatively. We defined VAS score from 0 to 10 scores which demonstrated pian level, 0 score indicated painless but 10 score indicated acute pain and could not endure any more. X-ray films were given to identify bone delayed union rate in 6 months and bone nonunion rate in 12 months. X-ray parameter was Voltage of 55 kV, course of 1 second, Current of 100mA, and distance between ball tube and sample of 80 cm.
Main Information between Experimental Group and Control Group
| Experimental Group | Control Group | P Value | |
|---|---|---|---|
| Gender (male/female) | 46(33/13 ) | 46(28/18) | P>0.05 |
| Mean age (year±variance) | 35.93±12.79 | 36.67±11.24 | P>0.05 |
| Tscherne & Oestern Type(No.) | 46 | 46 | P>0.05 |
| Type 0 | 20 | 22 | P>0.05 |
| Type 1 | 26 | 24 | P>0.05 |
| Intramedullary Nail Fixation (patients No.) | 46 | 46 | P>0.05 |
Defined P<0.05 to be statistically significant; Tscherne & Oestern Type was a type of closed fracture; variance is the standard deviation.
Lane-Sandhu Score
| Score | Callus Formation and Fracture Line |
|---|---|
| 0 | no callus formation and fracture line was clear; |
| 1 | 25% callus formation and fracture line was relatively clear; |
| 2 | 50% callus formation and fracture line was obscure; |
| 3 | 75% callus formation and fracture line was basically vanished; |
| 4 | 100% callus formation and fracture line was completely vanished; |
Statistical Analysis
These data were analyzed with SPSS16.0. Clinical bone healing time, Full weight bearing time, VAS score and Lane-Sandhu score were compared with t-test, while bone delayed union rate and nonunion rate were compared with χ2 test. Statistic significant level was set at 0.05.
Results
All 92 patients had been followed up (mean 14 months). Clinical bone healing time in experimental group was 88.78±8.80 days compared with 107.91±9.03 days in control group (P<0.05). Full weight bearing time was 94.07±9.81 days in experimental group while 113.24±13.37 days in control group (P<0.05). Bone delayed union rate was 4.3% in experimental group while 10.9% in control group and bone nonunion rate was 6.5% in experimental group while 19.6% in control group (P<0.05). In 3,6,12 months postoperatively VAS score and Lane-Sandhu score also had more significantly difference in experimental group than them in control group (Table 3).
Results between Experimental Group and Control Group
| Experimental Group | Control Group | P Value | |
|---|---|---|---|
| Clinical Bone Healing Time (days±variance) | 88.78±8.80 | 107.91±9.03 | P<0.05 |
| Full Weight Bearing Time (days±variance) | 94.07±9.81 | 113.24±13.37 | P<0.05 |
| VAS Score (mean±variance) | |||
| 3 months | 3.22±0.57 | 4.14±0.62 | P<0.05 |
| 6 months | 1.43±0.76 | 2.41±0.85 | P<0.05 |
| 12 months | 0.37±0.61 | 0.72±0.56 | P<0.05 |
| Lane-Sandhu Score (mean±variance) | |||
| 3 months | 1.46±0.69 | 1.02±0.65 | P<0.05 |
| 6 months | 2.57±0.81 | 2.11±0.88 | P<0.05 |
| 12 months | 3.57±0.78 | 3.15±0.92 | P<0.05 |
| Union Rate (%/No.) | |||
| Delayed Union (6 months) | 4.3/2 | 10.9/5 | P<0.05 |
| Nonunion (12 months) | 6.5/3 | 19.6/9 | P<0.05 |
Defined P<0.05 to be statistically significant; variance is the standard deviation.
Discussion
In orthopaedic trauma center lower tibial bone fractures are very common and many of the patients may cause bone delayed union or nonunion. Though fixation materials are abundance and strong, lower tibial bone delayed union or nonunion rates are still more than 5-10%[18]. During bone secondary healing, it needs early safe effective intermittent dynamic mechanical load to stimulate bone callus formation while lacking of essential physiological stress stimulation may easily cause bone delayed union and nonunion [19-21].
Researchers have studied out some methods to stimulate bone union, but many of them had somewhat disadvantages. Electromagnetic stimulation can stimulate bone union and avoid surgery[1], but the risk may be guide pin fractured, guide hole infectious and magnetic field produce eddy on metal fixation to make heat. Ultrasonicoscillation stimulation whether can stimulate bone union or not is still unknown[2]. Autologous bone graft can promote bone union but limited supply and large trauma to aggravate patients' pain[3]. Allogous bone graft is support-material for bone creeping substitution and to some extent can promote bone union[4, 5], but generate immunological rejection, infection and bone resorption. Bone tissue engineering is an exciting therapeutic method[5, 6], but therapeutic costs are too expensive and also generate immunological rejection, infection and other potential side effects. Mechanical engineering can prominently promote bone union without apparent side effect and produce axial pressure stress stimulation to induce fracture minute motion which can promote bone union[7], but majority of researches are still on animal experimental level. This clinical research can provide a strong mechanical engineering research evidence to promote bone union.
Bone union is an extremely complicated biological reconstituted procedure, exterior and interior factors such as age, blood supply of fracture end points, endocrine, fracture type, force environment may influence bone union rate. According to Wolff theorem[22, 23], it proved that mechanical load could promote bone callus formation and transform electrochemical circumstance of mesenchymal cell, which stimulated bone formation and promoted bone union and remodeling[24]. So this research group members designed the RSSS to found out that it could apply intermittent compressive stress to fracture site after intramedullary screw fixation, which verified that RSSS could stimulate bone callus formation, promote bone union and prevent osteoporosis[25, 26]. Domestic clinical preliminary application reveals a satisfactory effect, bone union rate almost come to 95%[27, 28]. This clinical research also reveals bone union rate in experimental group comes to 89.2% while bone union rate in control group just comes to 69.5%.
The optimal treatment time in this research is 7 days after intramedullary nail fixation regardless of male or female. Premature RSSS treatment may aggravate pain, fracture section swelling, wound exudation and inflammation. Latest results may confirm that optimal treatment time is the first week after fracture, just as Goodship[29] illustrates in his experiment that the optimal period for promoting bone union begins the first week after operation, Kershaw[30] and Sarmiento's theories[31] also confirm this finding. The optimal frequency of stimulation may be from 0.5 to 1 Hz regardless of male or female. The appropriate frequency, which adapts to humans walking frequency, has also illustrated in the literature from 0.5 to 2.5 Hz[32]. In force environment aspect, we consider the optimal stress should like patients' physiological mechanical load. In initial therapeutic stage the optimal stress should set 2-2.5N/Per Kilogram Body Weight regardless of gender. If patients can tolerate and have no complaint, stress may increase to 2.5-3N/ Per Kilogram Body Weight. Meanwhile, we find out that ankle-joint may absorb 0.5N/Per Kilogram Body Weight, so 2-2.5N/Per Kilogram Body Weight in initial therapeutic stage and 2.5-3N/Per Kilogram Body Weight in later stage may be the optimal stress and would not result in fixation loosening or bone secondary fracture. In Sarmiento's[33] research, 150N(60 Kilogram of Body Weight, equal to 2.5N/Per Kilogram Body Weight) stress is an optimal factor for bone callus formation and has a wonderful result.
We recommend RSSS to treat lower tibial bone fracture because it is safe, convenient, effective and non-trauma for patients during bone healing procedure. It imitates humans walking frequency and force environment, it has no potential side effects to ankle, wound, fractured site and can't result in screw loosening.
Authors' Contributions
SHEN Jia-zuo wrote the article. YAO Jian-fei and SHEN Jia-zuo contributed equally to the work and to be the same prima authors. The foundation was supported by key projects from Nanjing Military Region during the 11th Five-Year Plan Period (No.06MA106). All authors read and approved the final manuscript.
Abbreviations
RSSS: Rap System of Stress Stimulation; VAS: Visual Analogue Scales.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gossling HR, Bernstein RA, Abbott J. Treatment of ununited tibial fractures: a comparison of surgery and pulsed electromagnetic fields (PEMF). Orthopedics. 1992;15(6):711-9
2. Protopappas VC, Baga DA, Fotiadis DI, Likas AC, Papachristos AA, Malizos KN. An ultrasound wearable system for the monitoring and acceleration of fracture healing in long bones. IEEE Trans Biomed Eng. 2005;52(9):1597-608
3. Kanakaris NK, Paliobeis C, Nlanidakis N, Giannoudis PV. Biological enhancement of tibial diaphyseal aseptic non-unions: the efficacy of autologous bone grafting, BMPs and reaming by-products. Injury. 2007;38(Suppl 2):S65-75
4. Wilson CJ, Tait GR, Galea G. Utilisation of bone allograft by orthopaedic surgeons in Scotland. Cell Tissue Bank. 2002;3(1):49-53
5. Zou XH, Cai HX, Yin Z. et al. A novel strategy incorporated the power of mesenchymal stem cells to allografts for segmental bone tissue engineering. Cell Transplant. 2009;18(4):433-41
6. Uebersax L, Merkle HP, Meinel L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Control Release. 2008;127(1):12-21
7. Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14(2):179-86
8. Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparentdensity. J Biomech. 1988;21(2):155-68
9. Yamaji T, Ando K, Wolf S, Augat P, Claes L. The effect of micromovement on callus formation. J Orthop Sci. 2001;6(6):571-5
10. Kenwright J, Goodship AE. Controlled mechanical stimulation in the treatment of tibial fractures. Clin Orthop Relat Res. 1989(241):36-47
11. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349-55
12. Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57(5):344-58
13. Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349(1):1-5
14. Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88(1):104-12
15. Chen JC, Beaupre GS, Carter DR. An approach to quantifying bone overloading and hypertrophy with applications to multiple experimental studies. Bone. 2010;46(2):322-9
16. Moreau PG. Book review: Rockwood and Green's fractures in adults. Ann Saudi Med. 1994;14(1):75
17. Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18(2):213-25
18. Goldstein C, Sprague S, Petrisor BA. Electrical stimulation for fracture healing: current evidence. J Orthop Trauma. 2010;24(Suppl 1):S62-5
19. Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5-10 Hz. Bone. 2004;34(2):261-70
20. Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349(1):1-5
21. Cowin SC. The significance of bone microstructure in mechanotransduction. J Biomech. 2007;40(Suppl 1):S105-9
22. Wolff J. Das Gesetz der Transformtion der knochen. Berlin: Hirschwald[M]. 1892:11-13
23. Cowin SC, Sadegh AM, Luo GM. An evolutionary Wolff's law for trabecular architecture. J Biomech Eng. 1992;114(1):129-36
24. Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43(1):108-18
25. Zhenqi D, Guoshun D, Ping Z. The changing relationship between rap force of stimulator of bone stress and compressive stress at the fracture ends of lower limbs. Chinese Journal of Biomedical Engineering. 2010(16):351-354
26. Zhenqi D, Xigui Z, Mo S. The Research of Prevention of Denervation/Disuse Osteoporosis with Stimulator of Bone Stress. Chinese Journal of Osteoporosis. 2010(16):136-138
27. Zhenqi D, Jun G. Development of the rap system of stress stimulation and clinical study of on the promoting effect on the healing of closed tibia shaft ftacture. Journal of Clinical Orthopaedics. 2006(9):289-291
28. Zhenqi D, Jun G, Linxin G. Rap system of stress stimulation in treatment of delayed union and nonunion botained from traumatic femur osteomyelitis. Chinese Journal of Bone and Joint Injury. 2008;23:635-637
29. Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67(4):650-5
30. Kershaw CJ, Cunningham JL, Kenwright J. Tibial external fixation, weight bearing and fracture movement. Clin Orthop,London:Oxford. 1993;293:26-28
31. Sarmiento A, McKellop HA, Llinas A. et al. Effect of loading and fracture motions on diaphyseal tibial fractures. J Orthop Res. 1996;14(1):80-4
32. Hsieh YF, Turner CH. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16(5):918-24
33. Sarmiento A, McKellop HA, Llinas A. et al. Effect of loading and fracture motions on diaphyseal tibial fractures. J Orthop Res. 1996;14(1):80-4
Author contact
![]() Corresponding author: DING Zhen-qi, MD, Department of Orthopaedic Surgery, The Affiliated Southeast Hospital of Xiamen University, Orthopaedic Trauma Center of PLA, Zhangzhou, Fujian, 363000, China. Tel: +86-13063196989; Fax: +86-0596-2975543; E-mail: 24520091152983xmu.edu.cn. & shenjiazuo09com.
Corresponding author: DING Zhen-qi, MD, Department of Orthopaedic Surgery, The Affiliated Southeast Hospital of Xiamen University, Orthopaedic Trauma Center of PLA, Zhangzhou, Fujian, 363000, China. Tel: +86-13063196989; Fax: +86-0596-2975543; E-mail: 24520091152983xmu.edu.cn. & shenjiazuo09com.

 Global reach, higher impact
Global reach, higher impact