Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(2):94-100. doi:10.7150/ijms.7.94 This issue Cite
Research Paper
Factors affecting the long-term response to tacrolimus in renal transplant patients: Pharmacokinetic and pharmacogenetic approach
1. Department of Pharmacology, School of Medicine, University of Patras, Rion, Greece
2. Center for Cell Engineering, Molecular Pharmacology and Chemistry Program, Memorial Sloan-Kettering Cancer Center (MSKCC), New York, NY 10065, USA
3. Department of Medical Physics, School of Medicine, University of Patras, Rion, Greece
4. Department of Internal Medicine-Nephrology, School of Medicine, University of Patras, Rion, Greece
Received 2010-4-20; Accepted 2010-5-10; Published 2010-5-11
Abstract
Background: The aim of our study was to determine the impact of CYP3A5*1 and CYP3A5*3 on the kinetics of tacrolimus in renal transplant recipients.
Material and methods: Forty kidney recipients were selected to participate. Maintenance scheme consisted of tacrolimus, a purine inhibitor and a steroid. CYP3A5 genotyping was performed with PCR and RFLP. Pharmacokinetic model was developed with Linear Regression and General Linear Model repeated measures approach. The impact of sex, CYP3A5*1 allele, age at transplantation, hepatic and renal function on tacrolimus kinetics was examined.
Results: The frequency of CYP3A5*3/*3 and CYP3A5*1/*3 genotype was 35/40 and 5/40, respectively. No CYP3A5*1/*1 was detected. CYP3A5*1 variant was associated with significant lower TAC dose adjusted concentration at 3, 6, 12 and 36 months after transplantation. Hepatic and renal function showed a significant effect on tacrolimus dose adjusted concentration 3 months after transplantation (p=0.000 and 0.028, respectively). Sex did not show a significant impact on tacrolimus kinetics. Carriers of CYP3A5*1 allele had lower predicted measures for tacrolimus dose adjusted concentration and higher predicted measures for volume of distribution.
Conclusion: We proved that CYP3A5*1 carriers need higher tacrolimus dose than CYP3A5*3 homozygotes to achieve the target blood concentration.
Keywords: CYP3A5, general linear models, linear regression, tacrolimus, renal transplantation
Background
Tacrolimus, a calcineurin inhibitor, remains the centerpiece of the maintenance treatment scheme in renal transplant recipients. Both its narrow therapeutic index and its highly pharmacokinetic variance may lead to overtreatment and toxicity or insufficient treatment and transplant rejection, conditions that are usually seen in clinical practice. Thus, it is obvious that the optimal tacrolimus dose has to be achieved soon after transplantation and must be preserved thereafter.
In the recent years, effort has been made to determine the potential causes of inter- and intra-individual variability. Except for the characteristics of each individual and several environmental factors, the role of biological factors affecting the drug absorption, distribution, metabolism or deletion has been investigated [1-9]. Till now, the most significant biological factors known to affect pharmacokinetics are the drug transporters and the metabolizing enzymes [7,9].
CYP3A, the primary subfamily of the cytochrome P450 (CYP) enzymatic system, is responsible for the metabolism of tacrolimus [10]. Specifically; tacrolimus is mainly metabolized by CYP3A4 and CYP3A5 enzyme isotype. CYP3A5 polymorphisms seem to affect tacrolimus kinetics at a greater degree compared to CYP3A4 ones and thereby, these polymorphisms are thought to be the best candidates for pharmacogenetics' application in immunosuppresion [1,7,11-13]. CYP3A5 gene is expressed in a limited number of individuals. When it is expressed, it may count for the 50% of the total hepatic CYP3A protein [5,14].
Macphee et al were the first to detect the effect of a CYP3A5 polymorphism in the distribution of tacrolimus [11]. CYP3A5*3 (G6986A) polymorphism, located in intron 3, has been recognized as the most important CYP3A5 polymorphism. The alleles A and G are CYP3A5*1 and CYP3A5*3, respectively. Individuals carrying at least one CYP3A5*1 allele express CYP3A5 protein whereas individuals homozygotes for CYP3A5*3 are non-expressors [15-18].
There are grave indications that pharmacogenetic testing for CYP3A5 prior to transplantation improves the individualization of immunosuppressive therapy although epigenetic factors must be taken into account [7]. The aim of the present study was to determine the impact of CYP3A5*1 and CYP3A5*3 genotype on the kinetics of tacrolimus in renal transplant recipients. The drug dose and level, the drug dose-adjusted level and the drug volume of distribution values are analyzed based on the presence of CYP3A5*1 allele, sex, age, renal and hepatic function.
Material and Methods
Patient population
Forty renal transplant recipients (median age: 41 years, range: 13-69), who attended the Outpatient Clinic of Nephrology and treated with TAC as the primary immunosuppressant, were selected to participate in the study. The protocol was approved by the Institution Ethics Committee of our hospital and informed consent was obtained from all subjects.
Maintenance treatment scheme consisted of a combination of a calcineurin inhibitor (tacrolimus) with a purine inhibitor (mycophenolate mofetil or azathioprine) and a steroid (prednizolone). Tacrolimus was given twice a day in individually adjusted doses and its trough levels were measured 12 hours post dose.
Identification of CYP3A5 genotype
Five milliliter blood samples were drawn from each patient in a vacutainer tube containing ethylene-diaminetetracetic acid. Genomic DNA was extracted from 200μl whole blood by QIAamp DNA Blood kit (Qiagen GmbH, Hilden) and was analyzed on a 0.8% agarose/Tris-borate EDTA gel with ethidium bromide staining (Figure 1).
a. Genomic DNA. Lane M, base pair marker (λDNA 5μgr); lanes 1-9 genomic DNA from 9 blood samples. Analysis on a 0.8% agarose/Tris-borate EDTA gel. b. PCR for CYP3A5. Lane M, base pair marker (λDNA 5μgr); lanes 1-3 PCR product for CYP3A5 from 3 DNA samples. Analysis on a 2% agarose/Tris-borate EDTA gel. Figure is printed negative. c. RFLP for CYP3A5. Lane M, base pair marker (250-bp DNA ladder); lanes 1-10 SspI-digested PCR products from 10 PCR products. CYP3A5*1/*1 genotype gives 148-, 125- and 20- bp bands (lanes 1, 2, 3, 6, 7 and 9) and CYP3A5*1/*3 genotype gives 168-, 148-, 125- and 20- bp (lanes 4, 5, 8 and 10). CYP3A5*3/*3 genotype is not seen in this picture. The 20- bp band is not visible. Analysis on a 3.5% agarose/Tris-borate-EDTA gel.

PCR followed by RFLP was used for CYP3A5 genotyping. PCR primers for CYP3A5 were designed to amplify a 293 bp fragment (forward primer: 5'-CATCAGTTAGTAGACAGATGA-3', reverse primer: 5'-GGTCCAAACAGGGAAGAAATA-3') [19]. The 25μl PCR volume contained 2.5μl 10x PCR Buffer, 1μl MgCl2 50mM, 1μl dNTPs 10mM, 0.5μl forward primer 25pmol/μl, 0.5μl reverse primer 25pmol/μl, 0.25μl TAQ polymerase 5Units/μl, 1μl isolated DNA and 18.25 μl ddH20. PCR conditions were 1min at 94οC, 40 cycles of 1min at 94οC (template denaturation), 1min at 55οC (primer annealing), 1min at 72οC (primer extension) and finally, 7min at 72οC (final elongation). The PCR product was analyzed on a 2% agarose/Tris-borate EDTA gel with ethidium bromide staining (Figure 1).
Enzymatic digestion of PCR products was performed using SspI endonouclease (New England Biolabs). 25 μL PCR product was incubated for 2 hours at 37οC with 2μl SspI 5Units/μl and 3μl 10xBuffer. A 3.5% agarose/Tris-borate EDTA gel with ethidium bromide staining was used and bands were detected by a short wavelength UV transilluminator (Figure 1). CYP3A5*1/*1 genotype gives 148-, 125- and 20- bp bands, CYP3A5*3/*3 genotype gives 168- and 125-bp bands and CYP3A5*1/*3 genotype gives 168-, 148-, 125- and 20- bp [19].
Statistical analysis
Tacrolimus kinetics were estimated with the use of tacrolimus daily dose, concentration, dose adjusted concentration and volume of distribution. Transplant recipients weight (kgr) and daily dose (mgr/day) of TAC in 14 days, 1, 3, 6, 12, 24 and 36 months were recorded so that dose per weight (mgr/kgr/day) could be calculated. The tacrolimus dose adjusted concentration (concentration/dose ratio) and tacrolimus volume of distribution (dose/concentration ratio) were calculated. Statistical analysis was performed with the use of PASW Statistics 17.0 (SPSS Inc, Chicago, IL, USA). Pharmacokinetic parameters were firstly modeled with the use of Linear Regression (LR). The results were compared to General Linear Model (GLM) repeated measures results.
LR predicts the dependent variable value based on the values of at least one independent variable. It assumes that the model depends linearly on the unknown parameters and the conditional mean of Y variable given the value of X is an affine function of X. In GLM repeated measures approach, the dependent variable is measured at multiple times (n) and it is represented by n variables (within-subjects factors). Categorical factors are used to divide study population into subgroups (between-subjects factors) and continuous variables are used as control variables (covariates). Taking into account that linear relationships, normal distribution of the dependent variables and fixed effects exist, the effects of between- and within-subjects factors as well as the interaction between factors and covariates and its effects are analyzed.
The difference between LR and GLM repeated measures analysis is that LR does not take into account the sequel of the data and it encounters them as derived from different patients. Thus, GLM repeated measures approach seems to be a more accurate methodology for the analysis of such data but it is more demanding in the quantity of available data.
In the LR analysis, the kinetic parameters were modeled based on sex, presence of CYP3A5*1 allele, age at transplantation (<40 versus ≥40 years old), hepatic (AST≤40U/ml and ALT≤35U/ml versus AST>40U/ml and/or ALT>35U/ml) and renal (serum creatinine ≤1.4mgr/dl versus >1.5mgr/dl) function. In the GLM repeated measures approach, a multivariate model was developed. Sex and genotype were used to define the subgroups of our patient population whilst the age at transplantation served as a covariate. The threshold of statistical significance was set at 5% (a=0.05).
Results
The characteristics of the study population are shown in Table 1. The frequency of CYP3A5*3/*3 genotype was 87.5% (35/40) whereas the frequency of the CYP3A5*1/*3 genotype was 12.5% (5/40). No individual homozygote for CYP3A5*1 was detected. Patients' renal and hepatic function as well as tacrolimus trough concentration are presented in Table 2.
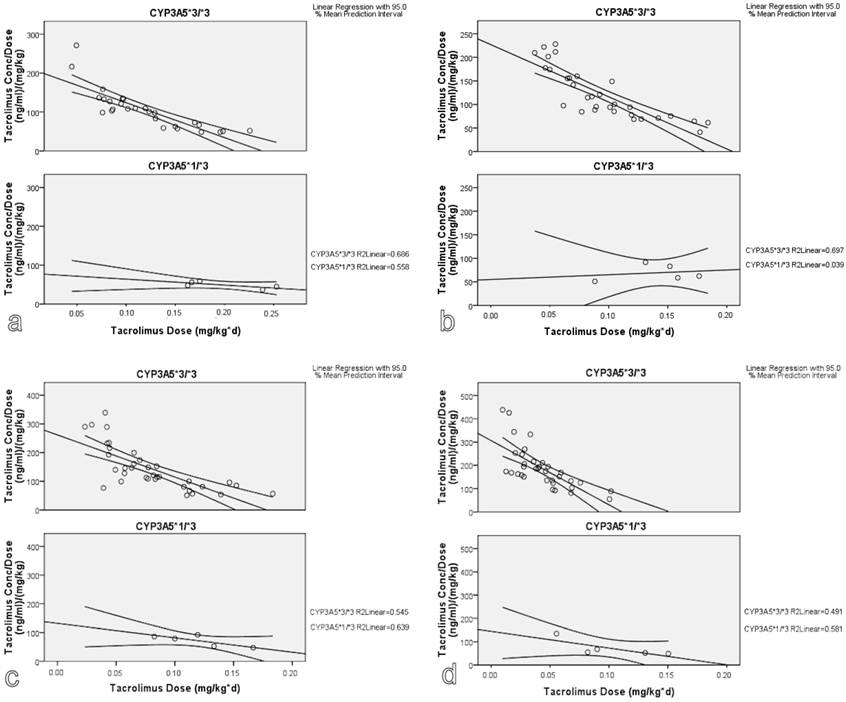
According to LR statistical approach, the presence of CYP3A5*1 variant was associated with lower tacrolimus dose adjusted concentration at 3, 6, 12 and 36 months after transplantation (Figure 2). Hepatic and renal function showed a statistically significant effect on tacrolimus dose adjusted concentration at 3 months after transplantation (p<0.001 and 0.028, respectively). There was no evidence that gender had a significant impact on tacrolimus kinetic parameters.
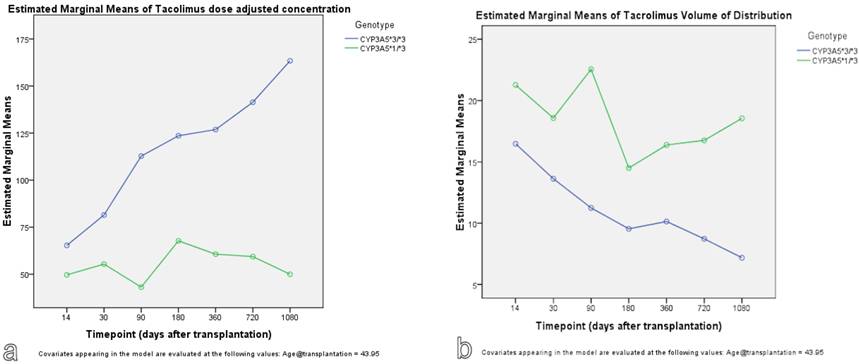
The findings were confirmed with the use of GLM repeated measures approach. As seen in Figure 3, carriers of CYP3A5*1 allele show lower predicted measures for tacrolimus dose adjusted concentration and higher predicted measures for tacrolimus volume of distribution. With GLM approach, the effect of timepoint, meaning the time after transplantation, on tacrolimus kinetic parameters was significant (p<0.001).
Genotype effect on tacrolimus dose adjusted concentration: A linear regression model. (a. 3 months, b. 6 months, c. 12 months and d. 36 months after transplantation).
Genotype effect on tacrolimus dose adjusted concentration (a) and volume of distribution (b): A GLM repeated measures approach.
Demographic characteristics of patient population (age is expressed as median value/25% quartile-75% quartile).
| Demographic characteristic | Number |
|---|---|
| Gender (female/male) | 13/27 |
| Age (years, median/range) | 41/35-54 |
| Age for females (years) | 42/37-53 |
| Age for males (years) | 41/35-54 |
| Transplantation number (first/second) | 28/12 |
| Place of transplantation (our centre/elsewhere) | 36/4 |
| Cadaver transplantation | 34 |
| Age at the onset of the chronic renal deficiency (years) | 38/24-50 |
| Age at the onset of the chronic renal deficiency for females (years) | 36/29-49 |
| Age at the onset of the chronic renal deficiency for males (years) | 38/23-50 |
| Primary kidney disease | |
| Unknown | 14 |
| Glomerulonephritis | 9 |
| Cystoureteral reflux | 4 |
| Polycystic kidney disease | 3 |
| Diabetic nephropathy | 2 |
| Alport syndrome | 1 |
| Eclampsia | 1 |
| Antiphospholipidic syndrome (Systemic lupus erythematosus) | 1 |
| Nephrolithiasis | 3 |
| Nephrosclerosis | 1 |
| Nephronophthisis | 1 |
Patients' renal and hepatic function, and tacrolimus trough levels at 14 days, 1, 3, 6, 12, 24 and 36 months after transplantation. Values are expressed as median (25% quartile-75% quartile).
| Timepoint | AST (U/ml) | ALT (U/ml) | Cr (mgr/dl) | Trough level (ngr/ml) |
|---|---|---|---|---|
| 14 days | 15 (11-31) | 27 (17-59) | 1.7 (1.3-2.7) | 11 (8.1-12.3) |
| 1 month | 13 (11-17) | 24 (16-34) | 1.8 (1.3-2.3) | 10.7 (9.2-13) |
| 3 months | 16 (12-23) | 20 (14-29) | 1.6 (1.2-2.1) | 10.4 (9.2-12.1) |
| 6 months | 13 (17-21) | 16 (13-22) | 1.6 (1.3-2.1) | 10 (8.5-11.2) |
| 12 months | 18 (15-23) | 18 (12-24) | 1.4 (1.2-2) | 9.1 (7.5-10.9) |
| 24 months | 17 (14-21) | 17 (11-25) | 1.4 (1.1-1.7) | 7.2 (6.1-8.7) |
| 36 months | 18 (16-22) | 19 (15-23) | 1.3 (1.1-1.6) | 6.7 (5.2-7.9) |
Discussion
Pharmacogenetics emerged during 1950s seeking to detect the potential relation between altered genetic basis and behavior after drug administration. However, several epigenetic factors such as age, co-morbidity, disease seriousness, co-administrated drug therapies and their interactions, renal and hepatic function, nutritional and other habbits, are often responsible for the intra- and inter-individual variability seen after drug administration [6,9]. For these reasons, therapeutic drug monitoring constitutes a crucial step in the administration of several drug agents. However, this empirical treatment algorithm carries increased risk of side effects or insufficient treatment.
Taking into account the critical clinical condition of renal transplant recipients and the limited number of renal transplants, the immunosuppressant treatment scheme prescribed to renal transplant patients must be adjusted in a way that precludes the possibility of under or over-treatment. The main drug in the immunosuppressant scheme that kidney recipients receive is tacrolimus. Most studies seek for the detection of polymorphisms that are associated with the individual's response to tacrolimus. However, in order to secure the appropriate administration of tacrolimus, both genetic and epigenetic factors must be taken into account [7,15]. For this reason, we attempted to model tacrolimus kinetics based on both genotype and epigenetic factors.
Disparity exists in the literature as to which genes and which genetic polymorphisms must be studied. We studied the presence of CYP3A5*1 in 40 kidney recipients. The genotype frequencies observed in our study are in agreement with the study of Arvanitidis et al, making us assume that our study sample is a representative one of the population [12]. Besides, the frequency of CYP3A5*3 allele in Caucasian Canadians is about 93%, and 83% of Dutch Caucasians are CYP3A5*3 homozygotes [19,20]. We chose to study this specific genetic polymorphism of CYP3A5 metabolizing enzyme as this isotype seems to critically affect the metabolism of TAC and this polymorphism is a frequent one in Greek population [3,5,19-21]. Besides, CYP3A5*1 has been proposed to regulate both the expression of CYP3A5 and CYP3A4. Other CYP3A5 alleles (*5, *6, *7) show low frequency in Caucasian population and thus, they are regarded as less suitable for screening purposes [19,20].
We failed to highlight the effect of the variants studied in all timepoints, probably due to the presence of several parameters affecting tacrolimus kinetics. The presence of CYP3A5*1 allele does not predict the amount of CYP3A5 protein accurately as it can be affected by other factors, genetic (expression of m-RNA, stability of CYP3A5 protein, dual metabolic pathway via CYP3A4 and CYP3A5, linkage disequilibrium with CYP3AP1 pseudogene etc) or not (drug interactions, environmental influences etc) [2,4,6,7,9,11,22,23]. Although trough concentrations of tacrolimus were corrected for dose, differences in diet, habits, comorbidity or concomitant treatment schemes were not inspected. Due to the limited patient population, we were not able to estimate the impact of concomitant drug agents and comorbidities on the kinetics of tacrolimus.
Till now, several studies with limited or extended patient populations have been carried out attempting to clarify the influence of various factors on tacrolimus kinetics. However, the results remain controversial with regard to pharmacokinetic and clinical effect of the studied parameters. Our main results are in alignment with the results of Zhao et al and Tsuchiya et al [14,17]. We found that individuals carrying one CYP3A5*1 allele need higher tacrolimus dose to achieve the target blood concentration, compared to CYP3A5*3 homozygotes.
Conclusions
We proved that CYP3A5 genotype affects individuals' response to tacrolimus both in the early and late phase after renal transplantation. However, given that most drug effects are polygenic in nature and that several epigenetic factors alter tacrolimus kinetics, the combination of population pharmacokinetic/pharmacodynamic and pharmacogenomic studies is deemed necessary.
Acknowledgements
The authors would like to thank the personnel of Department of Internal Medicine-Nephrology, University Hospital of Patras, Rion for their help throughout this study.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Fredericks S, Holt DW. Pharmacogenomics of immunosuppressive drug metabolism. Curr Opin Nephrol Hypertens. 2003;12:607-13
2. MacPhee IA, Fredericks S, Tai T. et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4:914-9
3. Mourad M, Wallemacq P, De Meyer M. et al. Biotransformation enzymes and drug transporters pharmacogenetics in relation to immunosuppressive drugs: impact on pharmacokinetics and clinical outcome. Transplantation. 2008Apr15;85(Suppl 7):S19-24
4. Anglicheau D, Flamant M, Schlageter MH. et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;118:2409-14
5. de Jonge H, Kuypers DR. Pharmacogenetics in solid organ transplantation: current status and future directions. Transplant Rev. (Orland). 2008;22(1):6-20
6. Evans WE. Pharmacogenomics: marshalling the human genome to individualise drug therapy. Gut. 2003;52(Suppl 2):S10-S18
7. Haufroid V, Mourad M, Van Kerckhove V. et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147-54
8. Brockmöller J, Tzvetkov MV. Pharmacogenetics: data, concepts and tools to improve drug discovery and drug treatment. Eur J Clin Pharmacol. 2008Feb;64:133-57
9. Christians U, Strom T, Zhang YL, Steudel W, Schmitz V, Trump S, Haschke M. Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2006;28:39-44
10. Daly AK. Significance of the minor cytochrome P450 3A isoforms. Clin Pharmacokinet. 2006;45:13-31
11. MacPhee IA, Holt DW. A pharmacogenetic strategy for immunosuppression based on the CYP3A5 genotype. Transplantation. 2008;85:163-5
12. Arvanitidis K, Ragia G, Iordanidou M. et al. Genetic polymorphisms of drug-metabolizing enzymes CYP2D6, CYP2C9, CYP2C19 and CYP3A5 in the Greek population. Fundam Clin Pharmacol. 2007;21:419-26
13. Macphee IA, Fredericks S, Mohamed M. et al. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation. 2005;79:499-502
14. Zhao Y, Song M, Guan D. et al. Genetic polymorphisms of CYP3A5 genes and concentration of the cyclosporine and tacrolimus. Transplant Proc. 2005;37:178-81
15. McCarthy AD, Kennedy JL, Middleton LT. Pharmacogenetics in drug development. Philos Trans R Soc Lond B Biol Sci. 2005;360:1579-88
16. Walker DK. The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development. Br J Clin Pharmacol. 2004;58:601-8
17. Tsuchiya N, Satoh S, Tada H. et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78:1182-7
18. Hesselink DA, van Schaik RH, van der Heiden IP. et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245-54
19. van Schaik RH, van der Heiden IP, van den Anker JN. et al. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48:1668-71
20. Roy JN, Lajoie J, Zijenah LS. et al. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005Jul;33:884-7
21. Tirelli S, Ferraresso M, Ghio L. et al. The effect of CYP3A5 polymorphisms on the pharmacokinetics of tacrolimus in adolescent kidney transplant recipients. Med Sci Monit. 2008;14:CR251-4
22. Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76:391-6
23. Anglicheau D, Legendre C, Thervet E. Pharmacogenetics of tacrolimus and sirolimus in renal transplant patients: from retrospective analyses to prospective studies. Transplant Proc. 2007;39:2142-4
Author contact
![]() Corresponding author: Paraskevi F. Katsakiori, Department of Pharmacology, School of Medicine, University of Patras, 26500 Rion, Greece, Tel: +30 6937 438208, Email: vkatsakupatras.gr; vkatsakcom
Corresponding author: Paraskevi F. Katsakiori, Department of Pharmacology, School of Medicine, University of Patras, 26500 Rion, Greece, Tel: +30 6937 438208, Email: vkatsakupatras.gr; vkatsakcom

 Global reach, higher impact
Global reach, higher impact