ISSN: 1449-1907International Journal of Medical Sciences
Int J Med Sci 2024; 21(5):817-825. doi:10.7150/ijms.90695 This issue Cite
Research Paper
The Outcomes of Patients with Omicron Variant Infection who Undergo Elective Surgery: A Propensity-score-matched Case-control Study
Department of Anesthesiology, Army Medical Center of PLA, Daping Hospital, Army Medical University, 10 ChangjiangZhilu, Yuzhong District, Chongqing 400042, China.
*These authors contributed equally to this work.
Abstract
Aim: To investigate whether it is safe for patients with Omicron variant infection to undergo surgery during perioperative period.
Methods: A total of 3,661 surgical patients were enrolled: 3,081 who were not infected with the Omicron variant and 580 who were infected with the Omicron variant. We conducted propensity score matching (PSM) with a ratio of 1:4 and a caliper value of 0.1 to match the infected and uninfected groups based on 13 variables. After PSM, we further divided the Infected group (560 cases) by the number of days between the preoperative Omicron variant infection and surgery: 0-7, 8-14, 15-30, and >30 days. Multivariate logistic regression analysis was subsequently conducted on the categorical variables and continuous variables with a P value below 0.05, thereby comparing the infected group (0-7, 8-14, 15-30, >30 days) and the uninfected group for perioperative complications.
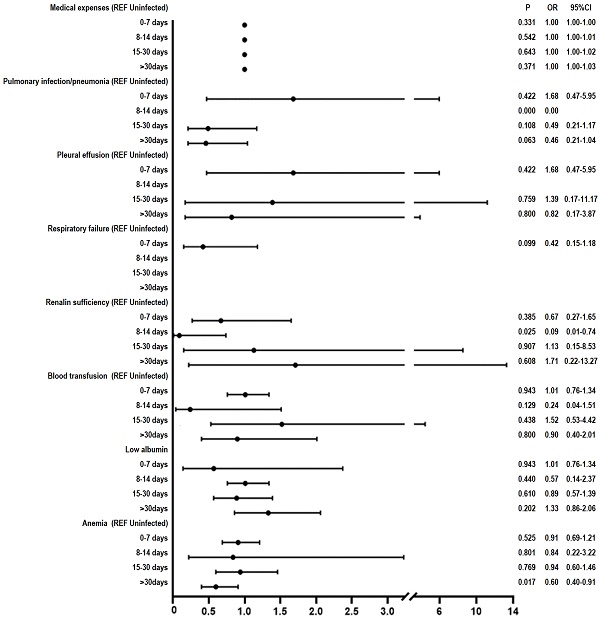
Results: Multivariate logistic regression analysis revealed that, compared to the uninfected group, among the four subgroups of the infected patients (0-7, 8-14, 15-30, >30 days), only renal insufficiency in the 8-14 days subgroup (OR: 0.09, 95%CI 0.01-0.74, P = 0.025) and anemia in the > 30 days subgroup (OR 0.6, 95%CI 0.4-0.9, P < 0.017) showed significant difference. However, there was no statistically significant difference in the incidence rate of blood transfusion, postoperative intensive care unit transfer, lung infection/pneumonia, pleural effusion, atelectasis, respiratory failure, sepsis, postoperative deep vein thrombosis, hypoalbuminemia, urinary tract infections, and medical expenses.
Conclusion: Omicron infection does not significantly increase the risk of perioperative major complications. The Omicron infection may not be a sufficient risk factor to postpone elective surgery.
Keywords: Omicron variant, postoperative complications, timing of surgery
Introduction
COVID-19 is a respiratory illness characterized by its sudden onset, caused by a specific coronavirus with a genetic material composed of single-stranded RNA and distinctive spike proteins on its surface [1]. The Omicron variant (B.1.1.529) is a worrisome, newly emerged form of the COVID-19 virus. It was initially detected in South Africa on November 26, 2021. Subsequently, this variant caused a significant surge in COVID-19 cases in Europe and the United States [2]. To November 8, 2023, there have been more than 771 million confirmed cases of COVID-19 worldwide, resulting in approximately 6.98 million deaths [3]. On January 8, 2022, Tianjin confirmed the first indigenous case of Omicron variant infection. The variant then spread across China [4]. In December 2022, there was a rapid surge in the number of Omicron variant infections [5] as the country loosened its control over COVID-19. In Chongqing, the dominant variant during that period was Omicron-BA.5.2 variant [6].
Maslo et al. observed that compared with patients infected with previous variants, those infected with the Omicron variant are generally younger, have fewer comorbidities, and experience lower severity and mortality rates [7]. Furthermore, asymptomatic infections and mild symptoms are more common among those infected with this variant [7]. Early research by Madhi SA et al. suggested that its pathogenicity was greatly reduced, and the morbidity and mortality were greatly reduced during the widespread transmission of the Omicron variant [8]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a rapidly evolving RNA virus that has recently mutated to form the Omicron variant. Compared to the Delta variant, the Omicron variant exhibits a significantly higher replication rate and notably greater infectivity [9]. According to the Association of Anesthetists in England, no evidence has been found to suggest how infection with the Omicron variant after receiving the SARS-CoV-2 vaccine might impact perioperative outcomes. Therefore, the advice to minimize elective surgery within seven weeks of contracting SARS-CoV-2 infection is still valid [10]. Sridhar et al. reported that individuals who have recuperated from asymptomatic or mild COVID-19 infections can safely undergo elective general surgical procedures after a waiting period of at least two weeks [11]. While a study conducted by Lieberman Nd et al. suggests that elective surgery can safely take place with 10 days delay from either the first day of symptom onset or the day of the first positive SARS-CoV-2 test result [12]. Dobbs TD et al. highlighted that asymptomatic patients who have been vaccinated can safely proceed with elective surgery within 5 to 10 days after being diagnosed [13].
Most of currently available studies suggest that patients infected with the Omicron variant ideally undergo surgery with a delay of 5 days to 7 weeks [10][11][12][13]. In the present study, we categorized our patients into four groups by time of surgery relative to their first infection (0-7, 8-14, 15-30, and >30 days), and compared the rate of perioperative complications, aiming to determine the best timing of surgery in patients with Omicron variant infection.
Materials and methods
Ethics approval
As this is a retrospective study, the Ethics Committee waived the requirements of obtaining the Informed Consent Forms from the patients. On May 4, 2023, the Army Medical Center of the PLA approved this study, with ratification number 2023 177.
Registration
The study registered in the WHO International Clinical Trial Registration (ChiCTR2300071913).
Inclusion and exclusion criteria
Inclusion criteria: All patients who had elective surgery at our hospital between October 2022 and January 2023.
Exclusion criteria: patients with no medical records pertaining to the Omicron variant between December 2022 and January 2023; patients under 18 years of age.
Research method
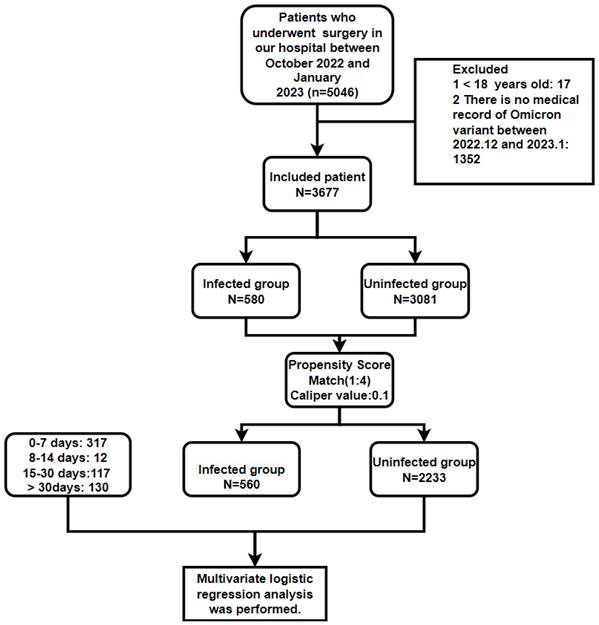
In total, 3,677 patients underwent surgery, comprising 3,081 uninfected patients (from October 2022 to January 2023) and 580 infected patients (from December 2022 to January 2023). Upon hospital admission, each patient underwent an rt-Polymerase chain reaction (PCR) test utilizing a nasopharyngeal swab specimen. Subsequently, patients were categorized into infected (Control group) and non-infected (Experimental group) groups based on these test results. The infected group and the non-infected group underwent propensity score matching (PSM) based on various factors, including age classification (≥ 60 years, < 60 years), gender (female, male), fractures, malignant tumors, hypertension (HBP), coronary heart disease (CHD), diabetes (DB), cerebral infarction, chronic obstructive pulmonary disease (COPD), preoperative deep vein thrombosis (DVT), surgical type (emergency surgery or elective surgery), methods of anesthesia (general anesthesia, spinal anesthesia, nerve block, local anesthesia), and American Society of Anesthesiologists (ASA) classification (I, II, III, IV, V) (Figure 1).
After PSM, the Infected group was further subdivided into four subgroups based on the time interval between Omicron variant infection and surgery: 0-7, 8-14, 15-30, and >30 days. A comparative analysis was subsequently performed between the Infected group and the Uninfected group in the following aspects: blood transfusion, postoperative patient transfer (regular ward or intensive care unit (ICU)), postoperative diagnosis (lung infection/pneumonia, pleural effusion, atelectasis, respiratory failure, renal insufficiency, sepsis, postoperative DVT, hypoalbuminemia, anemia, urinary tract infection), and medical expenses.
Data collection
We primarily acquired patients' clinical data by querying our hospital's clinical electronic medical record system and the relevant anesthesia-surgical systems. The collected information included: age, gender; preoperative medical records: HBP, diabetes, CHD, COPD, cerebral infarction, bone fracture, malignant tumor, preoperative DVT; during the surgery: surgery type (emergency or elective), method of anesthesia (general, spinal, or local anesthesia; nerve block), ASA classification, utilization of blood transfusion, postoperative patient transfer: regular ward or ICU; postoperative diagnosis: lung infection/pneumonia, pleural effusion, atelectasis, respiratory failure, renal insufficiency, sepsis, postoperative DVT, hypoalbuminemia, anemia, urinary tract infection, postoperative DVT, and medical expense. For the Infected group, the time interval between first Omicron variant infection and elective surgery was also collected. The diagnosis criteria for Omicron variant infection by our laboratory are as follows: (i) Before surgery, a positive rapid antigen test was conducted or a positive RT-PCR nasopharyngeal swab was performed; (ii) The preoperative chest computed tomography (CT) scan revealed findings consistent with pneumonitis associated with SARS-CoV-2 infection; (iii) A preoperative test indicating the presence of positive immunoglobulin G (IgG) or immunoglobulin M (IgM) antibodies [14]. According to the WHO criteria (and the reference values adopted by our laboratory), hemoglobin levels below 130 g/L for males or below 120 g/L for females are classified as anemia [15].
Statistical analyses
Statistical analysis was conducted by using SPSS26.0 (IBM Corp, Armonk, NY, USA). Patients after matching were categorized into the Infected group and the Uninfected group. PSM was subsequently employed for both groups with a ratio of 1:4 and a caliper value of 0.1. Categorical variables were compared using the chi-square test, while continuous variables were compared using the ANOVA test. Normally distributed enumeration data were represented by the mean plus or minus standard deviation (x ± s), and skewed enumeration data were represented by median (quaternary) [M (Q1, Q3)]. Multivariate logistic regression analysis was subsequently conducted on the categorical variables and continuous variables with a P value below 0.05, thereby comparing the infected group (0-7, 8-14, 15-30, >30 days) and the uninfected group for perioperative complications. P < 0.05 was considered statistically significant.
Results
Basic patient information
A total of 3,661 patients were enrolled (Figure 1). Before PSM, there were 2,153 males and 1,508 females, with a mean age of 49.5 years (in the Infected group) and 49.2 years (in the Uninfected group). There were 282 patients with bone fracture, and 979 patients with malignant tumor. The most common preoperative comorbidities in the patients included HBP (580 cases), DB (372 cases), and CHD (107 cases) (Table 1). Ages in each group after PSM are shown in Table 2.
Results of PSM analysis
PSM was applied to the Infected and Uninfected groups based on 13 variables: gender, age group, bone fracture, malignant tumor, HBP, DB, CHD, COPD, cerebral infarction, preoperative DVT, emergency or elective surgery, anesthesia method, and ASA classification. Results revealed that all variables had a P value > 0.05 and none had a standard deviation (|d|) greater than 0.25, suggesting that all the variables were balanced after the matching (Table 3). After PSM, the Infected group had 560 cases and the Uninfected group had 2,231, enabling a well-balanced comparison between the two groups.
Results of multivariate logistic regression analysis
After applying ANOVA test to the data following PSM, we observed a significant difference between the groups on the variable “medical expenses” (P < 0.05). We further performed Chi-square test and found that the following variables showed between-group differences (P < 0.05): pulmonary infection/pneumonia, low albumin, anemia, respiratory failure, renal insufficiency, and blood transfusion. The multivariable logistic regression analysis revealed the following results: renal insufficiency in the 8-14 days subgroup (OR: 0.09, 95% CI 0.01-0.74, P = 0.025) and in the > 30 days subgroup (OR 0.6, 95%CI 0.4-0.9, P < 0.017) (Figure 2).
Our study revealed no significant difference in the rate of perioperative complications (blood transfusion, postoperative ICU transfer, lung infection/pneumonia, pleural effusion, atelectasis, respiratory failure, sepsis, postoperative DVT, hypoalbuminemia, urinary tract infection, and medical expenses) in surgical patients with Omicron variant infection (0-7, 8-14, 15-30, > 30 days), as compared to uninfected patients. Although significant difference was observed in renal insufficiency for 8-14 days subgroup and anemia for > 30 days subgroup, it makes little difference.
Discussion
The global pandemic has exerted enormous pressure on healthcare systems worldwide, resulting in a sharp reduction in surgical activity. Within the first 12 weeks of pandemic outbreak [3], approximately 28 million surgeries were canceled, leading to millions of patients who were still in anticipation of their scheduled procedures [16]. Barie et al. emphasized that following the COVID-19 pandemic, there is an anticipated substantial increase in surgical procedures, potentially straining healthcare workers. Prolonged and distressing delays for particular patients may occur, ultimately resulting in frustration among surgical teams [17]. Given the persistent mutations of COVID-19 variants, alterations in transmissibility, virulence, and mortality rates are ongoing. Consequently, the scheduling of postponed surgeries should be adjusted accordingly [18]. El-Boghdadly et al. reported that perioperative risk returned to baseline at 7 weeks after SARS-CoV-2, prompting their recommendation for a 7-week delay post-infection before surgery [10]. However, it is crucial to recognize that none of the variants they investigated were the Omicron variant. In another study, Baiocchi G et al. found that for patients who had recovered from asymptomatic and mild COVID-19 infections, undergoing oncological surgeries after a waiting period of at least 2 weeks, with a median time of approximately 25 days (ranging from 12 to 84 days) following their COVID-19 diagnosis, did not result in a significantly higher risk of postoperative complications when compared to patients without COVID-19 [17]. However, it's worth mentioning that their study had a relatively limited sample size, consisting of just 49 cases. Codner et al. found that the timing of preoperative SARS-CoV-2 positivity relates to severe complications during the perioperative period. Their study involved 262 SARS-CoV-2 positive and 1,840 negative patients.
Distribution characteristics of covariates in surgical patients before and after PSM in the Infected group and Uninfected group
| Before matching (3661) | After matching (2824) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Infected group (580) | Uninfected group (3081) | X2 | P | Infected Group (560) | Uninfected group (2233) | X2 | P | ||||
| Gender | 0.34 | 0.297 | ||||||||||
| Male | 425 | 73.28% | 2231 | 72.41% | 0.70 | 0.22 | 327 | 58.39% | 1334 | 59.74% | ||
| Female | 155 | 26.72% | 850 | 27.59% | 233 | 41.61% | 899 | 40.26% | ||||
| Age group (year) | 0.18 | 0.36 | 1.59 | 0.114 | ||||||||
| ≤60 | 464 | 80.00% | 2231 | 72.41% | 412 | 73.57% | 1700 | 76.13% | ||||
| >60 | 86 | 14.83% | 850 | 27.59% | 148 | 26.43% | 533 | 23.87% | ||||
| Fracture | 6.77 | 0.01 | 1.83 | 0.104 | ||||||||
| Yes | 60 | 10.34% | 222 | 7.21% | 57 | 10.18% | 187 | 8.37% | ||||
| No | 520 | 89.66% | 2859 | 92.79% | 503 | 89.82% | 2046 | 91.63% | ||||
| Malignant tumor | 0.25 | 0.325 | 0.34 | 0.219 | ||||||||
| Yes | 160 | 27.59% | 819 | 26.58% | 158 | 28.21% | 608 | 27.23% | ||||
| No | 420 | 72.41% | 2262 | 73.42% | 402 | 71.79% | 1625 | 72.77% | ||||
| HBP | 0.09 | 0.402 | 0.98 | 0.178 | ||||||||
| Yes | 86 | 14.83% | 442 | 14.35% | 84 | 15.00% | 299 | 13.39% | ||||
| No | 494 | 85.17% | 2639 | 85.65% | 476 | 85.00% | 1934 | 86.61% | ||||
| DB | 1.84 | 0.101 | 0.13 | 0.381 | ||||||||
| Yes | 68 | 11.72% | 304 | 9.87% | 65 | 11.61% | 247 | 11.06% | ||||
| No | 512 | 88.28% | 2777 | 90.13% | 495 | 88.39% | 1986 | 88.94% | ||||
| CHD | 0.30 | 0.33 | 0.25 | 0.353 | ||||||||
| Yes | 19 | 3.28% | 88 | 2.86% | 18 | 3.21% | 63 | 2.82% | ||||
| No | 561 | 96.72% | 2993 | 97.14% | 524 | 93.57% | 2170 | 97.18% | ||||
| COPD | 0.06 | 0.462 | 0.2 | 0.385 | ||||||||
| Yes | 10 | 1.72% | 49 | 1.59% | 10 | 1.79% | 34 | 1.52% | ||||
| No | 570 | 98.28% | 3032 | 98.41% | 550 | 98.21% | 2199 | 98.48% | ||||
| Preoperative DVT | 0.18 | 0.396 | 0.65 | 0.27 | ||||||||
| Yes | 8 | 1.38% | 36 | 1.17% | 8 | 1.43% | 23 | 1.03% | ||||
| No | 572 | 98.62% | 3045 | 98.83% | 552 | 98.57% | 2210 | 98.97% | ||||
| Cerebral infarction | 3.82 | 0.042 | 0.8 | 0.224 | ||||||||
| Yes | 18 | 3.10% | 57 | 1.85% | 17 | 3.04% | 53 | 2.37% | ||||
| No | 562 | 96.90% | 3024 | 98.15% | 543 | 96.96% | 2180 | 97.63% | ||||
| Type of surgery | 0.01 | 0.482 | 0.46 | 0.273 | ||||||||
| Emergency surgery | 86 | 14.83% | 2629 | 85.33% | 80 | 14.29% | 292 | 13.08% | ||||
| Elective surgery | 452 | 77.93% | 452 | 14.67% | 480 | 85.71% | 1938 | 86.79% | ||||
| Methods of anesthesia | 8.61 | 0.035 | 0.55 | 0.908 | ||||||||
| General anesthesia | 432 | 74.48% | 2145 | 69.62% | 418 | 74.64% | 1674 | 74.97% | ||||
| Spinal anesthesia | 77 | 13.28% | 553 | 17.95% | 76 | 13.57% | 283 | 12.67% | ||||
| Nerve block | 29 | 5.00% | 178 | 5.78% | 28 | 5.00% | 110 | 4.93% | ||||
| Local anesthesia | 42 | 7.24% | 205 | 6.65% | 38 | 6.79% | 166 | 7.43% | ||||
| ASA Classification | 26.95 | <0.001 | 4.1 | 0.252 | ||||||||
| I | 93 | 16.03% | 721 | 23.40% | 91 | 16.25% | 426 | 19.08% | ||||
| II | 438 | 75.52% | 2194 | 71.21% | 426 | 76.07% | 1665 | 74.56% | ||||
| III | 39 | 6.72% | 145 | 4.71% | 36 | 6.43% | 126 | 5.64% | ||||
| IV | 9 | 1.55% | 21 | 0.68% | 7 | 1.25% | 16 | 0.72% | ||||
| V | 1 | 0.17% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | ||||
ASA: American society of Aneshesiologists; CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; DB: diabetes; DVT: deep vein thrombosis; HBP: hypertension; PSM: propensity score matching. P < 0.05 was considered statistically significant.
In the 0-15 day timeframe, SARS-CoV-2 positive patients were 1.88 times more likely to experience severe complications compared to SARS-CoV-2 negative surgical patients; within 15-30 days, it reduced to 0.43-fold, and within 31-50 days, it was 0.98-fold when compared to their SARS-CoV-2 negative counterparts [19]. In certain circumstances, they have found that postponing surgery by 14 days after testing positive for SARS-CoV-2 may be advisable [20]. But the sample size in their study is relatively small, potentially leading to biased results. Glasbey et al. reported that the time gap between asymptomatic SARS-CoV-2 infection and elective surgery could potentially be reduced to 5 days, but there is no supporting evidence from surgery-receiving patients [21].
Summary of patient characteristics after PSM
| Variance ± standard deviation | P-Value | |
|---|---|---|
| Age (years) | 0.423 | |
| Uninfected group | 48.96±16.45 | |
| 0-7 days | 50.91±16.2 | |
| 8-14 days | 50.92±10.32 | |
| 14-30days | 48.63±16.86 | |
| >30days | 46.89±17.39 | |
| Medical expenses (yuan) | 0.027 | |
| Uninfected group | 30859.24±857.5 | |
| 0-7 days | 39054.13±3386.6 | |
| 8-14 days | 31753.43±6891.7 | |
| 14-30days | 29852.9±2281.1 | |
| >30days | 29852.9 ±2442.6 |
PSM: propensity score matching. P < 0.05 was considered statistically significant.
Research scheme of propensity score matching analysis of uninfected and infected with Omicron variant in patients undergoing surgery.
Postoperative outcomes between SARS-CoV-2 negative patients and preoperatively positive SARS-CoV-2 patients stratified by timing of positivity before surgery with complication compared to SARS-CoV-2 negative patients
| Variables | Uninfected group | Infected Group (560) | |||||
|---|---|---|---|---|---|---|---|
| (2233) (REF) | 0-7 days (317) | 8-14 days (12) | 15-30 days (117) | >30 days (130) | X2 | P | |
| Pulmonary infection/pneumonia | 83(3.72%) | 22(6.94%) | 0 | 7(5.98%) | 9(6.92%) | 10.47 | 0.033 |
| Pleural effusion | 22(0.99%) | 3(0.95%) | 0 | 1(0.85%) | 2(1.54%) | 0.54 | 0.97 |
| Postoperative DVT | 30(1.34%) | 6(1.89%) | 0 | 2(1.71%) | 2(1.54%) | 0.82 | 0.935 |
| Urinary tract infection | 38(1.7%) | 5(1.58%) | 0 | 3(2.56%) | 6(4.62%) | 6.34 | 0.175 |
| Low albumin | 88(3.94%) | 19(5.99%) | 0 | 3(2.57%) | 11(8.46%) | 9.59 | 0.048 |
| Atelectasis of lung | 5(0.22%) | 1(0.32%) | 0 | 0 | 0 | 0.72 | 0.949 |
| Anemia | 164(7.34%) | 23(7.26%) | 0 | 5(4.27%) | 18(13.85%) | 10.31 | 0.035 |
| Respiratory failure | 14(0.63%) | 9(2.84%) | 0 | 0 | 1(0.77%) | 17.07 | 0.002 |
| Renal insufficiency | 24(1.07%) | 8(2.52%) | 1(8.33%) | 1(0.85%) | 1(0.78%) | 9.91 | 0.045 |
| Sepsis | 9(0.40%) | 4(1.26%) | 0 | 0 | 0 | 5.72 | 0.22 |
| Blood transfusion | 113(5.06%) | 28(8.83%) | 2(16.67%) | 4(3.42%) | 8(6.15%) | 11.32 | 0.023 |
| Transfer to ICU | 43(2.84%) | 9(2.84%) | 0 | 2(1.71%) | 2(1.54%) | 1.61 | 0.807 |
DVT: deep vein thrombosis; ICU: Intensive care unit; PSM: propensity score matching. P < 0.05 was considered statistically significant.
Multivariate logistic regression was performed to compare postoperative outcomes between SARS-CoV-2 negative patients and preoperatively positive SARS-CoV-2 patients stratified by timing of positivity before surgery with complications, as compared to SARS-CoV-2 negative patients after PSM.
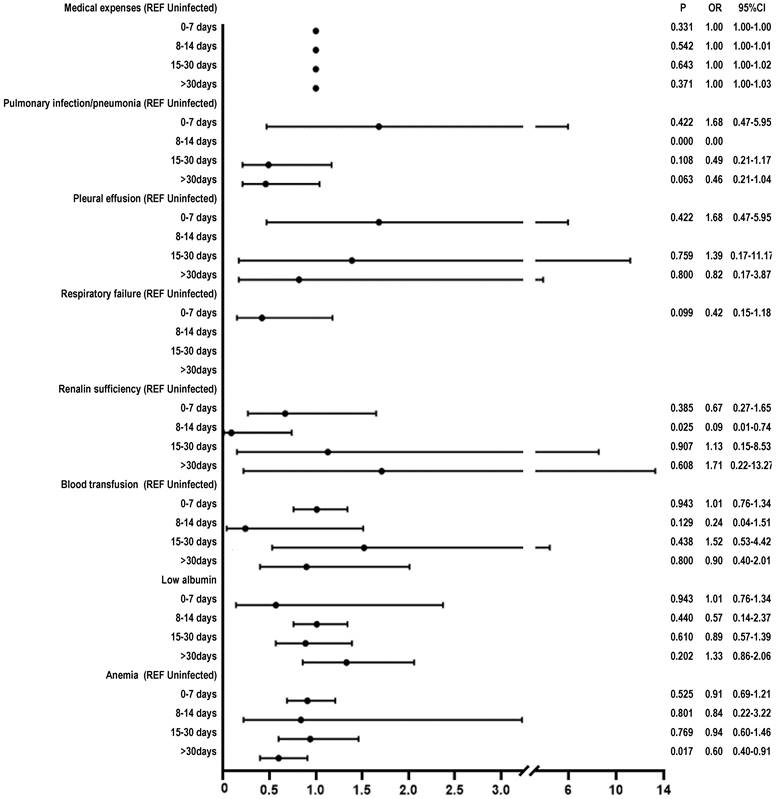
In contrast to these studies, our research is the first to reveal that patients infected with the Omicron variant (at different intervals post-infection, including 0-7, 8-14, 15-30, and >30 days) undergoing surgical treatment did not exhibit significant difference in perioperative complication rate (including transfusions, postoperative ICU admissions, lung infections/pneumonia, pleural effusion, lung collapse, respiratory failure, sepsis, postoperative deep vein thrombosis, hypoalbuminemia, urinary tract infections, and medical expenses), as compared to uninfected patients. We also observed significant difference in renal insufficiency in the 8-14 days subgroup (OR 0.09, 95% CI 0.01-0.74, P = 0.025) and anemia in the >30 days subgroup (OR 0.6, 95% CI 0.4-0.9, P < 0.017). In COVID-19 patients, the immune response to the viral infection can lead to elevated levels of serum cytokines, including Interleukin-6 and Tumor necrosis factor. These cytokines exhibit direct nephrotoxic effects [22][23]. Teng et al. observed that there was a mild decline in renal function after Omicron variant infection and returned to normal within six months. And most patient infected with Omicron variant exhibited a mild inflammation response [24]. Lechner-Scott et al. found that post-COVID-19 patients suffered persistent inflammation response [25], which may affect RBC production and RBC lifespan, and finally lead to the development of inflammatory anemia [26]. The mild inflammation observed after Omicron infection [24] results in a relatively minor effect on renal insufficiency and anemia. Therefore, it appears that Omicron variant infected patients do not need to postpone their surgery, whether it is elective or emergency. The reasons will be further explored below from the perspective of the Omicron variant virus and vaccination against COVID-19.
Omicron variant virus
Early research by South African scientists indicated that during the widespread transmission of the Omicron variant, its pathogenicity has significantly diminished, resulting in a substantial decrease in the occurrence of severe cases and mortality rate [8]. SARS-CoV-2, similar to other RNA viruses, can undergo mutations and genetic evolution over time as it adjusts to new human hosts, potentially leading to mutant variants exhibiting distinct characteristics compared to their original strains [27]. SARS-CoV-2 exhibits a relatively high mutation rate, and by 2022, several variant strains [28]. The Omicron strain has demonstrated higher transmissibility, evading immune system defenses, and exhibiting limited susceptibility to the COVID-19 vaccine [29]. Nevertheless, recent studies have reported that compared to other SARS-CoV-2 pathogen infections, Omicron variant infections are closely associated with a higher incidence of asymptomatic carriage, milder symptoms, lower hospitalization rate, and mortality [30]. Emerging data indicate that the Omicron variant tends to result in a higher number of asymptomatic cases, less severe symptoms, and lower rates of hospitalization and mortality when compared to previous variants [31]. In the analysis conducted by Desai et al., researchers have made a notable observation: the mortality rate among cancer patients diagnosed with COVID-19 during the Omicron phase is considerably lower than that of patients diagnosed during the early stages of the pandemic, with the acute mortality rate dropping from over 30% to below 10% [32].
Association with past COVID-19 vaccination
Cortellini et al. reported that previous SARS-CoV-2 immunization is an effective measure in safeguarding patients from COVID-19 sequelae, minimizing treatment disruptions, and reducing mortality rate [33]. Christensen et al. found that within their healthcare system, Omicron patients were notably younger, exhibited significantly higher vaccination rates, and had a significantly lower likelihood of hospitalization when compared to patients infected with the Alpha or Delta variants [31]. Furthermore, the Omicron variant group needed less intensive respiratory support and required shorter hospitalizations, aligning with an overall reduction in disease severity [31]. Moreover, it has been widely demonstrated that vaccinated surgical patients experience significantly reduced COVID-19 mortality compared to the general vaccinated population [34].
Large-scale vaccination campaigns have been implemented worldwide over the past two years to prevent and control the transmission of the Omicron variant. Specifically, the most widely administered SARS-CoV-2 vaccine is the inactivated one in mainland China [35]. Although domestic vaccines may not provide complete protection against Omicron variants, they do offer significant safeguards for adult patients [36]. Wang J et al. found that vaccinated adult patients had notably lower levels of Interleukin-6 and C-reactive protein compared to unvaccinated patients, revealing that the domestic vaccines provide significant protection against inflammation reduction in adult patients during the recovery phase [36]. In our study, over 90% of the patients had been vaccinated, which likely contributed to a notable reduction in the rate of perioperative complications. The Omicron variant has notably increased its transmissibility and possesses the capability to evade immunity acquired through previous infections, vaccination, or a combination of both [37]. Pilz et al. noted that compared to 2020, the severity of SARS-CoV-2 infections has noticeably diminished, with a significant reduction in associated complications. These trends may be attributed to the reduced virulence of prevalent variant strains, vaccination programs, and the natural immunity developed from previous infections [38]. Vaccination against COVID-19 stands as the most effective measure to reduce the severity of infection and minimize perioperative complications. Therefore, it is strongly encouraged to promote vaccination preoperatively [36]. Thus, Omicron variants exhibit high transmissibility but lower pathogenicity compared to other SARS-CoV-2 variants. Additionally, vaccination and previous infections have significantly enhanced population immunity, resulting in a decrease in severe cases of COVID-19. Consequently, SARS-CoV-2 is anticipated to circulate in a manner similar to seasonal coronaviruses [39].
In conclusion, the Omicron variant has reduced pathogenicity as it constantly evolves, leading to gradually attenuated inflammation within the body. In addition, as patients are generally vaccinated against SARS-CoV-2 and tend to be younger, the patients showed no significant difference in the rate of perioperative complications undergoing surgery at 0-7, 7-14, 14-30, and >30 days after infection, as compared to uninfected surgical patients. It is relatively safe for Omicron variant patients to undergo surgery, as the rate of perioperative complications showed no significant difference, thus maybe there is no need to postpone surgery.
We conducted this retrospective analysis primarily by collecting medical history and laboratory tests of patients who were uninfected or infected with the Omicron variant and underwent surgery. Nevertheless, there are certain limitations to this study. Firstly, it is a retrospective study and thus some data may be incomplete, including missing information in the medical records of some patients. In addition, because it is a single-center, retrospective, case-control study, there may be potential unaccounted confounding factors, necessitating further research with larger sample sizes.
Conclusion
We found no significant differences in the incidence of perioperative complications among patients with Omicron variant infection undergoing surgery at 0-7, 8-14, 15-30, and >30 days after infection compared to the uninfected patients.
Abbreviations
ACE: Angiotensin converting enzyme; ASA: American society of Aneshesiologists; CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; DB: diabetes; DVT: deep vein thrombosis; HBP: hypertension; ICU: Intensive care unit; OR: Odd ratio; PCR: Polymerase chain reaction; PSM: propensity score matching; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
Acknowledgements
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Contributed to the conception and design of the study: Qingxiang Mao, Rui Li and Hong Yan.
Contributed to the acquisition and analysis of data: Rui Li.
Responsible for statistical analysis: Rui Li and Xiaojuan Xiong.
Oversaw project conception and acts as overall guarantor: Qingxiang Mao and Hong Yan.
Wrote and revised the manuscript: Xiaojuan Xiong.
Read and approved the final manuscript, offering critical feedback: all members of the authorship group.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Harenberg J, Favaloro E. COVID-19: progression of disease and intravascular coagulation - present status and future perspectives. Clin Chem Lab Med. 2020;58(7):1029-1036
2. Sharma V, Rai H, Gautam DNS, Prajapati PK, Sharma R. Emerging evidence on Omicron (B.1.1.529) SARS-CoV-2 variant. J Med Virol. 2022;94(5):1876-1885
3. WHO Data. WHO COVID-19 dashboard. https://covid19.who.int (Accessed on November 8, 2023).
4. http://health.people.com.cn/n1/2022/0315/c14739-32375333.html (Accessed on March 15,2022)
5. http://www.nhc.gov.cn/xcs/zhengcwj/202212/6630916374874368b9fea6c2253289e1.shtml (Accessed on December 26,2022)
6. https://wsjkw.cq.gov.cn/ztzl_242/qlzhxxgzbdfyyqfkgz/yqtb/202301/t20230103_11447658.html (Accessed on January 3, 2023)
7. Maslo C, Friedland R, Toubkin M. et al. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA. 2022;327(6):583-584
8. Madhi SA, Kwatra G, Myers JE. et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N Engl J Med. 2022;386(14):1314-1326
9. Nishiura H, Ito K, Anzai A, Kobayashi T, Piantham C, Rodríguez-Morales AJ. Relative Reproduction Number of SARS-CoV-2 Omicron (B.1.1.529) Compared with Delta Variant in South Africa. J Clin Med. 2021;11(1):30
10. El-Boghdadly K, Cook TM, Goodacre T. et al. Timing of elective surgery and risk assessment after SARS-CoV-2 infection: an update: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, Centre for Perioperative Care, Federation of Surgical Specialty Associations, Royal College of Anaesthetists, Royal College of Surgeons of England. Anaesthesia. 2022;77(5):580-587
11. Sridhar RP, Titus DK, Surendran S. et al. Postoperative outcomes in patients undergoing elective general surgery after recovery from Covid-19 at a tertiary care centre: A one-year case series. Natl Med J India. 2022;35(4):197-200
12. Lieberman N, Racine A, Nair S. et al. Should asymptomatic patients testing positive for SARS-CoV-2 wait for elective surgical procedures?. Br J Anaesth. 2022;128(5):e311-e314
13. Dobbs TD, Gibson JAG, Fowler AJ. et al. Surgical activity in England and Wales during the COVID-19 pandemic: a nationwide observational cohort study. Br J Anaesth. 2021;127(2):196-204
14. COVIDSurg Collaborative; GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76(6):748-758
15. World Health Organization, 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity (No. WHO/NMH/NHD/MNM/11.1). World Health Organization.
16. The Lancet Rheumatology. Too long to wait: the impact of COVID-19 on elective surgery. Lancet Rheumatol. 2021;3(2):e83
17. Barie Philip S. et al. Surgical Infection Society Guidance for Restoration of Surgical Services during the Coronavirus Disease-2019 Pandemic. Surgical infections. 2021;22:818-827
18. Barie Philip S. et al. Omicron, Long-COVID, and the Safety of Elective Surgery for Adults and Children: Joint Guidance from the Therapeutics and Guidelines Committee of the Surgical Infection Society and the Surgery Strategic Clinical Network, Alberta Health Services. Surgical infections. 2023;24:6-18
19. Codner JA, Archer RH, Lynde GC, Sharma J. Timing is Everything: Surgical Outcomes for SARS-CoV-2 Positive Patients. World J Surg. 2023;47(2):437-444
20. Baiocchi G, Aguiar S Jr, Duprat JP. et al. Early postoperative outcomes among patients with delayed surgeries after preoperative positive test for SARS-CoV-2: A case-control study from a single institution. J Surg Oncol. 2021;123(4):823-833
21. Glasbey JC, Dobbs TD, Abbott TEF. Can patients with asymptomatic SARS-CoV-2 infection safely undergo elective surgery?. Br J Anaesth. 2022;128(6):909-911
22. Azkur AK, Akdis M, Azkur D. et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564-1581
23. Zipeto D, Palmeira JDF, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front Immunol. 2020;11:576745
24. Teng L, Song X, Zhang M. et al. The pattern of cytokines expression and dynamic changes of renal function at 6 months in patients with Omicron COVID-19. J Med Virol. 2023;95(2):e28477
25. Lechner-Scott J, Levy M, Hawkes C, Yeh A, Giovannoni G. Long COVID or post COVID-19 syndrome. Mult Scler Relat Disord. 2021;55:103268
26. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142
27. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 18. 2023
28. Malik JA, Ahmed S, Mir A. et al. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J Infect Public Health. 2022;15(2):228-240
29. Callaway E, Ledford H. How bad is Omicron?. What scientists know so far. Nature. 2021;600(7888):197-199
30. Vihta KD, Pouwels KB, Peto TE. et al. Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom. Clin Infect Dis. 2022;76(3):e133-e141
31. Christensen PA, Olsen RJ, Long SW. et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642-652
32. Desai A, Mohammed TJ, Duma N. et al. COVID-19 and Cancer: A Review of the Registry-Based Pandemic Response. JAMA Oncol. 2021;7(12):1882-1890
33. ortellini A, Tabernero J, Mukherjee U. et al. SARS-CoV-2 omicron (B.1.1.529)-related COVID-19 sequelae in vaccinated and unvaccinated patients with cancer: results from the OnCovid registry. Lancet Oncol. 2023;24(4):335-346
34. COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. Br J Surg. 2021;108(9):1056-1063
35. Lai DY, Xue JB, He P. et al. Longitudinal neutralization activities on authentic Omicron variant provided by three doses of BBIBP-CorV vaccination during one year. Proteomics. 2023;23(2):e2200306
36. Wang J, Dong H, Zhao J. et al. Effects of vaccines on clinical characteristics of convalescent adult patients infected with SARS-CoV-2 Omicron variant: A retrospective study. Front Microbiol. 2023;14:1096022
37. UK Health Security Agency, 2021. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing: Update on hospitalisation and vaccine effectiveness for Omicron VOC-21NOV-01 (B. 1.1. 529), 17.
38. Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209:112911
39. Jung C, Kmiec D, Koepke L. et al. Omicron: What Makes the Latest SARS-CoV-2 Variant of Concern So Concerning?. J Virol. 2022;96(6):e0207721
Author contact
![]() Corresponding authors: Hong Yan and Qingxiang Mao E-mail: cqyanhongcom; qxmaoedu.cn. Tel: (+86) 02368729729; Email address: 10 ChangjiangZhilu, Yuzhong District, Chongqing 400042, China.
Corresponding authors: Hong Yan and Qingxiang Mao E-mail: cqyanhongcom; qxmaoedu.cn. Tel: (+86) 02368729729; Email address: 10 ChangjiangZhilu, Yuzhong District, Chongqing 400042, China.
Received 2023-10-2
Accepted 2024-3-8
Published 2024-3-17
