3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(2):154-160. doi:10.7150/ijms.13649 This issue Cite
Research Paper
Functional Role of FcγRIIB in the Regulation of Mesenchymal Stem Cell Function
1. Department of Respiratory, Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
2. Department of Respiratory, The General Hospital of Shenyang Military, Shenyang, Liaoning, 110015, China;
3. Department of VIP Treatment, Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
4. Department of Orthopedics, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, 210000, China;
5. Department of Immunology, Zhejiang University, Hangzhou, Zhejiang310000, China;
6. Department of Respiratory, No. 1 People's Hospital of Yancheng, Yancheng, Jiangsu, 224000, China;
7. Department of Respiratory, The 174 Hospital, Xiamen, Fujian, 361000, China
8. Institute of Immunology, The Second Military Medical University, Shanghai, 200433, China
* These authors contributed equally to this study.
Received 2015-8-24; Accepted 2015-11-27; Published 2016-2-5
Abstract
Mesenchymal stem cells (MSCs) derived from bone marrow are plural-potent stem cells with immune regulatory functions. We aimed to evaluate role of FcγRIIB in the regulation of bone marrow-derived MSC function. MSCs were prepared from mouse bone marrow derived from wild-type (WT) or FcγRIIB-deficient (FcγRIIB-/-) mice. MSCs were co-cultured with bone marrow-derived dendritic cells (BMDCs), and BMDC maturation and function were evaluated by flow cytometric analysis and carboxyfluorescein succinimidyl ester-labeled OT-II T-cell addition. An acute asthma model was established by aeresol ovalbumin challenge in mice. Mice received WT or FcγRIIB-/- MSC therapy. Lung function was evaluated by histological examination and cytokine production measurement. mRNA and protein expression levels of target genes were examined by real-time quantitative polymerase chain reactionor western blotting. We found that MSCs derived from bone marrow exhibit a high level of FcγRIIB expression. FcγRIIB deficiency impaired the suppressive function of MSCs, as FcγRIIB deficiency efficiently reversed the inhibitory effect of MSCs on BMDC maturation and function. Additionally, FcγRIIB-/-MSCs were less potent at suppressing asthma in model mice, possibly through reduced expression of Smad2, Smad3, Cox-2, and prostaglandin E2 in FcγRIIB-/-MSCs. FcγRIIB might play an essential role in regulating the inhibitory effects of MSCs derived from bone marrow.
Keywords: mesenchymal stem cells, FcγRIIB, dendritic cell maturation, asthma.
Introduction
Mesenchymal stem cells (MSCs), multi-potential stem cells that reside largely within the bone marrow, have the capacity to differentiate into many cell lineages, such as adipocytes, osteocytes, and chondrocytes [1]. The MSCs derived from bone marrow exert immunomodulatory effects and have been widely studied in various human immune disorders, especially in autoimmune diseases [2-4]. Accumulating evidence suggests that MSCs derived from bone marrow have the ability to suppress allergic responses in asthma [5-7]. However, the underlying mechanism remains unclear.
Fcγ receptor II (FcγRII, also known as CD32) is a surface receptor mainly expressed on leukocytes, including B cells, follicular dendritic cells, macrophages, and neutrophils [8]. FcγRIIB is a low-affinity inhibitory Fcγ receptor that is highly homologous on its extracellular domain to the activating Fcγ receptor FcγRIIA [9]. Although FcγRIIB is known for its inhibitory effects on cell activation and cytokine production in many cell types, such as natural killer cells [10] and phagocytes [11], the potential role of FcγRIIB in the regulation of MSC function is still poorly understood.
In the present study, we investigated the influence of FcγRIIB on the biological functions of MSCs. To address this point, FcγRIIB-deficient (FcγRIIB-/-) mice were used, and the functions of MSCs derived from wild-type (WT) or FcγRIIB-/- mice were compared in vitro. Furthermore, a mouse acute asthma model was developed, and the differences in the in vivo function of MSCs derived from WT or FcγRIIB-/- mice were studied. Our findings may provide valuable insights into understanding the biological significance of FcγRIIB in mediating MSC function.
Materials & Methods
Animals
Balb/c and C57/B6 mice were purchased from BK Experimental Animal (Shanghai, China). FcγRIIB knockout (FcγRIIB-/-) mice were purchased from Jackson lab (Bar Harbor, ME, USA). All mice were housed in specific pathogen-free conditions. All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal experiments were performed with the approval of the Scientific Investigation Board of Second Military Medical University (Shanghai, China).
Reagents
Chicken ovalbumin (OVA) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Mouse fluorescein-conjugated antibodies against CD11c, I-Ab, CD80, CD86, CD40, and FcγRIIB were purchased from eBioscience. Enzyme-linked immunosorbent assay (ELISA) kits for the detection of mouse interleukin (IL)-12, IL-6, IL-4, and IL-13 as well as an enzyme immunoassay (EIA) kit for evaluation of prostaglandin E2 (PGE2) were bought from R&D Systems (Minneapolis, MN, USA).Rabbit anti-mouse Cox-2 and horseradish peroxidase (HRP)-conjugated anti-rabbit Ig secondary antibodies were purchased from Abcam (Cambridge, UK).
MSC culture and characterization
MSCs were prepared from mouse bone marrow (BM) derived from (WT) or FcγRIIB-/- C57/B6 mice as described previously [12]. Briefly, BM cells were flushed from femurs of mice and the red blood cells were lysed by incubating cells in Tris-NH4Cl solution for 2 min. BM cells were maintained in Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, and 6 ng/ml basic fibroblast growth factor (bFGF) for 7 days for in vitro differentiation. Cells were incubated in a 5%CO2-humidified incubator at 37°C. The adherent cells were detached using trypsin-ethylene diamine tetraacetic acid (EDTA) and harvested for a new passage. After three passages, cells were harvested and used for flow cytometric analysis. Adherent cells after three passages were able to differentiate into adipocytes and chondrocytes in vitro.
Bone marrow-derived dendritic cell (BMDC) culture
BMDCs were prepared from mouse BM as described previously [13] with a minor modification. Briefly, BM cells isolated and prepared from femurs of C57/B6 or FcγRIIB-/- mice were maintained in RMPI 1640 culture medium supplemented with 10%FBS, 10ng/ml granulocyte-macrophage colony stimulating factor (GM-CSF), and 5ng/ml IL-4. On day 7 following initial seeding, non-adherent cells and loosely adherent cells were collected, stained with phycoerythin (PE)-conjugated anti-mouse CD11c microbeads and purified using fluorescence-activated cell sorting (FACS) applied in a flow cytometric system (LSR II, BD).The purity of cultured BMDCs was greater than 80%.
Co-culture of MSCs with BMDCs
Passage 4 or 5 MSCs were cultured in six-well plates at a density of 3×105 cells/well. After incubation in a 5% CO2 incubator for 5-6 h, the culture medium was removed and replaced with 2 mL of BMDC suspension (0.5×105 cells/mL in RPMI-1640 medium containing 5% FBS). Cells were cultured in a 5% CO2-humidified incubator at 37°C until use in experiments.
Determination of DC maturation and function
BMDCs were stimulated with 100 ng/ml lipopolysaccharide (LPS) for 24 h. The DC surface markers including I-Ab and CD86 were analyzed using flow cytometric analysis. Data were analyzed by FlowJo software. To evaluate BMDC function, OT-II T cells were purified by staining of OTII splenocytes with anti-mouse CD4 microbeads (Miltenyi Biotech). Purified OT-II T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) by incubating the cells with 5µM CFSE dissolved in phosphate-buffered (PBS) for 5 min at 37°C. Then, BMDCs were incubated with CFSE-labeled OT-II T cells in the presence of 100µM OVA peptide for 72 h until flow cytometric analysis.
Mouse model of acute asthma
Balb/c mice were actively sensitized by intra-peritoneal injection of 100 µg OVA emulsified in aluminum hydroxide on days 1 and 14. Aerosol challenge was conducted from day 21 to day 24. Thirty minutes prior to challenge, mice were anesthetized with isofluorin and given 1 ×106 MSCs derived from either WT or FcγRIIB-/- mice intranasally. Intranasal administration of cell-free PBS was used as a control. During aerosol challenge, animals were treated once daily through a PARI Boy N (PARI GmbH, Germany) by holding 5 ml 1% OVA for 30 min from day 21 to day 24. After the last challenge, mice were euthanized and blood samples were collected through cardiac puncture. Serum samples were collected for ELISA analysis using an OVA-specific IgE ELISA kit. Bronchoalveolar lavage fluid (BALF) was prepared using 1ml PBS. Total cell numbers in BALF, including lymphocytes, macrophages, eosinophils, and neutrophils, were counted after Gimsa staining. Supernatants of BALF were collected for ELISA analysis of cytokine production. Lung tissues were carefully removed, fixed in formalin, embedded in paraffin, and prepared as 5-μm-thick sections. Lung sections were subjected to hematoxylin and eosin (HE) staining for histological examination.
Western blotting
A total of 1 ×107 BMDCs derived from WT or FcγRIIB-/-mice were collected and lysed with 0.5 ml lysis buffer containing 50 nM β glycerophosphate, 0.1 nM orthovanadate sodium, 2 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM DL-dithiothreitol (DTT), 1 mM phenylmethanesulfonyl fluoride (PMSF), 10 μg/ml aprotinin, and 20 μM leupeptin. Cell lysates were centrifuged at 12000 rpm for 10 min. A total of 30 μg denatured proteins were separated on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to a nitrocellulose (NC) membrane. Each membrane was blocked in 5% non-fat dry milk in PBS containing 0.1% Tween 20at room temperature for 2 h. Then, each membrane was probed with rabbit anti-mouse Cox-2 primary antibody and HRP-conjugated anti-rabbit Ig secondary antibody. Immunobands were visualized using an enhanced chemiluminescence (ECL) kit according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Statistical analysis
Data were analyzed by SPSS17.0 software (IBM SPSS), Chicago, IL, USA) and were presented as means ± standard deviation (SD). Statistical significance was determined using t test. A difference with P<0.05 was recognized as significant.
Results
Expression of FcγRIIB by MSCs
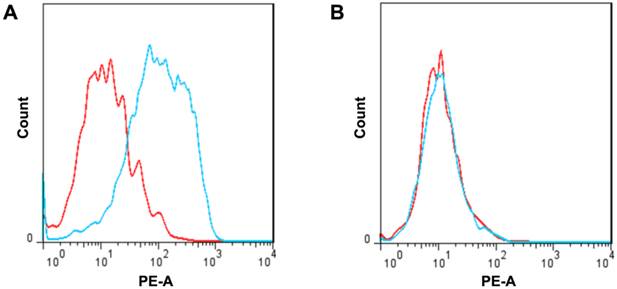
To characterize the expression of FcγRIIB in MSCs, MSCs were isolated from bone marrow and cultured in vitro. Flow cytometric analysis using specific cell surface markers demonstrated that the cultured cells were CD29-, CD44-, and Flk-positive and CD45- and CD34-negative. In addition, these cells had the capability to differentiate into adipocytes or chondrocytes in vitro, indicating these cells had characteristics of MSCs derived from bone marrow. Flow cytometric analysis showed a significantly increased level of FcγRIIB in MSCs based on negative isotype staining (Fig. 1A). Moreover, very little FcγRIIB could be detected in MSCs derived from FcγRIIB-/- mice (Fig. 1B).
FcγRII expression in MSCs derived from WT and FcγRIIB-/- mice. The level of FcγRII in MSCs derived from either WT (A) or FcγRIIB-/- (B) mice was evaluated by flow cytometric analysis using phycoerthryin-conjugated anti-FcγRIIB antibody. Blue curve, FcγRII; red curve, isotype control. Representative data are presented. n = 3.
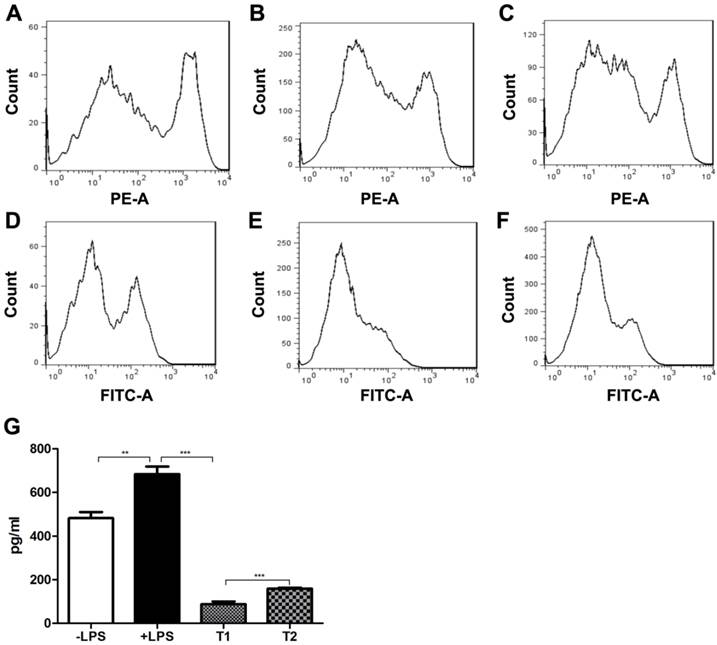
Effects of FcγRII on MSC-mediated DC maturation. BMDCs were cocultured without MSCs (A, D), with WT (B, E) or FcγRIIB-/- MSCs (C, F) in the presence of 100ng/ml LPS. Cells were firstly gated on CD11c+ population, and the expression of I-Ab (a-c) and CD86 (d-f) was analyzed. Data were acquired by flow cytometry and analyzed by FlowJo software. Representative data of three independent experiments are shown. (G) ELISA analysis of IL-12 level in BMDC culture supernatants. -LPS, BMDC culture supernatant without LPS stimulation; +LPS, BMDC culture supernatant with LPS stimulation; T1, LPS-stimulated BMDCs co-cultured with WT MSCs; T2, LPS-stimulated BMDCs co-cultured with FcγRIIB-/-MSCs. ** P<0.001; *** P<0.0001.
Effects of FcγRIIB on MSC-mediated DC maturation
We next investigated the influence of FcγRIIB deficiency on MSC function, such as the promotion of DC maturation. For this purpose, BMDCs were co-cultured with MSCs derived from WT mice. Upon LPS stimulation, the levels of the DC surface markers including I-Ab and CD86 were greatly increased as compared with control (Fig. 2). However, co-culture of BMDCs with WT MSCs significantly suppressed the I-Ab and CD86 expression. In addition, co-culture of BMDCs with MSCs derived from FcγRIIB-/- mice reversed the downregulation of I-Ab and CD86 induced by WT MSC co-culture. Similarly, production of the proinflammatory cytokine IL-12 was also elevated in BMDCs after LPS stimulation as compared with control (P<0.001). The IL-12 secretion was remarkably decreased in BMDCs co-cultured with WT MSCs (P<0.001), whereas co-culture of BMDCs with MSCs derived from FcγRIIB-/- mice reversed the reduction of IL-12 production induced by WT MSCs co-culture (P<0.001). These data indicate that MSCs play a suppressive role in DC maturation, and FcγRIIB deficiency efficiently reversed the inhibitory effect of MSCs on dendritic cell maturation.
Effects of FcγRIIB in MSC-regulated antigen-specific T-cell response
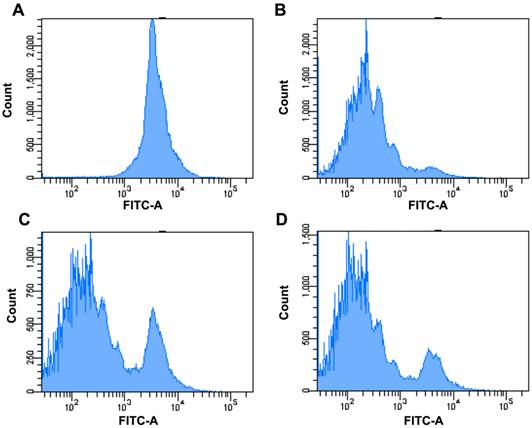
To evaluate the effects of FcγRIIB on the MSC-regulated antigen-specific T-cell response, CFSC-labeled OT-II T cells were added to BMDCs co-cultured with MSCs derived from WT or FcγRIIB-/- mice in the presence of OVA. Seventy-two hours after incubation, the CFSE division was examined using flow cytometric analysis. As revealed by Fig. 3, the division capability of OT-II T cells in BMDCs co-cultured with FcγRIIB-/- MSCs was higher than that of cells co-cultured with WT MSCs, suggesting FcγRIIB-/- MSCs are less potent at suppressing the antigen-specific T-cell response than WT MSCs.
FcγRIIB-deficient MSCs are less potent at suppressing mouse experimental asthma
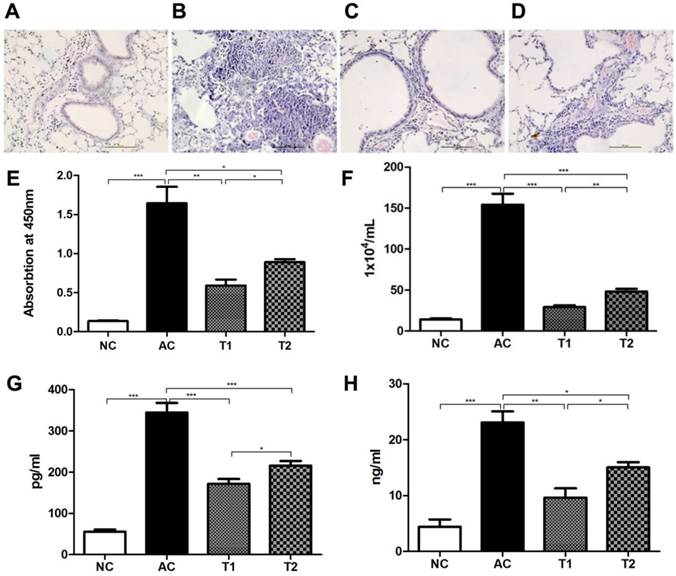
We next tested the biological functions of FcγRIIB-/-MSCs in vivo using a mouse model of allergic asthma. Histological examination showed a normal lung structure in control animals (Fig. 4A). However, massive lymphocyte infiltration in lung tissues was observed in model mice after aerosol OVA challenge (Fig. 4B). Animals receiving WT MSC therapy exhibited significantly reduced leukocyte infiltration, especially eosinophil infiltration in lung (Fig. 4C), whereas FcγRIIB-/- MSC therapy only moderately inhibited leukocyte infiltration in lung (Fig. 4D). ELISA analysis demonstrated that serum OVA-specific IgE level was significantly elevated in asthma model mice (P<0.001), whereas WT MSC therapy greatly reduced the serum OVA level as compared with that in model mice (P<0.001; Fig. 4E). Although FcγRIIB-/- MSC therapy also decreased the serum OVA level compared to that in model mice (P<0.05), its level was still higher than that in model mice that received WT MSCs (P<0.05). A similar trend was observed in the number of cells in BALF (Fig. 4F). As IL-4 and IL-13 are two distinctive Th2 cytokines involved in the pathogenesis of asthma, we also assessed the production of IL-4 and IL-13 in BALF. As shown by Fig. 4G and H, the levels of both IL-4 and IL-13 were elevated in asthma model mice (P<0.001). MSC therapy significantly reduced the IL-4 and IL-13 generation, and WT MSC therapy was more potent at decreasing their production as compared with FcγRIIB-/- MSC therapy (P<0.05).
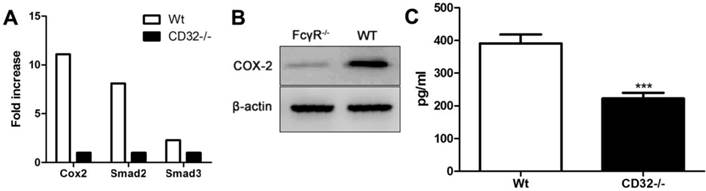
Reduced production of Smad2, Smad3, Cox-2, and PGE2 in FcγRIIB-deficient MSCs
To address the molecular mechanism underlying the compromised suppressive effect of FcγRIIB-/- MSCs, we compared the expression levels of Smad2, Smad3, and Cox-2, which are involved in inflammation response, between WT and FcγRIIB-/- MSCs by real-time quantitative PCR. Compared to FcγRIIB-/- MSCs, WT MSCs expressed a 10-fold enhancement in Smad2, Smad3, and Cox-2 mRNA (Fig. 5A). The protein expression of Cox-2 was also downregulated in FcγRIIB-/- MSCs compared to WT MSCs (Fig. 5B). Moreover, the PGE2 production in the supernatants of cultured WT MSCs was significantly higher than that in cultured FcγRIIB-/- MSCs (P<0.001; Fig. 5C). These results suggest that the compromised suppressive function of FcγRIIB-deficient MSCs might be related to the reduced expression of the molecules mentioned above.
Effects of FcγRIIB on MSC-regulated antigen-specific T-cell response. CFSE-labeled OT-II T cells were purified and cultured with BMDCs in the presence (B, C, D) or absence (A) of 100 μM OVA peptide. (a) No OVA peptide, MFI=4498. (B) BMDCs were incubated with OVA peptide. MFI=587. (C) BMDCs were co-cultured with WT MSCs in the presence of OVA. MFI=1233. (D) BMDCs were co-cultured with FcγRIIB-/- MSCs in the presence of OVA. MFI=921. Cells were first gated on CD4+ population and then the CFSE level was analyzed using flow cytometry. Data were acquired by LSRII and analyzed with FlowJo software. Representative data of three individual experiments were presented.
FcγRIIB-/-MSCs are less effective at controlling allergic asthma. Representative of three different animals are presented here. (A-D) Hematoxylin and eosin staining was used for histological examination of lung tissues. (A) Control mice; (B) asthma model mice; (C) asthma model mice that received WT MSC therapy; (D) and asthma model mice that received FcγRIIB-/- MSC therapy. (E) ELISA analysis of serum OVA-specific IgE production. (F) Total number of cells in BALF. Levels of IL-4 (G) and IL-13 (H) in BALF. NC, naive control; AC, asthma control; T1, asthma mice receiving WT MSC; T2: asthma mice receiving FcγRIIB-/- MSC. *P<0.05; **P<0.001; ***P<0.0001; NS, P>0.05.
Reduced production of Smad2, Smad3, Cox-2, and PGE2 in FcγRIIB-/-MSCs. (A) Cox2, Smad2, and Smad3 mRNA expression in WT and FcγRIIB-/- MSCs was analyzed using real-time quantitative PCR. (B) Western blotting analysis of Cox-2 expression. Representative data of three independent experiments are presented. (C) EIA analysis of PGE2 production in the culture supernatant of WT and FcγRIIB-/- MSCs. ***P<0.0001.
Discussion
MSCs are known to possess an array of immunosuppressive capabilities. For instance, MSCs suppress DC migration, maturation, and antigen presentation in vitro [14]. In addition, the immunosuppression properties of MSCs also include the MSC-mediated proliferation suppression of many immune cells, such as T lymphocytes, B lymphocytes, and natural killer cells [15]. Consistent with these findings, we also detected immunosuppression of DC maturation and function by MSCs derived from bone marrow. Moreover, intranasal administration of MSCs effectively modulated inflammation in an experimental model of asthma, which is in accordance with the results of a previous report [16].
FcγRIIB is known for its inhibitory role for B lymphocyte activation [17], and the absence of FcγRIIB facilitates the induction of autoimmune disease [18]. Baerenwaldt et al. demonstrated that FcγRIIB serves as a checkpoint of humoral tolerance in the human immune system, as impaired FcγRIIB function leads to the production of increased levels of serum immunoglobulins, the production of different autoantibody specificities, and an elevated generation of human plasmablasts and plasma cells in vivo [19]. In the present study, we found that MSCs derived from FcγRIIB-/- mice had restored function in mediating DC maturation. Our in vivo experiment further indicated that WT MSC therapy is more potent at decreasing inflammatory cytokine production than FcγRIIB-/- MSC therapy. These results suggest that FcγRIIB also plays a negative role in regulating MSC functions.
Smad2 and Smad3 are crucial intracellular mediators of airway inflammation that act through regulating transforming growth factor-β (TGF-β) signaling [20]. Prostaglandins (PGs), such as PGE2, are the metabolic products of the Cox pathway, which play important roles in inflammation and asthma [21]. Our previous study revealed that immune complex induced massive PGE2 production in macrophages through FcγRII induction [22]. Blockage of PGE2 production by celecoxib restored LPS-induced endotoxin shock in FcγRIIB-/- mice, indicating FcγRIIB-mediated PGE2 production is essential in immune suppression [22]. Here, we detected the reduced Smad2, Smad3, Cox-2, and PGE2 production in FcγRIIB-/- MSCs, implying the inhibitory effects of FcγRIIB on MSCs might be related to the decreased expression of these molecules. However, the precise mechanism involved in this process still needs to be further clarified.
Conclusions
In summary, our study demonstrated for the first time that FcγRIIB might play an essential role in regulating the inhibitory effects of MSCs derived from bone marrow. These findings may provide valuable insights into developing novel strategies to improve the suppressive functions of MSCs in cell-based clinical therapy.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (No. 81270073 and No. 81273319).
Author contribution
TYZ and RHC drafted the manuscript. ZL, JT and XXZ carried out the experiments. LRT and KDZ participated in the design of the study and performed the statistical analysis. YZY, CWD and CB conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
References
1. Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006:249-282
2. Ryan JM, Barry FP, Murphy JM. et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. (Lond). 2005;2:8
3. Barberini DJ, Freitas NP, Magnoni MS. et al. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res Ther. 2014;5:25
4. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736
5. Knight DA, Rossi FM, Hackett TL. Mesenchymal stem cells for repair of the airway epithelium in asthma. Expert Rev Respir Med. 2010;4:747-758
6. Kapoor S, Patel SA, Kartan S. et al. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy-induced asthma. J Allergy Clin Immunol. 2012;129:1094-1101
7. Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD. et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652-5657
8. Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093-1103
9. Selvaraj P, Fifadara N, Nagarajan S. et al. Functional regulation of human neutrophil Fc gamma receptors. Immunol Res. 2004;29:219-230
10. Trinchieri G, Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nat Immun. 1993;12:218-234
11. Joshi T, Butchar JP, Tridandapani S. Fcgamma receptor signaling in phagocytes. Int J Hematol. 2006;84:210-216
12. El Haddad N, Heathcote D, Moore R. et al. Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood. 2011;117:1176-1183
13. Idzko M, Hammad H, van Nimwegen M. et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935-2944
14. English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50-58
15. Shi Y, Hu G, Su J. et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510-518
16. Abreu SC, Antunes MA, de Castro JC. et al. Bone marrow-derived mononuclear cells vs. mesenchymal stromal cells in experimental allergic asthma. Respir Physiol Neurobiol. 2013;187:190-198
17. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34-47
18. Clynes R, Calvani N, Croker BP. et al. Modulation of the immune response in pristane-induced lupus by expression of activation and inhibitory Fc receptors. Clin Exp Immunol. 2005;141:230-237
19. Baerenwaldt A, Lux A, Danzer H. et al. Fcgamma receptor IIB (FcgammaRIIB) maintains humoral tolerance in the human immune system in vivo. Proc Natl Acad Sci U S A. 2011;108:18772-18777
20. Groneberg DA, Witt H, Adcock IM. et al. Smads as intracellular mediators of airway inflammation. Exp Lung Res. 2004;30:223-250
21. Claar D, Hartert TV, Peebles RS Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 2015;9:55-72
22. Zhang Y, Liu S, Liu J. et al. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fc gamma RIIb-dependent PGE2 production. J Immunol. 2009;182:554-562
Author contact
![]() Corresponding author: Prof. Chong Bai, Department of Respiratory, Changhai Hospital, Second Military Medical University, Shanghai 200433, China. Telephone: +86-21-31161312, Fax: +86-21-31161312. E-mail: bc7878com Or Prof. Yizhi Yu, Institute of Immunology, The Second Military Medical University, Shanghai 200433, China. Tel/Fax: +86021-81871101. Email: yuyz88com.
Corresponding author: Prof. Chong Bai, Department of Respiratory, Changhai Hospital, Second Military Medical University, Shanghai 200433, China. Telephone: +86-21-31161312, Fax: +86-21-31161312. E-mail: bc7878com Or Prof. Yizhi Yu, Institute of Immunology, The Second Military Medical University, Shanghai 200433, China. Tel/Fax: +86021-81871101. Email: yuyz88com.

 Global reach, higher impact
Global reach, higher impact