Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(10):805-810. doi:10.7150/ijms.12429 This issue Cite
Research Paper
Lithium Chloride Promotes Apoptosis in Human Leukemia NB4 Cells by Inhibiting Glycogen Synthase Kinase-3 Beta
1. Central Laboratory of Yong-chuan hospital, Chongqing Medical University, Chongqing 402160, China.
2. Key Laboratory of Laboratory Medical Diagnostics, Ministry of Education, Department of Laboratory Medicine, Chong-qing Medical University, Chongqing 400016, China.
Received 2015-4-16; Accepted 2015-9-2; Published 2015-9-20
Abstract
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML). With the application of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), APL becomes one of best prognosis of leukemia. However, ATRA and ATO are not effective against all APLs. Therefore, a new strategy for APL treatment is necessary. Here, we investigated whether lithium chloride (LiCl), a drug used for the treatment of mental illness, could promote apoptosis in human leukemia NB4 cells. We observed that treatment with LiCl significantly accelerated apoptosis in NB4 cells and led to cell cycle arrest at G2/M phase. Moreover, LiCl significantly increased the level of Ser9-phosphorylated glycogen synthase kinase 3β(p-GSK-3β), and decreased the level of Akt1 protein in a dose-dependent manner. In addition, LiCl inhibition of c-Myc also enhanced cell death with a concomitant increase in β-catnin. Taken together, these findings demonstrated that LiCl promoted apoptosis in NB4 cells through the Akt signaling pathway and that G2/M phase arrest was induced by increase of p-GSK-3β(S9).
Keywords: Lichium chloride, GSK-3β, leukemia cells, proliferation, apoptosis
Introduction
Acute promyelocytic leukemia (APL) is a unique model in cancer biology in that two therapeutic agents, ATRA and ATO. ATRA could induce differentiation and ATO could promote apoptosis in APL cells [1-6]. Although the complete remission rate of APL is over 90%, 10-30% of APL patients are not sensitive to ATRA and ATO [7]. Hence, it is important to explore new treatment strategies for APL.
Lithium chloride is one of the main drugs used for the treatment of bipolar disorder, and the antineoplastic effect of lithium is thought to be attributed to the inhibition of glycogen synthase kinase-3 (GSK-3), a type of multi-functional Silk/threonine protein kinase, generally exists in all eukaryotic cells, which plays an important role in multiple signaling pathway [8]. One of the most important ways regulated by GSK-3 is the Wnt singaling pathway, and GSK-3 also interacts with the Ras/PI3K/Akt/mTOR pathways. Previous studies have shown that lithium could increase the number of long-term hematopoietic stem cells in vivo [9]. Moreover, treatment with lithium results in an increase in peripheral blood CD34+ cells in patients [10], and it is also known to influence the prolifertion, apoptosis and cell cycle in different cell lines, such as esophageal cancer, medullary thyroid tumor, endometrial cancer and adenocarcinoma ductal pancreatic [11-15].
In this study, we demonstrate the apoptotic effect of lithium in an NB4 cell line, examine the potential mechanism underlying this effect, and elucidate the role of GSK-3β in this process. Our observations suggest that lithium chloride could provide a new therapeutic approach for the treatment of APL.
Materials and Methods
Materials
Lithium chloride (LiCl) and sodium chloride (NaCl) were purchased from Sigma (St Louis, MO). Antibodiy against Akt1 was purchased from Abcam (Hong Kong, China). Antibodies against β-catenin, GSK3β, p-GSK3-β (S9), c-Myc were from Cell Signaling Technology (USA).Goat anti-rabbit antibody, goat anti-mouse antibody and antibody against β-actin were purchased from Zhongshan Goldenbrige Biotechnology Co., Ltd (Beijing, China).
Cell line and culture
The human leukemia celll line were pruchased from the Shanghai Institute for Biological Science and maintained in RIPA-1640 supplemented containing 10% fetal bovine serum in an environment with 5% CO2 at 37 °C.
Cell viability assay
To assess cell viability, NB4 cells in each group were seeded in 96-well plates at a density of 5.0×103 cells/well. Then cells were incubated with viraous concentrations of Licl for 24h. In brief, 10 μl of CCK-8(7Sea Cell Counting Kit; Sevenseas Futai Biotechnology Co., Ltd.,Shanghai,China) was added to each well followed by incubation for 2h at 37 °C. The cell viability was assessed by detection of absorbance at 450 nm using a spectrophotometer. Cells growth curves were plotted. The experiment was repeated at least three times.
FACs analysis
Flow cytometry for cell apoptosis analysis
Cells with treatment of different concentrations of lithium chloride were harvested by centrifugation at 3000 g for 5 min. After washing twice with pre-cold PBS (pH 7.4), cells were re-suspended in Binding Buffer(Sungene Biotech Co., Ltd.,Tianjing,China), and then stained by Annexin V-PE and 7-AAD (Sungene Biotech Co., Ltd.,Tianjing,China) for 5-15 min at room temperature. At last, these cells were subjected to flow cytometer (Becton Dickinson, CA, USA) for detection of apoptotic cells. Each experiment was repeated at least three times.
Flow cytometry for cell style analysis
Cells were fixed in 75% ethanol overnight at -20℃ and washed in PBS and then further incubated with 50 ul propidiumiodide for 15min at room temperature.Then cells were analysis in flow cytometer. Each experiment was repeated at least three times.
Wertern blot analysis
1x106 cells in each group were washed with ice-cold phosphate-buffered saline and lysed in RIPA solution containing protease inhibitor cocktail. Protein concentration was determined with BCA method. A total of 80 μg of protein was added in 8% sodium dodecyl sulfate-polyacrylamide gel, and then transferred to polyvinylidene difluoride membrane. The membrane was blocked with 5% non-fat milk for 2 h, then incubated with specific antibodies(monoclonal antibody against Akt1;1:1000; Abcam,Hong Kong,China, monoclnal antibodies against β-catenin, GSK3β, p-GSK3-β (S9), c-Myc; 1:1000;Cell Signaling Technology,USA) overnight at 4 °C and then with secondary antibody(goat anti-rabbit antibody, 1:1000, Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China) for 1 h at 37 °C. After washing with Tris-Buffered Saline Tween-20 (TBST), the autoradiograms were scanned and subjected to densitometry. β-actin(mouse monoclonal antibody against β-actin, 1:500; Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China) was used as an internal control. Protein bands were visualized using the Quantity One Software (BIO-RAD, USA). Each experiment was repeated at least three times.
Statistical analysis
Data was expressed as means ± standard deviation (SD). Statistical analysis was performed with SPSS version 17.0. Independent sample t test was employed for comparing the means between two groups. A value of P<0.05 was considered statistically significant.
Results
Cytotoxic effect of LiCl in NB4 cells
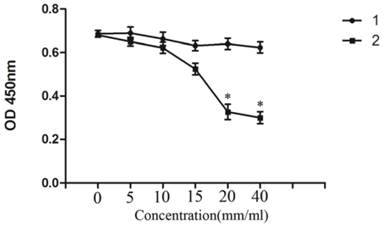
CCK-8 assay showed that, treatment of NB4 cells with 0-40 mM Licl for 24h induced was significantly inhibited in a dose-dependent manner and the highest inhibition is at the concentration of 40mM. NaCl was severed as a control (Figure 1).
Cell apoptosis
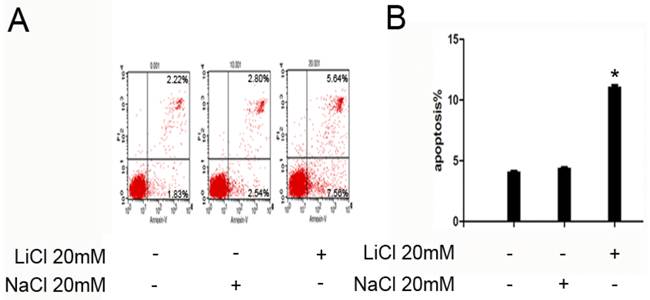
NB4 cells were treated with 20mM LiCl for 24h,untreated and treated with 20mM NaCl as control group.As is shown in Figure 2,the apoptosis rate of untreated/NaCl group was significantly lower than that of LiCl group.These results indicated that Licl could promote NB4 cells to apoptosis at the concentration of 20 mM (Figure 2).
LiCl inhibited the proliferation of NB4 cells. NB4 cells were cultured with 0-40mM LiCl for 24h in 96-well plate. Then the cytotoxic effect was detected by CCK-8 array.CCK-8 array showed that the proliferation of NB4 cells was inhibited by LiCl in a dose-depengdent manner compared with controls. Data was expressed as means ± SD.*P<0.05 (lane 1: NB4 cells treated with NaCl; lane 2: NB4 cells treated with LiCl).
Cell cycle
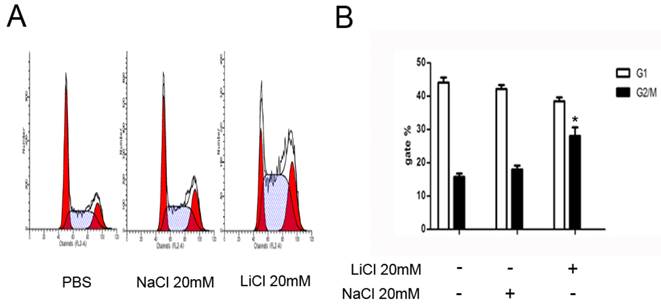
The cell cycle profiles of NB4 cells was detected by a flow cytometric assay. Compared with control groups, LiCl induced cell cycle arrest at G2/M phase. The assay showed that LiCl promoted cell cycle arrest at G2/M phase. NaCl was severed as a control (Figure 3).
Expression of related proteins in NB4 cells induced by LiCl
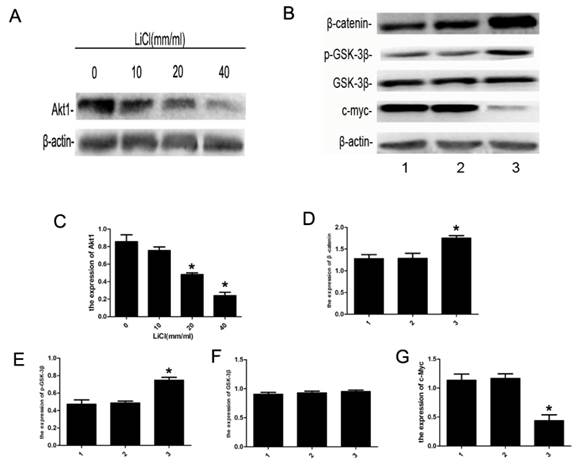
To futher investigate the effect of LiCl on the apoptosis in NB4 cells, the expression of proteins of relatived signaling pathway were detected by western blot assay. As is shown in Figure 4, the expression of Akt1 was inhibited by LiCl in a dose-dependent manner and that of c-Myc decreased significantly, and the level of p-GSK-3β(S9) increased effectively, accompanied with the increase of β-catenin, however, the expression of GSK-3β had no change.The results suggested LiCl could inhibit the expression of Akt1 and increase the level of p-GSK-3β(Ser9).
LiCl improve apoptosis in NB4 cells. A and B, 1x106 NB4 cells were treated with LiCl at the concentration of 20 mM in 6-well plate for 24h.After treatment, apoptosis was detected by flow cytometry. The number of apoptosis cells was expressed as % of total cell number. Data was expressed as means ± SD.*P<0.05
LiCl induces G2/M arrest. A and B NB4 cells were treated with 20mM LiCl/NaCl for 24h.PBS and NaCl was served as controls. Cells were harvested for analysis of cell cycle distribution by flow cytometry. Data was expressed as means ± SD.*P<0.05.
Expression of apoptosis-related proteins. A:NB4 cells were cultured with 0-40mM LiCl for 24h,B: NB4 cells were treated with 20mM LiCl/NaCl for 24h.Whole-cell extracts were then prepared and analyzed by western blot.C-G:detection of Akt1, β-catenin, p-GSK-3β, GSK-3β and c-Myc by western blot, β-actin was served as a control. Compared with the control, the expression of Akt1 was inhibited by LiCl in a dose-dependent manner, the expression of c-Myc decreased significantly, and that of β-catnin increased markly. The level of p-GSK-3β(S9) protein was increased remarkably, and the expression of GSK-3β had no significant differences. Data was expressed as means ± SD.*P<0.05 (lane 1: NB4 cells treated with PBS; lane 2: NB4 cells treated with NaCl 20mM; lane 3: NB4 cells treated with LiCl 20mM).
Discussion
The intensive study of APL pathogenesis has resulted in the current treatment breakthroughs. Although the prognosis for APL has been greatly improved, compared to that of other malignant tumor types, by the application of ATRA and ATO, leukocytosis and retinoic acid resistance do occur in some cases of ATRA treatment [1-7]. Hence, it is important to invest efforts into developing therapies that are more effective. GSK-3, an important regulator of multiple signaling pathways, has two isoforms in vivo, GSK-3α and GSK-3β, which are highly conserved in their catalytic domains but possess specific functions [16,17]. The level of p-GSK-3β at ser9 is high in oral squamous cell carcinoma, hepatocellular carcinoma cells and ovarian cancer cells [18-20], Since lithium is a specific inhibitor of GSK-3, it has been widely used in the study of the biological function of GSK-3β by increasing the phosphorylation of GSK-3β at Ser9 [21]. However, the mechanism by which LiCl inhibits the activity of GSK-3β is not fully understood at present. In addition, LiCl has been demonstrated to induce death in B-cell precursor acute lymphoblastic leukemia (BCP-ALL) cells [22]. Therefore, we sought to examine the effect of LiCl on NB4 cells.
The proliferation and apoptosis of leukemia cells treated with LiCl were examined in vitro. The CCK-8 array showed that the proliferation was significantly inhibited by LiCl in a dose-dependent manner, compared with that observed in the controls (Figure 1). These results indicated that LiCl could inhibite the proliferation of NB4 cells. Interestingly, lithium has been reported to induce apoptosis in pancreatic ductal adenocarcinoma cell lines[15] and G2/M arrest in hepatocellular carcinoma cells, endometrial cancer cell lines and non-small cell lung cancer A549 cells[14,15,23,24]. In addition, LiCl promoted apoptosis of NB4 cells, as deteced by flow cytometry (Figure 2). As shown in Figure 3, our data indicates that cell cycle arrest at the G2/M phase is induced by LiCl treatment.
In order to investigate the mechanism of LiCl-induced apoptosis, apoptosis-related proteins and components of the signaling pathways were detected by western blot analysis. Our results indicate the involvement of the Akt pathway. Akt1, also known as Akt or protein kinase B (PKB), plays an important role in controlling cell growth, apoptosis, cell cycly regulation, and other biological processes. Previous studies have demonstrated abnormal activation of the Akt signaling pathway in both acute and chronic leukemia [25-27]. GSK-3β, a key regulator of the Wnt signaling pathway, promotes the phosphorylation of β-catenin by forming a complex with a series of proteins including adenomatous polyposis coli (APC), casein kinase 1 (CK1), and Axin [28-30]. GSK-3β is also involved in various signal transduction pathway, it acts downstream of the Akt signaling pathway and regulates the expression of c-Myc [31], an important member of the Myc gene family that is involved in the development of a variety of tumors.c-Myc expression is up-regulated in different human tumor cell lines, such as myelogenous leukemia cell lines, breast cancer cell lines, and certain lung cancer cell lines [32-35]. Previous studies have shown that lithium could suppress the expression of c-Myc protein in intervertebral disc cells[36], the findings of our study show that LiCl inhibited the expression of Akt1 in a dose-dependent manner and the expression of c-Myc, also it enhanced the level of p-GSK-3β(S9) in leukemia NB4 cells (Figure 4). High level of p-GSK-β(S9) led to increased expression of β-catenin (Figure 4). However, the Akt pathway is dominant in leukemia cell proliferation. These results indicate that LiCl promotes apoptosis in NB4 cells by inhibiting GSK-3β. In addition, LiCl affects the Akt pathway, thus, decreasing c-Myc expression. However, further studies are required to elucidate the molecular mechanism underlying the effects of LiCl on signal transduction pathway in leukemia cells.
Conclusion
The results of our study indicate that LiCl can inhibit GSK-3β in human leukemia NB4 cells. The G2/M phase arrest in NB4 cells treated with LiCl may be due to inhibition of the Akt signaling pathway. These results suggest that treatment with LiCl may be an effective strategy for APL therapy. Further investigations may be helpful to develop novel therapeutic approaches for leukemia.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81171658) and Natural Science Foundation Project of CQ CSTC (No. 2011BA5037).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nichol JN, Garnier N, Miller WH Jr. Triple A therapy: the molecular underpinnings of the unique sensitivity of leukemic promyelocytes to anthracyclines, all-trans-retinoic acid and arsenic trioxide. Best Pract Res Clin Haematol. 2014;27:19-31
2. Ma H, Yang J. Insights into the All- trans -Retinoic Acid and Arsenic Trioxide Combination Treatment for Acute Promyelocytic Leukemia: A Meta-Analysis. Acta Haematol. 2015;134:101-108
3. Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114:5126-35
4. Brown G, Hughes P. Retinoid differentiation therapy for common types of acute myeloid leukemia. Leuk Res Treatment. 2012;2012:939021
5. ZY Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505-15
6. Chendamarai E, Ganesan S, Alex AA. et al. Comparison of newly diagnosed and relapsed patients with acute promyelocytic leukemia treated with arsenic trioxide: insight into mechanisms of resistance. PLoS One. 2015;10:e0121912
7. Kawasaki K, Akaike H, Miyauchi A. et al. Sivelestat relieves respiratory distress refractory to dexamethasone in all-trans retinoic acid syndrome: a report of two cases. Eur J Haematol. 2006;77:448-52
8. Gupta A, Schulze TG, Nagarajan V. et al. Interaction networks of lithium and valproate molecular targets reveal a striking enrichment of apoptosis functional clusters and neurotrophin signaling. Pharmacogenomics J. 2012;12:328-41
9. Huang J, Nguyen-McCarty M, Hexner EO. et al. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18:1778-85
10. Ballin A, Lehman D, Sirota P. et al. Increased number of peripheral blood CD34+ cells in lithium-treated patients. Br J Haematol. 1998;100:219-21
11. Wang JS, Wang CL, Wen JF. et al. Lithium inhibits proliferation of human esophageal cancer cell line Eca-109 by inducing a G2/M cell cycle arrest. World J Gastroenterol. 2008;14:3982-9
12. Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA. et al. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151-8
13. Adler JT, Hottinger DG, Kunnimalaiyaan M. et al. Inhibition of growth in medullary thyroid cancer cells with histone deacetylase inhibitors and lithium chloride. J Surg Res. 2010;159:640-4
14. Yin Y, Kizer NT, Thaker PH. et al. Glycogen synthase kinase 3β inhibition as a therapeutic approach in the treatment of endometrial cancer. Int J Mol Sci. 2013;14:16617-37
15. Peng Z, Ji Z, Mei F. et al. Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway. PLoS One. 2013;8:e61457
16. Gao F, Al-Azayzih A, Somanath PR. Discrete functions of GSK3α and GSK3β isoforms in prostate tumor growth and micrometastasis. Oncotarget. 2015;6:5947-62
17. McCubrey JA, Steelman LS, Bertrand FE. et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881-911
18. Chun KH, Lee HY, Hassan K. et al. Implication of protein kinase B/Akt and Bcl-2/Bcl-XL suppression by the farnesyl transferase inhibitor SCH66336 in apoptosis induction in squamous carcinoma cells. Cancer Res. 2003;63:4796-800
19. Desbois-Mouthon C, Blivet-Van Eggelpoël MJ, Beurel E. et al. Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells. Hepatology. 2002;36:1528-36
20. Cai G, Wang J, Xin X. et al. Phosphorylation of glycogen synthase kinase-3 beta at serine 9 confers cisplatin resistance in ovarian cancer cells. Int J Oncol. 2007;31:657-62
21. Stolovich M, Lerer T, Bolkier Y. et al. Lithium can relieve translational repression of TOP mRNAs elicited by various blocks along the cell cycle in a glycogen synthase kinase-3- and S6-kinase-independent manner. J Biol Chem. 2005;280:5336-42
22. Thiago LS, Costa ES, Lopes DV. et al. The Wnt signaling pathway regulates Nalm-16 b-cell precursor acute lymphoblastic leukemic cell line survival and etoposide resistance. Biomed Pharmacother. 2010;64:63-72
23. Wang XM, Li J, Feng XC. et al. Involvement of the role of Chk1 in lithium-induced G2/M phase cell cycle arrest in hepatocellular carcinoma cells. J Cell Biochem. 2008;104:1181-91
24. Li J, Xing M, Zhu M. et al. Glycogen synthase kinase 3beta induces apoptosis in cancer cells through increase of survivin nuclear localization. Cancer Lett. 2008;272:91-101
25. Evangelisti C, Evangelisti C, Chiarini F. et al. Therapeutic potential of targeting mTOR in T-cell acute lymphoblastic leukemia (review). Int J Oncol. 2014;45:909-18
26. Wang L, You LS, Ni WM. et al. β-Catenin and AKT are promising targets for combination therapy in acute myeloid leukemia. Leuk Res. 2013;37:1329-40
27. Robak T, Smolewski P. Novel target to kill CLL. Blood. 2015;125:211-2
28. Patel S, Woodgett J. Glycogen synthase kinase-3 and cancer: good cop, bad cop? Cancer Cell. 2008;14:351-3
29. Stamos JL, Weis WI. The b-Catenin Destruction Complex. Cold Spring Harb Perspect Biol. 2013;5:a007898
30. Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439-45
31. Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2012;37:7-16
32. Alves Rde C, Meurer RT, Roehe AV. MYC amplification is associated with poor survival in small cell lung cancer: a chromogenic in situ hybridization study. J Cancer Res Clin Oncol. 2014;140:2021-5
33. Seo AN, Yang JM, Kim H. et al. Clinicopathologic and prognostic significance of c-MYC copy number gain in lung adenocarcinomas. Br J Cancer. 2014;110:2688-99
34. Wu Y, Sato H, Suzuki T. et al. Involvement of c-Myc in the proliferation of MCF-7 human breast cancer cells induced by bHLH transcription factor DEC2. Int J Mol Med. 2015;35:815-20
35. Janghorban M, Farrell AS, Allen-Petersen BL. et al. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc Natl Acad Sci U S A. 2014;111:9157-62
36. Hiyama A, Sakai D, Arai F. et al. Effects of a glycogen synthase kinase-3β inhibitor (LiCl) on c-myc protein in intervertebral disc cells. J Cell Biochem. 2011;112:2974-86
Author contact
![]() Corresponding author: Bei-Zhong Liu, Department of Laboratory Medicine, Chongqing Medical University, 1#, Yixueyuan Road, Chongqing, 400016, China. Tel: +86 18716469741, Fax: +86 023-68485006; E-mail: liubeizhongedu.cn
Corresponding author: Bei-Zhong Liu, Department of Laboratory Medicine, Chongqing Medical University, 1#, Yixueyuan Road, Chongqing, 400016, China. Tel: +86 18716469741, Fax: +86 023-68485006; E-mail: liubeizhongedu.cn

 Global reach, higher impact
Global reach, higher impact