3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(10):1022-1028. doi:10.7150/ijms.8383 This issue Cite
Research Paper
Activated Hair Follicle Stem Cells and Wnt/β-catenin Signaling Involve in Pathnogenesis of Sebaceous Neoplasms
1. Department of Cell Biology, Third Military Medical University, Chongqing 400038, China.
2. Department of Oncology, Southwest Hospital, Third Military Medical University, Chongqing 400038, China.
3. “111” Project Laboratory of Biomechanics and Tissue Repair, College of Bioengineering, Chongqing University, Chongqing 400044, China.
Received 2013-12-18; Accepted 2014-7-14; Published 2014-7-30
Abstract
Sebaceous glands (SGs) undergo cyclic renewal independent of hair follicle stem cells (HFSCs) activation while HFSCs have the potential to differentiate into sebaceous gland cells, hair follicle and epidermal keratinocytes. Abnormalities of sebaceous gland progenitor cells contribute to the development of sebaceous neoplasms, but little is known about the role of HFSCs during sebaceous neoplasm development. Here, using dimethylbenzanthracene (DMBA) plus 12-o-tetradecanoyl phorbol-13-acetate (TPA) treatment developing sebaceous neoplasms (SNs) were identified with H&E and Oil red O staining. And then the molecular expression and activation of HFSCs and was characterized by immunostaining. Wnt10b/β-catenin signaling molecular which is important for activation of HFSCs were detected by immunostaining. We found hair follicle and epidermal cell markers were expressed in sebaceous neoplasms. Furthermore, SOX-9 and CD34-positive HFSCs were located in the basal layer of sebaceous lobules within the sebaceous neoplasms. Many appear to be in an active state. Finally, Wnt10b/β-catenin signaling was activated within the basal cells of sebaceous lobules in the sebaceous neoplasms. Collectively, our findings suggest that the abnormal activation of both HFSCs and Wnt10b/β-catenin signaling involves in the development of sebaceous neoplasms.
Keywords: Hair follicle, stem cell, Sebaceous neoplasm, Development, Wnt10b.
Introduction
Sebaceous glands (SG) undergo cyclic growth, degeneration, and rest, which depend on cyclical changes of sebaceous gland stem cell (SGSC) activity. But after grafting, hair follicle stem cells (HFSCs) are competent to differentiate into sebocytes [1-3]. HFSCs located in the hair follicle bulge, can be characterized by CD34 and SOX-9 expression [4-5]. HFSCs periodically provide cell resources for hair follicles. Previous studies also demonstrated that 12-O-tetradecanoyl-phorbol-13-acetate (TPA) can recruit HFSCs to maintain skin homeostasis and contribute to papilloma, squamous cell carcinoma and basal cell carcinoma [3,6,7]. However, it remains unknown whether the renewal of sebaceous neoplasms (SNs) induced by TPA is dependent on HFSCs.
The Wnt/β-catenin signaling pathway plays an important role in cell fate determination, proliferation and differentiation [8,9], and is involved in regulating SG development. When Lef-1 was functionally blocked, HFSCs differentiated into SGs [10]. Although β-catenin was up-regulated in SNs [11], how the Wnt signaling pathway affects SN development has not been extensively studied.
To better explore these questions, we used DMBA combined with TPA to induce SNs and investigated the morphological and differentiation characteristics of SNs. Next, we detected the activity of HFSCs and expression of key molecules in the Wnt10b/β-catenin signaling during neoplasm induction. Our results indicate that the abnormal activation of HFSCs involves in the development of SNs and activation of the Wnt10b/β-catenin signaling.
Materials and methods
Induction of SNs
Seven-week-old female C57BL/6 mice were obtained from the animal center of Third Military Medical University. The mouse dorsal skin was shaved and dosed with 100 μg DMBA for tumor initiation. A week later, all mice were treated with 4 μg TPA weekly for neoplasm promotion [12]. Six months later, the neoplasms and adjacent relatively normal tissues were harvested, and then the neoplasms were divided into young (size < 2 mm) and older (size > 2 mm) types [13]. Sebaceous neoplasms were characterized according to the histopathological criteria as follows [35,36]: (1) Sharp circumscription, symmetry, and smooth borders; (2) Aggregations of basaloid undifferentiated sebocytes surrounding mature vacuolated sebocytes; (3) small, monomorphous nuclei without pleomorphism or nuclear atypia in the constituent basaloid cells; (4) No palisade arrangement of the nuclei arround the aggregations. All the animal-related procedures were conducted in strict concert with the protocols of institutionally approved animal care and maintenance.
Oil Red O Staining
Specimens were fixed in 4% paraformaldehyde solution and rehydrated in 30% sucrose solution overnight. They then were placed in a small sealed box and frozen in liquid nitrogen for 20 seconds. Specimens then were kept at -20°C for 15 minutes, embedded in optimal cutting temperature compound and sectioned to 12μm thick slides in a cryostat. The slides were stained by Oil Red O solution for 10 min at room temperature, differentiated in 60% isopropyl alcohol, rinsed in distilled water and stained with hematoxylin for 1 min.
H&E Staining
Skin specimens were fixed in 4% paraformaldehyde overnight. They were dehydrated, embedded in paraffin and sectioned at 5 μm. Paraffin sections were rehydrated and stained with hematoxylin for 1 min. These sections were then rinsed with water, stained with eosin for another 1 min, and then embedded with resinene.
BrdU Insertion and Immunofluorescence
Intraperitoneal injection of BrdU was performed at a dose of 50 mg/kg per mouse. Four hours later, the neoplasms were isolated, fixed, dewaxed, dehydrated, embedded in paraffin and sectioned to 5 μm thickness. After dewaxing and rehydrating, skin specimens were microwaved for antigen retrieval. Specimens were rinsed twice in PBS for 5 min each, and blocked with 5% BSA in PBS at room temperature for 1 h. Specimens were incubated with primary antibodies diluted in PBS overnight at 4℃, rinsed three times in PBS for 10 min each, and incubated in secondary antibodies for 1h at 37℃. Next, DAPI was used to stain the nuclei for 5 min. The specimens then were washed three times in PBS for 10 min each and finally embedded with antifade mounting medium (Beyotime, China). Immunofluorescence images were scanned using a Nikon E-clipse E600 fluorescence microscope (Nikon, Japan). Primary antibodies were as follows: rabbit anti-human K10 (polyclonal IgG, 1:200, Bioss, China); mouse anti-human AE15 (monoclonal IgG, 1:2; gift); mouse anti-human AE13 (monoclonal IgG, 1:10; gift); rabbit anti-mouse CD34 (polyclonal IgG, 1:200, Boster, China); rabbit anti-human SOX-9 (polyclonal IgG, 1:100, Santa Cruz, USA); goat anti-mouse Ki67 (polyclonal IgG, 1:100, Santa Cruz, USA); mouse anti-BrdU antibody (monoclonal IgG, 1:100, Zhongshan, China); rabbit anti-human Wnt10b (polyclonal IgG, 1:100, Santa Cruz, USA); mouse anti-human β-catenin (monoclonal IgG, 1:100, Santa Cruz, USA). Secondary antibodies were as follows: Alexa Fluor®488 marked anti-mouse secondary antibody (IgG, 1:300, Invitrogen, USA); Cy3 marked anti-rabbit, anti-mouse and anti-goat secondary antibody (IgG, 1:300, Beyotime, China). Negative controls were performed by adding PBS instead of primary antibodies.
Results
Induction of the SNs by DMBA/TPA treatment
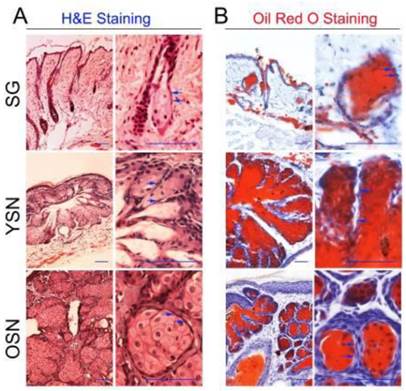
To investigate the role of HFSCs in SN development, we first induced neoplasms on the dorsal skin of mice using DMBA/TPA treatment. Given that SNs, papilloma and squamous carcinoma can be induced by two-stage skin carcinogenesis experiments, H&E and Oil Red O staining were used to identify the SNs. H&E staining showed that compared with SGs, the young and older SNs, were well-circumscribed, grew out of epidermis and developed plaque structures. The SNs consisted of basaloid cells intermingled with clusters of mature sebocyte-like cells (Fig. 1A). Moreover, Oil Red O stained mature sebocytes in SGs, adipocytes in subcutaneous tissues, as well as mature sebocyte-like cells in sebaceous lobules of the young and older SNs (Fig. 1B). Therefore, for the remainder of our study H&E and Oil Red O staining was used to identify young and older SNs. Seven SNs were identified, of which four were young neoplasms and others were older ones.
Hair follicles and epidermis differentiation markers in the SNs
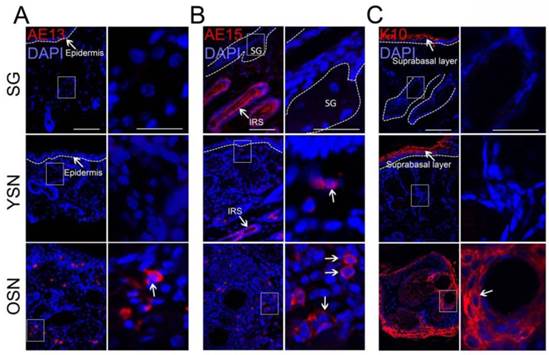
To further characterize the SNs, we detected the expression of hair follicle differentiation markers AE13 and AE15 and the epidermis differentiation marker K10 in SNs. Surprisingly, our data also showed that AE13, characteristically expressed in acidic keratins of the upper cortex and hair cuticle cells [14], was absent in SGs and young SNs but strongly expressed in older SNs (Fig. 2A). AE15, which stains three concentric layers of the inner root sheath (IRS) and medulla of the hair shaft [15], was expressed weakly in the basal cells in the young SNs and strongly in the older SNs (Fig. 2B). In addition, the epidermal differentiation marker K10 was not expressed in SGs or young SNs, but was strongly expressed in older SNs (Fig. 2C). Since HFSCs could differentiate towards SGs, epidermis and hair follicle cells [16,17], we speculate that HFSCs might play an important role in SN development.
Induction of the SNs. (A) H&E staining showed the young as well as older neoplasms contained many sebaceous lobules consisting of sebaceous differentiated cells and basaloid sebaceous undifferentiated cells (Blue arrow). (B) Oil Red O staining revealed that lipids were plentiful in sebaceous neoplasm lobules, SG and subcutaneous tissues. YSN: young sebaceous neoplasms. OSN: older sebaceous neoplasms. Scale bar: 50μm.
Activated HFSCs exist in the SNs
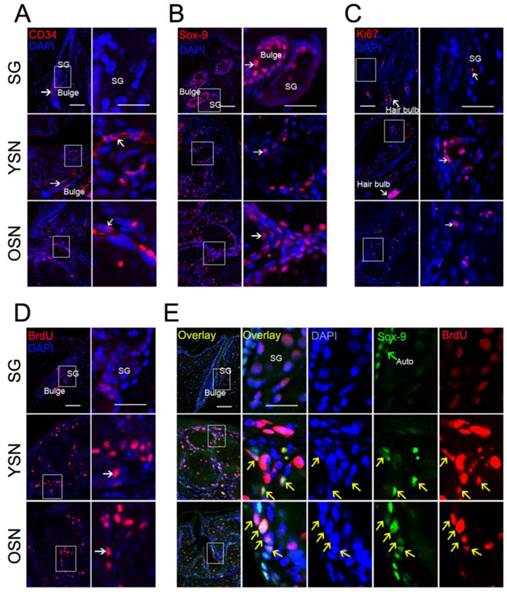
To explore whether HFSCs existed in the SNs, classical hair stem cell markers CD34 and SOX-9 were examined. Interestingly, unlike in normal SGs, both CD34 and SOX-9 were expressed in the basal cells of the young and older SNs (Fig. 3A, B). To further establish whether these HFSCs were activated in these SNs, immunostaining was used to detect Ki67 and BrdU. The results showed that Ki67 stained basal cells weakly in normal SGs but significantly in the basal cells of the young and older SNs (Fig. 3C). Moreover, consistent with Ki67 expression pattern, BrdU were not found in the basal cells in SGs but was obvious in the basal cells of the young and older SNs (Fig. 3D). Although the basal cells in neoplasms were highly proliferative, it remains unknown whether the HFSCs in SNs were actively proliferating. Next, we used double immunostaining to further address the co-expression of SOX-9 and BrdU in the young and older neoplasms. The results showed that SOX-9 partially co-localized with BrdU in the basal cells in the young and older SNs, but not in the normal SGs (Fig. 3E). Together, these findings suggest that HFSCs are indeed located in the basal cells of young and older SNs and a portion of HFSCs are in an activated state.
Up-regulation of Wnt10b/β-catenin signaling in the SNs
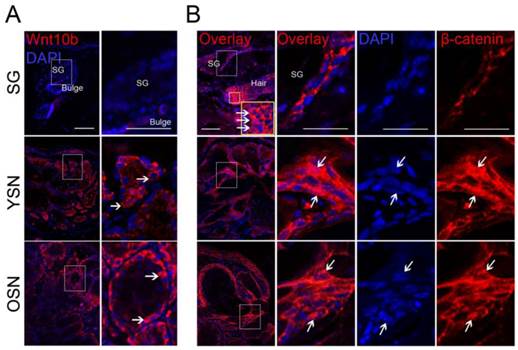
Previous research indicated that epithelial tumors initiating from abnormally activated HFSCs were regulated by the Wnt/β-catenin signaling pathway [11,18,19]. Recently, the Wnt/β-catenin signaling pathway was also reported to activate HFSCs to induce hair follicle renewal [20]. We thus speculated that Wnt/β-catenin signaling was also likely to play a role in SNs development, and therefore checked the expression of key molecules along the Wnt/β-catenin signaling pathway (Wnt10b and β-catenin) in SNs. Wnt10b was shown to be strongly expressed in the basal cells of young and older neoplasms while weakly in normal SGs (Fig. 4A). Moreover, nuclear β-catenin was expressed in the hair matrix but not in SGs (Fig. 4B). However, nuclear accumulation of β-catenin was detected in a subpopulation of the basal cells in both the young and older neoplasms (Fig. 4B). Taken together, these results suggest that Wnt10b and β-catenin are up-regulated in a subpopulation of SN basal cells.
Expression of hair follicule and epidermal differentiation markers in the SNs. (A) AE13 was not observed in SGs and YSNs but was positive in OSNs. (B) AE15 was detected in the normal inner root sheath (IRS) and hair shaft and in neoplasms (White arrow) but not observed in SGs. (C) K10 was expressed in suprabasal epidermis but not expressed in SGs and YSNs but was highly expressed in OSNs (White arrow). Scale bar: 50μm.
Activated HFSCs in the SNs. (A-B) Detection of HFSC markers CD34 and SOX-9 in SGs, YSNs and OSNs. CD34 and SOX-9 were located in the hair bulge region (Arrow) but not in SG, while in YSNs and OSNs, CD34 and SOX-9 were located in the basal cells (Arrow).(C-D) Ki67 and BrdU were detected in both SGs and the basaloid sebocytes in all SNs (Arrow). (E) BrdU and SOX-9 Co-localized in the basal cells of YSNs and OSNs but not in SGs (Yellow arrow). Scale bar: 50μm.
Immunostaining detection of Wnt signaling in the SNs. (A) Compared with SGs, Wnt10b was upregulated in the basaloid sebocytes in the YSNs and OSNs. (B) Nuclear accumulation of β-catnein was observed in normal hair matrix cells, and a subpopulation of the basaloid sebocytes in the YSN and OSN (White arrow) but not in SGs. Scale bar: 50μm.
Discussion
SGs undergo cyclic renewal, independent of HFSC activation. However, HFSCs can migrate towards SGs and provide a cell source for SGs when SG homeostasis is perturbed [21]. Continuous disorder of SGs will lead to SN development. But whether and how HFSCs contribute to SN development needs further investigation.
To address these questions, neoplasms were first developed on mouse skin using DMBA/TPA treatment. Morphologic identification showed that SNs consist of sebaceous lobules with Oil red O positive mature sebocytes located in their center. To further confirm the immune-phenotype of SNs, epidermis and hair follicle differentiation markers were examined. Interestingly, they were all expressed in the SNs. A neoplasm is thought to consist of the progeny of a single cell with accumulated mutations. Thus it is possible that these epidermis- and hair follicle-like cells result from cutaneous epithelial cells other than sebaceous gland cells. CD34 and SOX-9 positive cells are believed to be a population of multipotent stem cells with the capacity to differentiate into epithelial cells and sebocytes [5, 21]. Developmentally, SOX9-positive epithelial cells show key properties of adult HFSCs, which they contribute to HF, SG morphogenesis and repair of an injured interfollicular epidermis [5]. SOX9 is essential for maintaining the adult HFSCs [33]. SOX-9 was reported to function as an enhancer of Activin/TGFbeta signaling which inhibits the activation of HFSCs [34]. Thus we speculate that HFSCs may provide sebaceous gland and epithelial cell resources for the SNs. Although Noriyuki et al. reported that K15-marks cell populations in the bulge and base of a hair follicular infundibulum, K15 is also expressed in the SNs [22]. It remains unclear whether bulge HFSCs contribute to SN development. For the first time, our findings suggest that the bulge HFSCs marked by CD34 and SOX-9 were activated in SN development. Distinct from K15, CD34 was specifically expressed in the hair follicle bulge and SOX-9 in both the hair follicle bulge and secondary hair germ. In the present study, CD34 and SOX-9 were both expressed in the basal cells in the young and older SNs. Similarly, Ki67 and BrdU were located in the basal cells in the young and older SNs. Thus we wondered whether bulge HFSCs were activated to proliferate. Based on our data, SOX-9-positive HFSCs in SNs partially colocalized with BrdU-labeled proliferating sebocytes, indicating that these HFSCs were activated to proliferate during SN development. Accordingly, our results show that bulge HFSCs marked by CD34 and SOX-9 are located in the basal cells of SNs and provide a cell source for SN development. Wensheng et al. previously reported that continuous TPA treatment could induce HFSCs to migrate towards epidermis and differentiate into epidermal cells [23]. Additionally, long-term TPA treatment was also found to activate HFSCs and induce regeneration of hair follicles [6, 19]. Thus, TPA may play an important role in activating HFSCs in SN development and may recruit HFSCs towards SGs and promote these cells to differentiate into epithelial cells and sebocytes.
In the present study, we further confirmed that β-catenin was indeed up-regulated in the basal cells of SN sebaceous lobules. β-catenin is a key factor in Wnt/β-catenin signaling pathway. Upon Wnt stimulation, β-catenin degradation is inhibited [27]. β-catenin accumulates in the cytoplasm, travels to the nucleus and forms transcriptional complexes with TCF/LEF to activate Wnt target genes [28]. Nuclear β-catenin was thought as an indicator of activated Wnt/beta-catenin signaling pathway [29]. Wnt10b, a key ligand molecule in Wnt/β-catenin signaling, was also up-regulated in the basal cells. These data indicate that Wnt10b/β-catenin signaling is activated in the basal cells of sebaceous lobules in SNs. In support of our data, β-catenin was reported to be upregulated in the pathogenesis of sebaceous cell carcinoma of the eyelid [24]. In addition, prostaglandin E2-induced activation of the Wnt/β-catenin signaling pathway was reported to be implicated in regulation of the morphology, proliferation, and function of three-dimensional culture of sebaceous glands [30]. Expression levels of prostaglandin E2 are able to be increased by TPA [31]. Trempus et al. reported that up-regulated β-catenin was involved in the initiation and development of papilloma induced by DMBA/TPA by maintaining the activity of CD34-positive HFSCs [19]. Furthermore, β-catenin was up-regulated in cultured GT1-1 neuronal cells treated by TPA [25]. These results indicated that Wnt10b/β-catenin signaling is activated by continuous TPA treatment in a small population of basal sebaceous gland cells. However, it has also been reported that the inhibition of interaction between β-catenin and Lef-1 caused HFSCs to differentiate into sebocytes [26, 32]. Additionally, the Wnt/β-catenin signaling pathway plays a vital role in proliferation and differentiation of HFSCs towards hair follicular keratinocytes [20, 29]. In our study, differentiation markers of hair follicle keratinocytes were detected in SNs. Thus, these findings combined with our data suggest the Wnt/β-catenin signaling pathway may activate HFSCs in SNs and promote them to differentiate into hair follicle-like cells but not sebaceous gland-like cells during SN development.
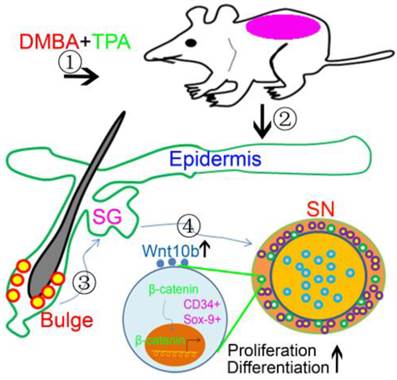
In conclusion, our study confirms that HFSCs marked by CD34 and SOX-9 are located in the basal cells of sebaceous lobules within SNs and involve in SN development. During SN development, HFSCs are activated to differentiate into sebocytes and epithelial cells, and this process is regulated by the activation of the Wnt10b/β-catenin signaling pathway (Fig.5). Our findings advance the understanding of the relationship between HFSCs and SN development, and also show important clinical implications for therapeutic advances in the treatment of SNs.
Schematic drawing showed activated HFSCs contributed to SN development. DMBA combined with TPA treatment disturbed the homeostasis of SGs and induced SN development during which HFSCs were activated and possibly induced to migrate towards SNs. And Wnt10b/β-catenin signaling pathway was activated to promote proliferation and differentiation of HFSCs in SNs.
Abbreviations
SG: Sebaceous gland; HFSC: hair follicle stem cell; SGSC: sebaceous gland stem cell; DMBA: dimethylbenzanthracene; TPA: 12-o-tetradecanoyl phorbol-13-acetate; SN: sebaceous neoplasm; YSN: young sebaceous neoplasms; OSN: older sebaceous neoplasms.
Acknowledgements
This study was supported by grant 30972645 from the National Nature Science Foundation of China. We thank Dr. Tung-Tien Sun for AE13 and AE15 antibodies. And we appreciate Dr. Randall B. Widelitz (University of Southern California) for carefully revising the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635-48 doi:10.1016/j.cell.2004.08.012
2. Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S. et al. Capturing and profiling adult hair follicle stem cells. Nature biotechnology. 2004;22:411-17 doi:10.1038/nbt950
3. Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233-45
4. Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92-105 doi:10.1016/j.cell.2010.11.049
5. Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell stem cell. 2008;3:33-43 doi:10.1016/j.stem.2008.05.009
6. Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241-55 doi:10.1242/dev.00703
7. Panteleyev AA, Rosenbach T, Paus R, Christiano AM. The bulge is the source of cellular renewal in the sebaceous gland of mouse skin. Archives of dermatological research. 2000;292:573-76
8. Sethi JK, Vidal-Puig A. Wnt signalling and the control of cellular metabolism. The Biochemical journal. 2010;427:1-17 doi:10.1042/BJ20091866
9. Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends in genetics: TIG. 1997;13:157-62
10. Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95-109
11. Li L, Zhang Z, Li B, Gao F, Jonas JB. E-cadherin and beta-catenin expression in sebaceous eyelid adenocarcinomas. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249:1867-73 doi:10.1007/s00417-011-1729-2
12. Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nature protocols. 2009;4:1350-62 doi:10.1038/nprot.2009.120
13. Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-11 doi:10.1038/nature07602
14. Baker CM, Verstuyf A, Jensen KB, Watt FM. Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation. Developmental biology. 2010;343:40-50 doi:10.1016/j.ydbio.2010.04.005
15. O'Guin WM, Sun TT, Manabe M. Interaction of trichohyalin with intermediate filaments: three immunologically defined stages of trichohyalin maturation. The Journal of investigative dermatology. 1992;98:24-32
16. Honeycutt KA, Roop DR. c-Myc and epidermal stem cell fate determination. The Journal of dermatology. 2004;31:368-75
17. Fu G, Gao QG, Lian XH, Yu J, Xiang MM, Yang T. Committed differentiation of hair follicle bulge cells into sebocytes: an in vitro study. International journal of dermatology. 2010;49:135-40 doi:10.1111/j.1365-4632.2009.04144.x
18. Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P. et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650-3 doi:10.1038/nature06835
19. Trempus CS, Morris RJ, Ehinger M, Elmore A, Bortner CD, Ito M. et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer research. 2007;67:4173-81 doi:10.1158/0008-5472.CAN-06-3128
20. Li YH, Zhang K, Yang K, Ye JX, Xing YZ, Guo HY. et al. Adenovirus-mediated Wnt10b overexpression induces hair follicle regeneration. The Journal of investigative dermatology. 2013;133:42-8 doi:10.1038/jid.2012.235
21. Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T. et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597-609 doi:10.1016/j.cell.2006.06.048
22. Misago N, Narisawa Y. Cytokeratin 15 expression in neoplasms with sebaceous differentiation. Journal of cutaneous pathology. 2006;33:634-41 doi:10.1111/j.1600-0560.2006.00500.x
23. Chen W, Ishikawa M, Yamaki K, Sakuragi S. Wistar rat palpebral conjunctiva contains more slow-cycling stem cells that have larger proliferative capacity: implication for conjunctival epithelial homeostasis. Japanese journal of ophthalmology. 2003;47:119-28
24. Jayaraj P, Sen S, Kashyap S, Sharma A, Pushker N, Bajaj MS. et al. Does beta-catenin have a role in pathogenesis of sebaceous cell carcinoma of the eyelid? The British journal of ophthalmology. 2011;95:284-7 doi:10.1136/bjo.2009.177204
25. Sun W, Lee H, Choe Y, Cho S, Kim DH, Kim K. Evidence for direct involvement of beta-catenin in phorbol ester-induced neurite outgrowth in GT1-1 hypothalamic neurones. Journal of neuroendocrinology. 2001;13:249-60
26. Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes & development. 2001;15:1688-705 doi:10.1101/gad.891401
27. He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663-1677
28. Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol. Biol. 2008;468:5-15 doi: 10.1007/978-1-59745-249-6_1
29. Rabbani P1, Takeo M, Chou W, Myung P, Bosenberg M, Chin L. et al. Coordinated Activation of Wnt in Epithelial and Melanocyte Stem Cells Initiates Pigmented Hair Regeneration. Cell. 2011;145:941-955 doi: 10.1016/j.cell.2011.05.004
30. Yoshida GJ1, Saya H, Zouboulis CC. Three-dimensional culture of sebaceous gland cells revealing the role of prostaglandin E2-induced activation of canonical Wnt signaling. Biochem Biophys Res Commun. 2013;438(4):640-6 doi: 10.1016/j.bbrc.2013.07.129
31. Hsu HC1, Lin WC, Chang PJ, Hong CZ, Chen CH. Propyl gallate inhibits TPA-induced inflammation via the nuclear factor-κB pathway in human THP-1 monocytes. Exp Ther Med. 2013;5(3):964-968
32. Niemann C. Differentiation of the sebaceous gland. Dermatoendocrinol. 2009Mar;1(2):64-7
33. Kadaja M1, Keyes BE, Lin M. et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014Feb15;28(4):328-41 doi: 10.1101/gad.233247.113
34. Niimori D, Kawano R, Felemban A. et al. Tsukushi controls the hair cycle by regulating TGF-β1 signaling. Dev Biol. 2012Dec1;372(1):81-7 doi: 10.1016/j.ydbio.2012.08.030
35. Troy JL, Ackerman AB. Sebaceoma. A distinctive benign neoplasm of adnexal epithelium differentiating toward sebaceous cells. Am J Dermatopathol. 1984;6:7
36. Misago N, Mihara I, Ansai S, Narisawa Y. Sebaceoma and related neoplasms with sebaceous differentiation: a clinicopathologic study of 30 cases. Am J Dermatopathol. 2002;24:294
Author contact
![]() Corresponding author: E-mail: 129hxcom. Tel: 86-023-68752264
Corresponding author: E-mail: 129hxcom. Tel: 86-023-68752264

 Global reach, higher impact
Global reach, higher impact