Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(2):158-163. doi:10.7150/ijms.7567 This issue Cite
Research Paper
A Nonsense Mutation of γD-crystallin Associated with Congenital Nuclear and Posterior Polar Cataract in a Chinese Family
1. Eye Center, Second Affiliated Hospital of Medical College, Zhejiang University, Hangzhou, Zhejiang, China
2. Key Laboratory of Ophthalmology of Zhejiang Province, China
3. Center for Structural Biology and Department of Chemistry and the Institute for Chemical Biology, Vanderbilt University Medical Center, USA
Received 2013-9-2; Accepted 2013-12-17; Published 2014-1-5
Abstract
Objective: The goal of this study was to characterize the disease-causing mutations in a Chinese family with congenital nuclear and posterior polar cataracts. Methods: Clinical data of patients in the family were recorded using slit-lamp photography and high definition video. Genomic DNA samples were extracted from the peripheral blood of the pedigree members and 100 healthy controls. Mutation screening was performed in the candidate genes by bi-directional sequencing of the amplified products. Results: The congenital cataract phenotype of the pedigree was identified by slit-lamp examinations and observation during surgery as nuclear and posterior polar cataracts. Through the sequencing of the candidate genes, a heterozygous c. 418C>T change was detected in the coding region of the γD-crystallin gene (CRYGD). As a result of this change, a highly conserved arginine residue was replaced by a stop codon (p. R140X). This change was discovered among all of the affected individuals with cataracts, but not among the unaffected family members or the 100 ethnically matched controls. Conclusions: This study identified a novel congenital nuclear and posterior polar cataract phenotype caused by the recurrent mutation p. R140X in CRYGD.
Keywords: congenital cataract, γD-crystallin, mutation, stop codon, Greek key motif
Introduction
Cataracts can be defined as complete or partial lens opacification, either congenital or acquired. A congenital cataract is especially severe, as it potentially impairs visual development, and is one of the leading causes of childhood blindness. The prevalence of congenital cataracts is approximately 6.31/100,000 [1], 27-39% of which are believed to be inherited [2]. Autosomal dominant (AD) inheritance is commonly observed among hereditary cataracts, while autosomal recessive and X-linked patterns have also been reported [3].
To date, scientists have identified more than 35 loci, including over 20 genes which are associated with isolated congenital cataracts [4]. Among the congenital cataract causative mutations discovered thus far, approximately half of the mutations are in the crystallin family genes, and one quarter of them are in gap junction genes. Other mutations have been identified in heat shock transcription factor-4 (HSF4), aquaporin-0 (AQP0, MIP), v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog (MAF), and beaded filament structural protein-2 (BFSP2), as well as other genes [5].
In this study, we investigated a three-generation family with congenital nuclear and posterior polar cataracts, and detected a R140X nonsense mutation in γD-crystallin, which cosegregated with the disease in this family.
Methods
The research protocols of this study adhered to the guidelines of the Declaration of Helsinki and were approved by the Medical Ethics Committees of the Second Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, China). Appropriate informed consent from each participant was obtained.
Clinical Evaluation
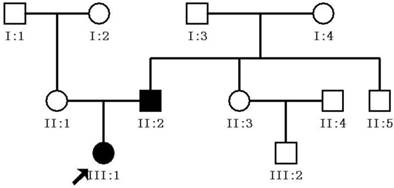
A three-generation Chinese pedigree consisting of eleven individuals, including two affected individuals, formed the basis for this study. All eleven family members participated in the study (two affected and nine unaffected individuals; Figure 1). The proband (III:1) was a 1 year-old girl who visited our eye center in June of 2013 for a cataract extraction surgery. Her father (II:1), diagnosed with congenital cataracts 30 years previously (at birth), had his cataract extraction and intraocular lens implantation 16 years previously at our eye center as well. All of the patients and family members underwent ophthalmic examinations that included visual function, slit-lamp examination, intraocular pressure measurement, and fundus evaluation with dilated pupils. In addition, 100 unrelated healthy subjects were recruited as a control group.
Pedigree of Chinese family with congenital cataracts. The proband is marked with an arrow. Squares and circles indicate males and females, respectively. Black and white symbols represent affected and unaffected individuals, respectively.
Mutation Screening
We used the functional candidate gene analysis approach for screening, and the detailed strategy was described in our previous study [6]. The experimental procedure is briefly described here. Peripheral blood was collected by venipuncture in EDTA-coated Becton-Dickinson Vacutainer tubes (BD, New Jersey, USA) and stored at -20 °C. Genomic DNA was extracted from peripheral lymphocytes using the QIAamp Blood kit (Qiagen, Dusseldorf, Germany). Polymerase chain reaction (PCR) was used to amplify the exons and the flanking intronic regions of the candidate genes: crystallin alpha A (CRYAA), crystallin alpha B (CRYAB), crystallin beta A3/A1 (CRYBA3/1), crystallin beta B1 (CRYBB1), crystallin beta B2 (CRYBB2), crystallin gamma C (CRYGC), crystallin gamma D (CRYGD), gap junction protein alpha 3 (GJA3), gap junction protein alpha 8 (GJA8), and major intrinsic protein (MIP). The primer sequences were described in our previous study [7], and the specific primer sequences for the CRYGD are listed below (Table 1). Bi-directional direct sequencing of the PCR products was performed afterwards.
Bioinformatics Analysis
The γD-crystallin amino acid sequences were obtained from the NCBI Gene Database (http://www.ncbi.nlm.nih.gov/gene/). We obtained the structure of wide-type human γD-crystallin from the Protein Data Bank (PDB) database (ID:1HK0), and visualized the protein structure using PyMOL software (DeLano Scientific LLC, San Francisco, CA, USA). We also used the BEST/COREX web server (http://best.bio.jhu.edu/BEST/index.php) to predict the stability of the native and mutant proteins.
Results
Clinical Evaluation
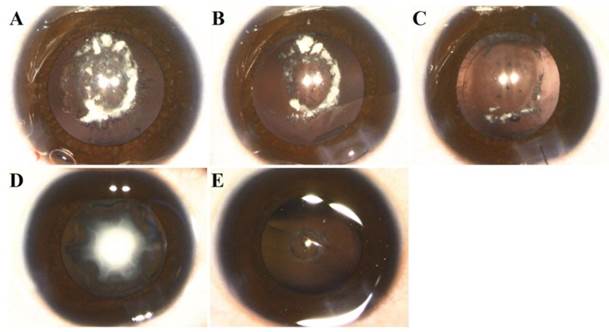
We identified isolated bilateral congenital nuclear and posterior polar cataracts in the Chinese family, and the two patients showed the same clinical symptoms: lens opacification, horizontal nystagmus and amblyopia. Individual II:2 was diagnosed with congenital cataracts 30 years previously, at his birth, and had phacoemulsification and intraocular lens implantation 16 years previously. According to his medical records, his lens opacities were located mainly at the lens nuclei and posterior poles. Individual III:1 was diagnosed with congenital cataracts at the age of 3 months. Slit-lamp examination revealed that III:1 had nuclear and posterior polar opacities with posterior lenticonus. Individual III:1 underwent surgeries for both eyes at one year of age. According to our observation, during the surgeries, both eyes of individual III:1 had nuclear and posterior polar opacities, with a posterior capsular thinning or defect, which are secondary complications to the posterior lenticonus [8]. However, each eye had a different appearance. Her right nuclear opacities had been reabsorbed, which made the remnants drop into the vitreous body through the posterior capsular defect. Her left eye had intact nuclear cataracts and posterior lenticonus with round posterior capsular thinning (Figure 2). We performed irrigation-aspiration combined with anterior vitrectomy on the right eye, and standard phacoemulsification on the left (high-definition video can be provided upon request). The postoperative best-corrected visual acuity (BCVA) of affected member II:2 was 0.1/0.1 due to amblyopia, and he still exhibited severe horizontal nystagmus. Individual III:1's BCVA was unable to be measured since she was still preverbal; however, the postoperative visual stimuli could be fixed, centered and followed. Intraocular lenses will be implanted when she is two years of age.
Primers used in PCR of CRYGD.
| Name | Prime Sequence (5′-3′) | Product length (bp) |
|---|---|---|
| Exon-1.2F | 5' CCTCGCCTTGTCCCGC 3' | 340 |
| Exon-1.2R | 5' TTAACTTTTGCTTGAAACCATCCA 3' | |
| Exon-3F | 5' TGCTTTTCTTCTCTTTTTATTTCTGGGTCC 3' | 400 |
| Exon-3R | 5' AGTAAAGAAAGACACAAGCAAATCAGTGCC 3' |
Phenotype of individual III:1. A: Photograph of right eye before surgery showing that lens was partially reabsorbed, with some dropping into the vitreous body through the posterior capsular hole. B: Photograph of right eye after irrigation/aspiration showing posterior capsular punctate opacities with central round hole; lens remnants remaining in the anterior segment of the vitreous. C: Photograph of right eye after anterior vitrectomy. D: Photograph of left eye before surgery showing nuclear opacities. E: Photograph of left eye after phacoemulsification and irrigation/aspiration showing round thinning of posterior capsule without rupture, protruding into the vitreous, and punctate opacities around the posterior pole.
Mutation Screening
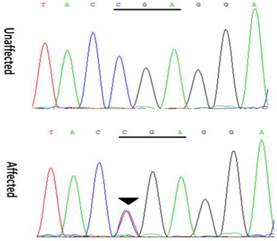
Mutation screenings were performed for all candidate genes, and a heterozygous change C>T at position 418 (c. 418C>T) was identified in exon 3 of the CRYGD gene (Figure 3). This mutation resulted in the substitution of a stop codon for a phylogenetically conserved arginine residue (p. R140X), and was only identified in the patients. It was not found in either the healthy family members or the 100 controls with same ethnic background. Moreover, this mutation was not present in either the 1000 Genomes Project database (http://browser.1000genomes.org/index.html) or the NHLBI Exome Variant Server (EVS, http://evs.gs.washington.edu/EVS/). With the exception of several nonpathogenic single nucleotide polymorphisms, no other mutations were detected.
Bioinformatics Analysis
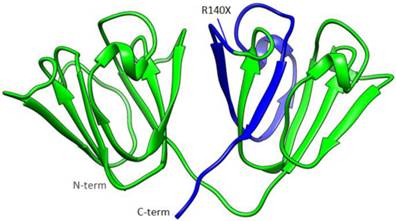
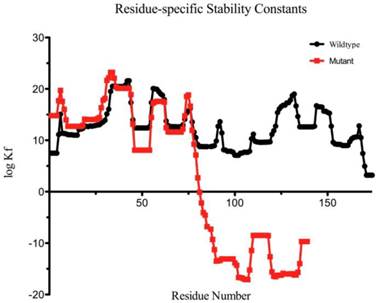
The mutant protein is 35 amino acids shorter than the wild-type one, and the fourth Greek key motif (GKM) at the C-terminus was partially absent (Figure 4). The BEST/COREX generated a coarse-grained energy evaluation at the residue level by calculating the structural thermodynamic ensembles from native and mutant γD-crystallin (Table 2). Compared to the mutant, the native γD-crystallin has a positive delta G for unfolding, while the mutant favors the C-terminal domain in the unfolded state by a negative delta G [9]. Moreover, the stability constant at the per residue level showed that the C-terminal domain of the mutant protein favors the unfolded states (Figure 5).
COREX/BEST scores
| Lowest energy state in ensemble | ||
|---|---|---|
| Native | Mutant | |
| Fraction Folded | 0.9769 | 0.5797 |
| Predicted delta G (Kcal/mol) | 2.053 | -9.209 |
| % total ensemble simulated | 95% | 83% |
| Residue States (F=Folded; U=Unfolded) | F(1-169)U(170-174) | F(1-80)U(81-139) |
Forward sequence analysis of the affected and unaffected individuals with congenital cataracts in this Chinese family, showing a heterozygous mutation (c. 418C>T) in exon 3 of CRYGD (black triangles).
The structural model of the γD-crystallin mutation. A structural model of γD-crystallin is shown in this illustration, in which the truncated portion resulting from the R140X mutation is highlighted in blue, while the rest of the protein is depicted in green.
Residue-specific Stability Constant for each residue of the protein as predicted by the BEST/COREX server. The logKf stability constant is the ratio of the summed probability of the residue in folded conformation to the summed probability of the residue in unfolded conformation.
Discussion
In this study, we report a nonsense mutation (c. 418C>T) in exon 3 of the CRYGD gene which caused congenital nuclear and posterior polar cataracts in a Chinese family. This mutation cosegregated with affected individuals, and was not detected in either the healthy family members or 100 ethnically matched controls.
Crystallins are structural proteins that play essential roles in lens development and the maintenance of transparency. Mammalian lens crystallins have a core set of three protein classes, the α-, β- and γ-crystallins, which are defined by the sizes of the oligomers that they form [10]. With the lowest molecular mass (20 kDa), the γ-crystallin family proteins are strictly monomeric [11]. The cDNA analyses show that most of the transcripts arising from this family among humans come from γC-crystallin and γD-crystallin, with the latter being the predominantly expressed species [12].
γD-crystallin is a member of the β/γ-crystallins super family, which shares the common feature of antiparallel β-sheets in the protein, called the ''Greek key motif'' [11]. In the γD-crystallin, the N-terminal and C-terminal halves of the sequence comprise two individual domains that each consist of two GKMs, which interact via the GKM2 and GKM4 [13]. Each GKM is composed of four antiparallel β-strands, and the R140X mutation in the present study is located in GKM4.
The transition of C>T at c. 418 in exon 3 of CRYGD is predicted to cause a premature stop codon, which leads to the deletion of the C-terminal residues 140-174. The overall γD-crystallin structure is depicted where the truncated portion from the R140X mutation is colored in blue (Figure 4).
As shown in the diagram, this deletion mutant loses its two β-strands of the fourth GKM, which destabilizes the C-terminal domain. Additionally, the missing residues may not only influence the folding of the domain, but also affect the aggregation state of the protein. A previous mutant investigation indicated that the R140X mutant exposed several buried residues to the surface, and displayed lower solubility and structural stability [14]. Venu Talla and co-workers reported that the loss of the C-terminal fragment in human γD-crystallin would lead to substantial intermolecular aggregates, which was expected to generate light-scattering particles, compromising the transparency of the cells and their assemblies [15]. The other two previously reported nonsense mutations in CRYGD, Y134X and W157X, also affected the fourth GKM and caused congenital cataracts.
Since γD-crystallin expression remains at a relatively high level in the human lens throughout one's lifetime, the CRYGD gene mutation has been associated with not only congenital cataracts, but also juvenile-onset cataracts. Mutations in the human CRYGD gene that cause childhood cataracts have been identified in more than 23 families thus far (Table 3) [16-37]. The R140X mutation was observed in our study as well as in another previously described family with congenital cataracts [35]. Our study indicates that the R140X is the actual causative mutation, and although the two studies revealed the same R140X amino acid alternation, the phenotypes were different. The previous study presented an exclusively nuclear congenital cataract family, and did not provide photographs of the affected individuals. Our study, however, suggests that the R140X mutation could present a novel nuclear and posterior polar cataract phenotype, and provides the photographic records of the patients.
Human CRYGD mutations associated with childhood cataracts.
| Year | Mutation | Amino acid change | Origin of family | Phenotype | Reference |
|---|---|---|---|---|---|
| 1999 | c.43C>T | R15C | American | Juvenile-onset punctate cataracts | [16] |
| 2006 | c.43C>T | R15C | Chinese | Congenital coralliform cataract | [17] |
| 2009 | c.43C>A | R15S | Chinese | Congenital coralliform cataract | [18] |
| 2007 | c.70C>T | P24S | Russian | Polymorphic congenital cataract | [19] |
| 2003 | c.70C>A | P24T | French | Congenital cerulean cataract | [20] |
| 2004 | c.70C>A | P24T | American | Congenital coral-like cataract | [21] |
| 2004 | c.70A>C | P24T | Chinese | Congenital fasciculiform cataract | [22] |
| 2011 | c.106G>C | A36P | Chinese | Congenital nuclear cataract | [23] |
| 2000 | c.109C>A | R37S | Czechs | Congenital crystal-like cataract | [24] |
| 2011 | c.109C>A | R37S | American | Congenital crystal-like cataract | [25] |
| 2005 | c.109C>A | R37S | Chinese | Congenital nuclear cataract | [26] |
| 2011 | c.110G>C | R37P | Chinese | Congenital nuclear cataract | [27] |
| 2011 | c.127T>C | W43R | Chinese | Congenital nuclear cataract | [28] |
| 2009 | c.168C>G | Y56X | Brazilian | Congenital nuclear cataract | [29] |
| 2005 | c.173G>A | R59H | Mexican | Congenital aculeiform cataract | [30] |
| 2008 | c.181G>T | G61C | Chinese | Congenital coralliform cataract | [31] |
| 2010 | c.229C>A | R77S | Indian | Juvenile-onset anterior polar cataract | [32] |
| 2006 | c.320A>C | E107A | Mexican | Congenital nuclear cataract | [33] |
| 2009 | c.418C>A | Y134X | Danish | Not mentioned | [34] |
| 2008 | c.418C>T | R140X | Indian | Congenital nuclear cataract | [35] |
| 2013 | c.418C>T | R140X | Chinese | Congenital nuclear and posterior polar cataract | Present study |
| 2002 | c.470G>A | W157X | German | Congenital nuclear cataract | [36] |
| 2007 | c.494delG | 165 new amino acid | Chinese | Congenital nuclear cataract | [37] |
Acknowledgements
We wish to thank this family for participating in our study. This work was supported by the Fundamental Research Funds for the Central Universities (Grant No. 2013FZA7013), the Key Program of the National Natural Science Foundation of China (Grant No. 81130018), the National Natural Science Foundation of China (Grant No. 81371001), the National Twelfth Five-Year Plan Foundation of China (Grant No. 2012BAI08B01), the Zhejiang Key Innovation Team Project of China (Grant No. 2009R50039), the Zhejiang Key Laboratory Fund of China (Grant No. 2011E10006) and the Project of the National Clinical Key Discipline of the Chinese Ministry of Health.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bermejo E, Martinez-Frias ML. Congenital eye malformations: clinical-epidemiological analysis of 1,124,654 consecutive births in Spain. American journal of medical genetics. 1998;75(5):497-504
2. Haargaard B, Wohlfahrt J, Fledelius HC, Rosenberg T, Melbye M. A nationwide Danish study of 1027 cases of congenital/infantile cataracts: etiological and clinical classifications. Ophthalmology. 2004;111(12):2292-2298
3. Hejtmancik JF. Congenital cataracts and their molecular genetics. Seminars in cell & developmental biology. 2008;19(2):134-149
4. Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Molecular vision. 2010;16:2007-2015
5. Huang B, He W. Molecular characteristics of inherited congenital cataracts. European journal of medical genetics. 2010;53(6):347-357
6. Zhu Y, Shentu X, Wang W, Li J, Jin C, Yao K. A Chinese family with progressive childhood cataracts and IVS3+1G>A CRYBA3/A1 mutations. Molecular vision. 2010;16:2347-2353
7. Li J, Wang Q, Fu Q, Zhu Y, Zhai Y, Yu Y, Zhang K, Yao K. A novel connexin 50 gene (gap junction protein, alpha 8) mutation associated with congenital nuclear and zonular pulverulent cataract. Molecular vision. 2013;19:767-774
8. Amaya L, Taylor D, Russell-Eggitt I, Nischal KK, Lengyel D. The morphology and natural history of childhood cataracts. Survey of ophthalmology. 2003;48(2):125-144
9. Vertrees J, Barritt P, Whitten S, Hilser VJ. COREX/BEST server: a web browser-based program that calculates regional stability variations within protein structures. Bioinformatics. 2005;21:3318-3319
10. Wistow G. The human crystallin gene families. Human genomics. 2012;6:26
11. Graw J. Genetics of crystallins: cataract and beyond. Experimental eye research. 2009;88(2):173-189
12. Wistow G, Bernstein SL, Wyatt MK, Behal A, Touchman JW, Bouffard G, Smith D, Peterson K. Expressed sequence tag analysis of adult human lens for the NEIBank Project: over 2000 non-redundant transcripts, novel genes and splice variants. Molecular vision. 2002;8:171-184
13. Blundell T, Lindley P, Miller L, Moss D, Slingsby C, Tickle I, Turnell B, Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981;289(5800):771-777
14. Vendra VP, Agarwal G, Chandani S, Talla V, Srinivasan N, Balasubramanian D. Structural Integrity of the Greek Key Motif in betagamma-Crystallins Is Vital for Central Eye Lens Transparency. PloS one. 2013;8(8):e70336
15. Talla V, Srinivasan N, Balasubramanian D. Visualization of in situ intracellular aggregation of two cataract-associated human gamma-crystallin mutants: lose a tail, lose transparency. Investigative ophthalmology & visual science. 2008;49(8):3483-3490
16. Stephan DA, Gillanders E, Vanderveen D, Freas-Lutz D, Wistow G, Baxevanis AD, Robbins CM, VanAuken A, Quesenberry MI, Bailey-Wilson J. et al. Progressive juvenile-onset punctate cataracts caused by mutation of the gammaD-crystallin gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):1008-1012
17. Gu F, Li R, Ma XX, Shi LS, Huang SZ, Ma X. A missense mutation in the gammaD-crystallin gene CRYGD associated with autosomal dominant congenital cataract in a Chinese family. Molecular vision. 2006;12:26-31
18. Zhang LY, Gong B, Tong JP, Fan DS, Chiang SW, Lou D, Lam DS, Yam GH, Pang CP. A novel gammaD-crystallin mutation causes mild changes in protein properties but leads to congenital coralliform cataract. Molecular vision. 2009;15:1521-1529
19. Plotnikova OV, Kondrashov FA, Vlasov PK, Grigorenko AP, Ginter EK, Rogaev EI. Conversion and compensatory evolution of the gamma-crystallin genes and identification of a cataractogenic mutation that reverses the sequence of the human CRYGD gene to an ancestral state. American journal of human genetics. 2007;81(1):32-43
20. Nandrot E, Slingsby C, Basak A, Cherif-Chefchaouni M, Benazzouz B, Hajaji Y, Boutayeb S, Gribouval O, Arbogast L, Berraho A. et al. Gamma-D crystallin gene (CRYGD) mutation causes autosomal dominant congenital cerulean cataracts. Journal of medical genetics. 2003;40(4):262-267
21. Mackay DS, Andley UP, Shiels A. A missense mutation in the gammaD crystallin gene (CRYGD) associated with autosomal dominant "coral-like" cataract linked to chromosome 2q. Molecular vision. 2004;10:155-162
22. Shentu X, Yao K, Xu W, Zheng S, Hu S, Gong X. Special fasciculiform cataract caused by a mutation in the gammaD-crystallin gene. Molecular vision. 2004;10:233-239
23. Sun W, Xiao X, Li S, Guo X, Zhang Q. Mutation analysis of 12 genes in Chinese families with congenital cataracts. Molecular vision. 2011;17:2197-2206
24. Kmoch S, Brynda J, Asfaw B, Bezouska K, Novak P, Rezacova P, Ondrova L, Filipec M, Sedlacek J, Elleder M. Link between a novel human gammaD-crystallin allele and a unique cataract phenotype explained by protein crystallography. Human molecular genetics. 2000;9(12):1779-1786
25. VanderVeen DK, Andrews C, Nihalani BR, Engle EC. Crystalline cataract caused by a heterozygous missense mutation in gammaD-crystallin (CRYGD). Molecular vision. 2011;17:3333-3338
26. Gu J, Qi Y, Wang L, Wang J, Shi L, Lin H, Li X, Su H, Huang S. A new congenital nuclear cataract caused by a missense mutation in the gammaD-crystallin gene (CRYGD) in a Chinese family. Molecular vision. 2005;11:971-976
27. Wang L, Chen X, Lu Y, Wu J, Yang B, Sun X. A novel mutation in gammaD-crystallin associated with autosomal dominant congenital cataract in a Chinese family. Molecular vision. 2011;17:804-809
28. Wang B, Yu C, Xi YB, Cai HC, Wang J, Zhou S, Zhou S, Wu Y, Yan YB, Ma X. et al. A novel CRYGD mutation (p.Trp43Arg) causing autosomal dominant congenital cataract in a Chinese family. Human mutation. 2011;32(1):E1939-1947
29. Santana A, Waiswol M, Arcieri ES, Cabral de Vasconcellos JP, Barbosa de Melo M. Mutation analysis of CRYAA, CRYGC, and CRYGD associated with autosomal dominant congenital cataract in Brazilian families. Molecular vision. 2009;15:793-800
30. Zenteno JC, Morales ME, Moran-Barroso V, Sanchez-Navarro A. CRYGD gene analysis in a family with autosomal dominant congenital cataract: evidence for molecular homogeneity and intrafamilial clinical heterogeneity in aculeiform cataract. Molecular vision. 2005;11:438-442
31. Li F, Wang S, Gao C, Liu S, Zhao B, Zhang M, Huang S, Zhu S, Ma X. Mutation G61C in the CRYGD gene causing autosomal dominant congenital coralliform cataracts. Molecular vision. 2008;14:378-386
32. Roshan M, Vijaya PH, Lavanya GR, Shama PK, Santhiya ST, Graw J, Gopinath PM, Satyamoorthy K. A novel human CRYGD mutation in a juvenile autosomal dominant cataract. Molecular vision. 2010;16:887-896
33. Messina-Baas OM, Gonzalez-Huerta LM, Cuevas-Covarrubias SA. Two affected siblings with nuclear cataract associated with a novel missense mutation in the CRYGD gene. Molecular vision. 2006;12:995-1000
34. Hansen L, Mikkelsen A, Nurnberg P, Nurnberg G, Anjum I, Eiberg H, Rosenberg T. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Investigative ophthalmology & visual science. 2009;50(7):3291-3303
35. Devi RR, Yao W, Vijayalakshmi P, Sergeev YV, Sundaresan P, Hejtmancik JF. Crystallin gene mutations in Indian families with inherited pediatric cataract. Molecular vision. 2008;14:1157-1170
36. Santhiya ST, Shyam Manohar M, Rawlley D, Vijayalakshmi P, Namperumalsamy P, Gopinath PM, Loster J, Graw J. Novel mutations in the gamma-crystallin genes cause autosomal dominant congenital cataracts. Journal of medical genetics. 2002;39(5):352-358
37. Zhang LY, Yam GH, Fan DS, Tam PO, Lam DS, Pang CP. A novel deletion variant of gammaD-crystallin responsible for congenital nuclear cataract. Molecular vision. 2007;13:2096-2104
Author contact
![]() Corresponding author: Prof. Ke Yao, Eye Center, Second Affiliated Hospital of Medical College, Zhejiang University, Hangzhou, Zhejiang 310009, China; Telephone: +86-571-87783897, Fax: +86-571-87783908, E-mail: xlrenedu.cn
Corresponding author: Prof. Ke Yao, Eye Center, Second Affiliated Hospital of Medical College, Zhejiang University, Hangzhou, Zhejiang 310009, China; Telephone: +86-571-87783897, Fax: +86-571-87783908, E-mail: xlrenedu.cn

 Global reach, higher impact
Global reach, higher impact