3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(10):1286-1294. doi:10.7150/ijms.5985 This issue Cite
Research Paper
Microcurrent Electrical Nerve Stimulation Facilitates Regrowth of Mouse Soleus Muscle
1. Laboratory of Physiology, School of Health Sciences, Toyohashi SOZO University, Toyohashi, Aichi, Japan;
2. Department of Sports Medicine, St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan;
3. Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University, Toyohashi, Aichi, Japan;
4. Faculty of Education, Yamaguchi University, Yamaguchi-City, Yamaguchi, Japan;
5. Graduate School of Medicine, Osaka University, Toyonaka, Osaka, Japan;
6. Hirosaki Gakuin University, Hirosaki, Aomori, Japan.
Received 2013-1-30; Accepted 2013-7-30; Published 2013-8-7
Abstract
Microcurrent electrical nerve stimulation (MENS) has been used to facilitate recovery from skeletal muscle injury. However, the effects of MENS on unloading-associated atrophied skeletal muscle remain unclear. Effects of MENS on the regrowing process of unloading-associated atrophied skeletal muscle were investigated. Male C57BL/6J mice (10-week old) were randomly assigned to untreated normal recovery (C) and MENS-treated (M) groups. Mice of both groups are subjected to continuous hindlimb suspension (HS) for 2 weeks followed by 7 days of ambulation recovery. Mice in M group were treated with MENS for 60 min 1, 3, and 5 days following HS, respectively, under anesthesia. The intensity, the frequency, and the pulse width of MENS were set at 10 μA, 0.3 Hz, and 250 msec, respectively. Soleus muscles were dissected before and immediately after, 1, 3 and 7 days after HS. Soleus muscle wet weight and protein content were decreased by HS. The regrowth of atrophied soleus muscle in M group was faster than that in C group. Decrease in the reloading-induced necrosis of atrophied soleus was facilitated by MENS. Significant increases in phosphorylated levels of p70 S6 kinase and protein kinase B (Akt) in M group were observed, compared with C group. These observations are consistent with that MENS facilitated regrowth of atrophied soleus muscle. MENS may be a potential extracellular stimulus to activate the intracellular signals involved in protein synthesis.
Keywords: skeletal muscle, atrophy, regrowth, microcurrent electrical nerve stimulation, intracellular signal, rehabilitation.
Introduction
Skeletal muscle injuries are common sports trauma. Not only professional but also recreational athletes having skeletal muscle injuries are forced to be inactive for a certain period. Muscle injuries-associated inactivity is one of major causes of skeletal muscle atrophy and weakness.1-3 Skeletal muscle atrophy is another serious problem for the recovery from sport injury. Various efforts have been made to establish effective rehabilitation therapy for the facilitation of recovery of injured and atrophied skeletal muscle.
Microcurrent electrical nerve stimulation (MENS) was developed as a physical therapy modality delivering current in the microampere range. MENS has a reducing effect of signs and symptoms of muscle damage.4,5 However, molecular mechanism for MENS-associated stimulation of the regenerative potential of injured skeletal muscle is still unclear because the regeneration of injured skeletal muscle is complicated process. Because increase in protein synthesis is essential during the regeneration of injured6-9 as well as atrophied10 skeletal muscle, it is strongly suggested that MENS may facilitate the recovery of atrophied skeletal muscle via stimulation of protein synthesis. However, there is no report regarding the effects of MENS on the regrowth of atrophied skeletal muscle.
Akt, also known as protein kinase B (PKB), is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes such as protein synthesis, glucose metabolism, apoptosis, cell proliferation, and other cell functions.11-14 It is generally accepted that not only Akt but also p70 S6 kinase (p70S6K) plays a key role in integrating intracellular signaling in protein synthesis of skeletal muscle cells.10,15-17 Phosphorylated form of both molecules are enzymatically active.12,14,18,19 Akt has been shown to be activated by mechanical stretch that could induce muscle hypertrophy.20 Akt is also known to regulate p70S6K indirectly via activation of the mTOR pathway.21 Up-regulation of phosphorylated p70S6K (p-p70S6K) has been implicated in elevated muscle protein synthesis by increasing mRNA translation through phosphorylation of ribosomal protein components.10 Increase in phosphorylated forms of Akt (p-Akt) and p-p70S6K was observed during muscle hypertrophy22 and regrowth of atrophied skeletal muscle.23 However, there is no evidence regarding the effects of MENS on these intracellular signals associated with muscle protein synthesis.
The purpose of the present study was to investigate the effects of MENS on the regrowth of atrophied skeletal muscle. Skeletal muscle atrophy with the loss of muscle mass and strength results from muscle unloading, which is associated with spaceflight24 and bed rest.25 It has been generally accepted that reduced generation of active and passive tensions26,27 in microgravitational environment most likely contributes to muscle wasting process.28 Ground-based hindlimb unloading rodent model has been widely used for space flight-induced muscle atrophy. Numerous studies have clearly shown that 7 to 14 days of hindlimb suspension (HS) of rodents causes atrophy of hindlimb skeletal muscle, especially antigravitational soleus muscle. In addition, it has been reported that inflammation, membrane lysis, and necrosis in reloading on unloading-associated atrophied rat soleus muscle were observed.29 On the other hand, unloading-associated atrophied soleus muscles of rats and mice are grown by reloading and muscle mass recovers to the control level.10,30,31 Therefore, in the present study, we investigate the effects of MENS on regeneration of muscle necrosis and regrowth of atrophied soleus muscle by using hindlimb unloading rodent model. We also discussed about the effect of MENS on the intracellular signals associated with protein synthesis during regrowth of atrophied soules muscle. Evidences from this study showed that MENS facilitated regrowth of atrophied skeletal muscle via the stimulation of muscle protein synthesis.
Materials and Method
Animals and Grouping
All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Bethesda, MD, USA) and were approved by the Animal Use Committee of Toyohashi SOZO University. Male C57BL/6J mice, aged 10 weeks old were used (n = 35). Mice were randomly divided into two groups: (i) untreated normal recovery (C, n = 25) and (ii) MENS-treated (M, n = 10) groups (Fig. 1).
Summary of the experimental protocol. C: untreated control group; M: microcurrent electrical nerve stimulation (MENS)-treated group. Pre: before hindlimb suspension; R0, R1, R3, R5, and R7: immediately after (0), 1, 3, 5, and 7 days after the hindlimb suspension.
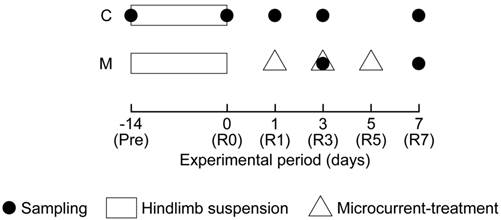
Five mice of C group were assigned as the cage control and killed immediately before the initiation of HS. To induce unloading-associated muscle atrophy, all mice in both groups, except the cage control, were subjected to HS for 2 weeks. The HS was performed following the methods described previously.32,33 Briefly, tails of the HS mice were cleaned, and were loosely surrounded by adhesive tapes cross-sectionally, fixing a string at the dorsal side of the tail, to maintain the blood flow intact. The string was fastened to the roof of the cage at a height allowing the forelimbs to support the weight, yet preventing the hindlimbs from touching the floor and the sides of the cage (20 x 31 cm and 13.5 cm height). The mice could reach food and water freely by using their forelimbs. Five mice of both groups, except for the period of HS, were housed in a home cage with the same size as for HS in a clean room controlled at approximately 23°C and at 55% humidity with a 12/12 hours light-dark cycle. Solid diet and water were provided ad libitum.
MENS treatment
Immediately after HS, ambulation recovery was performed on the mice in both groups. Following 1, 3 and 5 days after HS, the both hindlimbs of mice in M group were treated with MENS (intensity: 10 μA, frequency: 0.3 Hz, pulse width: 250 msec) by using an electical stimulator (Trio300, Ito Co., Ltd., Tokyo, Japan) for 60 min under anesthesia with i.p. injection of sodium pentobarbital (50 mg/kg). Mice in C group were also anesthetized for 60 min without MENS treatment. Epilation of mouse hindlimbs was performed by using a commercial hair remover for human. Two electrodes were placed on the distal posterior side of the knee joint and the proximal posterior side of ankle joint, respectively.34 The condition for MENS was determined considering the total current for animals, compared with the studies by using human35 and rats.36 In the present study, no muscle contraction in hindlimbs of mice was observed during MENS.
Sampling
Soleus muscles in C group were dissected from both hindlimbs before (Pre) and immediately after (R0), 1 (R1), 3 (R3), and 7 (R7) days after HS (n = 5 in each day). Soleus in M group were also dissected at R3 and R7 (n = 5 in each day). Previous study showed that reloading-associated necrotic muscle fibers were observed 2nd and 4th days of reloading on atrophied soleus muscle that was induced by 10 days of HS.37 In addition, immediately after HS and 7th day of reloading, necrotic area in soleus muscle was similar to untreated control.37 Regenerating fibers of soleus muscle were clearly observed 2nd and 4th days of reloading following HS.38 Unloading-associated atrophied soleus muscle mass is increased by reloading and the muscle weight recovers to the control levels 7th day of reloading following 10 days of HS.39 Judging from these evidences, we examined the effects of MENS on regeneration and regrowth of unloading-associated atrophied soleus muscle at R3 and R7.
After the experimental period, the animals of each group were sacrificed by cervical dislocation. Both soleus muscles were excised from both hindlimbs. Soleus was trimmed of excess fat and connective tissues, weighed, frozen in liquid nitrogen, and stored at -80°C until analyses.
Muscle Protein Content
Left soleus muscles were cross-sectionally cut into halves at the midbelly region. Half of the muscles were used for the measurement of muscular protein content. The muscles were homogenized in 0.4 ml of tissue lysis reagent (CelLyticTM-MT, Sigma, St. Louis, MO, USA) and completely solubilized by alkaline treatment with 2 N NaOH at 37°C for 1 h. Protein content of the tissue lysate was determined by using the Bradford technique (protein assay kit; Bio-Rad, Hercules, CA, USA) and bovine serum albumin (Sigma) as the standard. Total protein content in whole muscle was then calculated.
Western Blotting and Densitometry
The remaining half of the left soleus muscles were homogenized in 0.4 ml isolation buffer of tissue lysis reagent (CelLyticTM-MT, Sigma) with 10% (v/v) Protease Inhibitor Cocktail (P8340, Sigma) and 1% (v/v) Phosphatase Inhibitor Cocktail (524625, Calbiochem, San Diego, CA, USA) and centrifuged at 12,000 rpm (4°C for 10 min), then the supernatant was collected. The supernatant was mixed with sodium-dodecylsulfate (SDS) sample buffer: 30% (v/v) glycerol, 5% (v/v) 2-mercaptoethanol, 2.3% (w/v) SDS, 62.5 mM Tris-HCl, 0.05% (w/v) bromophenol blue and pH 6.8, at a concentration of 1 mg protein/ml and was boiled for 3 min. The SDS-polyacrylamide gel electrophoresis (PAGE) was carried out on 8 or 12% polyacrylamide containing 0.5% SDS at a constant current of 20 mA for 120 min. Equal amounts of protein (20 μg) were loaded on each gel. Molecular weight markers (Bio-Rad Precision Markers) were applied to both sides of 14 lanes as the internal controls for transfer process or electrophoresis.
Following SDS-PAGE, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (0.2 μm pore size, Bio-Rad) using a Bio-Rad mini trans-blot cell at a constant voltage of 100 V for 60 min at 4°C. After the transfer, the membranes were blocked for 1 h using a blocking buffer (5% skim milk with 0.1% Tween 20 in Tris-buffered saline with pH 7.5). Then, the membranes were incubated for 2 h with a polyclonal antibody (p-Akt, 9271, Cell Signaling Technology Inc. (CST), Beverly, Mass., USA; Akt, 9272, CST; p-p70S6K, 9205, CST; p70S6K, 9202, CST) and then reacted with a secondary antibody (goat anti-rabbit immunoglobulin G conjugate to horseradish peroxidase; CST) for 2 h. After the final wash, protein bands were visualized using chemiluminescence (ECL Advance Western blotting kit; GE Healthcare, Little Chalfont, UK), and signal density was measured by using Light-Capture (AE-6971) with CS Analyzer version 2.08b (ATTO Corporation, Tokyo, Japan). In the present study, the activation levels of Akt and p70S6K were evaluated by using the expression levels of the phosphorylated forms relative to total expression forms (phosphorylated and non-phosphorylated forms). Each sample was investigated in duplicate, at least, to ensure that results were not influenced by loading errors.
Histochemical Analyses
Frozen right soleus muscles were cut cross-sectionally into halves. Serial transverse cryosections (8 μm thick) of the midbelly region of the proximal side were sliced at -20°C and mounted on glass slides. The slides were air dried, fixed in citrate/acetone solution for 30 seconds at room temperature, and stained to analyze the necrotic area by using acid phosphatase staining. Histochemical staining of acid phosphatase reaction is generally used to evaluate muscle cell necrosis.40 The images of muscle sections in both stainings were incorporated into a personal computer (DP-BSW Ver.02.02, Olympus, Tokyo, Japan) by using a microscope (IX81 with DP70, Olympus). In acid phosphatase staining, the necrotic area and whole cross-sectional area of soleus muscle, respectively, were measured using the National Institutes of Health Image J 1.38X (NIH, Bethesda, MD, USA) software for Windows and then the percentage of necrotic area relative to the whole cross-sectional area of soleus was calculated.
Statistical Analysis
All values were expressed as means ± SEM. Statistical significance for changes of the measurements among Pre, R0, and R1 in C group was analyzed by using one-way analysis of variance (ANOVA) followed by the Scheffe's F post hoc test. Statistical significance for the changes of measurements at R3 and R7 in both groups was analyzed by using two-way (treatment x time) ANOVA followed by Scheffe's F post hoc test. When a significant interaction between two main effects (treatment and time) was observed, one-way ANOVA followed by Scheffe's F post hoc test was performed. The significance level was accepted at p < 0.05.
Results
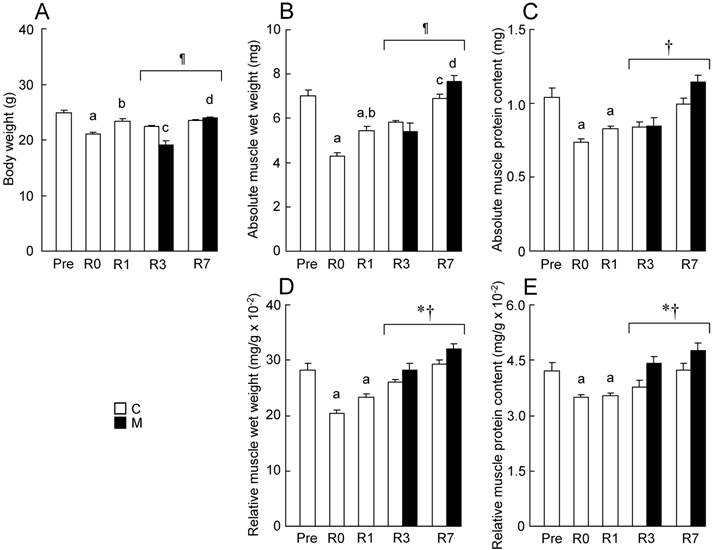
In the present study, body weight was significantly decreased by 2 weeks of HS (Fig. 2A, p < 0.05). The weight showed a trend to recover to the initial level (Pre) during the reloading period. Body weight at R3 in M group was lower than that in C group (p < 0.05). However, there was no significant difference in body weight between C and M groups at R7. Muscle wet weight and protein content of soleus were also significantly decreased by HS (Figs. 2B-E, p < 0.05). Then, a gradual and significant increase in muscle wet weight and protein content of soleus in both C and M groups was also observed during the reloading period. There was a significant difference in relative wet weight and protein content between R3 and R7 (Figs. 2D and E, p < 0.05). Relative wet weight and protein content in M group were significantly higher than those in C group during the reloading (p < 0.05).
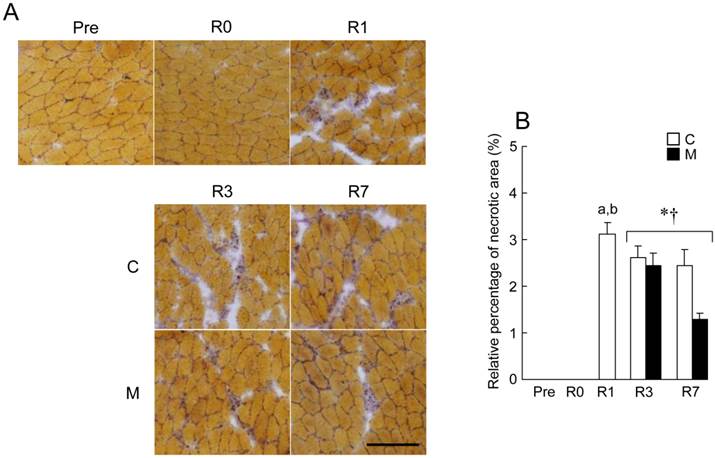
Necrotic region was observed in reloaded soleus muscle after HS (Fig. 3). The percentage of necrotic area relative to the whole cross-sectional area at R1 was approximately 3.1 ± 0.3%, although no necrotic area was observed at Pre and R0. The percentage of necrotic area of soleus muscle was gradually decreased during the recovery period. There is a significant difference in the percentage of necrotic area between R3 and R7 (Fig. 3B, p < 0.05). The percentage of necrotic area in M group was significantly lower than that in C group during the regrowing phase (p < 0.05).
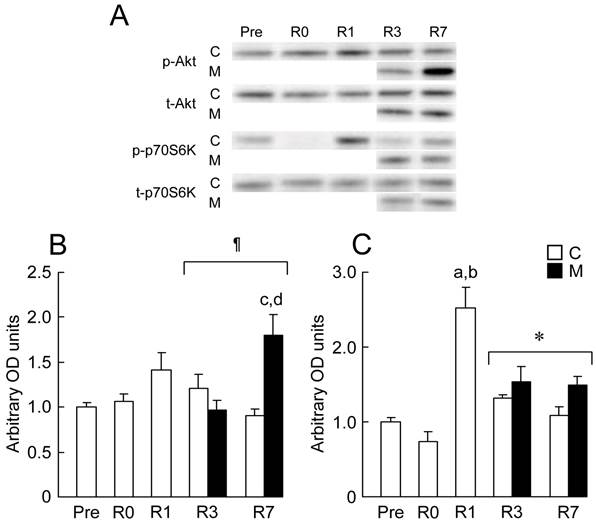
In the present study, the expression levels of Akt and p70S6K in response to 2 weeks of HS followed by 7days of reloading were investigated by using Western blotting analyses (Fig. 4). There were no significant changes in the mean expression level of Akt at R0 and R1 (Fig. 4B). There is no difference in the expression level of p-Akt between C and M groups at R3. However, the expression of p-Akt in M group was significantly increased within 7 days of reloading (p < 0.05). There were significant differences in the expression level of p-Akt between C and M group at R7 (p < 0.05), and between R3 and R7 in M group (p < 0.05).
There was also no significant change in the mean expression level of p70S6K at R0 (Fig. 4C). Relative expression level of p-p70S6K at R1 was significantly higher than that at Pre and R0 (p < 0.05). The expression levels of p-p70S6K in M groups were significantly higher than those in C group during 3rd and 7th days of reloading (p < 0.05).
Discussion
The present study demonstrated that the application of MENS facilitated regrowth of atrophied soleus muscle induced by hindlimb unloading. This is the first report concerning the effects of MENS on regrowth of atrophied skeletal muscle. MENS also promoted a decrease of the area of necrosis which was induced by reloading following HS. The levels of p-p70S6K (3rd and 7th day of reloading) and p-Akt (7th day of reloading) were up-regulated by MENS.
In the present study, losses of body weight, wet weight and protein content of soleus muscle were observed following 2 weeks of HS. These results were consistent with previously reported data in rat41 and mice.33 Decreased body weight showed a trend to recover to the initial level (Pre) during the reloading period. However, a significant decrease of body weight in MENS-treated M group was observed at R3, but not in C group. Thereafter, the weight recovered to control levels (C group) within 7 days of reloading. In the present study, MENS-induced muscle contraction was not observed. In addition, there is no report showing harmful effects of MENS on the skeletal muscle. A transient decrease of body weight in M group might be attributed to an immediately early response to MENS treatment.
We also found that muscle mass in C group was gradually increased by reloading following HS (Fig. 2). In addition, MENS facilitated the recovery of muscle mass during the regrowing phase of unloading-induced atrophied muscle. Reloading on unloading-induced atrophied skeletal muscle stimulates protein synthesis in the muscle, and recovers muscle mass.42 MENS may enhance the stimulating effects of reloading on protein synthesis of skeletal muscles during regrowth of unloading-associated atrophied muscle.
Effects of MENS on the body weight (A), absolute muscle wet weight (B), absolute muscle protein content (C), muscle wet weight relative to body weight (D), muscle protein content relative to body weight (E) of soleus. See figure 1 for abbreviations. Values are means ± SEM. n = 5 per group each day. *, †, and ¶: Significant main effect of treatment and time, and interaction, respectively, p < 0.05. a, b, c, and d: Significant different from Pre, R0, R3 in C group and R3 in M group, respectively, p < 0.05.
Effects of MENS on reloading-associated necrosis of soleus muscle. A: typical images of transverse cryosections of the midbelly region of soleus muscle stained with acid phosphatase. Scale bars = 100 μm. There was no necrosis before (Pre) and immediately after (R0) hindlimb suspension. B: the percentage of necrotic area relative to the whole cross-sectional area of soleus. See figure 1 for abbreviations. Values are means ± SEM. n = 5 per group each day. * and †: Significant main effect of treatment and time, respectively, p < 0.05. a and b: Significant different from Pre and R0 in C group, respectively, p < 0.05.
Representative expression patterns (A) and the mean expression levels of phosphorylated protein kinase B (Akt)/total Akt (B), phosphorylated p70 S6 kinase (p70S6K)/total p70S6K (C) in response to MENS. p-Akt: phosphorylated Akt; t-Akt: total Akt; p-p70S6K: phosphorylated p70S6K; t-p70S6K: total p70S6K. See figure 1 for other abbreviations. Values are means ± SEM. n = 5 per group each day. * and ¶: Significant main effect of treatment and interaction, respectively, p < 0.05. a, b, c, and d: Significant different from Pre, R0, R7 in C group and R3 in M group, respectively, p < 0.05.
As unloading-induced skeletal muscle atrophy results in muscle weakness, muscle inflammation, muscle membrane lysis and necrosis occur in reloaded skeletal muscle after unloading.29 In the present study, necrotic region in soleus muscle was also observed at R1. Thereafter, the necrotic area was gradually decreased during the reloading period. In addition, the application of MENS facilitated a decrease in the necrotic area (Fig 3). MENS may have a facilitating effect on the regeneration of injured skeletal muscle.
In the present study, no significant changes in the mean expression levels of p-Akt and p-p70S6K in soleus muscle were observed immediately after HS. There are controversial evidences regarding the responses of relative expression level of p-Akt or Akt activity to HS. Relative expression level of p-Akt in mice soleus43 and activity of Akt in rat soleus44 did not change following 7-14 days of HS. These observations were consistent with the results of the present study. On the other hand, there is other report that showed a decrease in relative expression of p-Akt in rat soleus muscle following 10 days of HS.10 It is still unclear that physiological function of p-Akt during unloading-associated muscle atrophy.
In the present study, the expression level of p-p70S6K in soleus muscle increased during the early recovery period (i.e., R1). The previous study showed the elevated level of phosphorylated ribosomal protein S6, which is a downstream of p70S6K, persisted several days following the increase in p-p70S6K in rat soleus muscle, resulting that muscle protein synthesis was facilitated during recovery from muscle atrophy.10 Reloading on unloading-induced atrophied skeletal muscle may stimulate protein synthesis via activation of p70S6K. In this study, we found that Akt-independent activation of p70S6K was induced by 1 day of reloading following unloading. Although Akt regulates the activation level of p70S6K via the mTOR pathway,21 Akt-independent regulation of p-p70S6K has been suggested observed by reloading on rat soleus10 and tibialis anterior muscles45 following hindlimb unloading.10,46 It has been suggested that p70S6K activation by mechanical stimulation on skeletal muscle is Akt-independent.46 Akt-independent activation of p70S6K in skeletal muscle may be induced by mechanical stress during reloading following unloading.
We also found that Akt-independent activation of p70S6K was induced by MENS, compared with untreated control group (i.e., R3). In addition, MENS-associated activations of both Akt and p70S6K were also observed at R7. There was no evidence regarding the effects of MENS as well as a traditional electrical stimulation, which could induce muscle contraction, on Akt/p70S6K signaling pathway in the regrowing phase of atrophied skeletal muscle following hindlimb unloading. It has been reported that HS-associated muscle atrophy of rat soleus could not be prevented by a traditional electrical stimulation even though the stimulation induced Akt-independent activation of p70S6K.45 Molecular mechanism for MENS-associated Akt-independent activation of p70S6K is still unclear. Collectively, all the aforementioned results suggested that MENS might have additional stimulating effects on muscle protein synthesis during the reloading period via activation of p70S6K, resulting that the recovery of atrophied skeletal muscle might be facilitated by MENS treatment. On the other hand, we have no clear explanation for Akt activation induced by MENS treatment. It is also still unclear whether MENS treatment could prevent or attenuate unloading-associated muscle atrophy.
It has been reported that electrical stimulation (0.1 ms pulses at 5 Hz) at ex tempore threshold amplitudes of between 3.0 and 5.0 V on denervated facial muscle reduces the number of innervated motor endplates in rats.47 Therefore, electrical stimulation and/or electrical stimulation-associated muscle contraction is not beneficial for recovery of denervated skeletal muscle by preventing reinnervation. On the contrary, in the present study, MENS was beneficial for regrowth of atrophied muscle. The discrepancy between these results may be attributed to the strength and amount of electrical current on skeletal muscle. In addition, it is generally accepted that there is no abnormality of innervations in unloading-associated atrophied skeletal muscle. Although the effect of MENS on denervated skeletal muscle remains unclear, muscle innervation might be one of key factors for the beneficial effects of MENS on skeletal muscle.
In conclusion, the present study demonstrated that MENS facilitated regrowth of unloading-induced atrophied soleus muscle. Decrease in the reloading-induced necrosis of atrophied soleus was also facilitated by MENS. MENS might be one of extracellular stimuli to induce intracellular signals involved in protein synthesis of atrophied and/or injured skeletal muscle. Application of MENS on skeletal muscle may be an effective rehabilitation tool for atrophied and injured skeletal muscles.
Acknowledgements
This study was supported, in part, by Grants-in-Aid for Young Scientists (B, 23700647, YO), Grants-in-Aid for Scientific Research (B, 20300218, KG; A, 22240071, TY), Grant-in-Aid for challenging Exploratory Research (24650411, KG) from the Japan Society for the Promotion of Science, and the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan (KG).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185-201
2. Booth FW. Effect of limb immobilization on skeletal muscle. J Appl Physiol. 1982;52:1113-8
3. Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1-12
4. Curtis D, Fallows S, Morris M, McMakin C. The efficacy of frequency specific microcurrent therapy on delayed onset muscle soreness. J Bodyw Mov Ther. 2010;14:272-9
5. Lambert MI, Marcus P, Burgess T, Noakes TD. Electro-membrane microcurrent therapy reduces signs and symptoms of muscle damage. Med Sci Sports Exerc. 2002;34:602-7
6. Jones GH. Protein synthesis in bupivacaine (marcaine)-treated, regenerating skeletal muscle. Muscle Nerve. 1982;5:281-90
7. Jones GH. Time course of changes in protein synthesis in marcaine-induced skeletal muscle regeneration. Mech Ageing Dev. 1984;27:373-81
8. Kojima A, Goto K, Morioka S, Naito T, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T. Heat stress facilitates the regeneration of injured skeletal muscle in rats. J Orthop Sci. 2007;12:74-82
9. Morioka S, Goto K, Kojima A, Naito T, Matsuba Y, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T. Functional overloading facilitates the regeneration of injured soleus muscles in mice. J Physiol Sci. 2008;58:397-404
10. Sugiura T, Abe N, Nagano M, Goto K, Sakuma K, Naito H, Yoshioka T, Powers SK. Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1273-8
11. Fan Y, Dickman KG, Zong WX. Akt and c-Myc differentially activate cellular metabolic programs and prime cells to bioenergetic inhibition. J Biol Chem. 2010;285:7324-33
12. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-74
13. Robey RB, Hay N. Is Akt the "Warburg kinase"?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25-31
14. Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243-50
15. Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120-7
16. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014-9
17. Zanchi NE, Lancha AH Jr. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol. 2008;102:253-63
18. An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. Am J Pathol. 2003;163:591-607
19. Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, Yonezawa K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J Biol Chem. 1999;274:34493-8
20. Sasai N, Agata N, Inoue-Miyazu M, Kawakami K, Kobayashi K, Sokabe M, Hayakawa K. Involvement of PI3K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve. 2010;41:100-6
21. Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427-31
22. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014-9
23. Childs TE, Spangenburg EE, Vyas DR, Booth FW. Temporal alterations in protein signaling cascades during recovery from muscle atrophy. Am J Physiol Cell Physiol. 2003;285:C391-8
24. Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915-30
25. Widrick JJ, Romatowski JG, Bain JL, Trappe SW, Trappe TA, Thompson JL, Costill DL, Riley DA, Fitts RH. Effect of 17 days of bed rest on peak isometric force and unloaded shortening velocity of human soleus fibers. Am J Physiol Cell Physiol. 1997;273:C1690-9
26. Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita-Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, Edgerton VR. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol. 1999;87:1776-85
27. Yamashita-Goto K, Okuyama R, Honda M, Kawasaki K, Fujita K, Yamada T, Nonaka I, Ohira Y, Yoshioka T. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol. 2001;91:417-24
28. Vandenburgh H, Chromiak J, Shansky J, Del Tatto M, Lemaire J. Space travel directly induces skeletal muscle atrophy. FASEB J. 1999;13:1031-8
29. Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol. 1999;65:492-8
30. Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol. 2007;102:1702-7
31. Yasuhara K, Ohno Y, Kojima A, Uehara K, Beppu M, Sugiura T, Fujimoto M, Nakai A, Ohira Y, Yoshioka T, Goto K. Absence of heat shock transcription factor 1 retards the regrowth of atrophied soleus muscle in mice. J Appl Physiol. 2011;111:1142-9
32. Goto K, Honda M, Kobayashi T, Uehara K, Kojima A, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T. Heat stress facilitates the recovery of atrophied soleus muscle in rat. Jpn J Physiol. 2004;54:285-93
33. Matsuba Y, Goto K, Morioka S, Naito T, Akema T, Hashimoto N, Sugiura T, Ohira Y, Beppu M, Yoshioka T. Gravitational unloading inhibits the regenerative potential of atrophied soleus muscle in mice. Acta Physiol (Oxf). 2009;196:329-39
34. Zhang BT, Yeung SS, Liu Y, Wang HH, Wan YM, Ling SK, Zhang HY, Li YH, Yeung EW. The effects of low frequency electrical stimulation on satellite cell activity in rat skeletal muscle during hindlimb suspension. BMC Cell Biol. 2010;11:87
35. Morinaga T. Microcurrent electro-therapy. 9th ed. Tokyo, Japan: Denshi & Igaku. 2007:11-41 in Japanese
36. Cheng N, Van Hoof H, Bockx E, Hoogmartens MJ, Mulier JC, De Dijcker FJ, Sansen WM, De Loecker W. The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin Orthop Relat Res. 1982;171:264-72
37. Tidball JG, St Pierre BA. Apoptosis of macrophages during the resulution of muscle inflammation. J Leukoc Biol. 1996;59:380-8
38. Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327-36
39. McClung JM, Davis JM, Carson JA. Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats. Exp Physiol. 2007;92:219-32
40. Minamoto VB, Grazziano CR, Salvini TF. Effect of single and periodic contusion on the rat soleus muscle at different stages of regeneration. Anat Rec. 1999;254:281-7
41. Ohira M, Hanada H, Kawano F, Ishihara A, Nonaka I, Ohira Y. Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn J Physiol. 2002;52:235-45
42. Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88:359-63
43. van der Velden JL, Langen RC, Kelders MC, Willems J, Wouters EF, Janssen-Heininger YM, Schols AM. Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3β. Am J Physiol Cell Physiol. 2007;292:C1636-44
44. Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-κB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529-38
45. Dupont E, Cieniewski-Bernard C, Bastide B, Stevens L. Electrostimulation during hindlimb unloading modulates PI3K-AKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK. Am J Physiol Regul Integr Comp Physiol. 2011;300:R408-17
46. Klossner S, Durieux AC, Freyssenet D, Flueck M. Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol. 2009;106:389-98
47. Sinis N, Horn F, Genchev B, Skouras E, Merkel D, Angelova SK, Kaidoglou K, Michael J, Pavlov S, Igelmund P, Schaller HE, Irintchev A, Dunlop SA, Angelov DN. Electrical stimulation of paralyzed vibrissal muscles reduces endplate reinnervation and does not promote motor recovery after facial nerve repair in rats. Ann Anat. 2009;191:356-70
Author contact
![]() Corresponding author: Katsumasa Goto, Ph.D. Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University, 20-1 Matsushita, Ushikawa, Toyohashi, Aichi 440-8511, Japan. TEL +81-50-2017-2272 FAX +81-532-55-0803 E-mail: gotokocn.ne.jp.
Corresponding author: Katsumasa Goto, Ph.D. Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University, 20-1 Matsushita, Ushikawa, Toyohashi, Aichi 440-8511, Japan. TEL +81-50-2017-2272 FAX +81-532-55-0803 E-mail: gotokocn.ne.jp.

 Global reach, higher impact
Global reach, higher impact