3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(9):1199-1208. doi:10.7150/ijms.5169 This issue Cite
Research Paper
Increased Expression of Pattern Recognition Receptors and Nitric Oxide Synthase in Patients with Endometriosis
1. East-West Medical Research Institute, Kyung Hee University
2. Department of Surgery, St. Vincent's Hospital, The Catholic University of Korea
3. Department of Obstetrics and Gynecology, St. Vincent's Hospital, The Catholic University of Korea
* Yeo, SG and Won, YS contributed equally to this work.
Received 2012-9-5; Accepted 2013-7-22; Published 2013-7-30
Abstract
Objective: Endometriosis is characterized by repeated inflammatory changes and serious adhesions, inducing innate and adaptive immune responses within the abdominal cavity. To assess these immune responses, we evaluated the levels of expression of Toll-like receptors (TLR)-1, -2, -4, -5, and -9; nucleotide-binding oligomerization domains (NOD)-1 and -2; interleukins-1β, -6, -8, -10, and -12; interferon-γ; tumor necrosis factor-α; inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS); and immunoglobulins (Igs) in patients with endometriosis.
Methods: The levels of TLRs, NODs, cytokines, and NOS mRNAs in peritoneal effusions were assessed by real time reverse transcription-polymerase chain reaction; and IgG, IgA and IgM concentrations were measured by enzyme-linked immunosorbent assays (ELISA) in 40 patients with and 40 without endometriosis. Findings from the two groups were compared.
Results: We observed expression of all pattern recognition receptors (PRRs), cytokines, and NOS mRNAs and Igs in the effusion fluid of patients with and without endometriosis. The levels of TLR-2 and -9; NOD-1 and -2; iNOS and eNOS mRNAs and CA 125 were significantly higher in the endometriosis than in the non-endometriosis group (p<0.05 each). Moreover, PRR, cytokine, and NOS expression showed significant correlations (p<0.05).
Conclusions: PRRs, cytokines, and NOS, which act cooperatively in the innate immune response, are closely associated with endometriosis. Increased expression of TLR-2, TLR -9, NOD-1, NOD-2, and NOS mRNA in peritoneal fluid may be associated with endometriosis.
Keywords: Endometriosis - Pattern Recognition Receptors, Toll-like receptors - Nitric oxide synthase, cytokine, Immunoglobulin
Introduction
Endometriosis is a disease related to chronic pelvic inflammation and associated pelvic pain that may be accompanied by, for example, dysmenorrhea, dyspareunia, infertility and menstrual irregularities. Although the pathogenesis of endometriosis has not been clearly defined, abnormal levels of immune system cells, including macrophages, dendritic cells and natural killer cells, have been observed within the abdominal cavities of patients with endometriosis. These cells, however, are unable to detect and eliminate ectopic endometrial cells. Moreover, immune system cells in the abdominal cavity were found to be dysfunctional (1). Complicated reactions may occur within the abdominal cavity, due to endometriosis-induced secretion or reactions of cytokines, chemokines, nitric oxide, immunoglobulins, and immune cells. Triggered immune reactions signify the host recognition of infectious agents, but, if pathogens are not swiftly recognized, immune reactions necessary to fight infections do not occur. Thus, recognition of infectious agents is regarded as one of the crucial processes in the host immune system.
Pattern recognition receptors (PRRs) recognize unique molecular characteristics of pathogens and induce appropriate immune responses. PRRs respond to distinct molecular motifs of pathogens, their sites of expression in microorganisms and signaling (2). Among the various types of PRRs in humans are Toll-like receptors (TLRs) and cytoplasmic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). The principal TLRs involved in endometriosis are TLR-4, present on cell surfaces, and TLR-3, present on lysosome/endosome membranes (3).
Increased concentrations of lipopolysaccharide (LPS) in the peritoneal cavity or endometriotic fluid can trigger pelvic inflammation and TLR-mediated endometriosis (4). TLRs are also activated by endogenous ligands, including heat shock protein, S100, fibronectin, fatty acid, oxidized LDL, neutrophil elastase and hyaluronan. TLRs stimulated by LPS or endogenous ligands and oxidative stress activate NF-κB, upregulating cytokine secretion as pro-inflammatory cascades (3, 5). As this process proceeds, the adaptive immune system becomes involved, along with the innate immune system. Although several studies have assessed the expression of PRRs, cytokines, NOS, and immunoglobulins separately in patients with endometriosis, no study to date has analyzed the relationships of these molecules by measuring all of them at the same time. We therefore analyzed the expression of PRRs, which are involved in inflammatory and immune responses; NOS, which are involved in the female reproductive process; and Igs, which are involved in the adaptive immune response, in patients with and without endometriosis. We also analyzed the relationships among these molecules in the peritoneal cavities of patients with and without endometriosis according to patient age, parity and serum CA125 concentration.
Subjects & Methods
Subjects
Intraperitoneal fluid samples were obtained from 80 patients who visited the Department of Obstetrics and Gynecology at our hospital between June 2011 and July 2012. Of these, 40 were positive for endometriosis on laparoscopy, a finding confirmed during histological examination after surgery. All patients enrolled in this study were in the proliferative stage. Of the patients with endometriosis, 27 had stage 1, 7 had stage 2, and 6 had stage 3 endometriosis; none had stage 4. The remaining 40 patients had benign tumors, with no evidence of endometriosis, including 26 patients with myomas, 5 with dermoid cysts, 2 with hydrosalphix, 3 with paratubal cysts, 1 with a serous borderline ovarian tumor, and 3 with non-pathologic specificities. During laparoscopy, peritoneal fluid was collected aseptically from the Douglas pouch, taking care to avoid bleeding. Patients were excluded if they had inflammatory diseases or hormone producing conditions, including pregnancy; if peritoneal fluid was contaminated with blood; or if no peritoneal fluid could be obtained. The samples were centrifuged at 1800 x g for 10 min; the supernatants were stored at -80°C in 1.5 ml aliquots; and the cell pellets were stored at -80°C in 1.5 ml aliquots after adding RNase inhibitor. The study protocol was approved by the institutional review boards (IRBs) of Vincent's Hospital, The Catholic University of Korea and Kyung Hee University Hospital, and informed consent was obtained from each patient (VC11TISI0091, KMC IRB 1236-02).
Real-time reverse transcription-polymerase chain reactions
Total RNA was extracted from peritoneal fluid using RNA-Bee solution kits (Tel-Test, Friendswood, TX, USA), as described by the manufacturer. First-strand cDNA was synthesized by reverse transcription in 20 μl of a reaction mixture containing 1 μg of RNA, 1x reaction buffer, 1 mM dNTP, 5 µM random primers, 20 units RNase inhibitor, and 20 units AMV reverse transcriptase (Promega, Madison, WI, USA). The reaction mixture was incubated at 42°C for 1 h, and the reaction was terminated by heating at 95°C for 5 min. Primers specific for Toll-like receptors (TLR)-1, -2, -4, -5, 6, and -9; nucleotide-binding oligomerization domains (NOD)-1 and -2; interleukins (IL)-1β, -6, -8, -10, and -12; interferon-γ; tumor necrosis factor-α; inducible nitric oxide synthase (NOS); and endothelial NOS are shown in Table 1. Real-time polymerase chain reactions (PCR) were performed using a Chromo4 Detector real-time system (Bio-Rad, Hercules, CA, USA) and the SsoFast EvaGreen supermix (Bio-Rad). Each 20‑μl PCR reaction contained 2 μl of cDNA, 10 μl SsoFast EvaGreen supermix, 2 μl of each primer and 6 μl PCR grade water. The amplification protocols consisted of an initial denaturation at 95°C for 30 sec, followed by 45 cycles of denaturation at 95°C for 5 sec and annealing and extension at 55°C to 64°C for 12 sec. The point at which expression of each cDNA crossed that of β-actin cDNA was applied to the formula, 2-(target gene- ß actin), and the relative amounts of each cDNA were quantitated.
Enzyme-Linked Immunosorbent Assay (ELISA)
IgG, IgA, and IgM concentrations in the supernatants of peritoneal fluid were measured by ELISA. Briefly, 50 μl 1:100 goat anti-human IgG, IgA, and IgM in coating buffer (1.59 g Na2CO3+2.93 g NaHCO3+5% NaN3, pH 9.6) were placed into each well of a 96 well plate and incubated overnight at 4°C. The wells were washed 6 times and incubated with blocking antibody. To each well was added 50 μl of sample, and the plates were incubated at room temperature for 3 hours. The wells were washed 6 times, followed by incubation at room temperature with purified goat anti-human IgG, IgA, or IgM conjugated to horseradish peroxidase in PBS/Tween/BSA solution. The plates were again washed 6 times and incubated with substrate solution (2, 2'-AZINO-Bis), and the optical absorbance of each well was measured at 450 nm (Bethyl Laboratories, Montgomery, TX).
Statistical analysis
The Kolmogorov-Smirnov test was used to assess normality and Levene's test was used to assess the equality of variances between groups. Between group differences in expression were determined using independent t-tests, with correlations assessed using Pearson correlation tests. Results according to endometriosis stage were analyzed using the Kruskal-Wallis test.
All statistical analyses were performed using SPSS version 13, with a p-value less than 0.05 considered statistically significant.
Primers for real-time RT-PCR
| Name | Sequences | Annealing temperature | Product size (bp) |
|---|---|---|---|
| TLR1 | F:5'-CTATACACCAAGTTGTCAGC-3' | 60 | 220 |
| R:5'-GTCTCCAACTCAGTAAGGTG-3' | |||
| TLR2 | F:5'-GCCAAAGTCTTGATTGATTGG-3' | 64 | 347 |
| R:5'-TTGAAGTTCTCCAGCTCCTG-3' | |||
| TLR4 | F:5'-TGGATACGTTTCCTTATAAG-3' | 56 | 507 |
| R:5'-GAAATGGAGGCACCCCTTC-3' | |||
| TLR5 | F:5'-CTAGCTCCTAATCCTGATG-3' | 56 | 438 |
| R:5'-CCATGTGAAGTCTTTGCTGC-3' | |||
| TLR6 | F:5'-CCTCCCAGGATCAAGGTACTTG-3' | 60 | 327 |
| R:5'-ATCAGGCCAGCCCTCTAACAC-3' | |||
| TLR9 | F:5'-CCCTCAACTTCACCTTGGATCT-3' | 64 | 408 |
| R:5'-CCACATATGGCCCAGTGCA-3' | |||
| IL-1β | F:5'-TGATGGCTTATTACAGTGGCAATG-3' | 140 | 60 |
| R:5'-GTAGTGGTGGTCGGAGATTCG-3' | |||
| IL-6 | F:5'-GTGTTGCCTGCTGCCTTC-3' | 60 | 194 |
| R:5'-AGTGCCTCTTTGCTGCTTTC-3' | |||
| IL-8 | F:5'-GACATACTCCAAACCTTTCCAC-3' | 60 | 160 |
| R:5'-CTTCTCCACAACCCTCTGC-3' | |||
| IL-10 | F:5'-GAACCAAGACCCAGACATC-3' | 60 | 137 |
| R:5'-CATTCTTCACCTGCTCCAC-3' | |||
| IL-12p40 | F:5'-TCGGCAGGTGGAGGTCAGC-3' | 60 | 77 |
| R:5'-CGCAGAATGTCAGGGAGAAGTAGG-3' | |||
| IFN-γ | F:5'-TGTGGAGACCATCAAGGAAGAC-3' | 60 | 121 |
| R:5'-TGCTTTGCGTTGGACATTCAAG-3' | |||
| TNF-α | F:5'-ATCTTCTCGAACCCCGAGTG-3' | 60 | 51 |
| R:5'-GGGTTTGCTACAACATGGGC-3' | |||
| iNOS | F:5'-TGGATGCAACCCCATTGTC-3' | 60 | 59 |
| R:5'-CCCGCTGCCCCAGTTT-3' | |||
| eNOS | F:5'-CGGCATCACCAGGAAGAAGA-3' | 67 | 60 |
| R:5'-CATGAGCGAGGCGGAGAT-3' | |||
| β-actin | F:5'-GCGAGAAGATGACCCAGATC-3' | 60 | 77 |
| R:5'-GGATAGCACAGCCTGGATAG-3' |
RT-PCR: real time-polymerase chain reaction; TLR: Toll-like receptor; NOD: nucleotide-binding oligomerization domain.
Results
Characteristics of Patients in the Endometriosis and Non-Endometriosis Groups
The 40 endometriosis patients included 18 nulliparas and 22 multiparas, of mean age 36.5±8.8 years. The 40 non-endometriosis patients included 13 nulliparas and 27 multiparas, of mean age 40.6±10.6 years. There were no between group differences in age, body mass index (BMI), fertility, or history of prior surgery (p>0.05 each). However, mean serum CA125 concentration was significantly higher in the endometriosis than in the non-endometriosis group (51.0±54.9 IU/ml vs 26.7±24.8 IU/ml, p<0.05).
Expression of TLR, NOD, IL, IFN-γ, TNF-α, iNOS and eNOS mRNAs in peritoneal fluid (Table 2)
We found that mRNAs encoding all PRRs, cytokines and NOS were present in the effusion fluid of patients in the endometriosis and non-endometriosis groups. The level of expression of each was higher in the endometriosis group, with the levels of expression of mRNAs encoding TLR-2 and -9; NOD-1 and -2; iNOS and eNOS being significantly higher in the endometriosis than in the non-endometriosis group (p<0.05).
Concentrations of Igs in effusion fluid
The concentrations of IgG (1759 ± 379 µg/ml vs 1670 ± 590 µg/ml) and IgA (875 ± 449 µg/ml vs 817 ± 359 µg/ml) were higher, whereas the concentration of IgM was lower (242 ± 128 µg/ml and 291 ± 151 µg/ml), in the endometriosis than in the non-endometriosis group. However, none of these differences was statistically significant (p>0.05 each).
Correlations of clinical manifestations with expression of PRRs, cytokines, NOS, and Igs (Table 3)
No significant correlation was observed between any clinical or demographic characteristic, including age, parity, or CA125 concentration, and the level of cytokine or NOS mRNA expression or Ig concentration, in the two patient groups (p>0.05 each).
Correlation of PRR, cytokine, and NOS mRNAs with Ig concentrations (Table 4)
Significant correlations were observed among the expression of mRNAs encoding PRRs, including TLRs-1, -2, -4, -5, 6, and -9; NODs-1 and -2; ILs-1 β, -6, -8, -10, and -12; IFN-γ; TNF-α, and iNOS and eNOS in the two groups (p<0.05 each) (Table 4). In the endometriosis group, however, none of these mRNAs was correlated with the concentrations of IgG, IgM, and IgA (p>0.05 each).
Expression of TLR, NOD, NOS, and cytokine mRNAs according to stage of endometriosis
In analyzing the expression of TLR, NOD, NOS, and cytokine mRNAs according to the stage of endometriosis, we found that the levels of expression of TLR2, TLR4, and TLR5 increased significantly with increasing stage of endometriosis (p< 0.05 each) (Table 5). Significant differences were also observed when these patients were divided into two groups, those with Stage 1 and those with Stage 2 and higher (p< 0.05 each) (Table 6).
TLR, cytokine and NOS mRNA expression in the peritoneal fluid of patients with and without endometriosis.
| Endometriosis group | Non-endometriosis group | P value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| TLR1 | 0.678 | 1.824 | 0.107 | 0.389 | 0.074 |
| TLR2 | 0.828 | 2.441 | 0.081 | 0.293 | 0.007 |
| TLR4 | 0.004 | 0.016 | 0.000 | 0.001 | 0.175 |
| TLR5 | 0.013 | 0.046 | 0.000 | 0.001 | 0.153 |
| TLR6 | 6.046 | 19.816 | 0.739 | 3.115 | 0.133 |
| TLR9 | 8.669 | 19.843 | 0.618 | 1.694 | 0.022 |
| NOD1 | 1.132 | 3.851 | 0.027 | 0.077 | 0.014 |
| NOD2 | 0.008 | 0.021 | 0.004 | 0.014 | 0.048 |
| iNOS | 0.131 | 0.330 | 0.011 | 0.016 | 0.036 |
| eNOS | 5.838 | 13.943 | 0.419 | 1.146 | 0.026 |
| IL-1β | 0.093 | 0.292 | 0.032 | 0.110 | 0.240 |
| IL-6 | 0.090 | 0.271 | 0.126 | 0.683 | 0.773 |
| IL-8 | 2.802 | 10.911 | 0.453 | 2.017 | 0.200 |
| IL-10 | 0.056 | 0.103 | 0.078 | 0.249 | 0.632 |
| IL-12 | 0.622 | 2.594 | 0.550 | 3.180 | 0.915 |
| IFN-ɤ | 0.004 | 0.007 | 0.019 | 0.084 | 0.322 |
| TNF-α | 0.085 | 0.408 | 0.086 | 0.465 | 0.991 |
SD; standard deviation
TLR: Toll-like receptor; NOD: nucleotide-binding oligomerization domain; iNOS: inducible nitric oxide synthase ; eNOS: endothelial NOS; IL: interleukin; IFN-ɤ: interferon-γ; TNF-α: Tumor necrosis factor-α
Correlations among age, parity, CA125 concentration, the expression of PRR, cytokines, and NOS mRNAs, and Ig concentrations
| Endometriosis group | Control group | ||||
|---|---|---|---|---|---|
| Pearson's Coefficients | P value | Pearson's coefficients | P value | ||
| Age | TLR1 | -.099 | .567 | -.248 | .171 |
| TLR2 | -.068 | .710 | -.263 | .176 | |
| TLR4 | -.095 | .619 | -.235 | .247 | |
| TLR5 | -.056 | .766 | -.192 | .338 | |
| TLR6 | -.048 | .789 | -.260 | .165 | |
| TLR9 | .089 | .611 | -.153 | .410 | |
| NOD1 | -.044 | .797 | -.146 | .410 | |
| NOD2 | -.045 | .796 | -.280 | .142 | |
| IL-1β | -.104 | .547 | -.145 | .412 | |
| IL-6 | .070 | .687 | -.087 | .625 | |
| IL-8 | -.167 | .325 | -.248 | .152 | |
| IL-10 | .098 | .558 | .063 | .721 | |
| IL-12 | -.075 | .655 | -.210 | .226 | |
| IFN-ɤ | .191 | .259 | -.014 | .935 | |
| TNF-α | .172 | .303 | .056 | .748 | |
| iNOS | .273 | .102 | -.196 | .267 | |
| eNOS | .202 | .223 | .062 | .719 | |
| Ig G | .209 | .195 | -.021 | .903 | |
| Ig A | .339 | .032 | .034 | .844 | |
| Ig M | .268 | .094 | .316 | .060 | |
| Parity | TLR1 | -.109 | .539 | -.182 | .319 |
| TLR2 | -.089 | .639 | -.197 | .315 | |
| TLR4 | -.122 | .537 | -.151 | .462 | |
| TLR5 | -.076 | .697 | -.116 | .565 | |
| TLR6 | -.062 | .738 | -.201 | .288 | |
| TLR9 | .036 | .843 | -.033 | .861 | |
| NOD1 | -.063 | .725 | .028 | .876 | |
| NOD2 | -.062 | .733 | -.211 | .271 | |
| IL-1β | -.125 | .481 | -.108 | .541 | |
| IL-6 | .009 | .961 | .077 | .666 | |
| IL-8 | .168 | .333 | -.198 | .255 | |
| IL-10 | .197 | .248 | -.032 | .853 | |
| IL-12 | .038 | .827 | -.191 | .272 | |
| IFN-ɤ | .202 | .244 | -.081 | .642 | |
| TNF-α | .159 | .356 | -.035 | .840 | |
| iNOS | .211 | .224 | -.182 | .302 | |
| eNOS | .186 | .278 | -.040 | .819 | |
| Ig G | .290 | .078 | .149 | .385 | |
| Ig A | .415 | .010 | -.119 | .491 | |
| Ig M | .198 | .232 | .136 | .430 | |
| CA125 | TLR1 | .089 | .660 | .345 | .148 |
| TLR2 | .165 | .440 | .311 | .224 | |
| TLR4 | .144 | .511 | .288 | .279 | |
| TLR5 | .127 | .553 | .290 | .258 | |
| TLR6 | .146 | .476 | .318 | .185 | |
| TLR9 | .372 | .056 | .298 | .216 | |
| NOD1 | .102 | .612 | .237 | .300 | |
| NOD2 | .116 | .574 | .308 | .214 | |
| IL-1β | .138 | .492 | .536 | .012 | |
| IL-6 | .368 | .059 | .198 | .389 | |
| IL-8 | .104 | .598 | .302 | .172 | |
| IL-10 | .113 | .561 | -.114 | .615 | |
| IL-12 | .149 | .440 | .287 | .195 | |
| IFN-ɤ | .126 | .515 | -.030 | .894 | |
| TNF-α | -.012 | .953 | -.127 | .565 | |
| iNOS | .169 | .390 | .310 | .171 | |
| eNOS | -.011 | .957 | -.119 | .588 | |
| Ig G | .067 | .719 | -.003 | .988 | |
| Ig A | .423 | .018 | .242 | .266 | |
| Ig M | .213 | .250 | .047 | .831 | |
TLR: Toll-like receptor; NOD: nucleotide-binding oligomerization domain; iNOS: inducible nitric oxide synthase ; eNOS: endothelial NOS; IL: interleukin; IFN-ɤ: interferon-γ; TNF-α: Tumor necrosis factor-α
Correlations between IgG, IgA, and IgM concentrations and mRNAs encoding TLRs-1, -2, -4, -5, -6, and -9; NODs-1 and -2; ILs-1β, -6, -8, -10, and -12; IFN-γ; and TNF-α in endometriosis group
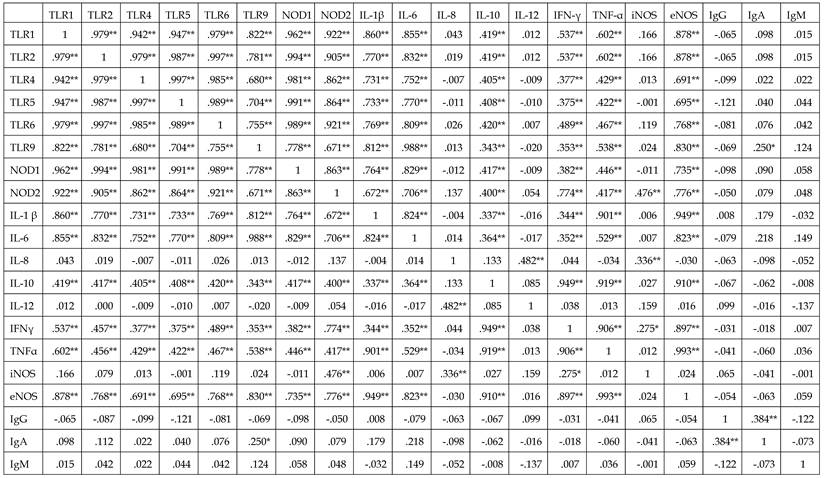
*p<0.05,** p<0.01.
TLR: Toll-like receptor; NOD: nucleotide-binding oligomerization domain; iNOS: inducible nitric oxide synthase ; eNOS: endothelial NOS; IL: interleukin; IFN-ɤ: interferon-γ; TNF-α: Tumor necrosis factor-α
Expression of TLR, NOD, NOS, and cytokines according to stage of endometriosis
| Number | Stage | Mean | SD | P value | |
|---|---|---|---|---|---|
| TLR1 | 25 | 1 | 0.539 | 1.988 | 0.189 |
| 6 | 2 | 1.037 | 1.686 | ||
| 5 | 3 | 0.941 | 1.204 | ||
| TLR2 | 23 | 1 | 0.679 | 2.686 | .016 |
| 5 | 2 | 1.518 | 2.193 | ||
| 4 | 3 | 0.818 | 1.136 | ||
| TLR4 | 21 | 1 | 0.004 | 0.019 | .003 |
| 5 | 2 | 0.007 | 0.012 | ||
| 4 | 3 | 0.002 | 0.001 | ||
| TLR5 | 22 | 1 | 0.012 | 0.052 | .032 |
| 5 | 2 | 0.019 | 0.036 | ||
| 4 | 3 | 0.008 | 0.013 | ||
| TLR6 | 23 | 1 | 5.438 | 22.677 | .115 |
| 6 | 2 | 9.196 | 16.527 | ||
| 5 | 3 | 5.061 | 7.192 | ||
| TLR9 | 24 | 1 | 4.747 | 14.425 | .191 |
| 6 | 2 | 19.807 | 34.838 | ||
| 5 | 3 | 14.129 | 17.826 | ||
| NOD1 | 25 | 1 | 0.982 | 4.286 | .096 |
| 6 | 2 | 1.791 | 3.441 | ||
| 5 | 3 | 1.091 | 1.987 | ||
| NOD2 | 24 | 1 | 0.007 | 0.024 | .191 |
| 6 | 2 | 0.012 | 0.016 | ||
| 5 | 3 | 0.009 | 0.011 | ||
| IL-1β | 25 | 1 | 0.072 | 0.261 | .561 |
| 6 | 2 | 0.203 | 0.347 | ||
| 5 | 3 | 0.341 | 0.557 | ||
| IL-6 | 25 | 1 | 3.135 | 10.364 | .191 |
| 6 | 2 | 14.708 | 25.143 | ||
| 5 | 3 | 8.706 | 9.613 | ||
| IL-8 | 26 | 1 | 0.100 | 0.340 | .510 |
| 6 | 2 | 0.132 | 0.165 | ||
| 5 | 3 | 0.013 | 0.015 | ||
| IL-10 | 27 | 1 | 0.113 | 0.318 | .075 |
| 6 | 2 | 0.041 | 0.065 | ||
| 5 | 3 | 0.025 | 0.032 | ||
| IL-12 | 27 | 1 | 3.739 | 12.885 | .195 |
| 6 | 2 | 0.785 | 0.955 | ||
| 5 | 3 | 0.160 | 0.165 | ||
| IFN-ɤ | 26 | 1 | 0.059 | 0.123 | .196 |
| 6 | 2 | 0.041 | 0.025 | ||
| 5 | 3 | 0.060 | 0.035 | ||
| TNF-α | 27 | 1 | 0.718 | 3.041 | .087 |
| 6 | 2 | 0.161 | 0.254 | ||
| 5 | 3 | 0.658 | 1.293 | ||
| iNOS | 26 | 1 | 0.003 | 0.008 | .061 |
| 6 | 2 | 0.006 | 0.007 | ||
| 5 | 3 | 0.005 | 0.006 | ||
| eNOS | 27 | 1 | 0.109 | 0.484 | .121 |
| 6 | 2 | 0.021 | 0.027 | ||
| 5 | 3 | 0.030 | 0.042 | ||
| IgG | 27 | 1 | 1739429.259 | 431206.009 | .447 |
| 7 | 2 | 1695337.143 | 148262.886 | ||
| 6 | 3 | 1922398.333 | 297959.256 | ||
| IgA | 27 | 1 | 816538.519 | 436065.112 | .423 |
| 7 | 2 | 979875.714 | 382088.450 | ||
| 6 | 3 | 1017103.333 | 596140.403 | ||
| IgM | 27 | 1 | 222407.778 | 107831.864 | .220 |
| 7 | 2 | 323938.571 | 157043.179 | ||
| 6 | 3 | 237848.333 | 166191.940 |
TLR: Toll-like receptor; NOD: nucleotide-binding oligomerization domain; iNOS: inducible nitric oxide synthase; eNOS: endothelial NOS; IL: interleukin; IFN-ɤ: interferon-γ; TNF-α: Tumor necrosis factor-α
Expression of TLR, NOD, NOS, and cytokines in patients with endometriosis stage 1 and with stage 2 or higher
| Stage 1 | Stage 2 or more | P value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| TLR1 | 0.539 | 1.988 | 0.994 | 1.416 | .080 |
| TLR2 | 0.679 | 2.686 | 1.207 | 1.739 | .004 |
| TLR4 | 0.004 | 0.019 | 0.014 | 0.009 | .001 |
| TLR5 | 0.012 | 0.052 | 0.014 | 0.028 | .008 |
| TLR6 | 5.438 | 22.677 | 7.317 | 12.725 | .038 |
| TLR9 | 4.747 | 14.425 | 17.226 | 27.253 | .078 |
| NOD1 | 0.982 | 4.286 | 1.473 | 2.763 | .035 |
| NOD2 | 0.007 | 0.024 | 0.011 | 0.013 | .078 |
| iNOS | 0.072 | 0.261 | 0.266 | 0.435 | .018 |
| eNOS | 3.135 | 10.364 | 11.980 | 19.049 | .041 |
| IL-1β | 0.100 | 0.340 | 0.078 | 0.132 | .523 |
| IL-6 | 0.113 | 0.318 | 0.034 | 0.051 | .095 |
| IL-8 | 3.739 | 12.885 | 0.501 | 0.757 | .339 |
| IL-10 | 0.059 | 0.123 | 0.050 | 0.030 | .028 |
| IL-12 | 0.718 | 3.041 | 0.386 | 0.877 | .076 |
| IFN-ɤ | 0.003 | 0.008 | 0.006 | 0.006 | .075 |
| TNF-α | 0.109 | 0.484 | 0.025 | 0.033 | .027 |
TLR: Toll-like receptor; NOD: nucleotide-binding oligomerization domain; iNOS: inducible nitric oxide synthase ; eNOS: endothelial NOS; IL: interleukin; IFN-ɤ: interferon-γ; TNF-α: Tumor necrosis factor-α
Discussion
Endometriosis is a complex inflammatory disease of the pelvis, characterized by disparate morphological, histological and biochemical properties. We evaluated endometriosis immunologically by analyzing the expression of PRRs, cytokines, nitric oxide, and immunoglobulins in the peritoneal cavity and determining whether expression of any of these molecules was correlated with the clinical characteristics of endometriosis.
The inability of the immune system to promptly recognize invading pathogens can lead to various diseases since the host immune response against these pathogens may not occur or may be inappropriate. Thus, recognizing pathogens during the initial stage of disease is crucial. This process is initiated by PRRs, a group of molecules distributed in the extracellular, membrane, and cytoplasmic compartments. Humans have various PRRs, including TLRs and NOD-like receptors (NLRs), which differ by location. For example, TLRs-1, -2, -4, -5, -6 and -10 are present on the cell surface; TLRs-3, -7, -8, and -9 are located on lysosome/endosome membranes; and NODs-1 and -2 are present in the cytoplasm (2,6-7). Pathogens that escape extracellular or membrane detection systems may be recognized by PRRs in the cytoplasm or on lysosome/endosome membranes (8).
We found that patients with endometriosis expressed mRNAs encoding TLRs-1, -2, -4, -5, 6, and -9 and NODs-1 and -2, all PRRs associated with immunity against infection. Moreover, the levels of expression of mRNAs encoding TLRs-2 and -9 and NODs-1 and -2 were significantly higher in patients with than without endometriosis. Up-regulation of PRR expression and accelerated endometrial proliferation can result in tumor formation (9). Human heat-shock protein 70 has been reported to induce pelvic inflammation, involving the TLR-3 and TLR-4-mediated growth of endometrial cells (5, 10), with TLR-4 having a significant role in innate immune reactions to bacterial endotoxin in patients with endometriosis (4). Moreover, a TLR-4 polymorphism associated with hypo-responsiveness of the receptor may result in peritoneal inflammation. Thus, endometrial cells tend to adhere to the peritoneum, a condition that may induce the initiation of endometriosis (11). We found that PRRs other than TLR-3 and TLR-4 are involved in immune reactions in patients with endometriosis of the peritoneal cavity.
NO reacts to homeostatic and pathologic stimuli and is secreted by neurons, endothelial cells, platelets and neutrophils. NO also plays a crucial role in female reproductive processes, including ovulation, menstruation, implantation, pregnancy maintenance, and labor and delivery (12). A higher level of activated peritoneal macrophages has been observed in women with endometriosis compared to those without the condition, with iNOS expression and NO production increased due to the activation of peritoneal macrophages (13). The participation of peritoneal macrophages in antimicrobial and antitumor activities increases as NO concentration increases. Furthermore, increased NO concentrations can change pro-inflammatory and peritoneal immune defense reactions and may be involved in the pathogenesis of endometriosis (14). Of the three NO-synthase isoenzymes, eNOS and nNOS are constitutively expressed, whereas iNOS, while not normally present in macrophages, can be upregulated depending on the stimulus. The latter enzyme is also involved in vasodilation and the destruction of pathogens such as bacteria (15). Although the level of NOS is dependent on endometrial phase, association with infertility, position in the menstrual cycle, and types of cells and tissues, we found that the levels of expression of iNOS and eNOS were higher in the endometriosis than the non-endometriosis group (16, 17).
Cytokines, a group of glycoproteins that participate in modulating inflammatory and immune reactions in many diseases, were recently found to be involved in endometriosis in humans and experimental animals. Although most studies have reported that the secretion of cytokines increases during and after the process of endometriosis, other studies have reported that cytokine secretion decreases or does not change significantly.
We observed no differences in the levels of expression of ILs-1β, -6, -8, -10, and -12, INF-γ, and TNF-α between our endometriosis and non-endometriosis groups. In contrast, other studies have reported higher expression of ILs-1ß, -6, -8, and -15, TNF-α, monocyte chemotactic protein-1 (MCP-1), eotaxin, regulated upon activation normal T-cell expressed and secreted (RANTES), and intercellular adhesion molecule-1 (ICAM-1), in patients with than without endometriosis (18-21). Several other studies, however, have found that the levels of expression of ILs-1ß, -10, -12, and -18, TNF-α, RANTES, VEGF, PDGF, sFas and sFasL, and vascular cell adhesion molecule-1 (VCAM-1) were similar, or in some cases lower, in patients with than without endometriosis (18, 22-24). Differences among study results may be due to the severity of endometriosis; polymorphisms in the genes encoding these molecules; types of samples, including endometriosis tissue, peritoneal fluid, or serum; the nature of the control group; or the use of medications by patients with endometriosis. Although none of our patients showed evidence of peritonitis or other inflammation before surgery, the abdominal cavities of all 80 patients were positive for IgG, IgA, and IgM secretion. The concentrations of IgG and IgA were higher, and the concentration of IgM was lower, in our endometriosis group, but none of these differences was statistically significant. Although B cells in the peritoneal cavity cannot produce antibodies in a sterile environment, without infection or external stimuli, immunoglobulins are spontaneously produced by B-1, not B-2, cells in the peritoneal cavity even in the absence of exogenous infection (25, 26). B-1 cells express the pan T-cell surface glycoprotein, CD5, and are localized in the peritoneal and pleural cavities, with few, if any, found in the spleen (27, 28). Although we did not separate B-1 from B-2 cells, the antibodies in the peritoneal cavity likely resulted from the immune responses of B-1 cells.
When B cells differentiate into plasma cells, the initial immunoglobulins produced are IgM and IgD, with IgG, IgA, and/or IgE produced following a process called “class-switching” (27). Thus, various antibodies can be present in the peritoneal cavity in the absence of any exogenous infection. We focused on three classes of antibody: IgG, which is primarily involved in chronic inflammation and auto-immune reactions; IgA, which is primarily involved in mucosal immunity; and IgM, which is primarily involved in acute inflammation. We excluded IgD and IgE, which are present at much lower concentrations. Previous findings showing that the concentration of a specific IgG autoantibody was increased in the peritoneal fluid of patients with endometriosis and that endometrial glandular epithelial staining for both IgG and IgA was significantly increased, suggests that endometriosis may be an autoimmune disease (29, 30). Moreover, the increase in IgG concentration suggests that these patients may have had a precursor condition of endometriosis requiring treatment (31).
We found that inflammatory, innate, and adaptive immune reactions in the peritoneal cavity were integrated, with significant correlations among mRNAs encoding PRRs such as TLRs-1, -2, -4, -5, -6, and -9, and NODs-1 and -2; cytokines such as ILs-1β, -6, -8, -10, and -12, INF-γ, and TNF-α; and iNOS and eNOS. These PRRs, cytokines, and NOS are induced by external stimuli, antigens, and pathogens, via distinct pathways; following which they stimulate and facilitate the production of other signaling molecules and/or act synergistically. The manifestations and correlations of PRRs, cytokines, and NOS may be involved in the pathogenesis of endometriosis and in endometriosis-induced immune reactions in the peritoneal cavity.
Our study had several limitations. For ethical reasons, our control group consisted of patients with lesions in the peritoneal cavity, rather than disease free normal subjects. Although none of the control patients had infections in the peritoneal cavity, the peritoneal lesions may have induced immune reactions. Second, we measured the levels of expression of mRNA instead of protein, since some proteins were not expressed. Third, the response of tissues other than the endometrium was not examined since the study samples consisted only of peritoneal fluid and there have been no comparative studies of serum and endometrial tissue.
We have shown here that mRNAs encoding various PRRs, cytokines, and NOS, as well as IgG, IgA, and IgM, were expressed in the peritoneal cavities of patients with endometriosis, with this expression due to diverse inflammatory and immune reactions. The expression of several PRRs and NOS increased significantly, suggesting that the cooperative interaction of PRRs, cytokines, and NOS in innate immune responses in the peritoneal fluid may be associated with endometriosis.
Acknowledgements
This work was supported by a research grant from the St. Vincent Hospital, The Catholic University of Korea, College of Medicine and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 20120009380).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Khoufache K, Michaud N, Harir N. et al. Anomalies in the inflammatory response in endometriosis and possible consequences: a review. Minerva Endocrinol. 2012;37(1):75-92
2. Cook DN, Pisesky DS, Schwarts DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975-79
3. Kajihara H, Yamada Y, Kanayama S. et al. New insights into the pathophysiology of endometriosis: from chronic inflammation to danger signal. Gynecol Endocrinol. 2011;27(2):73-9
4. Khan KN, Kitajima M, Hiraki K. et al. Toll-like receptors in innate immunity: role of bacterial endotoxin and toll-like receptor 4 in endometrium and endometriosis. Gynecol Obstet Invest. 2009;68(1):40-52
5. Midwood KS, Piccinini AM, Sacre S. Targeting Toll-like receptors in autoimmunity. Curr Drug Targets. 2009;10(11):1139-55
6. Sabroe I, Read RC, Whyte MK. et al. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003;171(4):1630-5
7. Mitchell JA, Paul-Clark MJ, Clarke GW. et al. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6(1):9-20
8. Lee MS, Kim YJ. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007;23(1):1-10
9. Yamada Y, Shigetomi H, Onogi A. et al. New insights into pattern recognition receptors and their ligands in gynecologic pathologies. Hum Immunol. 2011;72(3):213-8
10. Khan KN, Kitajima M, Imamura T. et al. Toll-like receptor 4-mediated growth of endometriosis by human heat-shock protein 70. Hum Reprod. 2008;23(10):2210-9
11. Latha M, Vaidya S, Movva S. et al. Molecular pathogenesis of endometriosis; Toll-like receptor-4 A896G (D299G) polymorphism: a novel explanation. Genet Test Mol Biomarkers. 2011;15(3):181-4
12. Wu MY, Chao KH, Yang JH. et al. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003;18(12):2668-71
13. Osborn BH, Haney AF, Misukonis MA. et al. Inducible nitric oxide synthase expression by peritoneal macrophages in endometriosis-associated infertility. Fertil Steril. 2002;77(1):46-51
14. Dong M, Shi Y, Cheng Q. et al. Increased nitric oxide in peritoneal fluid from women with idiopathic infertility and endometriosis. J Reprod Med. 2001;46(10):887-91
15. Conboy PJ, Jones NS. The nose and nitric oxide: a review. Clin Otolaryngol Allied Sci. 2000;25(5):337-41
16. Ota H, Igarashi S, Hatazawa J. et al. Endothelial nitric oxide synthase in the endometrium during the menstrual cycle in patients with endometriosis and adenomyosis. Fertil Steril. 1998;69(2):303-8
17. Wang J, Zhou F, Dong M. et al. Prolonged gonadotropin-releasing hormone agonist therapy reduced expression of nitric oxide synthase in the endometrium of women with endometriosis and infertility. Fertil Steril. 2006;85(4):1037-44
18. Bersinger NA, Dechaud H, McKinnon B. et al. Analysis of cytokines in the peritoneal fluid of endometriosis patients as a function of the menstrual cycle stage using the Bio-Plex® platform. Arch Physiol Biochem. 2012;118(4):210-8
19. Petraglia F, Arcuri F, de Ziegler D. et al. Inflammation: a link between endometriosis and preterm birth. Fertil Steril. 2012;98(1):36-40
20. Nishida M, Nasu K, Narahara H. Role of chemokines in the pathogenesis of endometriosis. Front Biosci (Schol Ed). 2011;3:1196-204
21. Chen QH, Zhou WD, Su ZY. et al. Change of proinflammatory cytokines follows certain patterns after induction of endometriosis in a mouse model. Fertil Steril. 2010;93(5):1448-54
22. Glitz C, Souza CA, Rodini GP. et al. Peritoneal and serum interleukin-18 levels are not increased in women with minimum or mild endometriosis. Braz J Med Biol Res. 2009;42(11):1039-43
23. Gmyrek GB, Sieradzka U, Goluda M. et al. A Flow cytometric evaluation of intracellular cytokine synthesis in peripheral mononuclear cells of women with endometriosis. Immunol Invest. 2008;37(1):43-61
24. Kalu E, Sumar N, Giannopoulos T. et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33(4):490-5
25. Rothstein TL. Commentary: Two B-1 or Not To Be One. J of Immunology. 2002;168:4257-61
26. Kim JB, Yeo SG, Kim SW. et al. Characteristic features of Immune B cells in Murine Cervical Lymph Node. Korean J Otolaryngol. 2005;48(2):241-6
27. Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 2003;21:205-30
28. Yeo SG, Tumang JR, Rothstein TL. Characteristic features of B cells in murine cervical lymph nodes. Acta Otolaryngol. 2006;126(1):56-61
29. Saifuddin A, Buckley CH, Fox H. Immunoglobulin content of the endometrium in women with endometriosis. Int J Gynecol Pathol. 1983;2(3):255-63
30. Gleicher N, el-Roeiy A, Confino E. et al. Is endometriosis an autoimmune disease? Obstet Gynecol. 1987;70(1):115-22
31. Topalski-Fistes N, Bujas M, Pjević M. et al. Immunologic characteristics of peritoneal fluid in endometriosis. Med Pregl. 1996;49(9-10):356-60
Author contact
![]() Corresponding author: Dong Choon Park, M.D., PhD. Department of Obstetrics and Gynecology, Saint Vincent's Hospital, The Catholic University of Korea, 93 Gi-dong, Paldal-ku, Suwon, Kyungki-do, Korea. Phone: 82-31-249-8221, Fax: 82-31-254-7481, E-mail: dcparkac.kr
Corresponding author: Dong Choon Park, M.D., PhD. Department of Obstetrics and Gynecology, Saint Vincent's Hospital, The Catholic University of Korea, 93 Gi-dong, Paldal-ku, Suwon, Kyungki-do, Korea. Phone: 82-31-249-8221, Fax: 82-31-254-7481, E-mail: dcparkac.kr

 Global reach, higher impact
Global reach, higher impact