3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(10):838-842. doi:10.7150/ijms.5094 This issue Cite
Short Research Communication
Inhibitory Effects of Anthocyanins on Secretion of Helicobacter pylori CagA and VacA Toxins
1. Department of Biomedical Laboratory Science, College of Health Science, Yonsei University, Wonju 220-710, Republic of Korea
2. Department of Clinical Laboratory Science, Semyung University, Jaecheon 390-711, Republic of Korea
3. Department of Biomedical Laboratory Science, Korea Nazarene University, Chenoan 331-718, Republic of Korea
4. Department of Functional Crops, National Institute of Crop Science, Rural Development Administration, Miryang 627-803, Republic of Korea
* These investigators contributed equally to this study.
Received 2012-8-24; Accepted 2012-10-25; Published 2012-11-1
Abstract
Anthocyanins have been studied as potential antimicrobial agents against Helicobacter pylori. We investigated whether the biosynthesis and secretion of cytotoxin-associated protein A (CagA) and vacuolating cytotoxin A (VacA) could be suppressed by anthocyanin treatment in vitro. H. pylori reference strain 60190 (CagA+/VacA+) was used in this study to investigate the inhibitory effects of anthocyanins; cyanidin 3-O-glucoside (C3G), peonidin 3-O-glucoside (Peo3G), pelargonidin 3-O-glucoside (Pel3G), and malvidin 3-O-glucoside (M3G) on expression and secretion of H. pylori toxins. Anthocyanins were added to bacterial cultures and Western blotting was used to determine secretion of CagA and VacA. Among them, we found that C3G inhibited secretion of CagA and VacA resulting in intracellular accumulation of CagA and VacA. C3G had no effect on cagA and vacA expression but suppressed secA transcription. As SecA is involved in translocation of bacterial proteins, the down-regulation of secA expression by C3G offers a mechanistic explanation for the inhibition of toxin secretion. To our knowledge, this is the first report suggesting that C3G inhibits secretion of the H. pylori toxins CagA and VacA via suppression of secA transcription.
Keywords: Helicobacter pylori, CagA, VacA, SecA, anthocyanin, cyanidin 3-O-glucoside.
Introduction
Helicobacter pylori is a Gram-negative helix-shaped bacterium that is found in about half of the world's population1. H. pylori colonization of the human stomach is consistently associated with gastric mucosal inflammation and is a risk factor for the development of peptic ulcer disease and distal gastric adenocarcinoma2,3. The most intensively studied virulence factors of H. pylori are cytotoxin-associated protein A (CagA) and vacuolating cytotoxin A (VacA), which are both involved in the pathogenesis of H. pylori4. The East Asian CagA strains of H. pylori are known to have a significant correlation with gastric ulceration and cancer development5,6. VacA causes gastric epithelial cell damage by inducing vacuoles7. Sustained damage caused by CagA and VacA over decades result in chronic superficial gastritis and chronic ulceration.
Numerous reports have demonstrated a correlation between consumption of anthocyanin-containing crops and fruits and disease prevention8-14. Data from these studies suggest that anthocyanins perturb peptic ulcer and gastric cancer development and at high doses anthocyanins exert antibacterial effects. Data from these studies suggest that anthocyanins may play a role in preventing ulceration and chronic inflammation due to H. pylori infection. In this study, we investigated the inhibitory role of four anthocyanins of cyanidin 3-O-glucoside (C3G), peonidin 3-O-glucoside (Peo3G), pelargonidin 3-O-glucoside (Pel3G), and malvidin 3-O-glucoside (M3G) on H. pylori toxin biogenesis and secretion.
Materials and Methods
Bacterial strains and culture
Helicobacter pylori reference strain 60190 (CagA+/VacA+) was purchased from ATCC (Manassas, VA, USA). Bacteria were grown under microaerophilic conditions at 37℃ on Brucella agar plates (Becton-Dickinson, Braintree, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Long Island, NY, USA). To examine the inhibitory effects of anthocyanins, H. pylori were cultured in Mueller-Hinton broth (Becton-Dickinson) containing 10% FBS.
Anthocyanin preparation
C3G, Peo3G, Pel3G, and M3G were obtained from Extrasynthese (Lyon, France). Each compound was dissolved in sterile dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis, MO, USA) in 50 mM. The various anthocyanins (C3G, Peo3G, Pel3G, and M3G) were added to the Mueller-Hinton broth at a final concentration of 100 μM.
Rabbit anti-H. pylori polyclonal antibodies production
Six-week old New Zealand White rabbits were purchased from Central Lab Animal Inc. (Seoul, Korea) and allowed to adapt to their new environment for two weeks before the first antigen inoculation. H. pylori 60190 bacteria (1 x 108/ml) were fixed in 0.2% formaldehyde/saline for 24 hr, washed with saline and then injected intravenously every week for a total of six weeks. Blood was allowed to clot at 4°C overnight and the serum isolated after centrifugation. Pre-immune serum was harvested prior to immunization. Collected anti-sera were purified and tested by ELISA
Western blot analysis
H. pylori were lysed in ice-cold RIPA lysis buffer (Millipore, Billerica, MA, USA) for 30 minutes on ice and sonicated for 2 minutes with 10 second intervals (Sonicator XL-2020, Heat Systems Ultrasonics, Pittsburgh, PA, USA). Protein concentration was determined using NanoQuant spectrophotometer (Infinite M200, TECAN, Austria). Protein extracts were resolved on 7.5 or 10% SDS-PAGE and then transferred to a nitrocellulose membrane (Millipore). Membranes were blocked with 5% skim milk for 30 minutes and then incubated with mouse anti-CagA monoclonal antibody (Santa Cruz Biotechnology, CA, USA), rabbit anti-VacA polyclonal antibody (Santa Cruz Biotechnology) or rabbit anti-Helicobacter pylori polyclonal antibody (this study). Appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA) were used and protein bands were visualized using enhanced chemiluminescence and X-ray film. The bacterial supernatant was concentrated 10-fold using a 3 kDa cut-off Centricon centrifugal filters (Millipore) at 3,000 rpm for 2 hours at 4°C.
RT-PCR analysis
H. pylori 60190 bacteria (1 x 108 CFU/ml) were grown in Mueller-Hinton broth at 37℃ under microaerophilic conditions with anthocyanins (100 μM) for three days. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and RNA concentration determined by spectrophotometry using Eppendorf BioPhotometer Plus (Eppendorf, Hamburg, Germany). Expression of cagA, vacA, secA, and galE (UDP-galactose 4 epimerase as an internal control) was determined by RT-PCR analysis. Total RNA (200 ng) was mixed with 0.25 ng of random hexamers (Invitrogen) and cDNA synthesized using 200 U of MMLV-RT (Invitrogen). PCR reactions were performed in a total volume of 20 μl consisting of 1/10 diluted cDNA, 2 μl of 10X PCR buffer (Tris-HCl [pH 9.0], 20 mM MgCl2, [NH4]2SO4), 2.5 mM dNTPs, 20 pmole of each primer, and 0.5 U G-Taq DNA polymerase (Cosmo Genetech, Seoul, Korea). PCR was performed using the GeneAmp PCR system 2700 (Perkin-Elmer Cetus, Boston, USA) and PCR products were analyzed by electrophoresis on a 2.0 % agarose gel containing 0.5 μg/ml of ethidium bromide. Gel images were captured and analyzed using the Quantity One System (Bio-Rad, Hercules, USA). Five independent cDNA samples were analyzed. The primer sequences and PCR conditions are listed in Table 1.
Used primers in this study.
Results and Discussion
Cyanidin 3-O-glucoside inhibits secretion of CagA and VacA
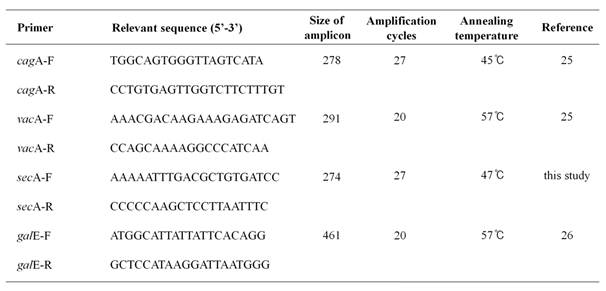
To test the inhibitory effects of anthocyanins, H. pylori 60190 bacteria were exposed to individual anthocyanins (100 μM) for three days and the levels of secreted CagA and VacA assessed by Western blot analysis. We found no change in the levels of secreted CagA and VacA when bacteria were cultured with peonidin 3-O-glucoside (Peo3G), pelargonidin 3-O-glucoside (Pel3G), and malvidin 3-O-glucoside (M3G). However, the levels of both secreted CagA and VacA decreased when bacteria were cultured with cyanidin 3-O-glucoside (C3G) (Fig. 1A). The bacterial numbers were unaffected by anthocyanins treatment (100 μM) suggesting that the decrease in CagA and VacA proteins was not due to decrease in bacterial numbers (data not shown). Furthermore, the levels of total secreted proteins were equivalent as determined by reactivity to rabbit anti-H. pylori polyclonal antibody showing that the decrease in CagA and VacA was specific to C3G treatment (Fig. 1B). The pre-immune serum showed negligible detection to H. pylori secreted proteins (data not shown).
Cyanidin 3-O-glucoside induces intracellular accumulation of CagA and VacA
If the secretion of CagA and VacA was inhibited by C3G then these proteins may accumulate inside the bacteria. Therefore, we assessed intracellular accumulation of CagA and VacA by Western blot analysis. We found an increase in both intracellular CagA and VacA when bacteria were treated with C3G but not with the other anthocyanins (Fig. 1C). The level of total intracellular proteins was equivalent as determined by reactivity to rabbit anti-H. pylori polyclonal antibody showing that the increase in CagA and VacA was specific to C3G treatment (Fig. 1D).
Cyanidin 3-O-glucoside decreases secA expression
In H. pylori, VacA is secreted via the type Va secretion system (T5aSS) apparatus. The cytoplasmic protein SecA facilitates the ATP-driven translocation of bacteria proteins out of the bacterial plasma membrane7,15,16. Therefore, VacA secretion is considered to be secA-dependent. We tested whether the intracellular accumulation of VacA after C3G treatment was due to decrease in secA expression. Due to a lack of SecA-specific antibodies, we examined for secA expression by RT-PCR analysis. RT-PCR analysis of C3G treated H. pylori showed decreased expression of secA (Fig. 2) whereas the other three anthocyanins had no effect. In addition, we found no changes in vacA expression in C3G treated bacteria supporting the notion that C3G prevented vacA secretion by inhibiting the secA-dependent pathway. It has been reported that CagA secretion occurs via the type IV secretion system (T4SS) apparatus17,18,19 and that CagA secretion is in part secA-independent20. In our studies we found that cagA expression was unaffected by C3G treatment (Fig. 2). Consistent with these observations, we found the possibility that C3G inhibits CagA secretion by inhibiting the sec-dependent pathway partially21-24.
There is a positive correlation between consumption of anthocyanin-containing foods and disease prevention8-13. The potential disease-preventive actions of anthocyanins include antibacterial effects10,11, inhibitory effects on the development of peptic ulcers and gastric cancer8,9,12, or reduction in interleukin-8 secretion13. However, these studies used anthocyanins contained in crude extracts and thus the beneficial effects attributed to anthocyanins are unclear 8-13. In this study, we tested four different pure anthocyanins for their inhibitory effects on the biogenesis and secretion of H. pylori toxins. In conclusion, our data suggest that C3G inhibits secretion of CagA and VacA in H. pylori via down-regulation of secA expression. Given that black fruits and crops are an important source of C3G, consumption of these foods may be beneficial in reducing gastric inflammation or stomach cancer due to H. pylori infection.
Effect of anthocyanins on secretion of H. pylori CagA and VacA. H. pylori was cultured with 100 μM of anthocyanin in Mueller-Hinton broth/10% FBS for 3 days and proteins assessed by Western blot. (A), secreted CagA and VacA in culture media. (B), secreted H. pylori proteins reacting with rabbit anti-H. pylori polyclonal antibody. (C), intracellular CagA and VacA. (D), intracellular H. pylori proteins reacting with rabbit anti-H. pylori polyclonal antibody. Representative image from five independent experiments.
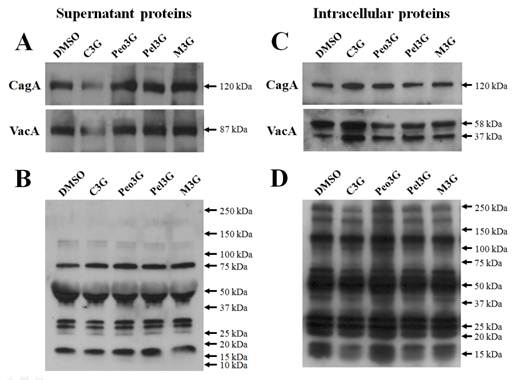
Effect of anthocyanins on secA transcription. H. pylori was cultured with 100 μM of anthocyanin in Mueller-Hinton broth/10% FBS for 3 days. After extensive washing, total RNA was extracted and cDNA synthesized. RT-PCR analysis was performed to assess expression of secA, cagA, and vacA. The galE (UDP-galactose 4-epimerase gene) was used as an internal control. No RT, no reverse transcriptase. A representative image from five independent experiments.
Acknowledgements
This work was supported by "Cooperative Research Program (Project No. PJ907017042012 and PJ907017022012)”, Rural Development Administration, Republic of Korea.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wiedemann T, Loell E, Mueller S. et al. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS One. 2009;4(3):e4754
2. Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136(6):1863-1873
3. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175-1186
4. Crabtree JE, Xiang Z, Lindley IJD. et al. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48(10):967-969
5. Covacci A, Telford JL, Del Giudice G. et al. Helicobacter pylori virulence and genetic geography. Science. 1999;284(5418):1328-1333
6. Hatakeyama M. Helicobacter pylori CagA-a potential bacterial oncoprotein that functionally mimics the mammalian Gab family of adaptor proteins. Microbes Infect. 2003;5(2):143-150
7. Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin Multifunctionality. Nat Rev Microbiol. 2005;3(4):320-332
8. Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50(2):145-149
9. Juranić Z, Zizak Z. Biological activities of berries: from antioxidant capacity to anti-cancer effects. Biofactors. 2005;23(4):207-211
10. Burger O, Ofek I, Tabak M. et al. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol Med Microbiol. 2000;29(4):295-301
11. Burger O, Weiss E, Sharon N. et al. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr. 2002;42(3 Suppl):279-284
12. Neto CC. Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 2007;137(1 Suppl):186S-193S
13. Zafra-Stone S, Yasmin T, Bagchi M. et al. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51(6):675-683
14. Abdel-Aal el-SM, Young JC, Rabalski I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J Agric Food Chem. 2006;54(13):4696-4704
15. Fiocca R, Necchi V, Sommi P. et al. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicle uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188(2):220-226
16. Feltcher ME, Braunstein M. Emerging themes in SecA2-mediated protein export. Nat Rev Microbiol. 2012;10:779-789
17. Shaffer CL, Gaddy JA, Loh JT. et al. Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathog. 2011;7(9):e1002237
18. Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nat Rev Microbiol. 2009;7(10):703-714
19. Delahay RM, Rugge M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2012(Suppl 1):9-15
20. Ripoll-Rozada J, Peña A, Rivas S. et al. Regulation of the type IV secretion ATPase TrwD by magnesium: implications for the catalytic mechanism of the secretion ATPase superfamily. J Biol Chem. 2012;287(21):17408-17414
21. Molnar B, Galamb O, Sipos F. et al. Molecular pathogenesis of Helicobacter pylori infection: The role of bacterial virulence factors. Dig Dis. 2010;28(4-5):604-608
22. Backert S, Clyne M, Tegtmeyer N. et al. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal. 2011;9(28):1-11
23. Andrzejewska J, Lee SK, Olbermann P. et al. Characterization of the pilin ortholog of the Helicobacter pylori type IV cag pathogenicity apparatus, a surface-associated protein expressed during infection. J Bacteriol. 2006;188(16):5865-5877
24. Pham KT, Weiss E, Jiménez Soto LF. et al. CagI is an essential component of the Helicobacter pylori Cag type IV secretion system and forms a complex with CagL. PLoS ONE. 2012;7(4):e35341
25. Boonjakuakul JK, Canfield DR, Solnick JV. Comparison of Helicobacter pylori virulence gene expression in vitro and in the Rhesus Macaque. Infect Immun. 2005;73(8):4895-4904
26. Kwon DH, Osato MS, Graham DY. et al. Quantitative RT-PCR analysis of multiple genes encoding putative metronidazole nitroreductases from Helicobacter pylori. Int J Antimicrob Agents. 2000;15(1):31-36
Author contact
![]() Corresponding author: Jong Bae Kim, PhD, e-mail: kimjb70ac.kr; phone: +82-33-760-2423; Department of Biomedical Laboratory Science, College of Health Sciences, Yonsei University, Wonju, Gangwon, 220-710, Republic of Korea. Or Woo Duck Seo, PhD; e-mail: swd2002kr; phone: +82-55-350-1166; Department of Functional Crops, National Institute of Crop Science, Rural Development Administration, Miryang, Gyeongnam, 627-803, Republic of Korea.
Corresponding author: Jong Bae Kim, PhD, e-mail: kimjb70ac.kr; phone: +82-33-760-2423; Department of Biomedical Laboratory Science, College of Health Sciences, Yonsei University, Wonju, Gangwon, 220-710, Republic of Korea. Or Woo Duck Seo, PhD; e-mail: swd2002kr; phone: +82-55-350-1166; Department of Functional Crops, National Institute of Crop Science, Rural Development Administration, Miryang, Gyeongnam, 627-803, Republic of Korea.

 Global reach, higher impact
Global reach, higher impact