3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(5):353-360. doi:10.7150/ijms.4276 This issue Cite
Research Paper
Analysis of the Mineral Composition of the Human Calcified Cartilage Zone
Center for Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing, 400038, China
Received 2012-2-24; Accepted 2012-6-28; Published 2012-7-5
Abstract
As the connecting tissue between the hyaline articular cartilage and the subchondral bone, calcified cartilage zone (CCZ) plays a great role in the force transmission and materials diffusion. However, the questions that remain to be resolved are its mineral composition and organization. In this study, 40 healthy human knee specimens were harvested; first the CCZ was dissected and observed by Safranin O/fast green staining, then CCZ chemical characteristics were measured by using amino acid assay and X-ray diffraction. The percentage of dry weight of type II collagen as an organic compound of CCZ was 20.16% ± 0.96%, lower than that of the hyaline cartilage layer (61.39% ± 0.38%); the percentage of dry weight of hydroxyapatite as an inorganic compound was 65.09% ± 2.31%, less than that of subchondral bone (85.78% ± 3.42%). Our study provides the accurate data for the reconstruction of the CCZ in vitro and the elucidation of CCZ structure and function.
Keywords: Calcified Cartilage, Subchondral Bone, Collagen
BACKGROUND
Calcified cartilage zone (CCZ) is a thin interlayer of hard tissue, between the hyaline articular cartilage and the subchondral bone (1). Hyaline cartilage is attached to the subchondral bone by this highly mineralized zone. Mechanical stress and biological stimuli are transmitted from the hyaline cartilage to the subchondral bone through CCZ. It is also limiting the hyaline cartilage to be divided from the subchondral bone (2).
The structure of CCZ plays a main role in its functions. Because of barrier-liked tissue between the hyaline cartilage and the subchondral bone, CCZ can transfer the interstitial fluid to each layer. Articular cartilage is one kind of avascular, alymphatic, and lacks nervous system input tissue. Cell growth and tissue repair within the cartilage is mediated by its hypoxic microenvironment (3). The lower permeability of the CCZ is also crucial for the stabilization of the physiological microenvironment within the hyaline cartilage (4).
Mechanical stress and stimuli are transferred by the CCZ between two different elastic materials, soft tissue (hyaline cartilage) and hard tissue (subchondral bone). The elastic modulus of compliant hyaline cartilage ranges from 1.9 MPa to 15 MPa (5), whereas subchondral bone is approximately 4 Gpa (6). Therefore, when a single incident of trauma to the joint happens, the CCZ is the first fracture site. CCZ collapse is also observed following repetitive injuries, such as overload traumatic osteochondrosis.
Since the connection between cartilage and bone reduces lateral extension, it has limited the development of cracks within the hyaline cartilage adjacent to the calcified cartilage-bone interlayer region (7,8). The stepwise transition from bone to cartilage is potential important for cartilage function and the distribution in CCZ stiffness. It plays as preventing failure of the cartilage-subchondral bone junction. In addition, the remodeling process of the CCZ is very active. This may be a key element in the development of disease, such as osteoarthritis. Therefore, the CCZ is of considerable clinical relevance (9,10).
To study CCZ function, it is necessary to understand its morphological structure and chemical composition. But to date, it has yet to be fully defined and understood. Type II collagen is well defined as the main compound of the hyaline cartilage, but little information about the CCZ is known (11). From previous reports about the calcium content, hydroxyapatiete (HA) seemed as the main dry weight in the calcified zone, accounting for 65-75% (2).
The hypothesis we wanted to test is whether the organic and inorganic compound in the CCZ had significant difference with hyaline cartilage and subchondrol bone. In this study, we investigated in the human biological and mineral composition of the CCZ by Safranin O/fast green staining, amino acid assay, and X-ray diffraction (XRD).
MATERIALS and METHODS
Statement
This study was in compliance with the Helsinki Declaration and was approved by the Ethics Committee of Southwest Hospital.
Preparation of experimental specimens
A total of 40 knee joints from 20 healthy individuals (10 male, 10 female, aged 20-45 y) were provided by the Bone Tissue Engineering Center of Southwest Hospital. These individuals were lost in traffic accidents, without any evidence of metabolic bone diseases in medical records. Osteochondral blocks were separated from the medial femoral condyle. From the part of weight-bearing regions of each knee joint, two osteochondral blocks (0.5 cm × 0.5 cm × 1.0 cm) were cut perpendicular to the articular surface using a bone chisel. Each specimen was examined for pathological conditions, such as cartilage defects or osteoarthritis.
Qualitative analysis of CCZ collagen
Preparation of histological sections: Osteochondral blocks were fixed in 4% neutral formaldehyde for 7 days at 4 °C, decalcified in 4.13% ethylenediaminetetraacetic acid (EDTA) for 14 days at 4 °C and then embedded in paraffin. Ten sections (5 µm) were cut from each paraffin block parallel to the articular surface using an automatic microtome (Vibratome-1500; Vibratome Company, USA).
Safranin O/fast green staining: After deparaffinizing, the sections were stained with hematoxylin for 3 min, and differentiated in 1% acid alcohol for 15s. This step was followed by staining in 0.02% aqueous Fast green for 3 min and counterstaining with 0.1% Safranin O for 3 min. Finally, the sections were dehydrated in an ethanol serial dilution, cleared in xylene, and mounted onto glass slides using neutral gum. Each of the stained sections were observed under light microscope and photographed using a photomicroscope equipped with a CCD video camera.
Type II collagen immunohistochemistry: Serial sections were digested in 0.05% trypsin for 20 min at 37°C, rinsed in distilled water, and soaked in phosphate buffered saline (PBS) for 5 min. Sections were then incubated in 3% H2O2 for 10 min at room temperature, rinsed in distilled water, and soaked in PBS for 5 min. Tissue was blocked in 10% normal goat serum for 15 min at room temperature. After washing, a 1:50 dilution of rabbit anti-human type II collagen antibody was added overnight at 4°C. Sections were rinsed in PBS 3 times for 5 min each and incubated in a 1:100 dilution of FITC (green fluorescence)-labeled goat anti-rabbit IgG secondary antibody for 1-2 h at 37°C. After another 3 rinses in PBS for 5 min each, the sections were dehydrated, cleared, mounted and observed as described above.
Quantitative analysis of CCZ collagen
Separation, collection and identification of the three layers of osteochondral composite tissue: Ten fresh osteochondral blocks were obtained from femoral condyles. First of all, the hyaline cartilage was removed as close to the CCZ as possible as the methods previous reported, and the remaining hyaline cartilage was discarded (14). The quality of hyaline cartilage removal was later determined by Safranin O/ fast green staining. After the hyaline cartilage was removed, the CCZ was rasped and collected with a bone file. Because the CCZ is harder than the subchondral bone, tissue collection stopped when the bone became loose and soft in texture. The quality of CCZ removal was determined histologically as described above. Finally, the subchondral bone was performed with a serial of sections. The tissue samples were stored at -70°C.
Detection of cartilage collagen content by amino acid assay: After each CCZ layer was freeze-dried at a low temperature, 2.0 mg of tissue was placed in a hydrolyzing tube containing 5 ml 6 M HCl. The tube was filled with nitrogen gas for 2 min. After gas evacuation, the tube was sealed, and the tissue was hydrolyzed for 22 h at 110°C. Samples were then placed in a water bath to evaporate the HCl and washed with ultra-pure water. After the samples were air-dried, 2 ml of a Na-S solution was added, and samples were filtered using 0.22-μm microporous membrane filters. Amino acids were detected using a 121-MB amino acid analyzer.
Qualitative analysis of the CCZ inorganic constituents
Von Kossa staining: The CCZ calcium salt content is higher than that of the subchondral bone. When incubated in 4.13% EDTA for 5-7 d, subchondral bone completely decalcifies, whereas CCZ retains calcium salts. After deparaffinizing, longitudinal sections were immersed in a 2% silver nitrate aqueous solution and irradiated under ultraviolet light for 60 min. The sections were then washed three times for 5-10 min each with distilled water. After washing, sections were immersed in a 5% sodium thiosulfate aqueous solution for 2 min and counterstained with 0.5% eosin for 2 min as described previously. Finally, the sections were dehydrated in an alcohol gradient, cleared in xylene, mounted onto glass slides using neutral gum, and observed under the light microscope.
Scanning electron microscopy: Ten fresh specimens were obtained from femoral condyles and sectioned (1 cm × 1 cm × 1 cm) using an osteotome. Sections were ignited and charred with the alcohol burner after freeze-drying at a low temperature to remove organic constituents. The coke remaining from combustion of the superficial hyaline cartilage was scraped off with a scalpel, and the tissue blocks were observed using a scanning electron microscope (XT30 ESEM-TMP).
Quantitative analysis of inorganic constituents using XRD
XRD qualitative analysis: Diffraction analysis was performed on the inorganic constituents of the CCZ and subchondral bone. The samples were prepared as described previously using an XRD-6000 diffractometer (Cu-target Kα X-ray, 40 kV, 30 mA, scanning speed = 4°/min, scanning range = 10°-85°). A PDF standard card (JCPDS 9-432) compiled by The International Centre for Diffraction Data (ICDD) was used to determine the crystal nature of the inorganic composition (13).
XRD quantitative analysis: Analysis was performed as described previously. Aluminum oxide (Al2O3) served as an internal parameter and the chemically synthesized nanometer hydroxyapatite ceramic (HAC) served as a standard for the test phase. X-ray diffraction was performed on a 4:1 (w/w) mixture to calculate the integral areas of two characteristic peaks. The correlation coefficient (Ksj) was calculated using Formula (1). Then, using the test sample to substitute for the standard, the HAC content in the CCZ inorganic constituents of the subchondral bone was calculated using Formula (2).
Basic formula for k-value method:
; (1)
(2)
where Ij = the intensity (integral area) of the characteristic diffraction peak of the test phase j; Is = the intensity (integral area) of the characteristic diffraction peak of the reference; Wj = the mass fraction of the test phase j in the test substance; W'j = the mass fraction of the test phase j in the sample mixture; Ws = the mass fraction of the reference in the sample mixture; and Ksj = the reference intensity (K value) of the test phase j to the reference, namely the correlation coefficient of two substance phases.
Statistical Analysis
All quantitative parameters were subjected to statistical analysis by one way analysis of variance (ANOVA) with the level of significance set to p < 0.05.
RESULTS
Qualitative analysis of CCZ collagen
After Safranin O/fast greenstaining, the CCZ appeared red, and the subchondral bone appeared blue (Fig. 1A). Immunohistochemical analysis showed that CCZ was positive for type II collagen, and the red Safranin O-stained calcified cartilage appeared as a fluorescent green. The subchondral bone was negative for type II collagen, and the blue Fast green stained-tissue was nonfluorescent (Fig. 1B). The results confirmed that the collagen in the CCZ is primarily type II collagen.
Separation, collection and identification of tissues within the cartilage
After the hyaline cartilage layer was removed with a wooden rasp, the remaining tissue blocks were decalcified, sectioned and stained with Safranin O/fast green. This treatment revealed the complete structure of the CCZ and SB (Fig. 1C). Removal of the CCZ was performed using the same method, and histological staining was performed to verify that only the SB remained (Fig. 1D). Our results demonstrated that this extraction method could obtain highly pure CCZ tissues.
Quantitative analysis of CCZ collagen
Based on amino acid assay of the cartilage (Table 1), 19 amino acids accounted for 61.39% ± 0.38% of the articular cartilage dry weight, 20.16% ± 0.96% of the calcification layer and 13.69% ± 0.45% of the subchondral bone. The amino acid content of each layer was significantly different (p<0.01). However, the proportion of Hydroxyproline (Hypro) accounting for the total content of amino acids between the groups (12.62% ± 0.31%) was not different in each layer (Table 1). In addition, the total amino acid content (Hypro accounted for 10-13%) in each tissue layer was similar to the collagen content (11). The Hypro content within each tissue layer was different. Hypro accounted for 4.28% ± 0.04% and 2.83% ± 0.17% of the total amino acid content within the cartilage and calcification layers, respectively, and these layers were characterized as type II collagen. Although Hylys accounted for only 1.31% ± 0.01% of the total amino acid content in the subchondral bone, which layer was characterized as type I collagen.
Qualitative analysis of the CCZ inorganic constituents
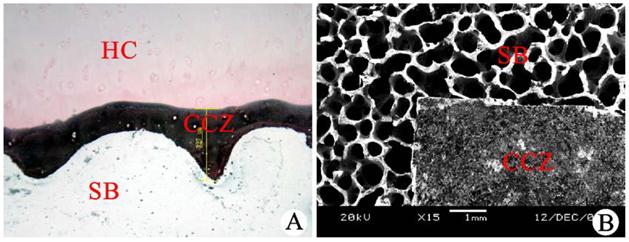
After von Kossa staining of the subchondral bone longitudinal sections, the hyaline cartilage was red, and cartilage cells were observed. The CCZ was stained black, and the margins were clearly observed. These structures were connected to the hyaline cartilage layer via the waveform tidal line structure in the upper margin and were anchored to the subchondral bone via rough and uneven comb-shaped structures in the lower margin. The subchondral bone was not completely stained due to calcification (Fig. 2A). After charring, the CCZ was observed as a dense structure using a scanning electron microscope. The CCZ was covered by the subchondral bone, which was loose and had a porous mesh shape after the CCZ was removed (Fig. 2B). Two independent detection methods confirmed that the CCZ contained inorganic salts.
Quantitative analysis of the CCZ inorganic constituents
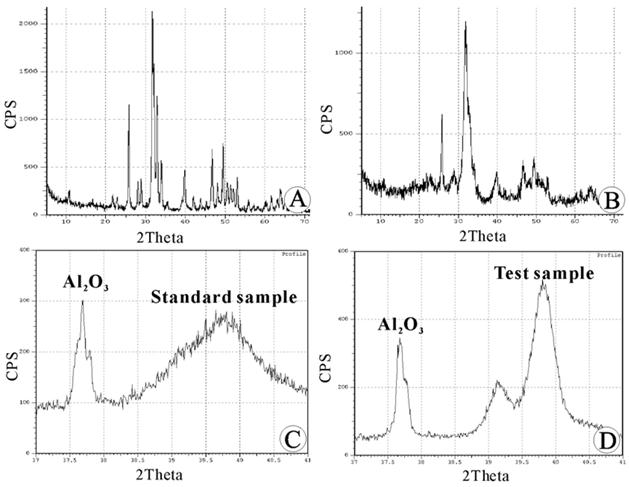
The HAC standard at a Ca/P ratio of 1.67 was used as a reference. X-ray diffraction was used to qualitatively analyze the inorganic constituents of the CCZ. The positions of each diffraction peak corresponded to the PDF standard card (12), suggesting that the CCZ contained HAC crystals. However, in comparison to a synthetic standard sample, the diffraction peaks of the CCZ had a low intensity (approximately 50% of the standard sample) (Fig. 3A, 3B). After scanning A12O3 and the HAC standard sample, the correlative characteristic diffraction peaks were obtained (Fig.3C). The difference in the intensity of the two substance phases was calculated based on the integral area of two characteristic peaks (Ksj =0.94). By substituting the pure HAC separately with the inorganic constituents of the CCZ and the subchondral bone (Fig. 3D); the percent values for the inorganic constituents were calculated (65.09% ± 2.31% and 85.78% ± 3.42%, respectively). The difference in percent composition was statistically significant (P<0.05), and the HAC content was significantly lower in the inorganic composition of the CCZ than that of the subchondral bone.
Total Amino acid assay of cartilage, CCZ and the subchondral bone (n=10, mean ±SD, %).
| Amino acid | Cartilage | CCZ | Subchondral bone |
|---|---|---|---|
| Asp. | 3.86±0.14 | 1.27±0.09 | 0.87±0.05 |
| Thr. | 1.91±0.1 | 0.6±0.04 | 0.35±0.01 |
| Ser. | 2.3±0.05 | 0.81±0.01 | 0.54±0.01 |
| Glu. | 8.07±0.08 | 2.64±0.04 | 1.65±0.03 |
| Pro. | 5.54±0.09 | 1.99±0.11 | 1.28±0.06 |
| Gly. | 5.28±0.1 | 1.94±0.09 | 1.52±0.07 |
| Ala. | 4.39±0.08 | 1.7±0.01 | 1.2±0.02 |
| Cys. | 0.24±0.05 | 0.05±0.01 | 0.05±0.01 |
| Val. | 1.71±0.02 | 0.41±0.16 | 0.48±0.07 |
| Met. | 1.42±0.13 | 0.39±0.02 | 0.27±0.02 |
| Ileu | 1.35±0.06 | 0.38±0.01 | 0.29±0.02 |
| Leu. | 2.87±0.06 | 0.93±0.04 | 0.62±0.02 |
| Tyr. | 1.42±0.1 | 0.35±0.01 | 0.2±0.01 |
| Phe. | 2.16±0.02 | 0.64±0.01 | 0.41±0.01 |
| Lys. | 2.68±0.09 | 0.94±0.07 | 0.67±0.01 |
| His. | 0.76±0.02 | 0.23±0.03 | 0.2±0.01 |
| Arg. | 5.3±0.05 | 1.79±0.14 | 1.16±0.05 |
| Hylys. | 2.63±0.04 | 0.57±0.06 | 0.18±0.01 |
| Hypro. | 7.53±0.17 | 2.55±0.19 | 1.77±0.13 |
| Total | 61.39±0.38 | 20.16±0.96 | 13.69±0.45 |
Hylys and Hypro analysis of hyaline cartilage, CCZ and the subchondral bone (n=10, mean ±SD, %).
| Amino acid | Hyaline Cartilage | CCZ | Subchondral bone |
|---|---|---|---|
| Hylys | 4.28±0.04* | 2.83±0.17* | 1.31±0.01* |
| Hypro | 12.27±0.49 | 12.65±0.36 | 12.93±0.49 |
*Statistical comparison between the groups, p<0.01
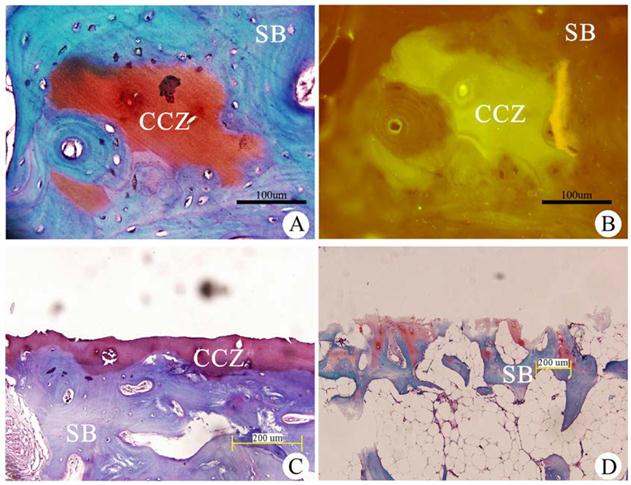
Qualitative analysis of the CCZ collagen and identification of the composition of each cartilage layer using Fast green/Safranin O staining. (A) Fast green/Safranin O staining. (B) Type Ⅱ collagen immunohistochemistry of the calcified cartilage zone (CCZ) and the subchondral bone (SB). Scale bar: 100 µm.(C) After the hyaline cartilage layer was removed, the section contained the CCZ and SB. (D) After the calcified cartilage zone (CCZ) was removed, the section only contained the subchondral bone (SB). Scale bar: 200 µm.
Qualitative analysis of the CCZ inorganic constituents. (A) von Kossa staining of longitudinal subchondral bone (SB) sections (scale bar: 327 µm). (B) Observation of cartilage tissue blocks using a scanning electron microscope (scale bar: 1 mm). HC: hyaline cartilage, CCZ: calcified cartilage zone.
Quantitative analysis of the calcified cartilage zone (CCZ) inorganic constituents using X-ray diffraction (XRD): (A) XRD pattern of the hydroxyapatite ceramic (HAC) standard sample. (B) XRD pattern of the calcified cartilage zone (CCZ) sample. The positions of each diffraction peak corresponded to the standard sample. (C) XRD pattern of the mixture of A12O3 and the HAC standard sample. (D) XRD pattern of the mixture of A12O3 and the sample to be tested.
DISCUSSION
In this study, we performed tissue section staining, immunohistochemistry, amino acid assay and XRD to qualitatively and quantitatively investigate the components of the CCZ. Forty specimens from 20 normal human femoral condyles were involved. The primary organic component of the CCZ was confirmed by type II collagen immunohistochemistry, and that was the same as the the hyaline cartilage. Amino acid assay showed that the amino acid content of the dry tissue was 20.16% ± 0.96%, which was significantly lower than the content of hyaline cartilage layer (61.39% ± 0.38%). Type I collagen was primarily proportion in the subchondral bone, and it accounted for 13.69% ± 0.45% of the total dry weight. Inorganic constituents exist in the CCZ by the von Kossa staining. XRD demonstrated that the main compound of the inorganic constituents was low-crystallizing hydroxyapatite; this substance accounted for 65.09% ± 2.31% of the dry weight, which was significantly lower than the subchondral bone hydroxyapatite content (85.78% ± 3.42%).
Calcified cartilage, the deepest layer of cartilage, was stained red by Safranin O. Therefore, the CCZ extracellular matrix contained type II collagen, similar to the hyaline cartilage. The result was coincidence with a previous study (11). However, the CCZ, in contrast to un-mineralized hyaline cartilage, had a high mineral content and was mechanistically different from hyaline cartilage due to differences in composition. These mechanical differences and the presence of a smooth waveform created an interface between the CCZ and the hyaline cartilage, increasing its vulnerability to shearing forces. This observation is in agreement with reports describing the mature bone-cartilage unit from interface fractures at the tidemark.
The cement line, described as the border between the CCZ and subchondral bone, is the second interface of the calcified cartilage (Figure 1C). Bilateral tissues within the cement line, the CCZ and subchondral bone, contain Ca+ particles. Scanning small-angle X-ray scattering analysis combined with qBEI demonstrated that the size, shape and orientation of the mineral particles were not significantly different (2). The interface between the CCZ and the subchondral bone was very close, almost seamless upon observation of the tissue sections.
It is kind of difficult to fully separate the CCZ, and there was also no standard protocol to describe how to do it yet. The CCZ is adjacent to the hyaline cartilage on one side and the subchondral bone on the other side (14). However, the CCZ and the subchondral bone contain type II and type I collagen, respectively, which can be identified by Safranin O/Fast green staining (CCZ stains red, and the subchondral bone stains blue). The difference in collagen matrices determines the mechanistic characteristics (15).
The extracellular matrix within the hyaline cartilage, CCZ and subchondral bone has particular components. The hyaline cartilage is predominantly made of Type II collagen and proteoglycans and does not contain calcium. The CCZ is primarily composed of calcium crystals and Type II collagen. And the subchondral bone contains Type I collagen and calcium. Therefore, specific staining methods that identify Type I collagen, Type II collagen and calcium are used to identify these layers. The hyaline cartilage and CCZ stain red in response to Safranin O, and the subchondral bone is stained blue by Fast green. Thus, Safranin O/fast green staining can be used to confirm that the dissected CCZ without presence of subchondral bone. In addition, the CCZ stains black, and the hyaline cartilage stains red by von Kossa staining. Von Kossa staining was used to confirm that the dissected CCZ did not contain hyaline cartilage. In our study, the CCZ was stained only red with Safranin O/fast green and was stained only black with von Kossa. The CCZ was stained red with Safranin O/fast green and did not contain any blue stain. After von Kossa staining, the dissected CCZ stained black without any reddish hyaline cartilage (Shown as Figure 2). We verified that the CCZ dissected by mechanical means did not contain hyaline cartilage or subchondral bone.
Composition and structure of tissue determined the function of CCZ (6,16). In order to further understand the structure and function of CCZ and facilitate in vitro construction of the CCZ, it is necessary to study the constituents of the CCZ. In the present study, the CCZ diffraction peaks identified by XRD highly corresponded with the diffraction pattern of the standard (JCPDS 9-432); however, the diffraction peak intensity was approximately half of that of the synthetic standard, demonstrating that the CCZ contained less crystallinity hydroxyapatite (HA). Quantitative analysis showed that minerals accounted for 60% ± 5% of the CCZ dry weight and 80% ± 5% of the subchondral bone dry weight. The CCZ inorganic composition was significantly lower than that of the subchondral bone; however, due to the high density of the CCZ, the inorganic content per unit volume of the CCZ was much higher than that of the subchondral bone. Because the CCZ contains a higher proportion of inorganic constituents, it can act as an isolation zone to divide cartilage and subchondral bone into two microenvironments that contain different amounts of oxygen and nutrients.
The organic constituents of an independently isolated CCZ were well analyzed. The hydroxyproline content was not different between the tissues (Table 1). Each tissue contained a similar amount of collagen and a similar amino acid composition (hydroxyproline accounted for 10%-13% of collagen). Tissues contained different percentages of hydroxylysines, of which the cartilage accounted for 4.28% ± 0.04%, suggesting the presence of type II collagen, which is rich in hydroxylysine. The subchondral bone contained only 1.31% ± 0.01% hydroxylysines, suggestive of type I collagen, which lacks hydroxylysine. The CCZ contained 2.83% ± 0.17% hydroxylysines; therefore, we speculate that CCZ may contain primarily type II collagen or other types of collagen.
Since CCZ was a thin layer but with a hard texture, complete dissection has been difficult. In this study, we utilized a layer-by-layer dissection method. The tissue organization was identified after dissection. However, the preparation inevitably contained some hyaline cartilage or subchondral bone. Therefore, the quantitative analysis was reliable albeit slightly inaccurate. In addition to type II collagen and hydroxyapatite, the CCZ contained other organic or inorganic components, such as collagen X, calcium phosphate, and calcium carbonate (17). Due to this rare content and a lack of appropriate analytical methods, we could not accurately determine its actual content.
CONCLUSION
To summarize, our study provides the foundation for the reconstruction of the CCZ in vitro and the elucidation of CCZ structure and function. The percentage of dry weight of type II collagen as an organic compound of CCZ was 20.16% ± 0.96%, lower than that of the hyaline cartilage layer (61.39% ± 0.38%); the percentage of dry weight of hydroxyapatite as an inorganic compound was 65.09% ± 2.31%, less than that of subchondral bone (85.78% ± 3.42%).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31070865, 31130021) and the Postdoctoral Science Foundation of China (201104756).
COMPETING INTERESTS
The authors have declared that no competing interest exists.
References
1. Koszyca B, Fazzalari NL, Vernon-Roberts B. Calcified cartilage, subchondral and cancellous bone morphometry within the knee of normal subjects. Knee. 1996;3:15-22
2. Zizak I, Roschger P, Paris O, Misof BM, Berzlanovich A. et al. Characteristics of mineral particles in the human bone/cartilage interface. J Struct Biol. 2003;141:208-217
3. Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477-486
4. Arkill KP, Winlove CP. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis and Cartilage. 2008;16:708-714
5. Bader DL, Kempson GE. The short-term compressive properties of adult human articular cartilage. Biomed Mater Eng. 1994;4:245-256
6. Mente PL, Lewis JL. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J Orthop Res. 1994;12:637-647
7. Keinan-Adamsky K, Shinar H, Navon G. The effect of detachment of the articular cartilage from its calcified zone on the cartilage microstructure, assessed by 2H-spectroscopic double quantum filtered MRI. J Orthop Res. 2005;23:109-117
8. Muir P, McCarthy J, Radtke CL. et al. Role of endochondral ossification of articular cartilage and functional adaptation of the subchondral plate in the development of fatigue microcracking of joints. Bone. 2006;38:342-349
9. Ea HK, Nguyen C, Bazin D. et al. Articular cartilage calcification in osteoarthritis: Insights into crystal-induced stress. Arthritis Rheum. 2011;63:10-18
10. Oegema TR, Carpenter RJ, Hofmeister F, Thompson RC. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc Res Tech. 1997;37:324-332
11. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30-35
12. Wang F, Ying Z, Duan X. et al. Histomorphometric analysis of adult articular calcified cartilage zone. J Struct Biol. 2009;168:359-365
13. Blanton TN, Barnes CL. Quantitative analysis of calcium oxide desiccant conversion to calcium hydroxide using x-ray diffraction. International Centre for Diffraction Data, Advances in X-ray Analysis. 2005;48:45-51
14. Lyons TJ, McClure SF, Stoddart RW, McClure J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet Disord. 2006;7:52
15. Gupta HS, Schratter S, Tesch W. et al. Two different correlations between nanoindentation modulus and mineral content in the bone-cartilage interface. J Struct Biol. 2005;149:138-148
16. Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomol Engineer. 2007;24:489-495
17. Lammi PE, Lammi MJ, Hyttinen MM. et al. Site-specific immunostaining for type X collagen in noncalcified articular cartilage of canine stifle knee joint. Bone. 2002;31:690-696
Author contact
![]() Corresponding author: Dr. Liu Yang, Center for Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing, 400038, China. Phone: +86 23 68765280. Fax: +86 23 68765293. E-mail: jointsurgerycom
Corresponding author: Dr. Liu Yang, Center for Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing, 400038, China. Phone: +86 23 68765280. Fax: +86 23 68765293. E-mail: jointsurgerycom

 Global reach, higher impact
Global reach, higher impact