3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(4):262-268. doi:10.7150/ijms.4243 This issue Cite
Research Paper
Keratocystic Odontogenic Tumors: Predictive Factors of Recurrence by Ki-67 and AgNOR Labelling
1. Istanbul University, Dentistry Faculty, Department of Oral and Maxillofacial Surgery, Istanbul, Turkey.
2. Istanbul University, Institute of Oncology, Department of Tumor Pathology & Cytology, Istanbul, Turkey.
Received 2012-2-16; Accepted 2012-5-3; Published 2012-5-23
Abstract
Purpose: The purpose of the present study was to investigate the possible role of Ki-67 and argyrophilic nucleolar organizing regions (AgNOR) between the recurrent and nonrecurrent keratocystic odontogenic tumors (KCOTs). Another aim was to compare the correlation between these two markers.
Materials and Methods: 22 KCOTs were evaluated retrospectively. The actual proliferative activity of the KCOT was measured by Ki-67 labelling index and argyrophilic nucleolar organizing regions AgNOR count per nucleus. Results: Recurrence occurred in 3 patients (13.6%) during the follow-up period (mean follow-up, 37.8 months) The Ki-67 and AgNOR counts were significantly higher in the recurrent lesions comparing to the non-recurrent lesions. (p=0,045; p=0,049) The correlation between Ki-67 and AgNOR counts was found to be positive (r=0,853 p=0,0001).
Conclusion: Within the limit of the present study, it is thought that Ki-67 and AgNOR might be helpful as a prognostic marker for the recurrences of KCOTs. These markers reinforced the meaning of the new classification of the lesion as an odontogenic tumor. Enucleation with curettage or decompression following enucleation with curettage is a simple and appropriate surgical model for the treatment of KCOT despite the relative high recurrence rate. On the other hand, the conservative treatment can be chosen only if there is no coronoid invasion, no interruptive cortical lysis and no tissular invasion.
Keywords: keratocystic odontogenic tumors, Ki-67, AgNOR
Introduction
Keratocystic odontogenic tumor (KCOT) defined by the World Health Organization (WHO), is a benign, intraosseous neoplasm of dental origin, with a characteristic lining of parakeratinized stratified squamous epithelium (1). Increased activity of the epithelium, has been confirmed by previous studies that have compared KCOTs with other odontogenic cysts may explain the high recurrence rates of KCOTs. Some are associated with nevoid basal cell carcinoma syndrome (2).
Immunohistochemical studies have examined KCOTs by using various markers of proliferation and of apoptosis. The proliferative activity of the epithelial lining of KCOTs has been the subject of various investigations by using different markers of proliferation as Ki-67 (2). Ki-67 is the prototypic cell cycle related nuclear protein, expressed by proliferating cells in all phases of the active cell cycle. It rapidly degrades after mitosis with a half-life of detectable antigen being an hour or less (3). Immunohistochemical detection of Ki-67 has been used to evaluate the proliferative potential of healthy cells as well as of preneoplastic and neoplastic lesions (4).
Nucleolar organizer regions (NORs) are loops of DNA which transcribes to ribosomal RNA. The NOR-related protein becomes visible in nucleus by a silver-staining technique under a light microscope, and it has been named argyrophilic protein of NOR (Ag-NOR) (5). Several different methods have been proposed to determine the proliferative rate in tumors. Silver staining of AgNORs is considered to be the best and most cost-effective marker to assess the proliferative behaviour of a lesion. The rapidity of the cell turnover is evaluated by speed of the cell cycle, so as to assess the growth rate of the lesion, which is easily assessed by AgNOR count per nucleus. The amount of AgNOR represents a cell kinetics parameter and can be used for prognostic purposes. AgNOR counts have been demonstrated to offer a predictive index in various malignancies (6,7).
Pathologists' interest in AgNOR proteins increased greatly around the end of 1980s following the observation that malignant cells frequently exhibit a greater AgNOR protein amount as compared with the corresponding benign or normal cells. AgNOR method has become widespread among pathologists, in various fields of tumour pathology (8).
The objective of the present study was to investigate clinical behavior of KCOTs regarding the recurrence, by evaluating Ki-67 and AgNOR staining. Another purpose was to investigate the correlation between these two markers and to demonstrate their possible prognostic role in these lesions.
Material and Methods
Tissue Samples
In the present study, KCOTs treated by two oral and maxillofacial surgeons from January 2004 to August 2010, were reviewed retrospectively. Clinical and histological information was recorded for each patient. The inclusion criteria of the study was the histopathological diagnosis of ''KCOT''. The cases which were previously treated in another service were excluded. The cases followed-up shorter than 1 year and which were associated with nevoid basal cell carcinoma syndrome (NBCCS) were also excluded from the study. The histopathological diagnosis was based on the criteria showing parakeratinization of the lining epithelium as described by WHO guidelines (1).
According to these criteria, a total of 22 histopathologically diagnosed KCOTs were selected for the study. Routinely panoramic radiographs were used before the operation. CT scans were also used according to the clinical features (size, anatomical location) of the tumor. The data included; age at diagnosis, gender of the patients, location of the lesion, clinical manifestation (pain, swelling, infection) treatment modality and recurrence (Table 1).
All subjects were evaluated clinically and radiographically at regular times. Panoramic radiographs were taken at 6 and 12 in the first post-operative year followed by once every year. The average follow-up period was 37.8 months.
Immunohistochemical staining
For immunohistochemistry, the parafin blocks were cut serially into approximately 5 μm thick sections on charged slides. Firstly, the sections were penetrated and dried overnight in an autoclave (56 0C). They were deparaffinized with xylene for 30 min, washed with 99% alcohol for 15 minutes, and then with 96% alcohol and distilled water. Histostain-Plus Bulk Kit (Zymed 2nd Generation, LAB-SA Detection System, 85-9043) was used in this study. For antigen retrieval, the sections were microwaved four times for 5 min in citrate buffer (Ph 6.0). Endogenous peroxidase activity was blocked by incubating the sections with 3% H2O2 and washed with distilled water and waited in PBS for 5 min. To prevent non-specific reactions, sections were incubated with block solution. Ki-67 with a dilution ratio of 1:50 (Zymed Laboratories, Mouse, Monoclonal, Clone 7B11) was used as the primary antibody. Slides were incubated with Ki-67 for 120 min. The secondary antibody was reacted for 25 min. AEC (Zymed Laboratories, 00-2007, Lot No:319293A) chromogen was used to visualize the reaction. Finally, the sections were counterstained with Mayer's hematoxylin, coverslipped and evaluated by a light microscope.
AgNOR staining
For AgNOR staining, the paraffin blocks were cut into approximately 5 μm thick sections. The sections were deparaffinised with xylene for 30 minutes, and washed with 99% alcohol for 15 minutes, then 96% alcohol and distilled water. The slides were immersed in a citric acid solution/ethanol solution (1:3). The 50% silver nitrate solution was mixed in 2 g/dL gelatin solution dissolved in 1g/dL formic acid in a 1:2 proportion and the sections were incubated at a dark room for 30 min. After washing off the distilled water, the sections were dehydrated with ethanol, cleared with xylene, and coverslipped and evaluated by a light microscope.
Patient demographics and characteristics of the lesions with Kİ-67 and Ag NOR.
| Gender | Age | Location | Follow-up (months) | Treatment method | Recurrence | Kİ-67 | AgNOR | |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 68 | Mandible anterior | 34 | decompression and enucleation with curettage | - | 4 | 3,60 |
| 2 | M | 25 | Mandible posterior | 17 | enucleation with curettage | - | 3,5 | 2,70 |
| 3 | M | 55 | Mandible posterior | 16 | enucleation with curettage | - | 3 | 3,30 |
| 4 | M | 58 | Mandible premolar and anterior | 46 | enucleation with curettage | - | 2,8 | 2,20 |
| 5 | M | 65 | Mandible premolar and posterior | 65 | enucleation with curettage | - | 2,3 | 3,10 |
| 6 | F | 51 | Maxilla premolar and anterior | 41 | enucleation with curettage | - | 4 | 3,51 |
| 7 | F | 32 | Mandible posterior, ramus | 29 | decompression and enucleation with curettage | + | 6 | 4,50 |
| 8 | F | 32 | Mandible posterior | 41 | enucleation with curettage | - | 4 | 3,60 |
| 9 | M | 50 | Mandible anterior | 38 | enucleation with curettage | - | 5,5 | 4,60 |
| 10 | M | 50 | Mandible premolar and anterior | 38 | enucleation with curettage | - | 4 | 3,70 |
| 11 | M | 37 | Maxilla, posterior, premolar, anterior | 37 | enucleation with curettage | + | 4,5 | 4,00 |
| 12 | F | 69 | Mandible premolar | 41 | enucleation with curettage | - | 4 | 3,90 |
| 13 | M | 24 | Maxilla premolar, posterior | 64 | enucleation with curettage | + | 4 | 4,10 |
| 14 | M | 50 | Mandible posterior | 19 | enucleation with curettage | - | 4 | 3,80 |
| 15 | F | 40 | Mandible posterior | 20 | enucleation with curettage | - | 4,5 | 4,30 |
| 16 | M | 62 | Mandible premolar | 12 | enucleation with curettage | - | 4,5 | 4,40 |
| 17 | M | 56 | Mandible premolar | 72 | enucleation with curettage | - | 5 | 4,45 |
| 18 | M | 53 | Maxilla, posterior, premolar, anterior | 53 | enucleation with curettage | - | 2,5 | 3,20 |
| 19 | M | 39 | Mandible posterior, premolar, | 38 | enucleation with curettage | - | 3 | 3,33 |
| 20 | F | 49 | Mandible posterior | 39 | enucleation with curettage | - | 3 | 4,50 |
| 21 | M | 58 | Mandible posterior | 38 | enucleation with curettage | - | 3 | 3,70 |
| 22 | F | 21 | Maxilla anterior | 34 | enucleation with curettage | - | 3,5 | 3,30 |
Evaluation Methods
Ki-67 immunostained slides were examined at 400x magnification in Olympus BX60 microscope. In epithelium, the positive and negative cells were counted in 5 contiguous and consecutive microscopic high-power fields. The number of positive cells was divided into the total number of cells counted in the whole layer. The result was multiplied by 100 to find the percentage of positive cells. AgNOR-stained slides were examined at 1000x magnification with immersion oil in Olympus BX60 microscope. The number of AgNORs in the nucleus was counted in 250 cells for each case. Black dots/aggregated clusters within cellular nucleoli were counted as one dot. The average number of AgNORs was divided into total number of cells.
Statistical analysis
Statistical analysis was aided by the NCSS (Number Cruncher Statistical System) 2007 Statistical Software (Utah, USA). Chi-square test and Fisher's exact test were used to assess qualitative parameters. Mann-Whitney-U test was used to evaluate descriptive statistics (median, interquartile range) (SD) and differences between groups. Probabilities of less than 0.05 were accepted as significant.
Results
The ages of recurrent group members were found to be statistically lower than the non reurrent group members (p=0,035). No statistically significant difference was observed between the recurrent and non recurrent groups regarding the follow-up. (p=0,472). Ki-67 values of recurrent group were found to be statistically higher than the non-recurrent group. (p=0,045). AgNOR values of recurrent group were found to be statistically higher than the non-recurrent group (p=0,049) (Table 2).
Comparison of recurrent and non recurrent lesions.
| Non recurrent | Recurrent | MW | p | |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| Age | 51 (40-58) | 32 (24-37) | 6,5 | 0,035 |
| Follow-up | 38 (34-50) | 42 (37-64) | 21 | 0,472 |
| Kİ-67 | 3,5 (3-4) | 4,5 (4-6) | 8 | 0,045 |
| AgNOR | 3,6 (3,3-3,9) | 4,1 (4-5,5) | 8 | 0,049 |
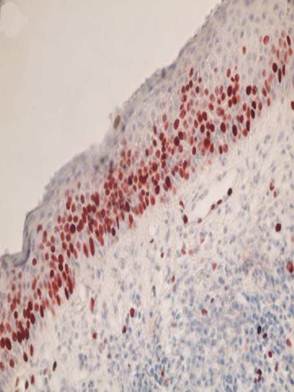
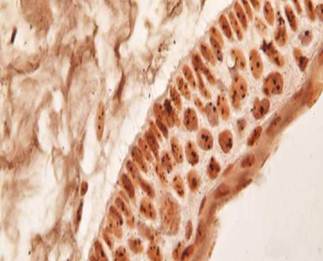
Most of the Ki- 67 cells being detected in the suprabasal layers (Figure 1) The AgNOR dots were higher in the nucleus of subrabasal cells than basal cells in KCOT (Figure 2).
No statistically significant difference was observed between the recurrent and non recurrent groups regarding the gender (p=0,952).
No statistically significant difference was observed between Kİ-67 and AgNOR regarding the age range.
Statistically significant positive correlation was found between Kİ-67 and AgNOR values. (r=0,853 p=0,0001). (Table 3).
Representative photomicrograph of keratocystic odontogenic tumor stained with Ki-67. Immunoreactivity was observed particularly in the suprabasal cell layers of lining epithelium. (x400)
Representative photomicrograph of argyrophilic nucleolar organizing regions (AgNOR) staining. The AgNOR dots were higher in the nucleus of subrabasal cells than basal cells in keratocystic odontogenic tumour. (x1000)
The correlation between AgNOR and Kİ-6.
| Kİ-67 | ||
|---|---|---|
| AgNOR | r | 0,853 |
| p | 0,0001 |
Discussion
In our study, no statistically significant difference was observed between the recurrent and non recurrent groups regarding the gender; although some authors consider that gender could play an important role in the recurrence rate (9,10). Others consider that the gender is not a significant determinant of recurrence (11,12,13).
The ages of recurrent group members were found to be statistically lower than the non-recurrent group members (p=0,035). This situation was similar to that found by Forssell et al and Gonzalez-Alva et al. who reported a higher recurrence rate in young patients (14, 15). This situation may be associated that younger patients often receive more conservative approach such as preserving the associated teeth as presented in our study.
Conservative and aggressive approaches have been reported about the management of KCOT. The choice of the treatment must take into account the patient's age, size of the lesion, previous recurrence history, soft tissue involvement and histological characteristics (16). In the conservative method, simple enucleation with or without curettage, marsupialization and decompression are suggested. Aggressive methods include; peripheral ostectomy, chemical curettage with Carnoy's solution, and periost, tissue or bone radical resection (17, 18). The treatment of KCOTs is still controversial. A recent Cochrane review demonstrated that there is a need for well conducted randomized controlled clinical trials about the management of the KCOTs (19).
The recurrence rates of reported KCOTs tend to vary from 0% to 100% (2, 18, 20, 21). These marked discrepancies are thought to be related to the different lengths of postoperative follow-up periods, operative techniques employed or inclusion of cases with nevoid basal cell carcinoma syndrome (NBCCS). Tumors also vary in their aggressiveness, contributing to variation in recurrence patterns (18). Recurrence rate is highest with the simple enucleation (without curettage) and range from 9% and 62.5% (22). Resection offers a high cure rate, but produces significant morbidity such as the loss of the jaw continuity or facial disfigurement. It should therefore be reserved only for aggressive or recurrent lesions, or for patients who cannot be closely followed up after conservative treatments (22). In our study, all the cases were treated with enucleation with curettage as reported Boffano et al (23). A surgical burr was used to remove about 2 mm of cortical bone all around the remained bone after enucleation. Only in 2 cases decompression was performed before the enucleation with curettage. The recurrence rate was 13.6%; as similar with the reports which showed the enucleation and curettage as a treatment of KCOT, as presented in our study (18, 23). On the other hand the recurrence rate in our study was found to be as rather low compared with previous reports which demonstrated the simple enucleation method as a treatment of KCOT (24,25,26, 27).
The patients are still under periodic observation in our institution. On the other it has been reported that KCOT may recur even after 6 to 25 years after enucleation (25). Therefore it is not possible to draw any long-term conclusions on recurrence rates regarding the mean follow-up time (37.8 months) of the present study.
It has been reported that KCOTs which were located in the mandibular molar region had significantly higher recurrence rates than those in other sites. Difficulty in removing all the traces of the epithelial lining is believed to be a major factor leading to recurrence (17). In our study, only one of the recurrent lesion was observed in the mandible (no 7 in table 1). The lesion size was more than 5 cm and located in the posterior part of the mandible, raising up to the condylar process. After 8 months of decompression the tumor was enucleated with curettage. 11 months after enucleation, the lesion recurred and treated again within the same conservative approach. The recurrence was associated with the difficult surgical access, large size and also biological aggressive behaviour of the lesion itself which was demonstrated with the highest Ki67 label index and AgNORs counts. It was not possible to remove the tumour in one piece. The other two recurrent lesions were seen in the maxilla. The lesions sizes were more than 4 cm and the lesions were removed in one piece. The sizes of the non-recurrent tumors vary from 1 to 5 cm. The recurrence may be explained with the conservative treatment (root resection) of the associated teeth. Extraction of teeth has been kept to a minimum. The patients denied multiple teeth extractions and accepted the risk of recurrence. Therefore it is thought that some tumor remnants which were associated with the teeth may cause the recurrence as reported Pogrel (28).
KCOTs arise from odontogenic epithelial cells and remnants of the dental lamina, and also from extensions of basal cells of the oral epithelium. Epithelial cells in KCOTs seem to have a different proliferative potential from those of other odontogenic lesions (4).
Ki-67 antibodies are useful in establishing the cell growing fraction in neoplasms. Ki-67 expression has been shown to be higher in the epithelium of KCOTs when compared with developmental and inflammatory cysts, with most of the Ki- 67 cells being detected in the suprabasal layers, as reported in our study (2, 3, 29).
Limited numbers of the studies were published about the evaluation of AgNORs in odontogenic cysts and tumors. Conflicting results have been observed in these studies (6, 30,31,32,33,34). Coleman et al. investigated whether AgNORs may be of value in distinguishing various odontogenic cysts from the unicystic ameloblastoma. They concluded that AgNOR counts are not of diagnostic significance and can not be used to distinguish the various odontogenic cysts from one another nor from the unicystic ameloblastoma (30). Allison and Spencer performed AgNOR counts on apical periodontal cysts, dentigerous cysts, odontogenic keratocysts, ameloblastomas and basal cell carcinomas. They reported significant differences between KCOT and apical periodontal cyst. In that study the mean AgNOR counts for all odontogenic cysts ranged between 2.02 and 2.65, and for ameloblastomas were 2.24. According the results they concluded that the method had neither a diagnostic nor a prognostic value in these lesions (34). In our study, the AgNOR dots were higher in the nucleus of subrabasal cells than basal cells in KCOT. The mean values of AgNORs were found higher regarding the count range reported in the literature (6, 32, 34).
The differences in the literature may be associated with the differences in staining method and counting protocol for AgNOR and because of the difference in simple size. Therefore we are agree with Gadbail et al and Eslami et al who recommended a standard protocol for AgNOR staining and counting protocol (6, 32).
There are several confounding factors for the analysis of relationship between recurrence of KCOT and clinicopathologic and immunohistochemical variables. There is no doubt that the surgical management is the most influential factor for the recurrences following any surgical interventions. However, it has not been studied for a group undergoing a single surgical procedure for KCOT to reduce the influence of confounding factors. Furthermore, little is known about any relationship between expression of cell proliferative markers and the recurrence in the KCOT in terms of recurrent risks for time dependent variables (18). The results of our study demonstrated higher expression of Ki-67 and AgNOR count in recurrent lesions comparing to non-recurrent lesion. On the other hand, Li et al, reported that no significant difference between simple (non-recurrent) and recurrent lesions regarding the expression of Ki-67 expression (35).
The limitation of this study is that only two markers have been evaluated. On the other hand, important point of this study is the demonstration of the positive correlation in between Ki-67 and AgNORs in suprabasal cell layers of KCOTs. This significant positive correlation was also reported by Gadbail et al (6).
Another limitation is the low sample size of the recurrent lesions. In our study the recurrence was present only in 3 out of 22 subjects. Similarly, Kuroyanagi et al, (18) reported 4 recurred lesions among 32 subjects diagnosed with KCOT. They reported that Ki-67 was found higher in the recurrent group and they suggested that this marker can be recommended as a prognostic factor. Our results are consisting with Kuroyanagi et al. (18) regarding the expression of Ki-67. Additionally, according to our results, we recommended that AgNOR might be useful as a prognostic marker in KCOTS.
Conclusion
Ki-67 and AgNOR might be useful as a prognostic marker in KCOTs. A standard protocol needed for the evaluation of AgNORs. The positive correlation of these markers reinforced the neoplastic character of the KCOT. The higher expression of these markers in recurrent lesions is important in order to consider additional surgical interventions to improve prognosis.
Acknowledgements
The authors thank to “ARK Biostatistical Office” for the statistical analyses of the present study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Philipsen HP. Keratocystic odontogenic tumour. In: (ed.) Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours: pathology and genetic of head and neck tumours. Lyon: IARC Press. 2005:306-7
2. Mendes RA, Carvalho JF, van der Waal I. A comparative immunohistochemical analysis of COX-2, p53, and Ki-67 expression in keratocystic odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:333-9
3. Ayoub MS, Baghdadi HM, El-Kholy M. Immunohistochemical detection of laminin-1 and Ki-67 in radicular cysts and keratocystic odontogenic tumors. BMC Clin Pathol. 2011;11:4
4. de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant'Ana Filho M. Odontogenic epithelium: immunolabeling of Ki-67, EGFR and survivin in pericoronal follicles, dentigerous cysts and keratocystic odontogenic tumors. Head Neck Pathol. 2011;5:1-7
5. Nakamura S, Takeda Y, Okabe Y, Yoshida T, Ohtake S, Kobayashi K, Kanno M, Matsuda T. Argyrophilic proteins of the nucleolar organizer region in acute leukemias and its relation to the S-phase fraction of leukemic cells. Acta Haematol. 1992;87(1-2):6-10
6. Gadbail AR, Chaudhary M, Patil S, Gawande M. Actual Proliferating Index and p53 protein expression as prognostic marker in odontogenic cysts. Oral Dis. 2009;15:490-8
7. Pillai KR, Sujathan K, Madhavan J, Abraham EK. Significance of silver-stained nucleolar organizer regions in early diagnosis and prognosis of oral squamous cell carcinoma: a multivariate analysis. In Vivo. 2005;19:807-12
8. Trerè D. AgNOR staining and quantification. Micron. 2000;31:127-31
9. Morgan TA, Burton CC, Qian F. A retrospective review of treatment of the odontogenic keratocyst. J Oral Maxillofac Surg. 2005;63:635-639
10. Ahlfors E, Larsson A, Sjogren S (1984) The odontogenic keratocysts. a benign cystic tumor? J Oral Maxillofac Surg. 1984;42:10-19
11. Habibi A, Saghravanian N, Habibi M, Mellati E, Habibi M. Keratocystic odontogenic tumor: a 10-year retrospective study of 83 cases in an Iranian population. J Oral Sci. 2007;49:229-235
12. Myoung H, Hong SP, Hong SD, Lee JL, Lim CY, Choung PH, Lee JH, Choi JY, Seo BM, Kim MJ. Odontogenic keratocyst: review of 256 cases for recurrence and clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:328- 333
13. Anand VK, Arrowood JP Jr, Krolls SO. Odontogenic keratocysts: a study of 50 patients. Laryngoscope. 1995;105:14-16
14. Forssell K, Forssell H, Kahnberg KE. Recurrence of keratocysts. A long-term follow-up study. Int J Oral Maxillofac Surg. 1988;17:25-28
15. González-Alva P, Tanaka A, Oku Y, Yoshizawa D, Itoh S, Sakashita H, Ide F, Tajima Y, Kusama K. Keratocystic odontogenic tumor: a retrospective study of 183 cases. J Oral Sci. 2008;50:205-12
16. Tolstunov L, Treasure T. Surgical treatment algorithm for odontogenic keratocyst: combined treatment of odontogenic keratocyst and mandibular defect with marsupialization, enucleation, iliac crest bone graft, and dental implants. J Oral Maxillofac Surg. 2008;66:1025-36
17. Hyun HK, Hong SD, Kim JW. Recurrent keratocystic odontogenic tumor in the mandible: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(2):e7-10
18. Kuroyanagi N, Sakuma H, Miyabe S, Machida J, Kaetsu A, Yokoi M, Maeda H, Warnakulasuriya S, Nagao T, Shimozato K. Prognostic factors for keratocystic odontogenic tumor (odontogenic keratocyst): analysis of clinico-pathologic and immunohistochemical findings in cysts treated by enucleation. J Oral Pathol Med. 2009;38:386-92
19. Sharif FNj, Oliver R, Sweet C, Sharif MO. Interventions for the treatment of keratocystic odontogenic tumours (KCOT, odontogenic keratocysts (OKC). Cochrane Database Syst Rev. 2010;8(9):CD008464
20. Zecha JA, Mendes RA, Lindeboom VB, van der Waal I. Recurrence rate of keratocystic odontogenic tumor after conservative surgical treatment without adjunctive therapies - A 35- year single institution experience. Oral Oncol. 2010;46:740-2
21. Mendes RA, Carvalho JF, van der Waal I. Characterization and management of the keratocystic odontogenic tumor in relation to its histopathological and biological features. Oral Oncol. 2010;46:219-25
22. Kukreja P, Godhi SS. Keratocystic odontogenic tumor: A review. J Maxillofac Oral Surg. 2009;8:127-131
23. Boffano P, Ruga E, Gallesio C. Keratocystic odontogenic tumor (odontogenic keratocyst): preliminary retrospective review of epidemiologic, clinical, and radiologic features of 261 lesions from University of Turin. J Oral Maxillofac Surg. 2010;68:2994-9
24. T.A. Morgan, C.C. Burton and F. Qian, A retrospective review of treatment of the odontogenic keratocyst. J Oral Maxillofac Surg. 2005;63:635-639
25. Stoelinga PJW. Long term follow up on keratocysts treated according to a defined protocol. Int J Oral Maxillofac Surg. 2001;30:14-25
26. Teronen O, Hietanen J, Lindqvist C. et al. Mast cell derived tryptase in odontogenic cysts. J Oral Pathol Med. 1996;25:376-381
27. Browne RM. The odontogenic keratocyst: Clinical aspects. Br Dent J. 1970;128:225-231
28. Pogrel MA. Treatment of keratocysts. The case for decompression and marsupialization. J Oral Maxillofac Surg. 2005;63:1667-73
29. Gurgel CA, Ramos EA, Azevedo RA, Sarmento VA, da Silva Carvalho AM, dos Santos JN. Expression of Ki-67, p53 and p63 proteins in keratocyst odontogenic tumours: an immunohistochemical study. J Mol Histol. 2008;39(3):311-6
30. Tsuneki M, Yamazaki M, Cheng J, Maruyama S, Kobayashi T, Saku T. Combined immunohistochemistry for the differential diagnosis of cystic jaw lesions: its practical use in surgical pathology. Histopathology. 2010;57(6):806-13
31. Savithri V, Sudha S, Shameena PM, Varghese I. A clinicopathologic study of odontogenic keratocyst and the role of AgNORs in cell proliferation. Oral and Maxillofac Pathol J. 2010;1:1
32. Eslami B, Yaghmaei M, Firoozi M, Saffar AS. Nucleolar organizer regions in selected odontogenic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(2):187-92
33. Coleman HG, Altini M, Groeneveld HT. Nucleolar organizer regions (AgNORs) in odontogenic cysts and ameloblastomas. J Oral Pathol Med. 1996;25(8):436-40
34. Allison RT, Spencer S. Nucleolar organiser regions in odontogenic cysts and ameloblastomas. Br J Biomed Sci. 1993;50(4):309-12
35. Li TJ, Browne RM, Matthews JB. Epithelial cell proliferation in odontogenic keratocysts: a comparative immunocytochemical study of Ki67 in simple, recurrent and basal cell naevus syndrome (BCNS)-associated lesions. J Oral Pathol Med. 1995;24(5):221-6
Author contact
![]() Corresponding author: Firat Selvi, D.D.S Ph.D. Istanbul University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Istanbul, Turkey. E-mail: fselviedu.tr; GSM: 0090 532 771 06 41.
Corresponding author: Firat Selvi, D.D.S Ph.D. Istanbul University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Istanbul, Turkey. E-mail: fselviedu.tr; GSM: 0090 532 771 06 41.

 Global reach, higher impact
Global reach, higher impact