3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(5):406-412. doi:10.7150/ijms.8.406 This issue Cite
Research Paper
Effects of Bolus Injection of 5-Fluorouracil on Steady-State Plasma Concentrations of 5-Fluorouracil in Japanese Patients with Advanced Colorectal Cancer
1. Kinki University Nara Hospital, Nara 630-0293, Japan
2. Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
3. School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women's University, Nishinomiya 663-8179, Japan
4. Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto 606-8501, Japan
5. Department of Clinical Pharmacy, Kyoto Pharmaceutical University, Kyoto 607-8414, Japan
Received 2011-4-15; Accepted 2011-6-24; Published 2011-7-1
Abstract
Objectives: The irinotecan (CPT-11) + 5-fluorouracil (5-FU)/leucovorin (LV) + UFT/LV chemotherapy, in which repetitive oral administration of UFT/LV replaces the infusion of 5-FU/LV in the FOLFIRI regimen, has been proposed previously. In this study, five of 10 patients were injected with a bolus of 5-FU and the other were not injected with it in order to examine the effect of omitting it in terms of pharmacokinetics of 5-FU.
Methods: The treatment consisted of the intravenous infusions of CPT-11 at 100 mg/m2 and l-LV at 15 mg/m2, and the injection of a bolus of 5-FU at 500 mg/m2 on day 1, and the repetitive oral administration of UFT/LV (300 mg/m2/day as tegafur + 75 mg/day of LV) on days 1-5. A total of 13 measurements of the plasma concentrations of uracil, 5-FU and tegafur were made per patient within 48 hr after the start of chemotherapy and the value of area under the concentration-time curve (AUC0-48) was evaluated. The plasma concentration was also determined at 2 weeks to assess long-term exposure to 5-FU.
Results: The plasma concentrations of 5-FU at 24 hr after the start of treatment were 27.4 ng/mL and 9.4 ng/mL in the patients with and without the bolus injection, respectively. At 48 hr, they were 31.3 ng/mL and 10.4 ng/mL with the AUC0-48 values of 22.16 mg*h/L and 0.65 mg*h/L, respectively. The 5-FU was detected in the plasma at 226 hr after the last administration of UFT/LV for the patients with the bolus injection, but not for those without.
Conclusion: A bolus of 5-FU on day 1 provided long-term exposure to 5-FU.
Keywords: 5-fluorouracil, UFT, bolus injection, constant infusion, pharmacokinetics
Introduction
The treatment of metastatic colorectal cancer has progressed significantly after developments of irinotecan (CPT-11) and oxaliplatin (L-OHP). Currently, the FOLFIRI or FOLFOX regimen with or without a targeted monoclonal antibody, is the standard treatment [1-4], and future improvements will likely require the incorporation of or substitution with a novel anticancer drug, personalization based on genetic profiling, or pharmacokinetically-guided administration.
The oral fluoropyrimidine UFT and capecitabine have been developed to improve tolerability and patient convenience, and have replaced continuous infusion of 5-FU in many treatment regimens [5]. UFT is a combination of tegafur, an oral prodrug of 5-FU, and uracil in a molar ratio of 1: 4. Tegafur produces a constant reserve of 5-FU and its active metabolites, and provides pharmacokinetics equivalent to the constant infusion of 5-FU. Uracil is an endogenous substrate for dihydropyrimidine dehydrogenase, the main enzyme responsible for the degradation of 5-FU, and enhances the anticancer effects of 5-FU. In reports published in 2002, UFT with LV proved comparable with bolus 5-FU/LV-based regimens in two multicenter, randomized phase III trials [6,7]. Subsequently, a number of phase II clinical trials have demonstrated the efficacy and tolerability of UFT in combination with CPT-11 or L-OHP in the first-line treatment of metastatic colorectal cancer [8-14]. The novel regimens have been referred to as TEGAFIRI and TEGAFOX, respectively.
Previously, the phase I study on the CPT-11 + 5-FU/LV + UFT/LV chemotherapy, in which repetitive oral administration of UFT/LV (300 mg/m2/day as tegafur + 75 mg/day of LV) replaces the infusion of 5-FU/LV (2,400 mg/m2/46h of 5-FU + 400 mg/m2/2h of LV) in the FOLFIRI regimen, has been conducted in the patients with advanced colorectal cancer [15]. The FOLFIRI regimen consists of a bolus injection of 5-FU, CPT-11 and infusion of 5-FU/LV, and recently critical evaluations have been performed with regard to the necessity of a bolus injection of 5-FU [1-4]. In this study, the effect of a bolus of 5-FU on its steady-state pharmacokinetics was examined in the CPT-11 + 5-FU/LV + UFT/LV chemotherapy.
Patients and Methods
Eligibility
Ten patients were enrolled from September 2004 to May 2006 in this pharmacokinetic study. All patients had histologically or cytologically confirmed advanced or metastatic colorectal adenocarcinoma. Patients had received no prior chemotherapy or only one regimen of previous chemotherapy (with a washout period of more than 4 weeks after the final day of the previous treatment). Adjuvant chemotherapy performed more than 6 months previously was not counted as previous treatment. Further eligibility criteria included: 1) age of 20-75 years; 2) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; 3) life expectancy of 3 months or more; 4) adequate hematological (absolute leukocyte count: 4,000/mm3-12,000/mm3, neutrophil count: 2,000/mm3 or more, platelets: 100,000/mm3 or more), hepatic (transaminases: 2.5 times or less of the upper limit of normal, serum bilirubin: 2.0 mg/dL or less) and renal (serum creatinine: less than the upper limit of normal) function; and 5) ability to take oral medication. Patients were excluded, if they had either brain metastases, a history of other neoplasms (except cured nonmelanoma skin carcinoma or cured carcinoma in situ), a history of severe drug allergy, interstitial pneumonitis or pulmonary fibrosis, severe pleural effusion or ascites, active infection, bowel obstruction, diarrhea, and serious uncontrolled comorbidity or medical conditions. Pregnant or lactating women or women not using an effective contraception were also excluded. This study was conducted at Kobe University Hospital, Japan. The institutional review board of the institute reviewed and approved the protocol before commencement of the study. Written informed consent was obtained from all patients in advance.
CPT-11 + 5-FU/LV + UFT/LV chemotherapy
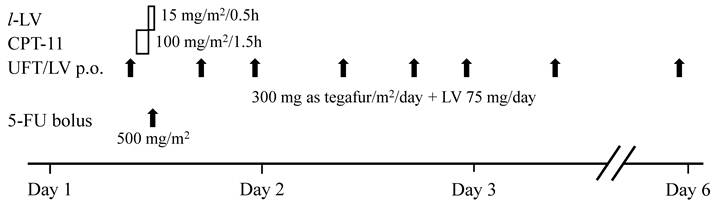
The protocol is presented in Figure 1. The treatment consisted of the intravenous infusion of CPT-11 at 100 mg/m2 for 90 min, the subsequent infusion of l-LV at 15 mg/m2 for 30 min, and the injection of a bolus of 5-FU at 500 mg/m2 immediately after completion of the l-LV infusion on day 1, and the repetitive oral administration of UFT/LV (300 mg/m2/day as tegafur + 75 mg/day of LV) on days 1-5. Five of 10 patients were not injected with a bolus of 5-FU, resulting in the reduction of dose (equivalent to 5-FU) from 1474.8 mg/ m2 to 974.8 mg/m2. The daily dosage of UFT/LV was divided into 3 doses and administered at either 1 hr before or 1 hr after food intake. The first dose of UFT/LV was given at 9:00 on day 1, 30 min before the infusion of CPT-11, and the second and third doses were administered at 17:00 and 23:00, respectively. It is noted that previously conducted phase I study resulted in the recommendation of 7 days treatment for repetitive oral administration of UFT/LV and a dose of 150 mg/m2 for CPT-11 [15].
Protocol of the CPT-11 + 5-FU/LV + UFT/LV chemotherapy. The treatment consisted of the intravenous infusion of CPT-11 at 100 mg/m2 for 90 min, the subsequent infusion of l-LV at 15 mg/m2 for 30 min, and the injection of a bolus of 5-FU at 500 mg/m2 immediately after completion of the l-LV infusion on day 1, and the repetitive oral administration of UFT/LV (300 mg/m2/day as tegafur + 75 mg/day of LV) on days 1-5. The daily dosage of UFT/LV was divided into 3 doses and administered at either 1 hr before or 1 hr after food intake.
Determination of Plasma Concentration of 5-FU
Aliquots of blood (5 mL) were collected into etylenediaminetetraacetic acid-treated tubes at 9:00 (prior to the 1st oral UFT/LV), 9:30, 10:00, 11:00, 11:30, 11:45, 12:00, 12:30, 13:00, 17:00 (prior to the 2nd oral UFT/LV) and 19:00 on day 1, at 9:00 on days 2, 3 and 15. The last sampling point was 226 hr after the final administration of UFT/LV. The plasma concentrations of uracil, 5-FU and tegafur were determined by high-performance liquid chromatography (HPLC), under conditions modified from the report by Chu et al [16]. To 100 μL of plasma were added 200 μL of internal standard solution, 1.0 μg/mL of 5-bromouracil in 50 mM phosphate buffer (pH 2.5) and 750 μL of ethylacetate, and the mixture was centrifuged at 3,000 rpm for 10 sec. The extraction by ethylacetate was repeated 3 times, and the ethylacetate layers collected were evaporated under a stream of nitrogen gas. The residue was solved in 200 μL of 50 mM phosphate buffer (pH 2.5), and injected into a HPLC system after filtration through a 0.45-μm membrane filter (Millipore Corp., MA, USA). The HPLC system (LC-20AT, Shimadzu Corp., Kyoto, Japan) was equipped with a variable-wavelength UV detector (SPD-20A, Shimadzu), adjusted to 260 nm, and an analytic C18 reverse-phase column (CHEMCOSORB 5-ODS-H, 4.6 mm x 25 cm, Chemco Scientific Co., Ltd., Osaka, Japan). The mobile phases were 10 mM phosphate buffer (pH 2.5) (A) and methanol (B), and the gradient program was set as follows: A: B = 100%: 0% at 0 min, 99%: 1% at 7 min, 90%: 10% at 20 min, 90%: 10% at 28 min, 100%: 0% at 29 min and 100%: 0% at 40 min. The calibration lines were constructed for each assay using standard solutions and based on the peak height ratio to 5-bromouracil. The calibration lines were satisfactory for exterminating the concentrations, with minimum within day or day-to-day variations. The 5-FU concentrations were validated by another method presented in other papers, and it was confirmed that this method provided the same values with high accuracy and precision [17-19].
Data Analysis and Statistics
All values reported are the mean±standard deviation (SD). Area under the plasma concentration-time curve (AUC) was calculated using the linear trapezoidal rule. The unpaired Student's t-test/Welch's test or Mann-Whitney's U test was used for two-group comparisons of the concentrations or AUC values. P values of less than 0.05 were considered to be significant.
Results
Table 1 lists the concentrations of 5-FU in plasma of patients with advanced colorectal cancer, who were treated with the CPT-11 + 5-FU/LV + UFT/LV regimen. “Time 0” means 9:00 on day 1, the time of the first oral administration of UFT/LV. The intravenous infusion of CPT-11 at 100 mg/m2 was administered from Time = 0.5 hr to 2 hr, and followed by the infusion of l-LV at 15 mg/m2 for 30 min. Immediately after, a bolus of 5-FU was injected at a dose of 500 mg/m2 in 5 of 10 patients (with bolus injection of 5-FU), the other 5 was not injected (without bolus injection of 5-FU). In the patients with bolus injection of 5-FU, the plasma concentration of 5-FU increased to 37.0±48.9 ng/mL at 1 hr after the oral administration of UFT/LV, then rapidly increased to more than 80,000 ng/mL on the injection of 5-FU, before dropping to 25.5±10.9 ng/mL 5.5 hr later. The trough levels of 5-FU were 27.4±12.0 ng/mL and 31.3±11.5 ng/mL prior to the 4th and 7th oral administration of UFT/LV, respectively, and 5-FU was detected at a concentration of 15.8±11.9 ng/mL at 336 hr after the start of treatment, that is, at 226 hr after the last administration of UFT/LV. On the other hand, in the patients without bolus injection of 5-FU, the trough levels of 5-FU were 9.4±8.0 ng/mL and 10.4±8.4 ng/mL prior to the 4th and 7th oral administration of UFT/LV, respectively, and 5-FU was not detected at 336 hr after the start of treatment, and these values were significantly lower than those with bolus injection of 5-FU.
Table 2 lists the plasma concentrations of uracil. The concentration of endogenous uracil in plasma was 0-48.5 ng/mL, and increased after the oral administration of UFT/LV. Due to extensive inter-individual variation of the concentrations, the effect of bolus injection of 5-FU was not clarified in this study. The data of tegafur is in Table 3. With or without bolus injection of 5-FU, the plasma concentration of tegafur was 2,000-10,000 ng/mL after repetitive oral administration of UFT/LV, and there was no statistical significance between 2 groups.
AUC0-48 values are listed in Table 4. The AUC0-48 values of uracil, 5-FU and tegafur were 8.50±3.68 mg*h/L, 22.16±6.57 mg*h/L and 227.83±50.91 mg*h/L, respectively, in the patients treated with the CPT-11 + 5-FU/LV + UFT/LV regimen. Omission of bolus injection of 5-FU did not affect the systemic exposure to uracil and tegafur, but AUC0-48 values of 5-FU decreased to 0.65±0.42 mg*h/L.
Discussion
5-FU has been widely used to treat metastatic colorectal cancer, and its efficacy is improved when administered with other drugs. Various doses of 5-FU, and schedules and routes of administration have been applied, and there is a consensus that response rates are improved with increased dose and prolonged infusion. With indwelling venous catheters and portable infusion pumps, patients can be treated on an out-patient basis. However, portable infusion pumps can still be inconvenient, and indwelling catheters can result in complications including thrombosis and infections. Oral fluoropyrimidine is a promising alternative to the constant infusion of 5-FU, and pharmacokinetic studies have found that the consecutive oral administration of UFT at 370 mg/m2/day as tegafur provided a steady-state concentration of 5-FU comparable to that achieved by a 5-day constant infusion at 250 mg/m2/day, and injecting a bolus of 5-FU results in ultra-high concentrations followed by rapid dissapperance [20,21].
We have proposed the CPT-11 + 5-FU/LV + UFT/LV chemotherapy, in which repetitive oral administration of UFT/LV replaces the infusion of 5-FU/LV in the FOLFIRI regimen [15]. In this study, the effect of a bolus injection of 5-FU on its steady-state pharmacokinetics was examined in the CPT-11 + 5-FU/LV + UFT/LV chemotherapy. The bolus injection of 5-FU increased its plasma concentrations at 24 hr or 48 hr 3-fold (Table 1), and the AUC0-48 values of 22.16 mg*h/L and 0.65 mg*h/L in the patients with and without the bolus injection, respectively (Table 4). It is noted that the dose of 5-FU for the first 48 hr was 889.9 mg/ m2 and 389.9 mg/m2, respectively, and there was no dose-linearity in the AUC0-48 values. Reportedly, it has been suggested that the toxicity and efficacy of 5-FU are correlated to 5-FU exposure quantified by the AUC in a steady-state, and the target level of 5-FU exposure to ensure a certain efficacy is in the range of 24-30 mg*h/L [22-30]. Therefore, the bolus injection of 5-FU was thought to be necessary to ensure the efficacy in the CPT-11 + 5-FU/LV + UFT/LV chemotherapy, and presumably also in the FOLFIRI and FOLFOX regimens consisting of both of bolus injection and continuous infusion of 5-FU. A review of preclinical reports suggests that short-term, high-dose administration results in growth inhibition refractory to thymidine protection, whereas long-term, low-dose exposure produces cytotoxicity [31]. Here, the injection of 5-FU was proved to alter the systemic exposure to 5-FU, which would contribute, in part, to the synergetic effects of the injection and continuous infusion of 5-FU.
On the other hand, critical evaluation of current treatment protocols in colorectal cancer have suggested that the bolus injection of 5-FU is not always necessary to ensure the efficacy [1-4]. The LV5FU2 regimen consisting of a bolus injection of 5-FU and infusion of 5-FU/LV resulted in a median survival time (MST) of 14.7 months in the first-line therapy [32,33]. The AIO regimen (the infusion of 5-FU/LV) provided the MST of 16.9 months [34], whereas the Mayo Clinic regimen (the bolus 5-FU/LV) [35] gave the MST of 16.1 months [1]. A bolus injection of 5-FU might be omitted in the FOLFIRI and FOLFOX regimens in the cases that the appearance of bolus-associated significant side effects would be expected, although no information on the effect of omission on efficacy. Consequently, further clinical investigations should be performed to clarify the necessity of a bolus injection of 5-FU.
Interestingly, 5-FU was detected at 336 hr after the start of treatment, that is, at 226 hr after the last administration of UFT/LV in the patients with bolus injection of 5-FU, whereas it was not detected for no bolus injection (Table 1). This observation can hardly be explained by the pharmacokinetic profile of 5-FU, i.e., an apparent half-life of about 10 min [36]. An intracellular pool of 5-FU might build up by a bolus injection of 5-FU, and the pooled 5-FU might be reabsorbed into systemic circulation with very slow speed for a long time. Nonclinical animal experiments might support this speculation.
Effect of bolus injection of 5-fluorouracil (5-FU) on the plasma concentration of 5-FU (ng/mL) in the CPT-11 + 5-FU/LV + UFT/LV chemotherapy a)
| Time (hr) | 5-FU dosing | with bolus injection of 5-FU (N = 5) | without bolus injection of 5-FU (N = 5) | P |
|---|---|---|---|---|
| 0 | 1st oral UFT/LV | 0.0±0.0 | 0.0±0.0 | NS |
| 0.5 | 14.0±31.2 | 1.5±3.4 | NS | |
| 1 | 37.0±48.9 | 19.8±18.7 | NS | |
| 2 | 28.5±18.7 | 79.3±64.7 | NS | |
| 2.5 | bolus injection b) | 81751.0±49135.1 | 67.3±45.2 | <0.05 |
| 2.75 | 21161.2±4592.7 | 41.4±36.9 | <0.05 | |
| 3 | 9326.7±2085.7 | 22.4±20.7 | <0.05 | |
| 3.5 | 1845.7±1308.2 | 14.0±7.6 | <0.05 | |
| 4 | 485.4±229.3 | 11.0±6.4 | <0.05 | |
| 8 | 2nd oral UFT/LV | 25.5±10.9 | 5.7±6.6 | <0.05 |
| 10 | 39.5±14.3 | 22.4±17.3 | NS | |
| 24 | 4th oral UFT/LV | 27.4±12.0 | 9.4±8.0 | <0.05 |
| 48 | 7th oral UFT/LV | 31.3±11.5 | 10.4±8.4 | <0.05 |
| 336 | 15.8±11.9 | 0.0±0.0 | <0.05 |
The values are the mean±SD.
a) Protocol is indicated in Figure 1. “Time 0” means 9:00 on day 1, the time of the first oral administration of UFT/LV at 100 mg x 3 as tegafur/m2/day and 25 mg x 3/day, respectively. The intravenous infusion of CPT-11 at 100 mg/m2 was done from Time = 0.5 hr to 2 hr, and followed by the infusion of l-LV at 15 mg/m2 for 30 min. Immediately thereafter, a bolus of 5-FU was injected at a dose of 500 mg/m2.
b) Five of 10 patients were not injected with a bolus of 5-FU.
Effect of bolus injection of 5-fluorouracil (5-FU) on the plasma concentration of uracil (ng/mL) in the CPT-11 + 5-FU/LV + UFT/LV chemotherapy a)
| Time (hr) | 5-FU dosing | with bolus injection of 5-FU (N = 5) | without bolus injection of 5-FU (N = 5) | P |
|---|---|---|---|---|
| 0 | 1st oral UFT/LV | 22.6±19.3 | 10.1±19.6 | NS |
| 0.5 | 1429.6±3133.5 | 26.6±41.6 | NS | |
| 1 | 983.9±1112.2 | 660.2±704.4 | NS | |
| 2 | 381.9±277.0 | 2169.6±2124.2 | NS | |
| 2.5 | bolus injection b) | 579.6±916.7 | 2031.7±2070.5 | NS |
| 2.75 | 287.7 (2450.3) | 622.0 (4232.1) | NS | |
| 3 | 325.1 (3447.7) | 224.6 (944.2) | NS | |
| 3.5 | 765.7±1207.9 | 66.7±94.0 | NS | |
| 4 | 137.9 (647.3) | 24.2 (41.8) | <0.05 | |
| 8 | 2nd oral UFT/LV | 53.1±9.8 | 22.9±29.1 | NS |
| 10 | 415.6±333.2 | 271.6±240.5 | NS | |
| 24 | 4th oral UFT/LV | 37.6±5.5 | 17.1±20.0 | NS |
| 48 | 7th oral UFT/LV | 30.9 (13.4) | 5.0 (25.9) | NS |
| 336 | 19.8±15.5 | 1.4±1.9 | NS |
The values are the mean±SD, when the normality assumption held, and are medians with interquartile ranges in parentheses, when failed.
a) Protocol is indicated in Figure 1. “Time 0” means 9:00 on day 1, the time of the first oral administration of UFT/LV at 100 mg x 3 as tegafur/m2/day and 25 mg x 3/day, respectively. The intravenous infusion of CPT-11 at 100 mg/m2 was done from Time = 0.5 hr to 2 hr, and followed by the infusion of l-LV at 15 mg/m2 for 30 min. Immediately thereafter, a bolus of 5-FU was injected at a dose of 500 mg/m2.
b) Five of 10 patients were not injected with a bolus of 5-FU.
Effect of bolus injection of 5-fluorouracil (5-FU) on the plasma concentration of tegafur (ng/mL) in the CPT-11 + 5-FU/LV + UFT/LV chemotherapy a)
| Time (hr) | 5-FU dosing | with bolus injection of 5-FU (N = 5) | without bolus injection of 5-FU (N = 5) | P |
|---|---|---|---|---|
| 0 | 1st oral UFT/LV | 0.0±0.0 | 0.0±0.0 | NS |
| 0.5 | 938.7 (4028.0) | 548.1 (1243.7) | NS | |
| 1 | 3456.2±2299.1 | 2669.5±2003.5 | NS | |
| 2 | 5453.3±2282.2 | 6394.3±2478.1 | NS | |
| 2.5 | bolus injection b) | 5571.8±1512.6 | 6770.0±1697.6 | NS |
| 2.75 | 5667.8±1305.9 | 6600.0±1611.4 | NS | |
| 3 | 5498.1±1246.9 | 6233.5±1621.3 | NS | |
| 3.5 | 4783.9±1528.9 | 5574.5±1313.9 | NS | |
| 4 | 5129.5±1339.3 | 4897.0±1607.2 | NS | |
| 8 | 2nd oral UFT/LV | 3556.0±1111.4 | 3292.4±1007.6 | NS |
| 10 | 7040.0±1721.2 | 7093.6±2711.9 | NS | |
| 24 | 4th oral UFT/LV | 3932.4±1042.7 | 3094.1±867.6 | NS |
| 48 | 7th oral UFT/LV | 5090.8±1794.3 | 4167.7±1639.4 | NS |
| 336 | 0.0±0.0 | 0.0±0.0 | NS |
The values are the mean±SD, when the normality assumption held, and are medians with interquartile ranges in parentheses, when failed.
a) Protocol is indicated in Figure 1. “Time 0” means 9:00 on day 1, the time of the first oral administration of UFT/LV at 100 mg x 3 as tegafur/m2/day and 25 mg x 3/day, respectively. The intravenous infusion of CPT-11 at 100 mg/m2 was done from Time = 0.5 hr to 2 hr, and followed by the infusion of l-LV at 15 mg/m2 for 30 min. Immediately thereafter, a bolus of 5-FU was injected at a dose of 500 mg/m2.
b) Five of 10 patients were not injected with a bolus of 5-FU.
Area under the plasma concentration-time curve from 0 hr to 48 hr (AUC0-48) of uracil, 5-fluorouracil (5-FU) and tegafur in patients treated with the CPT-11 + 5-FU/LV + UFT/LV chemotherapy
| AUC0-48 (mg*h/L) | uracil | 5-FU | tegafur |
|---|---|---|---|
| with bolus injection of 5-FU | 8.50±3.68 | 22.16±6.57 | 227.83±50.91 |
| without bolus injection of 5-FU | 6.17±2.49 | 0.65±0.42 * | 202.98±61.46 |
The values are the mean±SD.
* P < 0.05, compared with the data obtained with bolus injection of 5-FU.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Grothey A, Sargent D, Goldberg RM. et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-14
2. Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250-61
3. Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007;13:276-81
4. Sabharwal A, Kerr D. Chemotherapy for colorectal cancer in the metastatic and adjuvant setting: past, present and future. Expert Rev Anticancer Ther. 2007;7:477-87
5. Bennouna J, Saunders M, Douillard JY. The role of UFT in metastatic colorectal cancer. Oncology. 2009;76:301-10
6. Douillard JY, Hoff PM, Skillings JR. et al. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3605-16
7. Carmichael J, Popiela T, Radstone D. et al. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3617-27
8. Méndez M, Alfonso PG, Pujol E. et al. Weekly irinotecan plus UFT and leucovorin as first-line chemotherapy of patients with advanced colorectal cancer. Invest New Drugs. 2005;23:243-51
9. Delord J-P, Bennouna J, Artru P. et al. Phase II study of UFT with leucovorin and irinotecan (TEGAFIRI): first-line therapy for metastatic colorectal cancer. Br J Cancer. 2007;97:297-301
10. Ishida H, Miyake Y, Fukunaga M. et al. A Feasibility Study of UFT/LV and Irinotecan (TEGAFIRI) in Advanced or Metastatic Colorectal Cancer: Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG) PROG 0304. Jpn J Clin Oncol. 2009;39:601-5
11. Bajetta E, Di Bartolomeo M, Buzzoni R. et al. Uracil/ftorafur/leucovorin combined with irinotecan (TEGAFIRI) or oxaliplatin (TEGAFOX) as first-line treatment for metastatic colorectal cancer patients: results of randomised phase II study. Br J Cancer. 2007;96:439-44
12. Feliu J, Vicent JM, Garcia-Giro C. et al. Phase II study of UFT and oxaliplatin in first-line treatment of advanced colorectal cancer. Br J Cancer. 2004;91:1758-62
13. Rosati G, Cordio S, Tucci A. et al. Phase II trial of oxaliplatin and tegafur/uracil and oral folinic acid for advanced or metastatic colorectal cancer in elderly patients. Oncology. 2005;69:122-9
14. Bennouna J, Perrier H, Paillot B. et al. A phase II study of oral uracil/ftorafur (UFTs) plus leucovorin combined with oxaliplatin (TEGAFOX) as first-line treatment in patients with metastatic colorectal cancer. Br J Cancer. 2006;94:69-73
15. Chayahara N, Tamura T, Yamamori M. et al. Phase I and pharmacokinetic study of tegafur-uracil/leucovorin combined with 5-fluorouracil/leucovorin and irinotecan in patients with advanced colorectal cancer. Am J Clin Oncol. 2009;32:56-60
16. Chu D, Gu J, Liu W. et al. Sensitive liquid chromatographic assay for the simultaneous determination of 5-fluorouracil and its prodrug, tegafur, in beagle dog plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:377-82
17. Miki I, Tamura T, Nakamura T. et al. Circadian variability of pharmacokinetics of 5-fluorouracil and CLOCK T3111C genetic polymorphism in patients with esophageal carcinoma. Ther Drug Monit. 2005;27:369-74
18. Okuno T, Tamura T, Yamamori M. et al. Favorable genetic polymorphisms predictive of clinical outcome of chemoradiotherapy for Stage II/III esophageal squamous cell carcinoma in Japanese. Am J Clin Oncol. 2007;30:252-7
19. Sakaeda T, Yamamori M, Kuwahara A. et al. VEGF G-1154A is predictive of severe acute toxicities during chemoradiotherapy for esophageal squamous cell carcinoma in Japanese patients. Ther Drug Monit. 2008;30:497-503
20. Ho DH, Pazdur R, Covington W. et al. Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2'-tetrahydrofuryl)-5-fluorouracil. Clin Cancer Res. 1998;4:2085-8
21. Borner MM, Schoffski P, de Wit R. et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002;38:349-58
22. Gamelin E, Boisdron-Celle M, Delva R, Regimbeau C. et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol. 1998;16:1470-8
23. Gamelin EC, Danquechin-Dorval EM, Dumesnil YF. et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer. 1996;77:441-51
24. Vokes EE, Mick R, Kies MS. et al. Pharmacodynamics of fluorouracil-based induction chemotherapy in advanced head and neck cancer. J Clin Oncol. 1996;14:1663-71
25. Ychou M, Duffour J, Kramar A. et al. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol. 2003;52:282-90
26. Milano G, Etienne MC, Renée N. et al. Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol. 1994;12:1291-5
27. Hillcoat BL, McCulloch PB, Figueredo AT. et al. Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br J Cancer. 1978;38:719-24
28. Seitz JF, Cano JP, Rigault JP. et al. Chemotherapy of extensive digestive cancers with 5-fluorouracil: relation between the clinical response and plasma clearance of the drug. Gastroenterol Clin Biol. 1983;7:374-80
29. Fety R, Rolland F, Barberi-Heyob M. et al. Clinical impact of pharmacokinetically-guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res. 1998;4:2039-45
30. Highlights from. 5-fluorouracil drug management pharmacokinetics and pharmacogenomics workshop; Orlando, Florida; January 2007. Clin Colorectal Cancer. 2007;6:407-22
31. Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer-a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997;15:368-81
32. de Gramont A, Louvet C, André T. et al. A review of GERCOD trials of bimonthly leucovorin plus 5-fluorouracil 48-h continuous infusion in advanced colorectal cancer: evolution of a regimen. Groupe d'Etude et de Recherche sur les Cancers de l'Ovaire et Digestifs (GERCOD). Eur J Cancer. 1998;34:619-26
33. de Gramont A, Figer A, Seymour M. et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin. Oncol. 2000;18:2938-47
34. Köhne CH, Wils J, Lorenz M. et al. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. J Clin Oncol. 2003;21:3721-8
35. Poon MA, O'Connell MJ, Wieand HS. et al. Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol. 1991;9:1967-72
36. Sakaeda T, Yamamori M, Kuwahara A. et al. Pharmacokinetics and pharmacogenomics in esophageal cancer chemoradiotherapy. Adv Drug Deliv Rev. 2009;61:388-401
Author contact
![]() Corresponding author: Toshiyuki Sakaeda, Ph.D., Center for Integrative Education in Pharmacy and Pharmaceutical Sciences, Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto 606-8501, Japan, Tel: +81-75-753-9560, Fax: +81-75-753-9253, e-mail: sakaedatkyoto-u.ac.jp
Corresponding author: Toshiyuki Sakaeda, Ph.D., Center for Integrative Education in Pharmacy and Pharmaceutical Sciences, Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto 606-8501, Japan, Tel: +81-75-753-9560, Fax: +81-75-753-9253, e-mail: sakaedatkyoto-u.ac.jp

 Global reach, higher impact
Global reach, higher impact