3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(4):321-331. doi:10.7150/ijms.8.321 This issue Cite
Research Paper
Seroprevalence and Risk Factors for Hepatitis B Infection in an Adult Population in Northeast China
Department of Hepatology, First Hospital, Jilin University, Changchun 130021, China
* These authors contributed equally to this work.
Received 2011-3-29; Accepted 2011-5-16; Published 2011-5-20
Abstract
Background and aim: The prevalence of the hepatitis B virus (HBV) is higher in adults than in children. We determined the seroepidemiology of HBV infection in an adult population in JiLin, China, to guide effective preventive measures.
Methods: A cross-sectional serosurvey was conducted throughout JiLin, China. A total of 3833 people was selected and demographic and behavioral information gathered. Serum samples were tested for HBV markers and liver enzymes.
Results: The prevalence of the hepatitis B surface antigen (HBsAg), the antibody to the hepatitis B surface antigen (anti-HBs), the hepatitis B e antigen (HBeAg), the antibody to HBeAg (anti-HBe), and the antibody to the hepatitis B core antigen (anti-HBc) were 4.38%, 35.66%, 1.38%, 6.65%, and 40.88%, respectively. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were significantly higher among HBsAg (+) than HBsAg (-) subjects. By multivariate logistic regression analysis, independent predictors for chronic HBV infection were smoking, poor sleep quality; occupation as private small-businessmen, laborers, or peasants; male gender; family history of HBV; personal history of vaccination; and older age. Independent predictors for exposure to HBV were large family size, occupation as a private small-businessman, male gender, family history of HBV, personal history of vaccination, and older age. Independent predictors for immunity by vaccination were occupation as a private small-businessman, high income, personal history of vaccination, and young age. Independent predictors for immunity by exposure were drinking, male gender, personal history of vaccination, and older age.
Conclusions: The prevalence rate of HBV infection (4.38%) was lower than the previous rate of general HBV vaccination. However, 44.59% of the population remained susceptible to HBV. The prevalence of HBV infection was high in young adults, private small-businessmen, peasants, those with a family history of HBV, and males. Therefore, immunization of the non-immune population is reasonable to reduce hepatitis B transmission between adults.
Keywords: hepatitis B, immunity, seroepidemiologic study, vaccine.
Introduction
The Hepatitis B virus (HBV) causes liver infection that can be life-threatening and often leads to chronic liver disease, liver cirrhosis, and liver cancer. HBV infection is a major global health problem [1]. However, to date, relatively little data are available concerning HBV infection in China. An epidemic survey published in 2006 revealed that 7.18% of China's population was hepatitis B surface antigen (HBsAg)- positive. Therefore, China is still considered to be a highly endemic region [2-3]. There are about 93 million HBsAg-positive subjects in China, of whom about 20 million have chronic hepatitis B infection. Around half-a-million Chinese patients die from hepatitis B-related liver carcinoma and end-stage cirrhosis each year [2]. There is no ideal and specific cure for HBV infection. The economic burden of HBV infection is substantial because of high morbidity and mortality associated with end-stage liver disease, cirrhosis, and hepatocellular carcinoma (HCC) [4].
The modes of transmission of HBV vary between different regional, gender, and age groups [4]. The sources of infection of hepatitis B are mainly chronic hepatitis B patients and asymptomatic viral carriers. Infection and viral replication have been confirmed by the presence of HBV DNA in body fluids [5]. The main modes of transmission include mother-to-child transmission and blood and body fluids transmission, as well as sexual transmission. Perinatal transmission is believed to account for 35-50% of carriers although horizontal transmission is also important, particularly within families [6].
The national expanded program on immunization was instituted in China in 1992. As of December 2007, 171 counties reported that they had included the hepatitis B vaccine into their national infant immunization programs [7]. In China, babies are given the hepatitis B vaccine at birth. The national infant immunization program focuses on blocking mother-to-child transmission of hepatitis B. The vaccination program has produced encouraging initial results in China. The prevalence of HBsAg was 0.96% and 2.42% in children aged 1—4 and 5—14, respectively [8-10]. In addition to infant immunization, some adult members of China's population have been vaccinated voluntarily, outside national vaccination programs. However, older age groups, especially adults, have not received sufficient attention. Despite the availability of safe and effective HBV vaccines for over 20 years, strategies targeting risk groups have failed to sufficiently control hepatitis B transmission in the current population. HBV transmission has become an important mode of infection in adults, mainly because of difficulties in risk identification and in program implementation. Therefore, we conducted this study to determine the prevalence of HBV infection and the major independent risk factors for HBV transmission in an adult population in northeast China.
Materials and Methods
Design and study population
A cross-sectional seroepidemiologic study of HBV was carried out in Dehui, Jilin, China in 2007 (population approximately 410,600). Dehui is located 81 km from Changchun, the largest city in the area. There are 308 villages and 51 neighborhood committees in Dehui. Earnings of most inhabitants of Dehui are in the middle of the income range. The sex and age distribution of inhabitants are similar to those of JiLin in general. Therefore, Dehui City is representative of other areas in the province in terms of the level of economic and cultural development.
A two-stage, tiered-system sampling method was used. This survey was comprehensive and included geographic, economic, cultural, and other parameters. The first survey covered rural areas while the second covered urban areas. Each stage was divided into two layers. In the first layer, the populations of the villages or neighborhood committees were sorted, and the villages or neighborhood committees were selected by a computer according to the principle of equidistant random samples of the population size. We selected 9 villages and 11 neighborhood committees. In the second layer, the households were marked by the distance from the center of the villages or neighborhood committees, and they were selected according to the principle of equidistant random samples of the distance. Then, 150-200 or 80-100 households were computer-selected in villages or neighborhood committees, respectively. A sample of the general population in the selected households consisting of individuals who were at least 18 years of age and had lived in the same area for more than 10 years was selected using a systematic random 1-in-3 sampling procedure from the census list, which had been updated on February 1, 2007. We defined sample sizes of urban and rural groups according to the formula for the estimation of sample size: N = (t/d) 2 *(1-p) / p (t=1.96, p=0.09 and d=0.1.5) [11]. The samples were 1600 and 2400, based on the ratio of urban and rural populations of the area, respectively, and the total was 4000 (more than the value N).
In the end, 3833 people agreed to participate in the study, and their serum samples, demographic information, and behavioral factors were collected. The response rate was high (95.8%, 3833/4000). When we analyzed the relationship between HBV markers and liver enzymes, we excluded 75 people who had abnormal autoantibodies, ceruloplasmin, and iron tests. They consisted of 30, 29, 16 people, respectively. In addition, 98 people were excluded who reported consuming at least 40 g of alcohol per day, and 45 hepatitis C virus (HCV)-positive people were excluded in the analysis of the relationship between HBV markers and liver enzymes.
Data collection and blood sampling
The study team consisted of physicians and nurses who were trained in the survey methods in order to standardize the data collection, interviews, blood drawing, and handling of serum samples. The selected participants were asked to fast overnight (≥8 h) and attend the local health center during their scheduled appointment. The selected subjects were visited at home if they could not attend the local health center. An interview using a structured questionnaire was conducted at the time of the participant's visit. The questionnaire included the following questions: (1) Do you sleep well? (2) Do you smoke? (3) Have you stopped smoking? (4) How long have you been smoking? (5) Do you drink alcohol (the number and type of drinks per day)? (6) Have you donated or received blood? (7) How many people are in your family? (8) What is your ethnicity? (9) What is your occupation (peasant, laborer, small private businessman or cadre official)? (10) How many years did you study? (11) What is your yearly income? (12) Have you been vaccinated for HBV (yes/no)? (13) Do you have a family history of HBV? Information on demographics and behavioral factors was obtained. The study protocol was approved by the Institutional Review Board of the First Hospital of JiLin University. After written informed consent was obtained, blood samples were taken from each participant for seroprevalence analyses. Sera were stored at -200C until tested at the First Hospital of JiLin University. Anthropometric measurements including height, weight, and waist and hip circumference were conducted by well-trained examiners on individuals wearing light clothing. Waist circumference was measured to the nearest 0.1 cm at the midpoint between the lower borders of the rib cage and the iliac crest. Abdominal ultrasonography was performed to detect the presence of fatty infiltration in the liver by physicians specializing in diagnostic imaging, all of whom used standard criteria in evaluating the images for hepatic fat [12]. Fatty liver was diagnosed by concurrence of 3 ultrasonographers, who were unaware of the subjects' clinical and biochemical status. The results were supplemented by the liver-spleen density gradient (LSDG) determined with the non-contrast abdominal computer tomography (CT) in the local hospital.
Serological testing
In order to differentiate between the various possible stages of HBV infection, a combination of tests consisting of HBsAg, the antibody to hepatitis B surface antigen (anti-HBs), the hepatitis B e antigen (HBeAg), the antibody to HBeAg (anti-HBe), and the antibody to hepatitis B core antigen (anti-HBc) was performed using a commercial ELISA method. HCV antibody, ANA, ceruloplasmin, and iron studies were assayed by standard methods with kits from Ke Hua (Shanghai, China). Persons who tested negative for all HBV markers were classified as HBV-susceptible. Participants who were both anti-HBc- and anti-HBs-positive were classified as having been exposed to hepatitis B and possessed immunity (immunity by exposure). Persons who tested anti-HBc-negative and anti-HBs-positive were classified as most probably vaccinated (immunity by vaccination). Liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GTP), were evaluated by standard methods using kits from Ke Hua (Shanghai, China). Normal values were considered as: 10-40 IU/L for AST, and 5-40 IU/L for ALT, and <50 IU/L for γ-GTP. All laboratory analyses were performed at the First Hospital of JiLin University. Individuals having one or more of the four following criteria were defined as having hyperlipidemia: 1) hypertriglyceridemia (>1.7 mmol/L), 2) HDL-cholesterol (men, <1.04 mmol/L; women, <1.3 mmol/ L), 3) LDL-cholesterol (>4.3 mmol/L), 4) total cholesterol (>6.0 mmol/L).
Statistical analysis
Statistical analyses were performed using SAS software (version 8.0). The overall prevalence of HBV markers among the population in the country was calculated with 95% confidence intervals (CI). The association between the demographic and behavioral variables and the biochemical indicators and prevalence of hepatitis B markers was evaluated using the Chi-square test. Multivariate logistic regression analyses were used to assess the independent demographic and behavioral predictors of chronic infection (HBsAg seropositivity), lifetime exposure to HBV infection (anti-HBc seropositivity), immunity by vaccination, and immunity by exposure. In the immune-by-vaccination group, 9 patients had acute hepatitis B infection (HBsAg positive) and were excluded from the analysis.
The independent variables used were: region of residence, drinking, smoking, sleep quality, blood transfusion, blood donation, family size, ethnicity, occupation, education level, income, gender, personal history of vaccination, family history of HBV, and age. The family size in the household was categorized as small (less than five persons) vs. large (five or more persons per household). Educational level was defined by the highest educational level achieved and was classified based on years of education as group 1 (<8 years) or group 2 (≥9 years). Drinkers were defined as individuals whose alcohol consumption was more than 200 g per week for more than 4 consecutive years. Smokers were defined as individuals who smoked 10 or more cigarettes a day for more than 4 consecutive years. A two-sided P < 0.05 result was considered statistically significant.
Multivariate logistic regression analyses were also used to assess the relationship between the abnormal liver enzyme tests with HBsAg (+) vs. HBsAg (-), (HBsAg (+) and HBeAg (+)) vs. (HBsAg (+) and HBeAg (-)) groups. The other independent variables were region of residence, drinking, smoking, sleep quality, blood transfusion, blood donation, family size, ethnicity, occupation, education level, income, gender, body mass index (BMI), age, fatty liver, and hyperlipidemia. A two-sided P < 0.05 result was considered statistically significant.
Results
Demography of the study cohort
A total of 3833 serum samples was valid and was obtained from 1,778 males and 2,055 females in the age group 18-79 years with 37.7% (1445/3833) from urban and 62.3% (2388/3883) from rural areas.
Serological markers of hepatitis B immunity
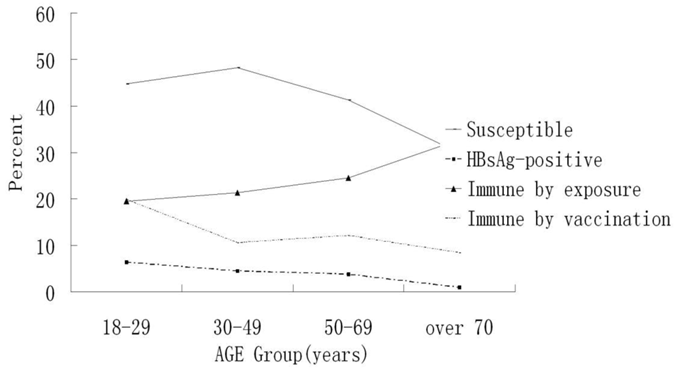
The results showed that the HBsAg (+) rate was 4.38%, 95% CI 3.74 -5.03, and 1.17% were carriers [HBsAg (+) and HBcAb (+)]. Anti-HBs was positive in 35.66% (95% CI, 34.15-37.18%) (n=1367) of the serum samples. Of the 1367 anti-HBs-positive participants, 471 (12.29%) were anti-HBc-negative, indicating that their antibody status was probably due to hepatitis B vaccination. Of the serum samples, 44.59% were negative for any HBV marker. The latter were non-HBV immune and needed hepatitis B vaccination; 0.52% of the samples were HBsAg, HBeAg, and anti-HBc positive, indicating active virus replication (Tables 1, 2). The seroprevalence of anti-HBc or immunity by exposure increased with age, but the percent of susceptible seroprevalence of HBsAg and immunity by vaccination had the opposite trend (Fig. 1, Table 5).
Univariate, multivariate analysis of serum tests (N=3833-218=3615)
We excluded 218 people who had abnormal autoantibodies, ceruloplasmin, or iron tests; those who were HCV-positive, or those who reported consuming at least 40 g of alcohol per day. There were 3165 people in total. Abnormal ALT and AST tests were elevated in persons in the HBsAg (+), HBsAg (+), and HBeAg (+) groups. However, the abnormalities in γ-GTP levels were not found to be significantly different between HBsAg (+) vs. HBsAg (-) or HBeAg (+) vs. HBeAg (-) with HBsAg (+) groups (Table 2). Multivariate logistic regression analyses were used to assess the relationship among the abnormal liver enzyme tests with HBsAg (+) vs. HBsAg (-) and HBeAg (+) vs. HBeAg (-) with HBsAg (+) groups. The results were not changed when other risk factors were considered (Table 3). The rate of abnormal ALT tests (23.81%) was higher than that of abnormal AST tests (17.26%) and γ-GT tests (7.7%) in HBsAg (+) subjects, especially those who were HBeAg (+). Abnormal ALT tests were more frequently abnormal compared with other liver enzyme tests in HBV subjects.
Multivariate logistic regression analysis of HBsAg
In univariate analysis, HBsAg was significantly associated with smoking, sleep quality, occupation, gender, personal history of vaccination, family history of HBV, and age (Table 5).
On multivariate analysis, seroprevalence of HBV remained negatively associated with personal history of vaccination and age. People in the 18-29 age group had a greater likelihood of having been infected by hepatitis B [HBsAg(+)], and a negative trend was seen with age. In contrast, a positive association was present with smoking, bad sleep quality, being a peasant, laborer, or private small-businessman, family history of HBV, or male (Table 6, Fig.1).
Risk factors such as region of residence, blood transfusion, blood donation, ethnicity, and education level are negative in all the analyses, and Tables 5 and 6 do not show them.
Multivariate logistic regression analysis of anti-HBc
In univariate analysis, anti-HBc seropositivity was significantly associated with smoking, large family size, family history of HBV, male gender, and personal history of vaccination and age (Table 5).
On multivariate analysis, there was a positive association with large family size, being a private small-businessman, male, personal history of vaccination, family history of HBV, and older age. People in the 18-29 age group had a lower likelihood of having been exposed to hepatitis B [anti-HBc (+)] and a positive trend was seen with age (Table 6).
Multivariate logistic regression analysis of serological markers consistent with vaccination (immune by vaccination)
In univariate and multivariate analysis, immunity by vaccination was significantly associated with cadre officials, high income, personal history of vaccination, and young adults. People in the 18-29 age group had a greater likelihood of having been immunized by vaccination for hepatitis B, compared with those over age 70. They had a 64.4% lower chance of having been vaccinated compared with the youngest group, and a negative trend was seen with age. (Table 6, Fig.1).
Multivariate logistic regression analysis of subjects with serology consistent with immunity post-infection (immune by exposure)
In univariate and multivariate analysis, immunity by vaccination was significantly associated with non-drinking, gender, personal history of vaccination, and young adult age. People in the 18-29 age group had the lowest likelihood of having been exposed to hepatitis B, and a positive trend was seen with age (Table 6, Fig. 1).
Seroprevalence of HBV markers in Northeast China by age groups
Hepatitis B Markers in the Study Population (n=3833)
| Marker | Number | Percent | 95%CI |
|---|---|---|---|
| HBsAg (+) | 168 | 4.38 | 3.74-5.03 |
| Anti-HBs (+) | 1367 | 35.66 | 34.15-37.18 |
| HBeAg (+) | 53 | 1.38 | 1.01-1.75 |
| Anti-HBe (+) | 255 | 6.65 | 5.86-7.44 |
| Anti-HBc (+) | 1567 | 40.88 | 39.33-42.43 |
| HBsAg (+) and HBeAg (+) | 24 | 14.29* | 8.99-19.58 |
HBsAg, hepatitis B surface antigen; anti-HBs, antibody to hepatitis B surface
antigen; HBeAg, hepatitis B e antigen; anti-HBe, antibody to HBeAg; anti-HBc, antibody to hepatitis B core antigen; CI: Confidence interval.
*HBsAg (+) and HBeAg (+) versus HBsAg (+)
Hepatitis B Marker Combinations in the Study Population (n=3833)
| MODE | Frequency | Percent | Cumulative Frequency | Cumulative Percent |
|---|---|---|---|---|
| HBsAg (+) | 13 | 0.34 | 13 | 0.34 |
| HBsAg (+) and HBeAg (+) | 1 | 0.03 | 14 | 0.37 |
| HBsAg (+) and HBeAg (+) and Anti-HBc (+) | 20 | 0.52 | 34 | 0.89 |
| HBsAg (+) and Anti-HBe (+) and Anti-HBc (+) | 69 | 1.80 | 103 | 2.69 |
| HBsAg (+) and Anti-HBc (+) | 45 | 1.17 | 148 | 3.86 |
| Anti-HBs (+) | 471 | 12.29 | 1169 | 30.50 |
| Anti-HBc (+) | 499 | 13.02 | 698 | 18.21 |
| Anti-HBe (+) | 35 | 0.91 | 3706 | 96.69 |
| Anti-HBs (+) and Anti-HBc (+) | 782 | 20.40 | 3660 | 95.49 |
| Anti-HBs (+) and Anti-HBe (+) | 11 | 0.29 | 3671 | 95.77 |
| Anti-HBe (+) and Anti-HBc (+) | 51 | 1.33 | 199 | 5.19 |
| None | 1709 | 44.59 | 2878 | 75.08 |
| Other | 127 | 3.31 | 3833 | 100.00 |
HBsAg, hepatitis B surface antigen; anti-HBs, antibody to hepatitis B surface antigen; HBeAg, hepatitis B e antigen; anti-HBe, antibody to HBeAg; anti-HBc, antibody to hepatitis B core antigen.
Comparison of Biochemical Data between Different Groups According to HBsAg and HBeAg Status (n=3615)
| n | Abnormal ALT test,n (%) | Abnormal AST test, n(%) | Abnormalγ-GT test, n(%) | |
|---|---|---|---|---|
| HBsAg (+) subjects | 168 | 40 (23.81)* | 29 (17.26)* | 13 (7.7) |
| HBsAg (-) subjects | 3447 | 320 (9.28) | 183 (5.3) | 200 (5.8) |
| HBsAg (+)and HBeAg (+)subjects | 24 | 11 (45.83)** | 8 (33.33)** | 4 (16.7) |
| HBsAg (+)and HBeAg (-)subjects | 144 | 29 (20.14) | 21 (14.58) | 9 (6.3) |
HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-glutamyl transpeptidase.
*P <0.001 vs HBsAg (-) subjects, ** P <0.001 vs HBsAg (-)and HBeAg (-) subjects
Risk for HBsAg and HBeAg Seropositivity Relative to Biochemical Markers among Study Participants
| OR(95% CI) | |||
|---|---|---|---|
| Abnormal ALT test | Abnormal AST test | Abnormal γ-GT test | |
| HBsAg (+) subjects | 2.66(1.80-3.05) | 3.88(2.62-4.15) | 1.06(0.52-1.46) |
| HBsAg (-) subjects | 1(reference) | ||
| HBsAg (+) and HBeAg (+) subjects | 3.12(2.12-4.05) | 2.81(2.01-3.79) | 1.14(0.35-4.50) |
| HBsAg (+) and HBeAg (-) subjects | 1(reference) | ||
HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-glutamyl transpeptidase;
Odds ratios with 95% confidence intervals were adjusted for region of residence, drinking, smoking, sleep quality, blood transfusion, blood donation, family size, ethnicity, occupation, education level, income, gender, BMI, age, fatty liver, and hyperlipidemia.
Prevalence of HBV Markers According to Sociodemographic Variables in the Jilin, China (n=3833)
| Parameters | N | HBsAg, n (%) | Anti-HBc, n (%) | Immune by vaccination, n (%) | Immune by exposure, n (%)a | |
|---|---|---|---|---|---|---|
| Drinking | No | 2532 | 102 (4.03) | 1051 (41.51) | 316 (12.37) | 603 (23.87) |
| Yes | 1301 | 66 (5.07) | 516 (39.66) | 155 (12.12) | 262 (20.18) | |
| P Value | NS | NS | NS | 0.0099 | ||
| Smoking | No | 2449 | 84 (3.43) | 905 (39.4) | 317 (12.94) | 554 (22.66) |
| Yes | 1384 | 84 (6.07) | 602 (43.5) | 154 (11.13) | 311 (22.55) | |
| P Value | 0.0001 | 0.0133 | NS | NS | ||
| Sleep quality | Bad | 915 | 57 (6.23) | 387 (42.3) | 98 (10.71) | 192 (21.06) |
| Good | 2918 | 111 (3.8) | 1180 (40.44) | 373 (12.78) | 673 (23.1) | |
| P Value | 0.0018 | NS | NS | NS | ||
| Family size | ≥5 people in house | 857 | 44(5.14) | 395(46.09) | 110(12.85) | 206(24.15) |
| <5 people in house | 2976 | 124(4.17) | 1172(39.38) | 361(12.13) | 659(22.64) | |
| P Value | NS | 0.0004 | NS | NS | ||
| Occupation | Peasant | 2277 | 104(4.57) | 924(40.58) | 277(12.17) | 521(23.93) |
| Laborer | 524 | 26(4.96) | 225(42.94) | 56(10.69) | 116(22.26) | |
| Small private businessmen | 278 | 22(7.91) | 122(43.38) | 25(8.99) | 63(21.74) | |
| Cadre officials | 754 | 16(2.12) | 293(39.26) | 113(14.99) | 165(21.88) | |
| P Value | 0.0004 | 0.0001 | 0.0271 | NS | ||
| Income | >800 RMB | 949 | 45(4.74) | 400(42.15) | 138(14.54) | 225(23.81) |
| ≤800 RMB | 2884 | 123(4.26) | 1167(40.46) | 333(11.55) | 640(22.23) | |
| P Value | NS | NS | 0.0146 | NS | ||
| Gender | Female | 2055 | 69(3.36) | 775(37.71) | 259(12.52) | 427(20.82) |
| Male | 1778 | 99(5.57) | 792(44.54) | 212(12.02) | 438(24.7) | |
| P Value | 0.0006 | 0.0001 | NS | 0.0042 | ||
| personal history of vaccination | No | 2691 | 146(5.1) | 970(34.2) | 194(6.8) | 306(11.4) |
| yes | 974 | 22(2.2) | 597(59.9) | 277(27.8) | 559(57.4) | |
| P Value | 0.001 | 0.001 | 0.001 | 0.001 | ||
| Family history of HBV | Yes | 572 | 106(18.5) | 273(47.7) | 79(13.8) | 119(20.8) |
| No | 3261 | 62(1.9) | 1294(39.7) | 392(12) | 746(22.9) | |
| P Value | 0.001 | 0.001 | NS | NS | ||
| Age (years) | 18-29 | 499 | 32(6.41) | 166(33.27) | 99(19.84) | 97(19.52) |
| 30-49 | 1749 | 79(4.52) | 677(38.71) | 184(10.52) | 371(21.29) | |
| 50-69 | 1478 | 56(3.78) | 658(44.52) | 179(12.11) | 362(24.51) | |
| 70 or older | 107 | 1(0.98) | 66(61.66) | 9(8.4) | 35(32.71) | |
| P Value | 0.0307 | 0.0001 | 0.0008 | 0.0033 |
NS: Not significant
a In the immunity by vaccination model, 9 patients had current infection for hepatitis B (HBsAg positive) and were excluded from the analysis.
Multivariate Analyses of Risk Factors for Serological Evidence of Immunity Among Study Participants
| HBsAg seropositivity | Anti-HBc seropositivity | Immunity by vaccination | Immune by exposure | ||
|---|---|---|---|---|---|
| Parameters | Odds ratios with 95% confidence intervals | ||||
| Drinking | Yes | 0.58*(0.47-0.72) | |||
| No | 1.00 (reference) | ||||
| Smoking | Yes | 1.61*(1.13-2.29) | |||
| No | 1.00 (reference) | ||||
| Sleep equality | Bad | 1.79*(1.27-2.524) | |||
| Good | 1.00 (reference) | ||||
| Family size | ≥5 people in house | 1.35*(1.14-1.60) | |||
| <5 people in house | 1.00 (reference) | ||||
| Occupation | Peasant | 2.25*(1.12-4.50) | 1.11(0.86-1.42) | 0.95(0.66-1.369) | |
| Laborer | 2.26*(1.14-4.48) | 1.22(0.95-1.58) | 0.73(0.50-1.07) | ||
| Private small-businessman | 3.17*(1.56-6.43) | 1.39*(1.03-1.88) | 0.55*(0.33-0.89) | ||
| Cadre officials | 1.00 (reference) | ||||
| Income | >800 RMB | 1.30*(1.20-1.69) | |||
| ≤800 RMB | 1.00 (reference) | ||||
| personal history of vaccination | No | 1.00 (reference) | |||
| yes | 0.49*(0.21-0.58) | 3*(2.2-3.4) | 6.2*(4.9-6.31) | 4.2*(3.5-5.6) | |
| Family history of HBV | Yes | 2.41*(2.10-2.71) | 1.38*(1.16-1.65) | ||
| No | 1.00 (reference) | ||||
| Gender | Male | 1.79*(1.21-2.68) | 1.61*(1.36-1.90) | 1.64*(1.35-1.99) | |
| Female | 1.00 (reference) | ||||
| Age (years) | 18-29 | 1.00 (reference) | |||
| 30-49 | 0.65(0.41-1.00) | 1.35*(1.09-1.67) | 0.46*(0.35-0.61) | 1.17(0.91-1.51) | |
| 50-69 | 0.488*(0.30-0.78) | 1.64*(1.32-2.05) | 0.55*(0.41-0.72) | 1.39*(1.07-1.80) | |
| 70 or older | 0.15*(0.02-0.85) | 3.33*(2.14-5.17) | 0.35*(0.17-0.73) | 2.02*(1.26-3.23) | |
CI: Confidence interval. *P <0.001 vs reference
Immunity by exposure: anti-HBc (+) and anti-HBs (+); Immunity by vaccination: anti-HBc (-) and anti-HBs (+);
Discussion
Dehui City is representative of JiLin in general because of most of the inhabitants' earnings in the middle of the income range, the sex and age distribution, and the level of economic and cultural development in the province. A multistage, tiered-system sampling method used to control selection bias provided a study sample that was a good representative of the target population in JiLin.
HBV infection is considered a major public health problem in China [13]. The present study was conducted with a systematic epidemiological approach to the current prevalence of HBV infection in northeast China. At present, according to our findings, it seems that 40.88% of the population of northeast China was anti-HBc (+), implying they had been exposed to HBV. Our findings indicate that the seroprevalence of HBsAg as measured in the present study (4.38%) has been reduced by more than two-fold in China compared with the rate (9.75%) in the pre-vaccination era, and was lower than the rate (7.18%) in the National Epidemiological Survey from 2006 [14]. Northeast China, thus, currently qualifies as an intermediate endemic region [3]. The presence of HBeAg in serum indicates active viral replication [15]. Young adults (18-29 years) have been reported to have high rates of HBeAg-positivity (1.62%, 8/499), and the rate of positivity decreased with age due to the spontaneous seroconversion to the antibody against HBeAg. Older carriers have been shown to be more likely than younger carriers to clear HBeAg [16].
Comparisons between various groups with chronic HBV infection showed that the increased liver enzyme levels were significantly higher in HBsAg (+) people compared with HBsAg (-) people. Moreover, people positive for both HBsAg and HBeAg were more likely to have abnormal values of liver enzymes compared with HBsAg carriers negative for HBeAg. Thus, measurement of aminotransferase levels remains the most common and convenient way to identify liver inflammation in patients with chronic HBV infection. But the relationship may be better established by serial observations and analysis rather than by a single examination of aminotransferase levels [17]. ALT levels have been correlated positively with liver inflammation, and patients with persistently normal ALT levels had significantly lower liver histology scores compared with patients with either persistently or intermittently elevated ALT levels [18-19].
The higher prevalence of HBV infection among males in northeast China is in agreement with recent seroprevalence studies conducted in Hawaii [20]. HBsAg positivity rates were higher in private small-businessmen, peasants and laborers, persons with risky social activities or unhygienic living habits, and other factors. These factors may increase the risk of contact and rate of infection compared with cadre officials [20]. These differences were correlated with a high infection rate, but further investigations are needed for better understanding the mechanisms of these relationships. The relationship between age and HBsAg prevalence that was found in the current study has also been reported elsewhere, most notably by serologic surveys in Korea [21]. Significantly lower risk for HBsAg positivity in older persons might have resulted from a spontaneous clearance of HBsAg over time (a 40% cumulative rate of HBsAg seroclearance has been observed among HBV carriers after 25 years) [22]. In addition, mortality due to HBV-related sequelae may lead to decreased prevalence in older age (probability of survival is 84% at 5 years and 68% at 10 years for HBsAg (+) patients with compensated cirrhosis) [20].
Regarding family size and family history of HBV, HBV is more frequently transmitted in the families with hepatitis B patients [23]. The difference in sex ratio in persons aged 18-29 years versus 70 or older, age 30-49, or age 50-69 was statistically significant. There were more males in the younger than older age groups (data not shown). The factors of male gender, personal history of vaccination, and being a private small-businessman had interactive effects with age in HBV infection and exposure to HBV. There may have been a confounding effect in the independent risk factors which were synergistic. The reason for the sex difference is unclear. It could have been due to differences in the immune response to the HBV infection [24]. Infection at birth, risky sexual behavior, or drug use were the main reasons identified for HBV infection of young people in other parts of the world [25]. These data may also explain why the risk in older persons was lower than that in the younger group [24].
The prevalence of HBV infection was found not to be different between rural and urban areas (data not shown). On the other hand, living in a rural area was not found as a risk factor for transmission of hepatitis B. Some studies have reported that there was a significant seropositivity difference between rural and urban regions [20, 26]. There are 56 ethnic groups in China and the lifestyle of the minority ethnic population is considerably different from the Han majority ethnic population. Ethnic differences and HBV infection have not been well described in northeast China. We investigated possible differences among ethnic groups, but found no association between HBV contact, infection, and ethnicity. However long-term poor quality of sleep may inhibit the immune response, resulting in poor defense, and HBV infection [27].
In the current study, 44.59% were negative for all hepatitis B markers. This high proportion of the population is disturbing, but not very surprising because the impact of the national infant immunization program would not have been seen in the age groups studied. The large number of residual susceptible individuals found in this study reflects the inadequacy of voluntary adult vaccination that occurred outside the national vaccination campaign. Hepatitis B vaccine failure has been reported to occur in 5%-10% of individuals completing a full course of three doses [28], but this would explain less than half of the seronegative results.
Adult vaccination has already had a substantial effect on the general level of hepatitis B immunity in China. In this cohort of people from JiLin province, almost 35.66 % (1367/3833) were immune, and 12.28% of this immunity was attributable to vaccination rather than natural infection. The immunity rate from past infection is higher than the 5% rate reported in blood donors in 1987—before vaccination became widespread [29].
The substantial impact of adult vaccination was greatest among people who were aged 18-29, had high income, were cadre officials, had a personal history of vaccination, and were not concentrated in the demographic or lifestyle risk groups for whom vaccination is officially recommended. This represents an ongoing public health challenge. At the same time, primary hepatitis B vaccine failure and rapidly declining anti-HBs titres after vaccination may also affect the effectiveness of the immunity program [28]. People in the youngest age group had a greater likelihood of being currently immune by vaccination against hepatitis B, compared with those aged over 70 having significantly (64.4%) less chance of being immune by vaccination. But males in the older age group likely became immune by exposure. The reason may be related to immune maturation and opportunities for exposure to HBV to generate antibodies, resulting in seroconversion [23]. Booster doses of the hepatitis B vaccine are regarded as unnecessary on the premise that a rapid anamnestic response will occur on challenge [30], and this policy is supported by the demonstration of antibody production after booster doses of the vaccine and the lack of reports of acute icteric hepatitis among “high risk” vaccinees [31-32]. Personal history of vaccination was determined by patient recall which affects the accuracy and impact of the study. We do not know vaccination age, Hep B genotype, vaccination doses, number of inoculated, and duration of HBV infection. However personal history of vaccination was associated with decreasing HBsAg-positivity and increasing HBsAb positivity. The positive results increase our confidence in the use of the vaccine [8].
The multivariate logistic regression analysis showed that independent predictors for chronic HBV infection and exposure to HBV were family history of HBV, and large family size. This does not identify a mode of transmission, but suggests that vertical and sexual transmission may be involved as they are well known risk factors. Further studies will be required to determine the actual modes of transmission.
Therefore, vaccination program for all newborns should be continued. Susceptible adults whose HBV markers are all negative should have repeat or catch-up immunization, especially those individuals who have risk factors of having poor sleep quality, being private small-businessmen, having a family history of HBV, having no personal history of vaccination, and being a young male adult. HBV carriers need to be closely monitored or treated. The findings of the current study add to the knowledge of hepatitis B epidemiology in areas with sizable high-risk groups, demonstrating the importance of screening programs for hepatitis B. Mass screening permits baseline estimates of prevalence and provides insight into appropriate vaccination strategies. Furthermore, because of China's high incidence of hepatitis B-related hepatocellular carcinoma, screening programs are clinically significant for facilitating referral of newly diagnosed cases to appropriate medical care [31, 33].
Conclusions
Strong associations between HBV infection or immunity were observed regarding the gender, occupation, personal history of vaccination, and age in adults. There has been a decrease in the prevalence of HBV infection since the National Expanded Program on Immunization. Mother-to-fetal vertical transmission of HBV is well controlled. Transmission between adults has become the most common mode of HBV spread. While the survey focused on a region in northeast China, it is likely that similar results may be found elsewhere in the country. Identifying groups at risk for susceptibility can assist in the development of national strategies to target specific groups for cost-effective salvage vaccination programs for adults in the future.
Abbreviations
HBV: hepatitis B virus; HBsAg: hepatitis B surface antigen; anti-HBs: antibody to hepatitis B surface antigen; HBeAg: hepatitis B e antigen; anti-HBe: antibody to HBeAg; anti-HBc: antibody to hepatitis B core antigen; ALT: alanine aminotransferase; AST: aspartate aminotransferase; γ-GTP: γ-glutamyl transpeptidase.
Acknowledgements
We sincerely thank those at the First Hospital of JiLin University who contributed to the survey work and to Medjaden.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;250:1118-29
2. Qu JB, Zhang ZW, Shimbo S. et al. Urban-rural comparison of HBV and HCV infection prevalence in eastern China. Biomed Environ Sci. 2000;13:243-53
3. Kurugöl Z, Koturoğlu G, Akşit S. et al. Seroprevalence of hepatitis B infection in the Turkish population in Northern Cyprus. Turk J Pediatr. 2009;51:120-6
4. Liaw YF. Management of patients with chronic hepatitis B. J Gastroenterol Hepatol. 2002;17:406-8
5. Weinbaum CM, Mast EE, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. Hepatology. 2009;49:s35-44
6. Chang MH. Hepatitis B virus infection. Semin Fetal Neonatal Med. 2007;12:160-7
7. Mossong J, Putz L, Patiny S. et al. Seroepidemiology of hepatitis A and hepatitis B virus in Luxembourg. Epidemiol Infect. 2006;134:808-13
8. Liang JH, Wang M, Liu JH. et al. [Study on the immunization coverage and effects of hepatitis B vaccine in the 20 - 59 years-old population in Guangzhou city.]. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:1336-9
9. Liang X, Bi S, Yang W. et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-7
10. Liang X, Bi S, Yang W. et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39-47
11. Zhang ZW, Shimbo S, Qu JB. et al. Hepatitis B and C virus infection among adult women in Jilin Province, China: an urban-rural comparison in prevalence of infection markers. Southeast Asian J Trop Med Public Health. 2000;31:530-6
12. Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-25
13. Merican I, Guan R, Amarapuka D. et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-61
14. Zhang LP, Yang P, Li FH. et al. [Hepatitis viruses infection situation in Mianyang of the Sichuan province]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008;22:449-51
15. Yang HI, Lu SN, Liaw YF. et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-74
16. McMahon BJ, Holck P, Bulkow L. et al. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759-68
17. Davaalkham D OT, Nymadawa P. Seroepidemiology of hepatitis B virus infection among children in Mongolia: results of a nationwide survey. Pediatrics International. 2007;49:368-74
18. Davaalkham D, Ojima T, Nymadawa P. et al. Significance of HBV DNA levels in liver histology of HBeAg and Anti-HBe positive patients with chronic hepatitis B. Am J Gastroenterol. 2004;99:2032-7
19. Zhang H, He SM, Sun J. et al. Prevalence and etiology of abnormal liver tests in an adult population in jilin, china. Int J Med Sci. 2011;8:254-62
20. Tsai NC, Holck PS, Wong LL. et al. Seroepidemiology of hepatitis B virus infection: analysis of mass screening in Hawaii. Hepatol Int. 2008;2:478-85
21. Lee DH, Kim JH, Nam JJ. et al. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002;17:457-62
22. Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45:1187-92
23. Tawk HM, Vickery K, Bisset L. et al. The impact of hepatitis B vaccination in a Western country: recall of vaccination and serological status in Australian adults. Vaccine. 2006;24:1095-106
24. Tswana S, Chetsanga C, Nyström L. et al. A sero-epidemiological cross-sectional study of hepatitis B virus in Zimbabwe. S Afr Med J. 1996;86:72-5
25. Mast EE, Weinbaum CM, Fiore AE. et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1-33
26. Lee A, Cheng FF, Chan CS. et al. Sero-epidemiology and risk factors of positive hepatitis B surface antigen amongst Chinese adolescents. Asia Pac J Public Health. 2001;13:30-5
27. Cakirbay H, Bilici M, Kavakçi O. et al. Sleep quality and immune functions in rheumatoid arthritis patients with and without major depression. Int J Neurosci. 2004;114:245-56
28. Wood RC, MacDonald KL, White KE. et al. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA. 1993;270:2935-9
29. Ismay SL, Thomas S, Fellows A. et al. Post-transfusion hepatitis revisited. Med J Aust. 1995;163:74-7
30. Datta SD, Fiore AE, Mast E. et al. Routine booster doses of hepatitis B vaccine for health care workers are not necessary. Arch Intern Med. 2000;160:3170-1
31. Keating GM NS. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63:1021-51
32. Keating GM, Noble S. A review of the efficacy, immunogenicity and tolerability of a combined hepatitis A and B vaccine. Expert Rev Vaccines. 2004;3:249-67
33. Su FH, Chen JD, Cheng SH. et al. Waning-off effect of serum hepatitis B surface antibody amongst Taiwanese university students: 18 years post-implementation of Taiwan's national hepatitis B vaccination programme. J Viral Hepat. 2008;15:14-9
Author contact
![]() Corresponding author: Dr. Shumei He, Department of Hepatology, First Hospital, Jilin University, Changchun 130021, China; Tel: +86-431-85612708; Fax: +81-431-85612708; E-mail: hsm19642003com.cn. Dr. Junqi Niu, Department of Hepatology, First Hospital, Jilin University, Changchun 130021, China; Tel: +86-431-85612708; Fax: +81-431-85612708; E-mail: junqiniucom.cn
Corresponding author: Dr. Shumei He, Department of Hepatology, First Hospital, Jilin University, Changchun 130021, China; Tel: +86-431-85612708; Fax: +81-431-85612708; E-mail: hsm19642003com.cn. Dr. Junqi Niu, Department of Hepatology, First Hospital, Jilin University, Changchun 130021, China; Tel: +86-431-85612708; Fax: +81-431-85612708; E-mail: junqiniucom.cn

 Global reach, higher impact
Global reach, higher impact