3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(6):338-347. doi:10.7150/ijms.6.338 This issue Cite
Research Paper
High-Resolution Flow Cytometry: a Suitable Tool for Monitoring Aneuploid Prostate Cancer Cells after TMZ and TMZ-BioShuttle Treatment
1. German Cancer Research Center, Dept. of Medical Physics in Radiooncology, INF 280, D-69120 Heidelberg, Germany
2. University of Heidelberg, Institute of Pathology, INF 220, D-69120 Heidelberg, Germany
3. German Cancer Research Center, Central Peptide Synthesis Unit, INF 580, D-69120 Heidelberg, Germany
4. Radiation Oncology, University of Heidelberg, INF 400, D-69120 Heidelberg, Germany
5. German Cancer Research Center, Division of Biophysics of Macromolecules, INF 580, D-69120 Heidelberg, Germany
# The authors contributed by equal parts to this work
Received 2009-8-17; Accepted 2009-11-16; Published 2009-11-18
Abstract
If metastatic prostate cancer gets resistant to antiandrogen therapy, there are few treatment options, because prostate cancer is not very sensitive to cytostatic agents. Temozolomide (TMZ) as an orally applicable chemotherapeutic substance has been proven to be effective and well tolerated with occasional moderate toxicity especially for brain tumors and an application to prostate cancer cells seemed to be promising. Unfortunately, TMZ was inefficient in the treatment of symptomatic progressive hormone-refractory prostate cancer (HRPC). The reasons could be a low sensitivity against TMZ the short plasma half-life of TMZ, non-adapted application regimens and additionally, the aneuploid DNA content of prostate cancer cells suggesting different sensitivity against therapeutical interventions e.g. radiation therapy or chemotherapy. Considerations to improve this unsatisfying situation resulted in the realization of higher local TMZ concentrations, sufficient to kill cells regardless of intrinsic cellular sensitivity and cell DNA-index. Therefore, we reformulated the TMZ by ligation to a peptide-based carrier system called TMZ-BioShuttle for intervention. The modular-composed carrier consists of a transmembrane transporter (CPP), connected to a nuclear localization sequence (NLS) cleavably-bound, which in turn was coupled with TMZ. The NLS-sequence allows an active delivery of the TMZ into the cell nucleus after transmembrane passage of the TMZ-BioShuttle and intra-cytoplasm enzymatic cleavage and separation from the CPP. This TMZ-BioShuttle could contribute to improve therapeutic options exemplified by the hormone refractory prostate cancer. The next step was to syllogize a qualified method monitoring cell toxic effects in a high sensitivity under consideration of the ploidy status. The high-resolution flow cytometric analysis showed to be an appropriate system for a better detection and distinction of several cell populations dependent on their different DNA-indices as well as changes in proliferation of cell populations after chemotherapeutical treatment.
Keywords: TMZ-BioShuttle, Prostate Cancer Cells, Flow Cytometry
Introduction
Prostate cancer (PCa) is the most common solid tumor in men. In 2007, there will be approximately 220.000 men diagnosed with prostate cancer in the U.S. [1]. The annual incidence of the male population in West-Europe and the U.S.A averages approximately 88 per 100.000 men [2]. As the wide range of the PCa's aggressiveness shows: while some patients are being able to live symptom-free and without any treatment for many years, there are aggressive forms with rapid growth and early metastatic spreading. The current therapeutic options in the treatment of PCa are: i) Radical excision of prostate and seminal vesicles [3, 4]. ii) Percutaneous radiation therapy with high energetic photons (6-23 MeV) [5, 6]. iii) Interstitial radiation therapy with temporarily or permanent radioactive implants (brachytherapy) [7]. iv) The standard initial systemic therapy for locally advanced or metastatic disease is hormonal or androgen deprivation therapy (ADT) that may be performed by bilateral orchiectomy or pharmaceutical means. The androgen-sensitive period in patients with metastatic disease lasts a median of 14-30 months [8]. v) Chemotherapy for hormone refractory prostate cancer (HRPC) is possible using mitoxantrone plus prednisone [9] or with taxane-containing agents [10]. Despite manifold combined therapeutic approaches for a successful HRPC control in the past, metastatic AIPC and HRPC is difficult to treat [11-18]. Unfortunately, all current therapeutic options for patients with HRPC turned out to be poorly effective and chemotherapy had low response rates with median survivals of up to only 12 months [14]. Therefore, chemotherapy has not traditionally been offered to patients with HRPC as a routine treatment due to its treatment-related toxicity and poor response [19]. Despite initially encouraging results of tumor growth control were reported in other tumor types than brain tumors [20], the results of a phase II study of TMZ in PCa have been discouraging [21]. One of the reasons for this could be the presence of aneuploid cell fractions, which offers a broad spectrum of cells from highly sensitive up to therapy resistant [22]. In addition, the therapy resistant cell fraction gains a selective advantage after therapeutic intervention. The DU 145 cell line, presenting a cellular heterogeneity, suggests behaviour's similarity to advanced HRPC tumors. As demonstrated by karyotypic analyses DU 145 show an aneuploid human karyotype with a modal chromosome number of 64. Marker chromosomes like translocated Y chromosome, metacentric minute chromosomes and three large acrocentic chromosomes have been identified [23, 24] which in their entirety can be responsible for the constricted sensitivity against alkylating agents.
It is clear that effort is necessary to search for new forms of treatment modalities like drug delivery and targeting systems realizing high local TMZ concentrations in the nuclei of target cells. To eradicate the target cells, without considering the HRPC cells resistance against therapeutic interventions it is necessary to find convenient methods which allow the monitoring of the therapy progress and success at the cellular level. Flow cytometric analysis should be able to detect several cell populations dependent on their different DNA-indices, which are corresponding to different amount in chromosome numbers. By this method, the genetic integrity and stability can be analyzed [25].
Materials & methods
Cell culture
The hormone refractory adherent prostate cancer cell line DU 145 [24] was cultivated and maintained in RPMI cell medium (Gibco, Germany) supplemented with 5% fetal calf serum and 4 mM glutamine (Biochrome, Germany) at 37°C in 5% CO2 atmosphere.
Chemical procedures
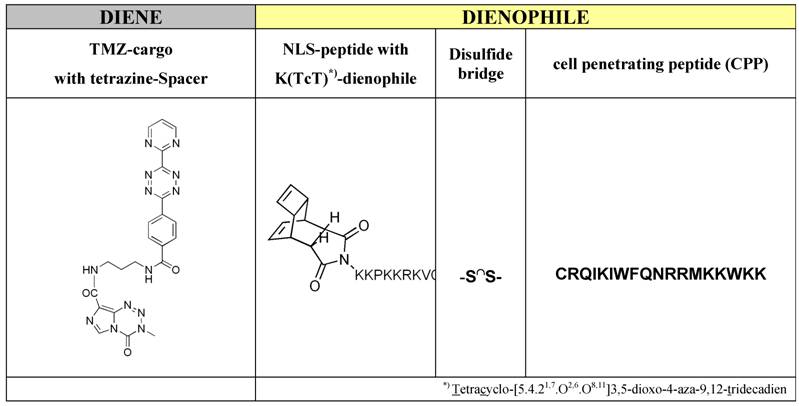
The syntheses of the investigated peptide-based functional modules like the cell penetrating peptide (CPP) and the nuclear localization sequence (NLS) as well as the syntheses of tetracyclo-[5.4.21,7.O2,6.O8,11]3,5-dioxo-4-aza-9,12-tridecadien acting dienophile and the tetrazoline-derivatization of the active compound temozolomide to (TMZ-tetrazine diene) (Table 2, left column), and in the end, the both ligation procedures, firstly the ligation of the dienophile-NLS module (Table 1, upper row) with cell penetrating peptide (CPP) (Table 1, lower row) by a reversible disulfide-bridge formation, and secondly, the compounds for the ligation in virtue to the Diels-Alder-Reaction with inverse electron-demand extensive described by Braun [26] and Pipkorn [27] are illustrated in Table 2.
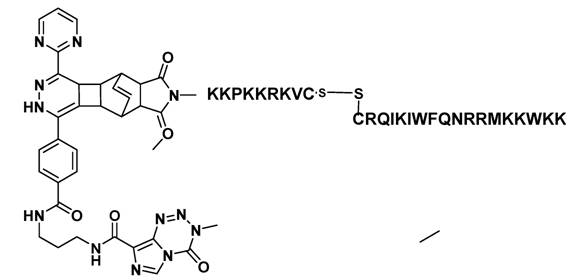
All reactions and procedures were carried out under normal atmosphere conditions. The Figure 1 exhibits the constitutional formula of investigated active TMZ-NLS component.
Schematic ligation pattern of the K(TcT)-NLS-Cys & CPP-Cys modules by disulfide bridge formation. Itemized modules of the investigated TMZ-BioShuttle. The upper line shows the chemical structure of the tetracyclo-[5.4.21,7.O2,6.O8,11]3,5-dioxo-4-aza-9,12-tridecadien (TcT) acting as dienophile compound in the DARinv. It is connected via the ε-amino-coupling of the lysine spacer to the nuclear address sequence (NLS). At the right site of the table the CPP module in the single letter code mode is represented. In the upper line the corresponding module in the single code mode is represented.
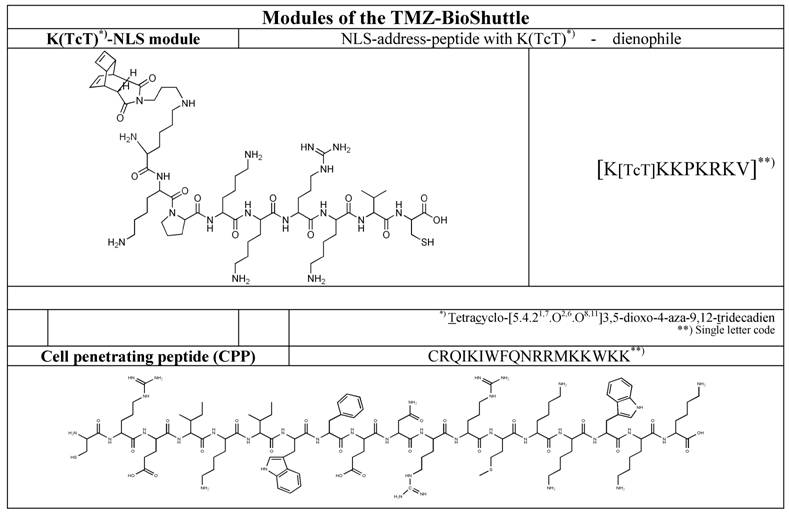
Middle and right part of the table represents the dienophile-educt for the DARinv: K(TcT)-NLS-S∩S-CPP and the TMZ tetrazine spacer derivatized acts as a diene compound as a cargo (left column).
Constitutional formula of the investigated TMZ-BioShuttle
Cell Cycle and Cell Death Analysis
The effects on the cell viability and the cell cycle distribution were determined by DNA flow cytometry.
Flow cytometric analyses were performed using a PAS II flow cytometer (Partec, Munster/Germany) equipped with mercury lamp 100 W and filter combination for 2, 4-diamidino-2-phenylindole (DAPI) stained single cells. From native sampled probes the cells were isolated with 2.1% citric acid/ 0.5% Tween 20 according to the method for high resolution DNA and cell cycle analyses [28] at room temperature with slightly shaking. Phosphate buffer (7.2g Na2HPO4 × 2H2O in 100ml H2O dist.) pH 8.0 containing 2, 4-diamidino-2-phenylindole (DAPI) for staining the cell suspension was performed. Each histogram represents 30.000 cells for measuring DNA-index and cell cycle. For histogram analysis we used the Multicycle program (Phoenix Flow Systems, San Diego, CA).
Human lymphocyte nuclei from healthy donors were used as internal standard for determination the diploid cell population. The mean coefficient of variation (CV) of the diploid lymphocytes was 0.8 - 1.0.
Cell viability and apoptotic cells were assessed by flow cytometry with propidium iodide (PI)-method. For detection of apoptotic cells and viability a FACS Calibur flow cytometer (Becton Dickinson Cytometry Systems, San Jose, CA) was used with filter combinations for propidium iodide. For analyses and calculations the Cellquest program (Becton Dickinson Cytometry Systems, San Jose, CA) was used. Each histogram represents 10.000 cells. After preparation according to Nicoletti with modifications [29, 30] measurements were acquired in Fl-2 in logarithmic mode and calculated by setting gates over the first three decades to detect apoptotic cells. The effect of the used solvent acetonitrile on the viability of lymphocytes was without pathological findings.
Preparation of short term lymphocytes
Human lymphocytes were isolated from 30ml native venous blood from a healthy donor by a lymphocyte preparation with LymphoprepTM gradient (AXIS-Shield PoC AS, Norway) under sterile conditions. A short term culture was established with lymphocytes using RPMI 1640 medium, containing 10% foetal calf serum and Phytohemagglutinin (PHA-P) (5mg/ml) in phosphate buffer solution PBS (Sigma, Germany) at 37°C and 5% CO2 for 144 hours.
Treatment of lymphocytes was followed by identical procedures according to the DU 145 cells. The suspension culture was harvested by centrifugation at 800rpm for 10min, rinsed in PBS and marked with propidium iodide (PI) before measurement in a FACS-calibur flow cytometer (Becton & Dickinson, Germany) equipped with a 488nm argon laser and emission filter combinations for red fluorescence (610nm) using the Cell Quest acquisition and analyses software (Becton & Dickinson, Germany). For analysis a minimum of 10.000 cells were counted and the results were presented as histograms in logarithmic-modus. The specific fluorescence intensity was calculated as the ratio of the geometric mean fluorescence values obtained with the specific PI-uptake.
Results
The aims of the study were:
- to achieve a rapid and high local concentration and an accumulation of TMZ derivative within the cell nuclei using the TMZ-BioShuttle as delivery and targeting system.
- the presentation, distinction and evaluation of the cell cycle behaviour of aneuploid DU 145 prostate cancer cells after treatment with TMZ alone and with its TMZ-BioShuttle derivative.
Control DU 145 cells form a continuous monolayer, while treated DU 145 cells show loss of adhesion primarily in the TMZ-BioShuttle treated cells combined with spread and attached to the well-plate. Whereas in the TMZ treated cells, the cell closeness was declined and accompanied with an increase of amount of dead cells in the supernatant.
Using the flow cytometry technique not only a differentiation in the cell cycle state of DU 145 cells but also a schedule line of the diploid (red) and an aneuploid (blue) DNA content of the DU 145 cells is demonstrated as shown in Figure 2 and table 3 A, graphically visualized in 3B.
The figure shows the cell cycle distribution of DU 145 cells: In the left part of the figure the plot of the untreated control is demonstrated, the middle and the right plot show the cell cycle distribution 144 h after treatment with TMZ-BioShuttle and TMZ respectively. The prostate cancer cell line exhibits two cell fractions: a diploid (DNA-index of 1.0)[red coloured] and an aneuploid fraction (DNA-index 1.1)[blue coloured], close to the diploid. The G1 and the G2M peaks show a diploid and an aneuploid DNA content respectively. S-phase: After TMZ treatment (right plot) the cell number of the aneuploid cells is 10% higher (59 %) compared to aneuploid S-Phase cells in the TMZ-BioShuttle treated probe (middle plot). This in turn was 1.8 fold increased (49 %) compared to the corresponding cell fraction of the control (27 %). The relative amount of the diploid cell fraction differs from the aneuploid fraction: The control shows 35 % diploid cells. The aneuploid fractions reveal decreased amounts 15% (TMZ-BioShuttle) and 3% (TMZ). G2/M phase: The cell cycle behaviour of both cell fractions, the diploid and the aneuploid, show partly opposing effects. In comparison to the control which shows identical percentage of the diploid and the aneuploid cells, the TMZ and the TMZ-BioShuttle treated probes display an increased diploid cell fraction in which the TMZ-BioShuttle probe shows the highest cell contingent. The fraction of aneuploid cells is reduced to 11% whereas the diploid part is increased to 28% in the TMZ-BioShuttle treated probe. The amount of cells in the G2/M phase is increased in a similar ratio of 25 % diploid and 24 % aneuploid cells in the TMZ probe. G1-phase: The comparison of the amount of aneuploid and diploid cells in the G1 phase shows a ratio of 49 % and 54%. The cells in the G1 phase of the TMZ treated probe was increased to 73 % (diploid) and 57 % in the TMZ-BioShuttle probe. The aneuploid cell fractions exhibit an opposite result: the aneuploid cell fraction is decreased to 41% (TMZ-BioShuttle) and 17 % (TMZ).
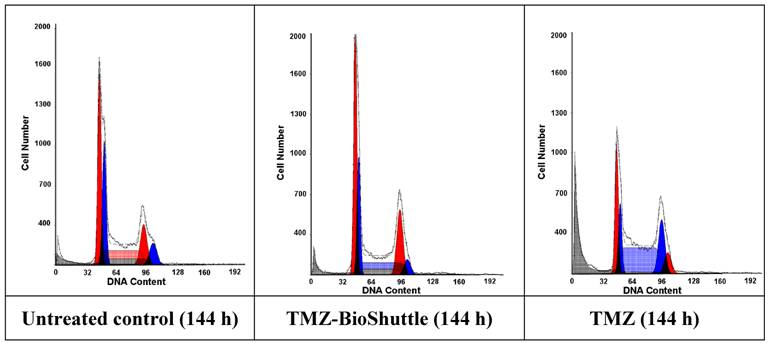
The relative appointment [%] of the particular cell fractions of the cell cycle in DU 145 cells is listed in the table 3A and vertical bar chart 3B. Diploid (red) and aneuploid (blue) DNA contents are demonstrated. The varying cell fraction's properties are clarified by connecting lines.
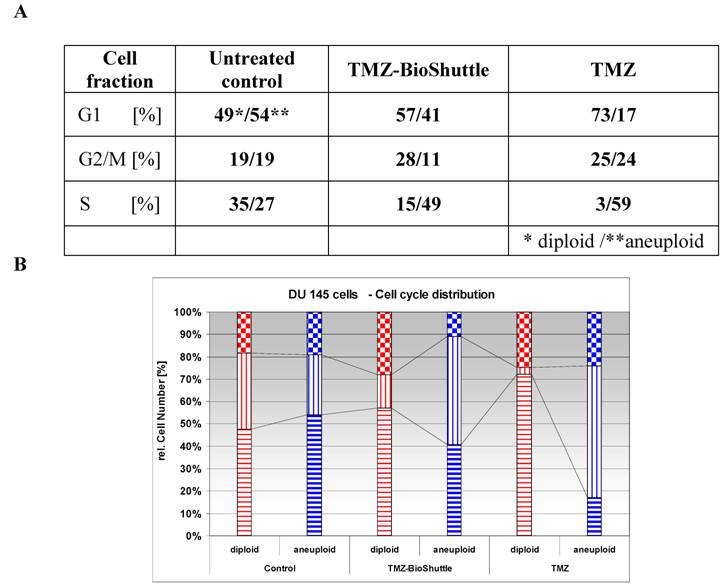
The cell cycle distribution of the untreated control cells shows two different cell fractions harbouring a diploid (49%) and an aneuploid part (54%) in the G1 phase. Both cell fractions have a rate of 19% in the G2/M phase respectively. The cell fraction in the S phase possesses a diploid / aneuploid ratio of 35% to 27%.
The cell cycle distribution of the diploid and aneuploid cell fractions after treatment with TMZ alone and with TMZ-BioShuttle shows different patterns. The amount of the diploid cell fraction and of the aneuploid fraction is opposed in untreated cells compared with the cells in the G1 phase of the TMZ-BioShuttle and TMZ cell fractions (table 3A).
In contrast to the untreated control cell fractions in the G1-phase, the diploid part of the TMZ-BioShuttle treated cells in the G1 phase is slightly increased to 57% whereas the aneuploid fraction is decreased to 41%. This different behaviour could be caused by an arrest of diploid cells in the G1 phase, whereas the cells of the aneuploid fraction pass from the G1 into the S phase.
This finding is confirmed by the investigation of DU 145 cells in the S phase: TMZ-BioShuttle treated cells show in the S phase cell fraction a decreased amount of diploid (15%) but an increased amount of aneuploid cells (49%). The increase of diploid cells to 28% in the G2/M phase and a parallel decrease of the amount of aneuploid cells to 11% measured after TMZ-BioShuttle treatment exhibit a similar cell cycle behaviour of both cell fractions. This amount of diploid cells suggests an arrest in the G2/M phase, while the aneuploid cell fraction runs through this phase into the S phase.
The DU 145 cells treated with TMZ show different cell cycle behaviour and a different DNA-index-dependant sensitivity. The treatment results in increasing amounts of cells of the diploid (25%) and of the aneuploid cell (24%) fraction in the G2/M phase.
The amount of diploid TMZ treated cells increased to 73 % (!) featuring an arrest of diploid cells in the G1 phases in contrast to the aneuploid fraction which is reduced to 17%. This could be a hint for a block by TMZ-sensitivity in the G1 phase. The aneuploid cell fraction however proves to be insensitive against TMZ and the G1 phase seems to be reduced because the cells reach the S phase as shown in the measurements. The diploid cells in the S phase are reduced to 3%, whereas the part of aneuploid cells exhibits an extreme increase to 59%!
Moreover, this strong increase of the aneuploid cell fraction in the S phase could be an evidence for a cell cycle block in the S phase.
Lymphocytes treatment
Undesired effects of the TMZ on peripheric lymphocytes of patients often show a leukopenia like hemogram. A reformulation of TMZ should circumvent these adverse reactions, limiting the therapeutic outcome.
Under the aspect of a potential future use of the TMZ-BioShuttle in patients, we investigated fresh human lymphocytes for survival of the treatment in the used concentrations and with our solvent. The life/dead cells data are depicted in Figure 3.
For studies cells were seeded at a density of 1.8 × 106 cells/ml. After incubation with TMZ and TMZ-BioShuttle in a final concentration of 50µM respectively, DU 145 cells were incubated and measured after 48 hours.
Histograms of lymphocytes of one healthy proband is represented exemplarily. The histogram shows in a log-mode the relative fluorescence intensities of human lymphocytes marked with PI. By setting the gate M1 apoptotic cells are marked, morphologically intact cells with intact DNA content could be observed with higher relative fluorescence intensity in the gate M2.
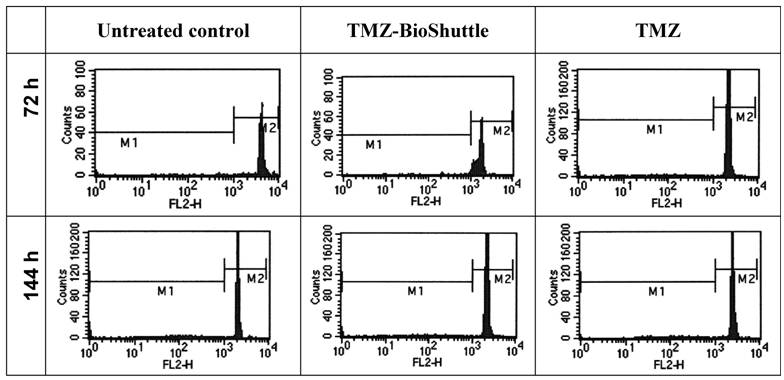
The observation of effects of the TMZ molecule and its TMZ-BioShuttle respectively in blood of four healthy test persons permits an estimation of the toxicity. 72 hours after treatment the ratio of the fraction of dead cells was nearly constant at a median of 4.7 % and 4.5 % in the untreated control as well as in the TMZ-BioShuttle treated cells. The average of the TMZ treated lymphocytes was increased to 6.2 %. Six days after treatment the amount of dead cells was increased but constant on the untreated lymphocytes and in the TMZ-BioShuttle at 10.2 % whereas the TMZ-probe showed 14.2 % dead cells.
It turned out that high-resolution flow cytometric measurements are suitable for monitoring the therapeutic success at the cellular level.
The achievement of relevant high local concentrations of therapeutic substances at the side of action, like in the nuclei of tumor cells was realized using delivery systems like the modularly composed peptide-based BioShuttle which brings chemical agents like TMZ into cells and in a second step into the cell nuclei. Regardless the cell's aneuploid state, responsible for the restricted pharmacological effects of chemotherapeutic agents, the local concentration is sufficient to overcome the intractability for cell killing under protection non-affected cells and the surrounding healthy tissue.
Aneuploidy is considered as the primary cause of the high rates and wide ranges of drug resistance in cancer cells.
Discussion
The outcome after chemotherapy still shows poor results with respect to overall survival in the treatment of advanced HRPC [31-33]. The resistance against therapeutic interventions is not completely understood, but various factors may be considered:
The behaviour of this form of PCas could be explained by either the loss of the homoeostasis's controls between cell proliferation and programmed cell death (apoptosis) [34, 35]. Tumorigenesis and progression are independent processes initiated and boosted by aberrant activation of cell cycle activating pathways but also by the inactivation of cell death associated signals resulting in the loss of the proliferation control and in the augmented resistance against apoptosis respectively [36]. Additionally recent data indicate that the inhibition of apoptosis is not associated with the transformation process to malign cells [37]. But affected cells show a prolonged cellular survival time and rate [38] compared with normal tissue. Both events can be detected in highly aggressive prostate cancer resistant to chemo- or /and radiation therapy. Anti-angiogenesis strategies avoiding the disappointing results are discussed [39]. The increasing understanding of molecular mechanisms and of the complex regulatory cellular network gives reason for several molecular approaches with high sensitivity and specificity for successful therapeutic intervention with lower side effects. Several approaches, like siRNA [40], Human-Antigen R (HuR) [41], [A+U]-rich element (ARE) [42], opener/closer mediated [43, 44] gene regulation [45] could be promising strategic approaches [46] in the treatment of HRPC.
But we are at the beginning and up to the clinical practice large scores of hurdles must to be taken. Until then, it must be resorted to reliable currently available drugs like the alkylating agent temozolomide (TMZ). The TMZ new-formulation with the focus enhancing the TMZ transport into the almost untreatable glioblastoma multiforme (GBM) is well documented and revealed higher pharmacological effect as TMZ alone.[47] Recent data indicate, compared to different primary glioblastoma cells, a lower sensitivity of DU 145 prostate cancer cells against TMZ treatment whereas all probes TMZ-BioShuttle treated showed dramatic cell cycle responses and diminished cell viability. [48]
The increased amount of diploid cells in the G1 phase after TMZ treatment suggests a cell trapping of the diploid cells in the G2/M phase. It is well documented that both cell cycle phase points G1 and G2/M represent check points for control and repair maintaining the genomic DNA-integrity.[49-51] Therefore among other things, both phases are characterized by low sensitivity against DNA-damaging interventions like exposition to ionizing radiation and after chemotherapeutic alkylation [52], whereas the latter part of the S phase is highly sensitive against DNA-damaging effectors.[53] Presumably the block of the diploid cell fraction of the DU 145 cells in the G1 phase allows the DNA-repair and subsequently the re-entry in the cell cycle. Therefore these cells turn out to be refractory against TMZ as shown in the TMZ-based therapy of advanced CaP.
In contrast to the described DNA fragmentation of glioma cells after TMZ treatment [54] the TMZ-BioShuttle treated glioma cells exhibited a deviant pattern: no comet formation indicating DNA single-strand breaks but cells swollen were observed.[48] This results from a loss of plasma membrane integrity which suggests nuclear chromatin decondenzation considered as necrosis biomarker.[55, 56] The observation, that the TMZ and the TMZ-BioShuttle influence unequally the cell cycle behaviour of DU 145 prostate cancer cells could indicate a mode of action different from the documented methylation of the O6 position of guanine in the genomic DNA [57].
It remains to speculate to which extent the aneuploidy state of the DU 145 cells could influence the pharmacological effect of the TMZ on the proliferation behaviour. Flow cytometric cell cycle studies exhibit an enhanced fraction of S phase cells of the numeric aberrant chromosomes harbouring cells. DU 145 karyotype analyses show the threefold existence of the chromosome 8. The impact of alterations of chromosome 8 and the high-grade state of advanced prostate carcinoma is well documented and appears to be associated with poor prognosis [58]. We would like to emphasize that alterations of the chromosome 8 indicate an early critical step in the prostate tumorigenesis [59]. C-myc is localized at chromosome 8 [60] and its overrepresentation is associated with prostate cancer progression [61]. Due to the fact that prostate cancer is one of the leading causes of death in the industrialized world, the need of new approaches for control the CaP is the major goal in the current research.
As the results show the DNA-cytometry proves to be a dedicated diagnostic tool in the cytopathology by measurements of the DNA-content in cells and tissues. Within the scope of the tumor diagnostics, objective and valid gradiations of the malignant potential of cells of different tumors and inside of a tumor (in process control) are possible. For the purpose of the malignant grading the extent of the DNA-aneuploidy must be quantified. Given the fact that different tumor identities present different secondary and tertiary aberrations of chromosomes during the tumor progression, the prognostic interpretation of the DNA-distribution must be realized tumor-specifically. In case of CaP (early state) the DNA malignant grading allows relevant early therapeutic decisions. High resolution flow cytometry is an appropriate tool not restricted to the monitoring of the therapeutic effect. DNA aneuploidy, as determined with high-resolution flow cytometry, has been shown to be an excellent and independent predictor of cell survival [62].
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. 2007. CA Cancer J Clin. 2007;57:43-66
2. Li Y, Cozzi PJ. Targeting uPA/uPAR in prostate cancer. Cancer Treat. Rev. 2007;33(6):521-7
3. Catalona WJ, Smith DS. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol. 1994;152:1837-1842
4. Walsh PC. The status of radical prostatectomy in the United States in 1993: where do we go from here? J. Urol. 1994;152:1816
5. Hanks GE, Hanlon AL, Epstein B, Horwitz EM. Dose response in prostate cancer with 8-12 years' follow-up. International Journal of Radiation Oncology Biology Physics. 2002;54:427-435
6. Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, Venkatramen ES, Reuter VE, Fair WR, Ling CC, Fuks Z. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1998;41:491-500
7. Bratt O. The urologist's guide to low dose-rate interstitial brachytherapy with permanent seed implants for localized prostate cancer. BJU. Int. 2007;99:497-501
8. Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238-244
9. Bloomfield DJ, Krahn MD, Neogi T, Panzarella T, Smith TJ, Warde P, Willan AR, Ernst S, Moore MJ, Neville A, Tannock IF. Economic evaluation of chemotherapy with mitoxantrone plus prednisone for symptomatic hormone-resistant prostate cancer: based on a Canadian randomized trial with palliative end points. J. Clin. Oncol. 1998;16:2272-2279
10. Autorino R, Di LG, Damiano R, De PS, D'Armiento M. Role of chemotherapy in hormone-refractory prostate cancer. Old issues, recent advances and new perspectives. Urol Int. 2003;70:1-14
11. Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de WR, Eisenberger M. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J. Clin. Oncol. 2007;25:3965-3970
12. Garrett-Mayer E, Parmigiani G, Zhong X, Cope L, Gabrielson E. Cross-study validation and combined analysis of gene expression microarray data. Biostatistics. 2008Apr;9(2):333-54
13. Hainsworth JD, Meluch AA, Spigel DR, Barton J, Simons L, Meng C, Gould B, Greco FA. Weekly docetaxel and bortezomib as first-line treatment for patients with hormone-refractory prostate cancer: a Minnie Pearl Cancer Research Network phase II trial. Clin. Genitourin. Cancer. 2007;5:278-283
14. Di LG, Autorino R, Giuliano M, Morelli E, Giordano A, Napodano G, Russo A, Benincasa G, D'Armiento M, Altieri V, De PS. Phase II trial of gemcitabine, prednisone, and zoledronic acid in pretreated patients with hormone refractory prostate cancer. Urology. 2007;69:347-351
15. Ueda T, Suzuki H, Akakura K, Ishihara M, Kamiya N, Komiya A, Shimbo M, Suyama T, Sakamoto S, Ichikawa T. Bisphosphonate and low-dose dexamethasone treatment for patients with hormone-refractory prostate cancer. Hinyokika Kiyo. 2006;52:515-521
16. Abratt RP, Brune D, Dimopoulos MA, Kliment J, Breza J, Selvaggi FP, Beuzeboc P, Demkow T, Oudard S. Randomised phase III study of intravenous vinorelbine plus hormone therapy versus hormone therapy alone in hormone-refractory prostate cancer. Ann. Oncol. 2004;15:1613-1621
17. Reilly DS, Tomassini N, Bevins CL, Zasloff M. A Paneth cell analogue in Xenopus small intestine expresses antimicrobial peptide genes: conservation of an intestinal host-defense system. J. Histochem. Cytochem. 1994;42:697-704
18. Small EJ, Srinivas S, Egan B, McMillan A, Rearden TP. Doxorubicin and dose-escalated cyclophosphamide with granulocyte colony-stimulating factor for the treatment of hormone-resistant prostate cancer. J. Clin. Oncol. 1996;14:1617-1625
19. Mike S, Harrison C, Coles B, Staffurth J, Wilt TJ, Mason MD. Chemotherapy for hormone-refractory prostate cancer. Cochrane. Database Syst. Rev. 2006Oct18(4):CD005247
20. Trudeau ME, Crump M, Charpentier D, Yelle L, Bordeleau L, Matthews S, Eisenhauer E. Temozolomide in metastatic breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada - Clinical Trials Group (NCIC-CTG). Ann. Oncol. 2006;17:952-956
21. van Brussel JP, Busstra MB, Lang MS, Catsburg T, Schroder FH, Mickisch GH. A phase II study of temozolomide in hormone-refractory prostate cancer. Cancer Chemother. Pharmacol. 2000;45:509-512
22. Duesberg P, Li R, Sachs R, Fabarius A, Upender MB, Hehlmann R. Cancer drug resistance: the central role of the karyotype. Drug Resist. Updat. 2007;10:51-58
23. Dahiya R, Yoon WH, Boyle B, Schoenberg S, Yen TS, Narayan P. Biochemical, cytogenetic, and morphological characteristics of human primary and metastatic prostate cancer cell lines. Biochem. Int. 1992;27:567-577
24. Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer. 1978;21:274-281
25. Ehemann V, Hashemi B, Lange A, Otto HF. Flow cytometric DNA analysis and chromosomal aberrations in malignant glioblastomas. Cancer Lett. 1999;138:101-106
26. Braun K, Peschke P, Pipkorn R, Lampel S, Wachsmuth M, Waldeck W, Friedrich E, Debus J. A biological transporter for the delivery of peptide nucleic acids (PNAs) to the nuclear compartment of living cells. J. Mol. Biol. 2002;318:237-243
27. Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. Inverse-electron-demand Diels-Alder reaction as a highly efficient chemoselective ligation procedure: Synthesis and function of a BioShuttle for temozolomide transport into prostate cancer cells. J. Pept. Sci. 2009;15:235-241
28. Ehemann V, Sykora J, Vera-Delgado J, Lange A, Otto HF. Flow cytometric detection of spontaneous apoptosis in human breast cancer using the TUNEL-technique. Cancer Lett. 2003;194:125-131
29. Singer S, Ehemann V, Brauckhoff A, Keith M, Vreden S, Schirmacher P, Breuhahn K. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007Sep;46(3):759-68
30. Tschaharganeh D, Ehemann V, Nussbaum T, Schirmacher P, Breuhahn K. Non-specific Effects of siRNAs on Tumor Cells with Implications on Therapeutic Applicability Using RNA Interference. Pathol. Oncol. Res. 2007;13:84-90
31. Stathopoulos GP, Koutantos J, Vaslamatzis MM, Athanasiadis A, Papadopoulos G, Labrodimou G, Stathopoulos J, Rigatos S. Survival after cytotoxic chemotherapy in patients with advanced hormone-resistant prostate cancer: A phase II study. Oncol. Rep. 2009;22:345-348
32. Chambers HF, Moreau D, Yajko D, Miick C, Wagner C, Hackbarth C, Kocagoz S, Rosenberg E, Hadley WK, Nikaido H. Can penicillins and other beta-lactam antibiotics be used to treat tuberculosis? Antimicrob. Agents Chemother. 1995;39:2620-2624
33. Newton RU, Taaffe DR, Spry N, Gardiner RA, Levin G, Wall B, Joseph D, Chambers SK, Galvao DA. A phase III clinical trial of exercise modalities on treatment side-effects in men receiving therapy for prostate cancer. BMC. Cancer. 2009;9:210
34. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342-348
35. Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr. Opin. Immunol. 1994;6:279-289
36. Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366-374
37. Jurgensmeier JM, Bauer G. Interference of bcl-2 with intercellular control of carcinogenesis. Int. J. Cancer. 1997;71:698-704
38. Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225-3236
39. Li Y, Cozzi PJ. Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev. 2009 [Epub ahead of print]
40. Mu P, Nagahara S, Makita N, Tarumi Y, Kadomatsu K, Takei Y. Systemic delivery of siRNA specific to tumor mediated by atelocollagen: Combined therapy using siRNA targeting Bcl-xL and cisplatin against prostate cancer. Int. J. Cancer. 2009;125(12):2978-90
41. Cherry J, Karschner V, Jones H, Pekala PH. HuR, an RNA-binding protein, involved in the control of cellular differentiation. In Vivo. 2006;20:17-23
42. Zhang T, Kruys V, Huez G, Gueydan C. AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors. Biochem. Soc. Trans. 2002;30:952-958
43. Niesporek S, Kristiansen G, Thoma A, Weichert W, Noske A, Buckendahl AC, Jung K, Stephan C, Dietel M, Denkert C. Expression of the ELAV-like protein HuR in human prostate carcinoma is an indicator of disease relapse and linked to COX-2 expression. Int. J. Oncol. 2008;32:341-347
44. Meisner NC, Hackermuller J, Uhl V, Aszodi A, Jaritz M, Auer M. mRNA openers and closers: modulating AU-rich element-controlled mRNA stability by a molecular switch in mRNA secondary structure. Chembiochem. 2004;5:1432-1447
45. Lammers T, Subr V, Peschke P, Kuhnlein R, Hennink WE, Ulbrich K, Kiessling F, Heilmann M, Debus J, Huber PE, Storm G. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br. J. Cancer. 2008;99:900-910
46. Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, Hennink WE, Storm G. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30:3466-3475
47. Braun K, Wiessler M, Ehemann V, Pipkorn R, Spring H, Debus J, Didinger B, Koch M, Muller G, Waldeck W. Treatment of glioblastoma multiforme cells with temozolomide-BioShuttle ligated by the inverse Diels-Alder ligation chemistry. Drug Design. Development and Therapy. 2008;2:289-301
48. Waldeck W, Wiessler M, Ehemann V, Pipkorn R, Spring H, Debus J, Didinger B, Mueller G, Langowski J, Braun K. TMZ-BioShuttle--a reformulated temozolomide. Int. J. Med. Sci. 2008;5:273-284
49. Powell SN, Bindra RS. Targeting the DNA damage response for cancer therapy. DNA Repair (Amst). 2009;8:1153-1165
50. Shimada M, Nakanishi M. DNA damage checkpoints and cancer. J. Mol. Histol. 2006;37:253-260
51. Damia G, Broggini M. Cell cycle checkpoint proteins and cellular response to treatment by anticancer agents. Cell Cycle. 2004;3:46-50
52. Fournier C, Taucher-Scholz G. Radiation induced cell cycle arrest: an overview of specific effects following high-LET exposure. Radiother. Oncol. 73 Suppl. 2004;2:S119-S122
53. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:928-942
54. Trog D, Moenkemann H, Breipohl W, Schueller H, Schild H, Golubnitschaja O. Non-sufficient cell cycle control as possible clue for the resistance of human malignant glioma cells to clinically relevant treatment conditions. Amino. Acids. 2007;32:373-379
55. Lukusa T, Fryns JP. Human chromosome fragility. Biochim. Biophys. Acta. 2008;1779:3-16
56. Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin. Nephrol. 1998;18:505-518
57. Tisdale MJ, Antitumor imidazotetrazines--XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem. Pharmacol. 1987;36:457-462
58. Sato K, Qian J, Slezak JM, Lieber MM, Bostwick DG, Bergstralh EJ, Jenkins RB. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J. Natl. Cancer Inst. 1999;91:1574-1580
59. Emmert-Buck MR, Vocke CD, Pozzatti RO, Duray PH, Jennings SB, Florence CD, Zhuang Z, Bostwick DG, Liotta LA, Linehan WM. Allelic loss on chromosome 8p12-21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995;55:2959-2962
60. DePinho RA, Schreiber-Agus N, Alt FW. myc family oncogenes in the development of normal and neoplastic cells. Adv. Cancer Res. 1991;57:1-46
61. Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57:524-531
62. van Heerden WF, Dreyer L, Swart TJ, van Heerden MB, Boy SC. The suitability of paraffin-embedded material to predict metastatic potential of oral squamous cell carcinoma. Anticancer Res. 2002;22:4147-4150
Author contact
![]() Correspondence to: Dr. Klaus Braun, Medical Physics in Radiology, Deutsches Krebsforschungszentrum (DKFZ), Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. Tel: +49 6221 42 2495; Fax: +49 6221 42 3326. k.braunde
Correspondence to: Dr. Klaus Braun, Medical Physics in Radiology, Deutsches Krebsforschungszentrum (DKFZ), Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. Tel: +49 6221 42 2495; Fax: +49 6221 42 3326. k.braunde

 Global reach, higher impact
Global reach, higher impact