3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(3):135-136. doi:10.7150/ijms.6.135 This issue Cite
Short Communication
Laboratory diagnosis of Toxoplasma gondii infection
Department of Pathology and Laboratory Medicine,Section of Microbiology, University of Parma, Parma (Italy)
Published 2009-3-19
Toxoplasma gondii is an obligate intracellular protozoan responsible for infections throughout the world in a wide range of hosts including humans. Primary infection is usually subclinical but in some patients cervical lymphadenopathy or ocular disease can be present [1]. Infection acquired during pregnancy may cause severe damage to the fetus. In immunocompromised patients, reactivation of latent infection can cause life-threatening encephalitis [1]. T. gondii infection can be diagnosed indirectly with serological methods and directly by Polymerase Chain Reaction (PCR), hybridisation, isolation, and histology. Whereas indirect serological methods are widely used in immunocompetent patients, definitive diagnosis in immunocompromised people is mostly undertaken by direct detection of the parasite [1].
In our laboratory the diagnosis of infection by T. gondii is carried out by the detection of specific anti-Toxoplasma immunoglobulin (IgM and IgG) and to discriminate chronic from reactivated infection IgG avidity is also determined with VIDAS instrument (bioMerieux, France) [2]; moreover, the diagnosis of toxoplasmosis using bioptic tissue samples, blood, and urine is done detecting T. gondii DNA by a Real-Time PCR Fluorescence Resonance Energy Transfer (FRET), targeting T. gondii 529 bp repeated region [3]. The extraction of DNA is performed from samples by the MagNA Pure LC instrument (Roche Diagnostics, Mannheim, Germany), according to the manufacturer's specifications. For FRET PCR, we used the LightCycler Fast-StartDNA MasterPLUS Hybridization Probes Kit (Roche Diagnostics), as previously described [3].
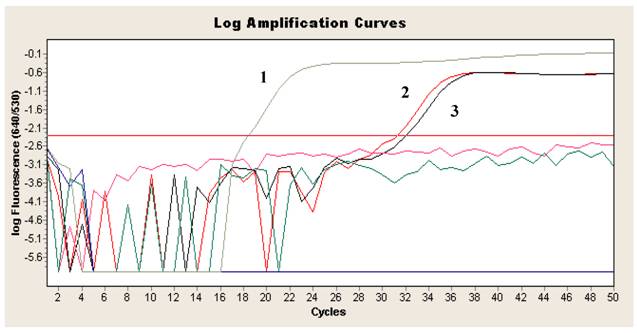
During the period 2006-2008 a total of 34,603 sera were sent to our laboratory for the diagnosis of infection by T. gondii. Specific IgM were detected in 1,287 sera (3.7%), while 33,135 sera (95.8%) were negative and 181 (0.5%) equivocal. Specific IgG were detected in 7,328 sera (21.2%), while 26,951 sera (77.9%) were negative and 324 (0.9%) equivocal. Among the 34,603 samples analyzed, 88 were from ophthalmological clinic of our University Hospital, with 43 samples (48.9%) positive, and 1 (1.1%) equivocal for IgG, 4 samples (4.5%) positive and 1 (1.1%) equivocal for IgM, 44 samples (50%) negative for IgG and 83 samples (94.3%) negative for IgM. In two Human Immunodeficiency Virus (HIV) infected patients with IgG positive and IgM negative for T. gondii, the use of Real-Time FRET PCR was successfully used to diagnose T. gondii retinochoroiditis. For both patients serological assays revealed the absence of IgM and the presence of IgG anti-Cytomegalovirus. Antibodies anti-Toxocara spp. were negative for one patient and not done for the other one. In both patients eosinophilia was not detected. The first patient presented with a progressive deterioration of sight and conjunctival hyperaemia associated with pain and at the fundus oculi examination, the ophthalmologist found out the presence of an active retinochoroiditis. The second patient had a deterioration of sight associated with the presence of a retinic scar, subjected to a biopsy to define its aetiology. For both patients the detection of T. gondii DNA by FRET PCR performed on a small volume of aqueous humor (<100 μl) gave a positive result (Figure 1), allowing to confirm the clinical suspect of T. gondii retinochoroiditis. In conclusion, as already reported in the literature, amplification of T. gondii DNA is a useful tool to support or confirm the diagnosis of toxoplasmosis in subjects with IgG positive for T. gondii as the cause of the retinal lesions in both aqueous humor and vitreous fluid in humans, considering that the detection of local specific antibodies may only provide indirect evidence of ocular infection [4]. Moreover, it's important to highlight that FRET PCR is able to detect the presence of T. gondii DNA on a small amount of sample as it was in our experience (<100 μl) from ocular compartment.
Log amplification curve of Real-time PCR FRET for the detection of T. gondii DNA in aqueous humor showing positive result. 1 = T. gondii DNA positive control; 2, 3 = patient sample (aqueous humor) tested in duplicate.
References
1. Montoya J.G. Liesenfeld O. Toxoplasmosis. The Lancet. 2004;363:1965-1976
2. Calderaro A, Piccolo G, Peruzzi S, Gorrini C, Chezzi C, Dettori G. Evaluation of Toxoplasma gondii Immunoglobulin G (IgG) and IgM Assays Incorporating the New Vidia Analyzer System. Clin. Vaccine Immunol. 2008;15:1076-1079
3. Calderaro A, Piccolo G, Gorrini C, Peruzzi S, Zerbini L, Bommezzadri S, Dettori G, Chezzi C. Comparison between two Real-time PCR assays and a nested-PCR for the detection of Toxoplasma gondii. Acta Biomed. 2006;77:75-80
4. Contini C, Seraceni S, Cultrera R, Incorvaia C, Sebastiani A, Picot S. Evaluation of a Real-time PCR-based assay using the lightcycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int J Parasitol. 2005;35:275-283

 Global reach, higher impact
Global reach, higher impact