Impact Factor
ISSN: 1449-1907
Int J Med Sci 2007; 4(4):196-202. doi:10.7150/ijms.4.196 This issue Cite
Research Paper
Inhibition by Natural Dietary Substances of Gastrointestinal Absorption of Starch and Sucrose in Rats and Pigs: 1. Acute Studies
1. Georgetown University Medical Center, Department of Physiology, Washington, D.C. 20057, USA
2. Department of Pharmacy Sciences, Creighton University Medical Center, Omaha, NE 68178, USA
3. Advocare International, Carrollton, TX 75006, USA
Received 2007-3-1; Accepted 2007-8-3; Published 2007-8-6
Abstract
Rapid gastrointestinal absorption of refined carbohydrates (CHO) is linked to perturbed glucose-insulin metabolism that is, in turn, associated with many chronic health disorders. We assessed the ability of various natural substances, commonly referred to as “CHO blockers,” to influence starch and sucrose absorption in vivo in ninety-six rats and two pigs. These natural enzyme inhibitors of amylase/sucrase reportedly lessen breakdown of starches and sucrose in the gastrointestinal tract, limiting their absorption. To estimate absorption, groups of nine SD rats were gavaged with water or water plus rice starch and/or sucrose; and circulating glucose was measured at timed intervals thereafter. For each variation in the protocol a total of at least nine different rats were studied with an equal number of internal controls on three different occasions. The pigs rapidly drank CHO and inhibitors in their drinking water. In rats, glucose elevations above baseline over four hours following rice starch challenge as estimated by area-under-curve (AUC) were 40%, 27%, and 85% of their internal control after ingesting bean extract, hibiscus extract, and l-arabinose respectively in addition to the rice starch. The former two were significantly different from control. L-Arabinose virtually eliminated the rising circulating glucose levels after sucrose challenge, whereas hibiscus and bean extracts were associated with lesser decreases than l-arabinose that were still significantly lower than control. The glucose elevations above baseline over four hours in rats receiving sucrose (AUC) were 51%, 43% and 2% of control for bean extract, hibiscus extract, and L-arabinose, respectively. Evidence for dose-response of bean and hibiscus extracts is reported. Giving the natural substances minus CHO challenge caused no significant changes in circulating glucose concentrations, indicating no major effects on overall metabolism. A formula combining these natural products significantly decreased both starch and sucrose absorption, even when the CHO were given simultaneously. These results support the hypothesis that the enzyme inhibitors examined here at reasonable doses can safely lower the glycemic loads starch and sucrose.
Keywords: starch blockers, bean and hibiscus extracts, sucrose blockers, L-arabinose, hibiscus extract
1. INTRODUCTION
The overweight state and obesity are now recognized as attaining epidemic proportions in the United States and throughout the world [1-5]. Although the potential for excess fat accumulation and perturbed metabolism from ingesting diets high in refined CHO content has been recognized for many years [6-9], it is only recently that the general public, medical community, and food industry have taken this possibility to heart [10-12]. Seeking a remedy, many of the afflicted have turned to caloric-restricted diets proportionately low in refined carbohydrates (CHO) [13-15]. Some individuals have successfully lost weight on “low carb diets,” others are not prepared to accept this life style change. Issues ranging from the wisdom of replacing CHO with fat to the palatability of a diet severely depleted in CHO have led to procrastination. Accordingly, continual emergence of data supporting a positive correlation between excess refined CHO intake and obesity has made many investigators seek more practical means to duplicate results found with the stringent depletion of CHO in the diet. One alternative is to reduce the rapid gastrointestinal absorption of CHO in a manner similar to reports of decreased fat absorption with various fibers [16-18].
Numerous natural dietary substances possess inhibitory effects on enzymes that influence CHO absorption in the gastrointestinal tract -- the theory being that ingested starches and sucrose not broken down into smaller units will pass through the small intestines instead of being reabsorbed. Subsequently, the unabsorbed CHO are fermented distally by intestinal microbiota that can lead to a multitude of effects – some that may be beneficial toward body fat loss [19]. While the approach seems simple, what appears to be a sound hypothesis remains an elusive one to prove. Conclusive, difficult-to-refute results concerning the inhibitory and/or hypoglycemic effects of natural constituents such as bean extract, hibiscus extract, and L-arabinose are limited. Bean and hibiscus extracts have been reported to inhibit amylase [20-25], while L-arabinose inhibits sucrase [26-28].
The major purpose of the present study is to examine the potential of certain natural substances alone and combined in a formula to decrease or at least slow the gastrointestinal absorption of CHO. As a first approximation, we examined the ability of three natural ingredients known to inhibit amylase and/or sucrase – bean extract, hibiscus extract, and L-arabinose, as well as a formulation containing these three ingredients to influence starch and sucrose absorption in Sprague-Dawley rats.
2. METHODS AND PROCEDURES
Animals
The Animal Welfare Board at Georgetown University Medical Center approved the protocol for the investigation. Ninety-six male Sprague-Dawley rats (SD) were obtained from Taconic Laboratories (Germantown, NY). Rats ate regular rodent chow and drank water ad libitum and were maintained in a facility with constant temperature and a 12 hour light-dark phase. Adult rats, obtained at varying times, weighed between 344-442 grams at the start of this acute study. Two Yorkshire pigs, initially weighing approximately 20 Kg, were obtained from Thomas D. Morris, Inc., Reisterstown, MD and were allowed free access to food and water.
Protocols
In the studies, there were two variables. The first variable was the oral CHO challenge that consisted of no CHO (control), rice starch, sucrose, or combined rice starch and sucrose. The second factor was the potential blocker to be examined such as bean extract, hibiscus extract, L-arabinose, or a formula containing these three ingredients.1
Rats were deprived of food the night before each testing (approximately 17 h). A baseline blood was then drawn. One half hour prior to the CHO challenged, SD were gavaged with either two ml of water alone of two ml of water containing the inhibitor(s), i.e., 0.5 grams of each ingredient(s) (bean and hibiscus extracts, L-arabinose, and the formulation described below) were given. At the moment of CHO challenge, rats again received either a gavage of two milliliters of water alone or two milliliters of water containing the same inhibitor(s) as in the preceding one-half hour plus either two grams rice starch, sucrose, or combined rice starch (2 g) and sucrose (2 g). Thus, each test rat received a total of one gram of an inhibitor or the formulation. A drop of blood was obtained from the tail at baseline (time 0), 1 hour, 2 hours, 3 hours and 4 hours after the final challenge for glucose determinations. The total amount of blood drawn in a rat for a given study was below 0.5 ml. Glucose was estimated using commercial glucose strips (Lifescan, One Touch Ultra, Melitas, CA).
In a given daily procedure, three rats were examined in a test situation. Three additional SD received a comparable volume of water and served as internal controls to account for any daily variations in test results. Since each test situation was examined at three different time intervals, nine datum points were obtained for both control and test in any given situation. The same rat was not tested more than once during a three-week interval, or more than four times in all.
Two Yorkshire pigs, weighing approximately 70 and 90 kg at the initiation of study, were deprived of food and water for 2 hours at the time of study. Then, they were given challenges of 200 g sucrose (table sugar) and/or 100 g rice starch individually or combined in enough drinking water to solubilize the constituents. This fluid mixture was consumed totally within minutes. To complete an investigation on each challenge, two separate procedures were run on the two pigs. In the first, pig 1 was control and pig 2 was the test animal receiving the CHO blocker. In the second, the roles were reversed. Thus, each pig could serve as his own control. When a pig served as test, it was given the contents of four capsules of the formulation described below in the drinking water along with the CHO challenges. At baseline and the selected times, a drop of blood from the ear was used to measure circulating glucose concentrations. The total amount of blood obtained at a single testing amounted to less than 0.5 ml.
Ingredients
The individual test ingredients as well as the formulation were obtained from AdvoCare International, Carrollton, Texas. The formula was composed of w/w: dry bean extract (seed - Phaseolus vulgaris) 19%, hibiscus extract (flower - Hibiscus sabdariffa) 31%, L-arabinose 31%, gymnema extract ((leaf - Gymnema sylvestre) 12%, green tea extract leaf - (Camellia sinensis) 6%, and apple extract (fruit - Malus sylvestris) 1% plus the addition of Lactobacillus acidophilus and Bifidobacterium bifidum.
Statistical Analyses
Results are presented as mean ± SEM. Where a significant effect of regimen was detected by ANOVA (repeated measures) (p<0.05), the Dunnett t test was used to establish which differences between means reached statistical significance [29]. When the data from two columns of data were analyzed at a single time point, Student's t test was used. Statistical significance was set at p < 0.05.
3. RESULTS
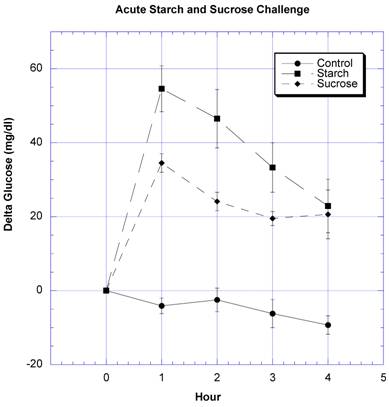
To develop a testing procedure, rice starch or sucrose challenges were carried out individually on SD rats and compared to the control situation in which rats received a similar volume of fluid (water only) (Fig. 1). Following the respective challenges of rice starch or sucrose, the appearance of glucose above baseline (delta) increased significantly, the highest measured point at one hour with a decrease over the remaining three hours. The circulating glucose levels decreased below baseline over the course of the four hours in the control rats, which had been fasted overnight and received only water, i.e., no CHO challenge.
The changes in circulating glucose at timed intervals after challenges with water (control), rice starch and sucrose are shown. Mean ± SEM is depicted for a minimum of 9 rats in each group.
All rats were gavaged with 2 ml water – no CHO challenge. One half hour prior to the water challenges and at the time of challenges a total of 2 ml of water (control), or 1 gram of bean extract, hibiscus, or L-arabinose in 2 ml water was given. The change in circulating glucose at timed intervals after various challenges is depicted. Mean ± SEM is depicted for nine rats. * Significantly different at that time point when compared to control.
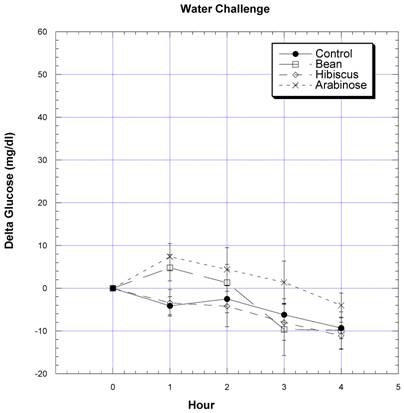
Bean extract, hibiscus extract, and L-arabinose were tested for their effects on rats receiving only water (no CHO challenge) (Fig. 2). In these rats starved overnight and deprived of food for the four hour study, the blood glucose levels of rats receiving only water tended to decrease, resembling the earlier findings depicted in Fig. 1. The circulating glucose pattern was essentially no different than control after the SD rats had been given bean extract, hibiscus extract, or L-arabinose.
The effects of three natural elements, bean extract, hibiscus extract, and L-arabinose, on glucose appearance in the circulating blood after sucrose challenge are depicted in Table 1. The average circulating glucose level after the 17 h deprivation of food was 88.4 mg/dl ± 1.4 (SEM) with a range of 72 mg/dl to 105 mg/dl. L-Arabinose proved to be very effective, i.e., the appearance of glucose in the blood stream after gavage of sucrose was essentially non-existent. Area under the curve was only 2% of control. Interestingly, both hibiscus and bean extracts also decreased glucose appearance compared to control after sucrose challenge over the first three hours, although at comparable doses, bean and hibiscus extracts were not as effective as L-arabinose. The glucose elevations above baseline at two hours (mg/dl ± SEM) were: 24.1±2.5 for control, -5.7±3.7 for L-arabinose, 9.8±8.5 for bean, and 8.1±2.3 for hibiscus. All interventions were statistically significantly different from control. Areas under the curve averaged 51% for bean extract and 43% for hibiscus extract compared to control.
The effects of three natural products (bean extract, hibiscus extract, and L-arabinose) on glucose appearance in the circulating blood after rice starch challenge are also depicted in Table 1. L-Arabinose had only a small, insignificant effect on the appearance of blood glucose after the rice starch challenge, i.e., there were no statistically significant differences at any of the time points compared to control. Area under the curve was 85% of the control. In contrast, both bean and hibiscus extracts significantly lowered the appearance of circulating glucose compared to control following the rice starch challenge -- at the first and second hours for bean extract and at the first, second and third hours for hibiscus. The glucose elevations above baseline at two hours (mg/dl ± SEM) were: 46.5±7.9 for control, 14.7±10.0 for bean, 5.9±3.3 for hibiscus, and 39.0±8.7 for L-arabinose. Findings for the bean and hibiscus extracts were statistically significantly different from control. Area under the curve was 40% for bean and 27% for hibiscus extracts after starch challenge compared to the control situation in which no natural inhibitor was given.
In additional studies, effects of increasing the doses of bean and hibiscus extracts by 50% to 100% compared to the original doses were examined (Table 2). For bean extract, a 50% increase and a doubling of the initial dose caused further lowering of the absorption of rice starch compared to the standard dose after one and two hours as estimated by the appearance of circulating glucose. Although glucose appearance for all doses was statistically lower than control, the differences among the various doses did not prove statistically significant. Results with hibiscus extract were somewhat similar in these studies, except at the original dose (1X) the value at the two hour period was not different from the one hour period, unlike the previous studies. This was not the case for the higher doses.
Carbohydrate Challenge Tests in Rats Using Different CHO Blockers
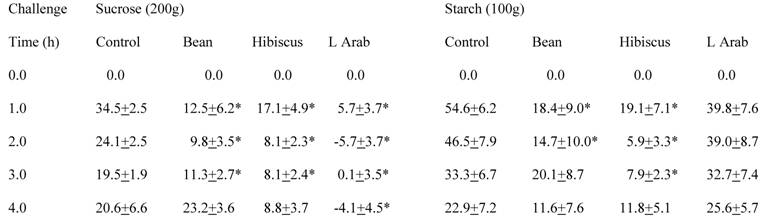
Circulating glucose levels above or below baseline after CHO challenge specified in heading.
Each number represents the average change in glucose concentrations (mg/dl) ± SEM of 9 rats.
*Statistically significantly different from control at that time point.
Dose-Response for Bean and Hibiscus Extracts in Rats One and Two Hours after Challenge
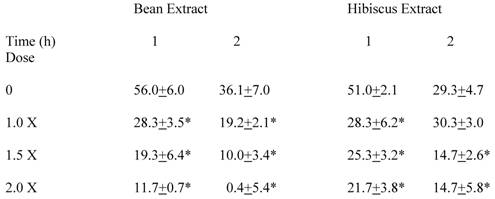
Circulating glucose levels above baseline after starch challenge at specified times.
Each value represents the average change in glucose concentrations (mg/dl) ± SEM of 9 rats.
* Statistically significantly different from control (Zero Dose).
Two doses of a formula of natural ingredients containing bean and hibiscus extracts and L-arabinose were examined, and these data are presented in Table 3. A one gram dose, designated “low dose”, and a “high dose”, composed of twice as much, were studied. Concerning the rice starch challenge, the higher dose was so effective that there was virtually no elevation of circulating glucose levels following the starch challenge. The area under the curve was negative to baseline. Despite not being as effective as the high dose, the lower dose of the formulation was still effective over the first two hours, but inexplicably the circulating glucose levels were higher than the control situation by the fourth hour. The area under the curve was 48% of control. After sucrose challenge, both doses were effective over three hours. At one and three hours, the higher dose caused a statistically greater lowering than the low dose. Similar to the case with the rice starch challenge, the high dose virtually prevented any rise in the circulating glucose levels after sucrose challenge. Area under the curve for the low dose was 47% and for the high dose was 6% of control.
The contents of four capsules of the CHO enzyme-inhibiting formulation were given when the pigs were challenged. The human dose is three to four capsules at one time. In Table 4, it can be seen that the addition of the formula containing the enzyme inhibitors significantly lowered the appearance of glucose in the circulating blood whether the challenge was starch alone, sucrose alone, or a combination of the two CHO. For example, 30 minutes after the starch challenge, the blood glucose increased above baseline by an average of approximately 25 mg/dl in the pigs in the absence of the enzyme-inhibiting formula, with essentially no increase in blood glucose when the formula was co-administered with the starch. Similar results were observed at the one hour time points following the sucrose challenge and combined starch plus sucrose challenge.
Carbohydrate Challenge Tests in Rats Using Two Doses of Formula
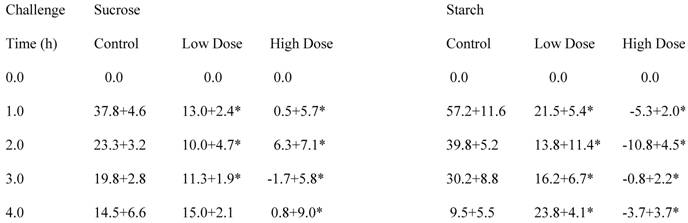
Circulating glucose levels above or below baseline after CHO challenge in control rats and two groups receiving different doses of formula
Each number represents the average change in glucose concentrations (mg/dl) ± SEM of 9 rats.
* Statistically significantly different from control.
Carbohydrate Challenge Tests on Two Pigs
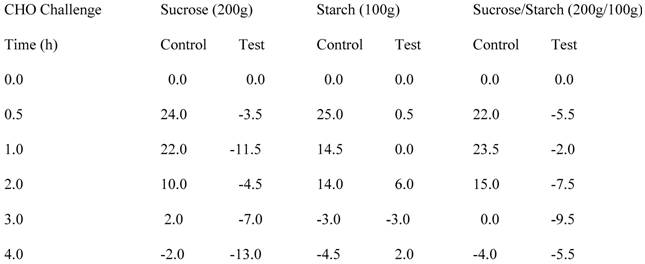
Circulating glucose levels above or below baseline (mg/dl) after CHO challenge specified in heading.
Test pigs received the equivalent of 4 capsules of the formula.
Each number represents the average values of the two pigs.
4. DISCUSSION
Few well-controlled animal studies (in vivo) of so-called CHO blockers are available [26,27,30,31]. Even less information exists comparing different inhibitors and examining dose-responses. The gavage of rice starch or sucrose causes a rapid appearance of glucose in the blood as depicted in Fig. 1. We chose this appearance to estimate the gastrointestinal breakdown of rice starch and sucrose. The hypothesis tested was that natural starch and sucrose enzyme inhibitors (amylase and sucrase) would diminish and/or slow the breakdown of starch and sucrose in the gastrointestinal tract, diminishing glucose entrance into the blood stream.
The actions of the bean and hibiscus extracts and L-arabinose on CHO absorption in the gastrointestinal tract differed somewhat. After the rice starch challenge, bean and hibiscus extracts at the same dose significantly decreased the appearance of glucose in the circulating blood to a reasonably similar extent (Table 1). In contrast, L-arabinose had no significant effect on this appearance after the starch challenge. The results were different when sucrose provided the challenge. L-Arabinose proved to be highly effective in preventing a rise in circulating glucose after sucrose challenge (Table 1). In fact, there was virtually no appearance of glucose after sucrose challenge when L-arabinose was given. Although less effective than L-arabinose, both bean and hibiscus decreased the absorption of sucrose significantly. When the doses of bean and hibiscus extract were increased, less glucose appeared in the circulation over the first and second hour following the higher doses compared to the lower doses (Table 2). These data suggest that there is a dose-response with bean and hibiscus extracts on circulating glucose after rice starch challenge.
Just as postulated, we believe that changes in the appearance of circulating glucose are due to the effects on CHO breakdown in the gut [20-28]. This concept was strengthened when it was shown that these natural ingredients did not affect circulating glucose levels unless the rats were challenged with rice starch or sucrose, i.e., these natural ingredients did not affect circulating glucose levels after a water challenge (Fig. 2). The fact that bean and hibiscus extracts blocked the appearance of glucose after sucrose challenge suggests the possibility that they may also have the additional ability to inhibit sucrase.
When a formula containing the above three ingredients was given to the rats, the acute appearance of glucose was diminished significantly whether the challenge was rice starch or sucrose (Table 3). When the dose of the formulation was doubled, i.e., two grams, the appearance of glucose was essentially nonexistent. In the latter case, the amounts of L-arabinose and hibiscus extract in the formula were only about one-third of the amounts in the direct challenge. Bean extract was only 19% by weight of the straight dosage. Therefore, combining ingredients might be useful to increase the over all effects. The formulation contained other ingredients not examined here (Gymnema sylvestre, apple extract, and green tea). We cannot state what role they played in the results.
In calculating human doses based on the doses used in our rats, the levels of inhibitors seemed unreasonable for common use. Therefore, we examined two pigs that possessed weights in a range common for human adults. In these studies, we accomplished significant decreases in glucose appearance in the blood stream from starch and/or sucrose challenge when using doses equivalent to those recommended in humans. Thus, our studies indicate that gastrointestinal absorption of starches and sugars can be lessened significantly with reasonable doses of CHO enzyme inhibitors.
In conclusion, examining extracts from bean and hibiscus at similar doses in rats shows them to be comparably effective in blocking rice starch absorption in rats. A positive dose-response was noted. Interestingly, these same ingredients also delayed sucrose absorption based on their ability to influence the appearance of circulating glucose after sucrose challenge. L-Arabinose slowed the absorption of sucrose, but not that of rice starch. The inability of any of these agents to influence circulating glucose when there was no CHO challenge confirms that they work mostly via affecting CHO absorption rather than on overall metabolism. When combined in a formula, these ingredients could slow absorption after the simultaneous challenge of sucrose and starch. When the formula was given to large pigs at the suggested human dosing, the inhibitors were quite effective in lowering the appearance of glucose in the circulation after sucrose and starch challenges alone and in combination. Accordingly, these findings lend support to the concept that natural, safe supplements can influence the glycemic load favorably and perhaps be beneficial for many aspects of health.
Acknowledgements
The investigation was supported with funds from AdvoCare International, Carrollton, Texas. Dr. Preuss is a member of the Scientific/Medical Advisory Board, and Dr. Stohs is the Senior Vice President for Research and Development of Advocare International.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
References
1. Bray GA. Obesity. Present Knowledge in Nutrition. Ziegler EE and Filer LJ, eds. Washington DC: ILSI Press. 1996:19-32
2. Guterman L. Obesity problem swells worldwide. The Chronicle of Higher Education. 2002 :A18
3. US Department of Health and Human Services. The Surgeon General's call to action to prevent and decrease over weight and obesity 2001. Washington, DC: US General Printing Office. 2001
4. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. Br Med J. 2000;320:1240-1243
5. Popkin BM, Paeratakul S, Zhai F, Keyou G. A review of dietary and environmental correlates of obesity with emphasis on developing countries. Obes Res. 1995;3:145S-153S
6. Szanto S, Yudkin J. The effect of dietary sucrose on blood lipids, serum insulin, platelet adhesiveness, and body weight in human volunteers. Postgrad Med J. 1969;45:602-607
7. Yudkin J. The low carbohydrate diet in the treatment of obesity. Postgrad Med. 1972;51:151-154
8. Yudkin J. Sugar and obesity. Lancet. 1983;2:794
9. Yudkin J. Sucrose, coronary heart disease, diabetes, and obesity. Do hormones provide a link? Am Heart J. 1988;115:493-498
10. Acheson KJ. Carbohydrate and weight control: where do we stand? Curr Opin Clin Nutr Metab Care. 2004;7:485-492
11. Harper A, Astrup A. Can we advise our obese patients to follow the Atkins diet? Obes Rev. 2004;5:93-94
12. Ornish D. Was Dr Atkins right? J Am Diet Assoc. 2004;104:537-542
13. Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homeostasis, and plasma lipids in animals. Lancet. 2004;364:778-785
14. Brehm BJ, Seeley RJ, Daniels SR, D'Allessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Fam Pract. 2003;52:515-516
15. Meckling KA, Gauthier M, Grubb R, Sanford J. Effects of a hypocaloric, low carbohydrate diet on weight loss, blood lipids, blood pressure, glucose tolerance, and body composition in free-living overweight women. Canad J Physiol Pharmacol. 2002;80:1095-1105
16. Ganji V, Kies CV. Psyllium husk fiber supplementation to soybean and coconut oil diets of humans: effect of fat digestibility and faecal fatty acid excretion. Eur J Clin Nutr. 1994;48:595-597
17. Wadstein J, Thom E, Heldman E, Gudmunsson S, Lilja B. Biopolymer L112, a chitosan with fat binding properties and potential as a weight reducing agent. In: (ed.) Muzzarelli RAA. Chitosan Per Os; From Dietary Supplement to Drug Carrier. Grottammare, Italy: Atec. 2000:65-76
18. Preuss HG, Kaats GR. Chitosan as a dietary supplement for weight loss. A review. Current Nutrition Reviews. 2006;2:297-311
19. Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, Jones CK, Tulley RT, Melton S, Martin RJ, Hegsted M. Effects pf resistant starch, a non-digetible fermentable fiber, on reducing body fat. Obesity. 2006;14:1523-1534
20. Udani J, Hardy M, Madsen DC. Blocking carbohydrate absorption and weight loss: a clinical trial using Phase 2 brand proprietary fractionated white bean extract. Altern Med Rev. 2004;9:63-69
21. Santimone M, Koukiekolo R, Moreau Y, Le Berre V, Rouge P, Marchis-Mouren G, Desseaux V. Porcine pancreatic alpha-amylase inhibition by the kidney bean (Phaseolus vulgaris) inhibitor (Alpha-AII) and structural changes in the alpha-amylase inhibitor complex. Biochim Biophys Acta. 2004;1696:181-190
22. Frels JM, Rupnow JH. Purification and partial characterization of two alpha-amylase inhibitors from black bean (Phaseolus vulgaris). J Food Biochem. 1984;1:385-401
23. Gibbs B, Alli I. Characterization of a purified alpha-amylase inhibitor from white kidney bean (Phaseolus vulgaris). Food Research International. 1998;31:217-225
24. Hansawasdi C, Kawabata J, Kasai T. Alpha-amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci Biotechnol Biochem. 2000;64:1041-1043
25. Hansawadi C, Kawabata J, Kasai T. Hibiscus acid as an inhibitor of starch digestion in the Caco-2 cell model system. Biosci Biotechnol Biochem. 2001;65:2087-2089
26. Seri K, Sanai K, Matsuo N, Kawakubo K, Xue C, Inoue S. L-arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppresses glycemic response after sucrose ingestion in animals. Metabolism. 1996;45:1368-1374
27. Osaki S, Kimura T, Sugimoto T, Hizukuri S, Iritani N. L-arabinose feeding prevents increases due to dietary sucrose in lipogenic enzymes and triacylglycerol levels in rats. J Nutr. 2001;131:796-799
28. Brudnak MA. Weight-loss drugs and supplements: are there safer alternatives? Medical Hypotheses. 2002;58:28-33
29. Dunnett C. A multiple comparison procedure for comparing several treatments with control. J Am Statis Assoc. 1955;50:1096-1121
30. Tormo MA, Gil-Exojo I, Romero de Tejada A, Campillo JE. Hypoglycemic and anorexigenic activities of an alpha-amylase inhibitor from white kidney beans (Phaseolus vulgaris) in Wistar rats. Br J Nutr. 2004;92:785-790
31. Deglaire A, Moughan PJ, Bos C, Tome D. Commercial Phaesolus vulgaris extract (starch stopper) increases ileal endogenous amino acid and crude protein losses in the growing rat. J Agric Food Chem. 2006;54:5197-5202
Author contact
![]() Correspondence to: Harry G. Preuss M.D., Georgetown University Medical Center, Basic Science Bldg, Room 231B, 4000 Reservoir Rd, NW, Washington, D.C. 20057, USA
Correspondence to: Harry G. Preuss M.D., Georgetown University Medical Center, Basic Science Bldg, Room 231B, 4000 Reservoir Rd, NW, Washington, D.C. 20057, USA
Footnotes
Carb-EaseTM, Advocare International, Dallas, TX

 Global reach, higher impact
Global reach, higher impact