3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2007; 4(3):159-163. doi:10.7150/ijms.4.159 This issue Cite
Research Paper
Efficiency of vibration exercise for glycemic control in type 2 diabetes patients
1. Institut für Physiologie und Anatomie, Deutsche Sporthochschule Köln, Germany and Trainingsinstitut Prof. Dr. Baum GmbH, Köln, Germany
2. Trainingsinstitut Prof. Dr. Baum GmbH, Köln, Germany
Received 2007-2-28; Accepted 2007-5-29; Published 2007-5-31
Abstract
Although it is well documented that persons suffering from diabetes type 2 profit from muscular activities, just a negligible amount of patients take advantage of physical exercises. During the last decade, vibration exercise (VE) could be established as an effective measure to prevent muscular atrophy and osteoporosis with low expenditure of overall exercise-time. Unfortunately, little is known about the metabolic effects of VE. In the present study we compared VE with the influence of strength training and a control group (flexibility training) on glycemic control in type 2 diabetes patients. Forty adult non-insulin dependent patients participated in the intervention. Fasting glucose concentration, an oral glucose tolerance test (OGTT), haemoglobin A1c (HbA1c), the isometric maximal torque of quadriceps muscles, and endurance capacity were evaluated at baseline and after 12 weeks of training with three training sessions per week. The main findings are: Fasting glucose concentrations remind unchanged after training. The area under curve and maximal glucose concentration of OGTT were reduced in the vibration and strength training group. HbA1c values tended to decrease below baseline date in the vibration training group while it increased in the two other intervention groups. Theses findings suggest that vibration exercise may be an effective and low time consuming tool to enhance glycemic control in type 2 diabetes patients.
Keywords: diabetes, vibration exercise, strength training, HbA1c, glycemic control
1. Introduction
Since some decades it is known that beside pharmacological treatments and body weight reduction endurance related exercises are able to enhance glycemic control in type II diabetes patients [13,18,22]. More recently, strength training also became an established treatment in that world-wide spreading metabolic disease [2,3,4,9]. However, till today only a negligible amount of patients take advantage of any sport activity. There are some reasons to explain that phenomenon, one of the most important may be that nearly all patients are obese and follow a lifelong sedentary life style. Obviously, these patients can hardly be motivated for longer lasting physical activities.
Vibration exercise is a new and effective measure to prevent muscular atrophy and osteoporosis [14,15,19,21]. It is assumed that vibrations with an amplitude of 2 to 6 mm and a frequency of 20 to 30 Hz evoke muscle contractions probably induced via the monosynaptic stretch reflex [17]. Compared to traditional training regimes, VE needs significant less time and, therefore, can be expected to reach a higher compliance in previously inactive patients. Unfortunately, rare information exists about the metabolic consequences of VE. It is just known that oxygen consumption increases with body weight as well as frequency and amplitude of VE [14]. In the present study we investigated the influence of a three month vibration-exercise period on parameters of glucose metabolism in type II diabetes patients. The results were compared to a control group (FT-group) and a group performing strength training (ST).
2. Research Design and Methods
Subjects characteristics and general experimental design
Prior to recruitment of subjects the study protocol was approved by the ethic committee of the German Sports University. Volunteers were included if they met three conditions: A diagnosed type II diabetes, not insulin depending, and not regularly involved in sport activities. All patients were under oral medication. Subjects were excluded if they suffered from retinopathy or other medical problems which did not allow for participating in vibration exercises or strength training.
Patients were encouraged to follow their habitual life style including medication throughout the whole investigation period. After verbal introduction of the study 40 subjects (24 male, 16 female) gave their written consent and participated in the study (Table 1). Subjects were randomly divided into three groups: A flexibility training group, a strength training group and a vibration training group. They trained for 12 weeks at three days per week. All sessions were supervised and participation assessed. Training volume and intensity were stepwise increased after 6 and 9 weeks. The detailed training regimen was as follows:
Flexibility training
Each FT session consisted of eight static exercises which involved main muscles of the upper and lower body. During the initial six weeks, one set was performed with the positions kept for 20 s each. From week 7 to 9 volume was increased by one more set. During the last three weeks two sets with exercise durations of 30 s were applied. The total training duration did not exceed 15 minutes per session.
Strength training
Commercially available weight machines (Conex© multiform) were used for strengthening muscle groups of the upper and lower body. Eight stations were included in each session, e.g. leg extension, seated leg flexion, leg press, seated calf raise, lat pulley, horizontal chest press, butterfly, and rowing. Subjects performed dynamic contractions with intermittent relaxations after each concentric-eccentric phase in order avoid critical blood pressure responses [1]. After familiarization with the correct movements, the one repetition maximum (1RM) was established prior to the training period. During the first six weeks of training, 1 set with 12 repetitions at 70 % of 1 RM was performed. From week 7 to 9 volume was increased by an additional set. In weeks 10 to 12, 3 sets with 10 repetitions at 80 % of 1 RM were realized. About 45 minutes was needed for a training session of the last three weeks.
Vibration exercise
Subjects exercised on a horizontal swinging platform with an amplitude of 2 mm (Vibrogym Professional©). Vibration frequency was set to 30 Hz from weeks 1 to 9 and to 35 Hz during the last three weeks. The duration of a single exercise bout was constant throughout the training period and amounted to 30 s. A training session consisted of 8 different exercises including muscles of the whole body (Fig. 1). Subjects were encouraged to work isometricaly against the swinging platform.The number of sets was identical with the strength training regimen. It took about 20 minutes to fulfill a training session of the last three weeks.
Baseline subjects characteristics. Mean ± SD
| Intervention group (number of subjects) | Age (years) | Weight (kp) | Height (cm) | Systolic blood pressure (mm Hg) | Diastolic blood pressure (mm Hg) |
|---|---|---|---|---|---|
| Stretching (13) | 63,3 ± 5,9 | 88,6 ± 24,1 | 173 ± 14,2 | 136 ± 13,8 | 83 ± 7,0 |
| Strength (13) | 62,9 ± 7,3 | 86,5 ± 14,7 | 172 ± 6,7 | 142 ± 16,2 | 87 ± 10,4 |
| Vibration (14) | 62,2 ± 4,0 | 83,3 ± 13,4 | 177 ± 7,2 | 137 ± 15,1 | 79 ± 7,3 |
Images of the eight exercises during vibration training.

Test procedure and Outcome Measures
Before and three to four days after the training period, subjects entered the laboratory after 12 h fasting and an oral glucose tolerance test was performed. Time of drug ingestion was individually kept constant prior to both tests. Micro-blood samples were taken from an ear lobe before and for 2,5 h every 30 min after administering a 75 g glucose drink (Dextro© O.G-T., Roche Diagnostics Ltd). Blood samples were analyzed by means of HemoCue© Glucose 201+ (HemoCue Ltd). HbA1C levels were determined by a HPLC-System (Tosoh G7, Eurogenics) from a blood sample taken from the antecubital vein.
On separate days, maximal torque of quadriceps muscles and endurance capacity were tested. Maximal torque was detected isometrically with participants in an upright sitting posture and the hip and knee joint flexed to 90o. A force transducer (Digimax© , Mechatronic Ltd) was used and the lever arm calculated as the distance between knee joint space and contact point of force transduction. The best of three trials of each leg were taken for further computation.
Endurance capacity was determined by an incremental cycle ergometry (Ergoline Ergoscript 2012 EL). Load was increased every 3 min for 25 w until lactic acid concentration exceeded 4 mmol/l. Heart rate (ECG leads) and lactic acid concentration (Accutrend© Lactate, Roche Mannheim) were measured at rest and within the last 30 s of each load.
In addition, body weight and blood pressure were measured before each training session. The mean values of the initial and last five sessions were taken for further computations.
Statistical analysis
Statistical analysis was conducted using SPSS version 12.0 for Windows. If not otherwise stated data are expressed as mean and standard deviation. The data were analyzed by analysis of variance for repeated measurements (factors: time and training form). In case that the two-factorial analysis yielded a significant result (p < 0,05), a Newman-Keuls test was performed as a posteriori test.
3. Results
Subjects characteristics
Body weight did not significantly change during the 3-month intervention. A mean reduction of 1,68 kp ± 4,57, 1,30 kp ± 2,36, and 0,86 kp ± 1,77 could be obtained for FT, ST, and VT, respectively. Systolic blood pressure decreased significantly (p < 0,05) in all intervention groups to 126 mm Hg ± 7,4, 133 mm Hg ± 16,4, and 123 mm Hg ± 12,5 for FT, ST, and VT, respectively. Diastolic blood pressure did not change significantly. Three subjects decreased their oral hypoglycemic medication dosage (two persons in ST and one person in VT group). No major complications or injuries were reported from either stretching, strength, or vibration training.
Endurance performance parameters
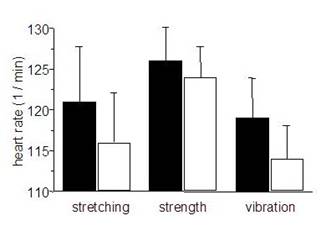
No significant differences in endurance capacity could be detected between pre and post-training in any group. Subjects reached 4 mmol [lactate] at loads of 89 w ± 8,2 (pre) and 86 w ± 9,7 (post), 99 w ± 14,8 (pre) and 95 w ± 13,3 (post), 89 w ± 6,2 (pre) and 92 w ± 5,9 (post) for FT, ST, and VT, respectively. In contrast, at these loads heart rate was reduced after the training intervention in all groups (Fig. 2), which became significant for VT.
Mean heart rates at loads corresponding to a lactic acid concentration of 4 mmol / l. gray bars = pretraining, black bars = posttraining. mean ± SE.
Strength
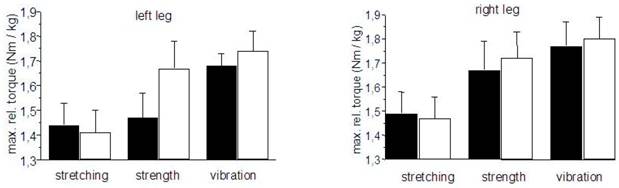
The relative maximal isometric torque of the quadriceps muscles increased after training in the ST and VT groups. A significant increase of 14 % could be obtained in subject`s left leg of the ST group (Fig. 3).
Relative maximal isometric torques of the quadriceps muscles. black bars = pre-training, hollow bars = post-training. mean ± SE.
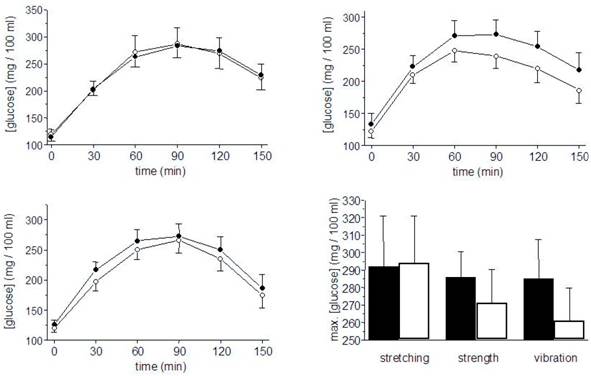
Glucose plasma-concentrations during OGTT before (black) and after the training period (hollow). Left top: Flexibility training group, left bottom: strength training group, right top: vibration training group. Right bottom: mean of individual maximal glucose concentrations. Mean ± SE.
Fasting glucose concentration and OGTT
After training intervention, fasting glucose concentrations were slightly reduced in all groups (n.s., Tab. 2). Within the 150 minutes observation period of OGTT, pre- and posttraining results in the FT group were nearly identical. In both ST and VT the integrals were reduced by 5,6 % and 6,3 %, respectively (p < 0,05 for both groups with no significant differences between both groups). Fig. 4 shows the time courses of all three training groups as well as the mean of individual maximal glucose concentrations.
Plasma fasting glucose concentrations before and after training intervention. Mean ± SD
| Intervention group | Plasma [glucose] pretraining (mg / 100 ml) | Plasma [glucose] posttraining (mg / 100 ml) | Significance |
|---|---|---|---|
| Flexibility | 120 ± 25 | 115 ± 22 | n.s. |
| Strength | 126 ± 23 | 120 ± 22 | n.s. |
| Vibration | 133 ± 57 | 122 ± 35 | n.s. |
HbA1C
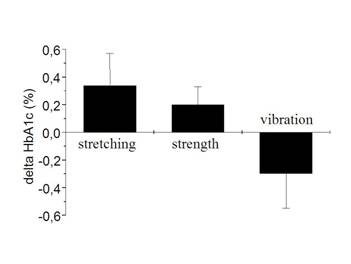
At baseline the HbA1c amounted to 6,7 % ± 0,26 (FT), 6,8 % ± 0,17 (ST), and 7,3 % ± 0,66 (VT) (Differences not significant). After training intervention a small decrease in HbA1c occurred in the VT-group (n.s.). In contrast, HbA1c values in the FT and ST group were elevated.
Net changes in HbA1c. Mean ±SE
4. Discussion
Twelve weeks of strength training increased muscular strength and did not affect the endurance capacity while stretching and vibration exercise improved neither strength nor endurance significantly. These results on physical performance parameters reflect just in part the outcome of glycemic control parameters: As it could be expected flexibility training failed to improve glycemic control and strength training showed beneficial effects. Although the duration of VT training sessions were about half of thoses of the strength group, the effect on OGGT was comparable. Obviously, there is a beneficial effect of vibration-exercise on glycemic control without detectable changes in physical performance parameters. A dominant influence of body weight changes appears unlikely since weight reduction was weakest in the VT group and strongest in the FT group. Vibrations applied on skeletal muscles activate muscle spindle receptors [10] and so enlarge the drive to alpha-motoneurons via the monosynaptic reflex [17]. Therefore, compared to exercises without vibrations it may be true that a higher number of motor units are activated. Beside some general health-related beneficial effects of exercise on skeletal muscles such as improvements of endothelial function [8] and an increased enzyme capacity of energy metabolism [13] there are two specific effects of contracting skeletal muscle cells on the ability to transport glucose into these cells: First, a regularly performed training increases the content of the glucose transporter protein GLUT-4 within the cells [9,12]. Secondly, a single bout of muscle contractions leads to a translocation of GLUT-4 to the sarcolemmal membrane, which acutely enhances glucose transport capacity [6,7,11]. Evidence of acute training effects on glycemic control rather then a chronic adaptation to training originates from the findings of Fenicchia et al. [5] and Ostergard et al. [13]. In the first study a single bout of resistance exercise was sufficient to improve glycemic control. In the endurance-training intervention of Ostergaard et al. [13], no correlation between changes in maximal oxygen uptake and insulin sensitivity could be detected. The authors discussed that improvements of insulin sensitivity are dissociated from muscle mitochondrial function.
In spite of significantly reduced peak glucose concentrations and area under curve during OGTT in the post-training intervention, the 4 % reduction of HbA1c levels obtained after VT intervention failed to reach statistical significance. Moreover, in the control group (flexibility training) and the strength training group HbA1c increased by 5 % and 3 %, respectively. This finding is clearly inconsistent with the outcome of other strength-training related interventions [2,4,5]. It is well known that HbA1c reflects glycemia over the preceding two to three month [16]. The obviously missing long-lasting beneficial effect on glycemic control in the present study may be in part due to the fact that we initially used a training of low-volume load. In contrast to other studies our subjects performed only one set per session during the initial 6 weeks and three sets were applied just for the last three weeks. This slow increase in training load was utilized to enhance subject's compliance to physical activity.
Beside a dose-dependent phenomenon an alternative explanation originates from the results of Tseng et al. [20]. They reported a seasonal influence on HbA1c with higher HbA1c values during winter. This epidemiological study included more that 280.000 patients living in different climate conditions. Interestingly, the strongest summer-winter contrast appeared in the regions with an intermediate winter climate (winter temperatures between 0oC to 4.4oC). That is close to the conditions of our region. If the assumption holds that seasonal influences provoked the HbA1c increases in the FT and ST groups the 5 % HbA1c decrease in the vibration-exercise group may become even more meaningful.
The present paper shows, as a pilot study, that vibration exercise may be an effective measure to improve glycemic control in non insulin dependent diabetes type 2 patients. Further studies should be encouraged to optimize frequency, amplitude, and duration of vibration exercises.
Since the time to treat is far beyond traditional training forms, patients without any affinity to traditional sports activities may prefer vibration training as a part of an intended lifestyle modification.
Acknowledgements
This work was funded by a grant of International Biotechnological Future Knowledge GmbH, Krefeld, Germany. We are grateful to Dr. Hiemer for excellent cooperation, Susanne Schuster for her practical assistance during training sessions, the kind cooperation of the study participants, Vibrogym Ltd and Roche Diagnostics Ltd for the supply of equipments.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Baum K, Rüther T, Essfeld D. Reduction of blood pressure response during strength training through intermittent muscle relaxations. Int J Sports Med. 2003;24:441- 4
2. Brooks N, Layne JE, Gordon PL. et al. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4:19- 27
3. Cauza E, Hanusch-Enserer U, Strasser B. et al. The relative benefits of endurance and strength training on metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527- 33
4. Dunstan DW, Daly RM, Owen N. et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes care. 2002;25:1729- 36
5. Fenicchia LM, Kanaley JA, Azevedo JL. et al. Influence of resistance training on glucose control in women with type 2 diabetes. Metabolism. 2004;53:284- 9
6. Gao J, Ren J, Gulve EA, Holloszy JO. Additive effect of contractions and insuline on GLUT-4 translocation into the sarcolemma. J Appl Physiol. 1994;77:1587- 1601
7. Goodyear LJ, Hirshman MF, Horton ES. Exercise-induced translocation of skeletal muscle glucose transporters. Am J Physiol Endocrinol Metab. 1991;261:E795- 9
8. Green DJ, Maiorana AJ, Tschakovsky ME. et al. Relationship between changes in brachial artery flow-mediated dilation and basal release of nitric oxide in subjects with Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2006;291:H1193- 9
9. Holten MK, Zacho M, Gaster M. et al. Strength training increases insulin-mediated glucose uptake, GLUT-4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53:294- 305
10. Kasai T, Kawanishi M, Yahagi S. The effects of wrist muscle vibration on human voluntary elbow flexion-extension movements. Exp Brain Res. 1992;90:217- 20
11. Kennedy JW, Hirshman MF, Gervino EV. et al. Acute exercises induces GLUT4 translocation in skeletal muscle of normal human subjects with type 2 diabetes. Diabetes. 1999;48:1192- 7
12. Kim HJ, Lee JS, Kim CK. Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur J Appl Physiol. 2004;93:353- 8
13. Ostergard T, Andersen JL, Nyholm B. et al. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;290:E998- 1005
14. Rittweger J, Ehrig J, Just K. et al. Oxygen Uptake in Whole-Body Vibration Exercise: Influence of Vibration Frequency, Amplitude, and External Load. Int J Sports Med. 2002;23:428- 32
15. Roelants M, Delecluse C, Verschueren SM. Whole body vibration training increases knee-extension strength and speed of movement in older women. JAGS. 2004;52:901- 8
16. Rohlfing CL, Wiedmeyer HM, Little RR. et al. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275- 8
17. Rotmuller C, Cafarelli E. Effects of vibration on antagonist muscle coactivation during progressive fatigue in humans. J Physiol (London). 1995;485:857- 64
18. Segal KR, Edano A, Abalos A. Effects of exercise training on insuline sensitività and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol. 1991;71:2402- 11
19. Torvinen S, Sievänen H, Järvinen TAH. et al. Effect of a 4-min Vertical Whole Body Vibration on Mucle Performance and Body Balance. Int Sports Med. 2002;23:374- 9
20. Tseng CL, Brimacombe M, Xie M. et al. Seasonal patterns in monthly haemoglobin A1c values. Am J Epidemiol. 2005;161:565- 74
21. Verschueren S, Roelants M, Delecluse C. et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352- 9
22. Young JC. Exercise prescription for individuals with metabolic disorders. Practical considerations. Sports Med. 1995;19:43- 54
Author contact
![]() Correspondence to: Klaus Baum, Prof. Dr., Trainingsinstitut, Wilhelm-Schlombs-Allee 1, 50858 Köln, Germany. Telephone (0049) 221 28558550 Fax 285585525 E-mail baumde
Correspondence to: Klaus Baum, Prof. Dr., Trainingsinstitut, Wilhelm-Schlombs-Allee 1, 50858 Köln, Germany. Telephone (0049) 221 28558550 Fax 285585525 E-mail baumde

 Global reach, higher impact
Global reach, higher impact