3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2026; 23(1):243-252. doi:10.7150/ijms.123212 This issue Cite
Research Paper
Oral administration of soybean okara extract reduces osteoarthritis pain and progression in rats
1. Department of Post-Baccalaureate Medicine, National Chung-Hsing University, Taichung, Taiwan.
2. Department of Orthopedics, Taichung Veterans General Hospital, Taichung, Taiwan.
3. Sinying Hospital, Ministry of Health and Welfare, Tainan, Taiwan.
4. Department of Pharmacology, School of Medicine, China Medical University, Taichung, Taiwan.
5. Chinese Medicine Research Center, China Medical University, Taichung, Taiwan.
6. Department of Medical Laboratory Science and Biotechnology, College of Medical and Health Science, Asia University, Taichung, Taiwan.
7. Graduate Institute of Biomedical Sciences, China Medical University, Taichung, Taiwan.
8. Translational Medicine Center, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan.
9. Master in Senior Heath and Exercise Sciences, Tunghai University, Taichung, Taiwan.
10. Department of Physiology, School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan.
#These authors contributed equally to this work.
Received 2025-8-6; Accepted 2025-10-6; Published 2026-1-1
Abstract
Osteoarthritis (OA) is a common inflammatory degenerative disease that causes joint pain and irreversible bone damage, affecting many middle-aged and elderly people. Currently, there is no effective treatment available. Okara, a byproduct of soybean processing, contains bioactive compounds with antioxidant and anti-inflammatory properties similar to those found in soybeans, making it a promising candidate for reuse as a food supplement to provide health benefits. In this study, we explored the therapeutic potential of soybean okara extract (SOE) in OA using a rat surgical anterior cruciate ligament transection (ACLT) OA model. Oral administration of SOE significantly alleviated bone pain associated with ACLT, as demonstrated by a weight-bearing behavioral assay. Histopathological analysis revealed that oral SOE ameliorated ACLT-induced bone destruction, improved cartilage and synovium integrity, and reduced the levels of proinflammatory cytokines IL-1β, TNF-α, and the chondrolytic enzyme MMP-3. This, in turn, led to a decrease in the degradation of aggrecan and collagen type II, thereby preserving cartilage. These findings suggest that oral administration of SOE could be a promising approach for the prevention and treatment of OA.
Keywords: soybean okara, antioxidant, anti-inflammatory agents, osteoarthritis
Introduction
With advances in medicine and increased life expectancy, degenerative diseases have become some of the most common health challenges, with osteoarthritis (OA) being among the most prevalent. According to the Global Burden of Disease report, approximately 528 million individuals worldwide were living with OA, representing a 114.5% increase in prevalence between 1990 and 2019 [1]. The pathological features of OA include subchondral bone sclerosis, cartilage degradation, and synovial tissue inflammation, which are often irreversible at the time of diagnosis and result in joint pain and stiffness [2, 3]. OA is currently incurable, with treatment options limited to slowing disease progression or alleviating pain.
Chronic inflammation of the synovial tissues is closely associated with joint pain and the development of structural damage and with synovial release, playing an essential role in driving inflammation and tissue destruction in OA [4, 5]. The elevated expression of proinflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α, along with synovium-secreted mediators, such as matrix metalloproteinase (MMP)-3, is markedly correlated with the severity of knee OA and may be associated with OA progression [6-9]. Relevant studies indicate that anti-inflammatory approaches are a potential therapeutic strategy for OA [4, 10].
Soybean, a widely consumed plant-based protein source, is rich in essential nutrients, comprising approximately 35-40% protein, 20% lipids, 9% dietary fiber, and 8.5% moisture [11]. Soybeans are abundant in bioactive compounds, including isoflavones, saponins, and bioactive peptides and proteins (such as the Bowman-Birk protease inhibitor) [11-14]. The consumption of soybeans has been associated with a reduced risk of various chronic diseases, including obesity, cardiovascular disease, neurodegenerative disorders, and osteoporosis [9, 12]. Among the isoflavones, genistein and daidzein, which together constitute about 90% of soybean-derived isoflavones, have been extensively reported for their health benefits. Notably, genistein has demonstrated protective effects against bone and cartilage-related disorders, including OA, rheumatoid arthritis, osteoporosis, and intervertebral disc degeneration [15].
Soybeans, due to their low cost and high nutritional value, are processed into various products, such as soy milk, tofu, firm tofu, and tofu skin, which have been integral to Asian diets for millennia. This processing results in the production of large quantities of soybean residue, known as okara. Okara retains many of the nutrients found in soybeans, including bioactive isoflavones, albeit in different proportions [16, 17]. Recent studies have reported that oral consumption of okara can attenuate cognitive decline, mirroring the preventive effects of soy isoflavones against cognitive deficits and Alzheimer's disease [16, 18-20]. Given its nutritional composition, similar to that of soybeans, okara presents a valuable opportunity for repurposing as a nutritious food supplement, contributing to sustainable food production [21].
The high moisture content and rich nutritional profile of raw okara contribute to its short shelf life, typically limited to a few days under refrigeration. In this study, we aimed to extend the shelf life of okara by preparing dried okara using an oven dryer. Additionally, we investigated the protective effects of soybean okara extract (SOE) against OA in a preclinical rat model induced by anterior cruciate ligament transection (ACLT). We focused on the analysis of key inflammatory mediators, such as IL-1β, TNF-α, and the chondrolytic enzyme MMP-3, which play crucial roles in OA progression [22]. Our findings demonstrate that SOE significantly reduces inflammation and offers protective effects against OA in the ACLT-induced rat model.
Materials and Methods
Materials
Antibodies for immunohistochemistry were obtained from various suppliers: IL-1β (MAB601) from R&D Systems, Inc. (Minneapolis, MN, USA); TNF-α (A11534) and collagen II (A1560) from ABclonal, Inc. (Woburn, MA, USA); MMP-3 (SC-21732) from Santa Cruz Biotechnology (Dallas, TX, USA); and aggrecan (ab3778) from Abcam (Cambridge, UK). Other chemicals and reagents were sourced from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of Soybean Okara Extract
Okara was obtained from Maido Co., Ltd, Taiwan. Organic, non-GMO soybeans from Canada or the USA were soaked overnight in reverse osmosis (RO) water. They were then ground, and the juice (soy milk) was extracted. This grinding and extraction process was repeated three times to obtain moist crude okara with a moisture content of approximately 80-85%. The crude okara was baked in an oven dryer (Maido Co., Ltd, Taiwan) until it turned dark brown, then crushed to achieve a consistent particle size after cooling.
The dark brown dried okara was soaked in hot RO water (40 g/L) and boiled for 15 minutes. After cooling to room temperature, the solution was filtered using Whatman Grade 4 filter paper to remove the crude okara. The filtered SOE was then lyophilized into a powder using a freeze dryer. This SOE powder was reconstituted with double-distilled water and sterilized using a 0.2 µm syringe filter before being administered orally to the rats.
ACLT-induced OA model
Eight-week-old Sprague Dawley (SD) rats (300-350 g) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and maintained in the animal center of China Medical University (Taichung, Taiwan). All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC). The ACLT surgery for OA induction was performed as previously described [23, 24]. Rats in the control group (Control) underwent arthrotomy without transection of the ACL.
Two days after ACLT surgery, the rats were randomly divided into three groups and orally administered daily either 1 mL of RO water alone (the ACLT group), celecoxib at a dose of 50 mg/kg (the ACLT+Celecoxib group), or SOE at a dose of 40 mg/kg (the ACLT+SOE group), five days per week. After 6 weeks, following the final weight-bearing test, the rats were euthanized.
Pain Behavior Weight Bearing Incapacitance Test
The static weight-bearing incapacitance test (Bioseb, Vitrolles, France) was conducted weekly to analyze spontaneous pain following ACLT, as previously described [25, 26]. The difference in dynamic weight bearing (expressed in grams) over a 10-second period between the left and right hind limbs was measured using separate sensor plates. For each animal, three consecutive measurements were taken on each test day to calculate the average score. To account for variations in body weight among animals, the results are presented as a percentage of body weight.
Micro-CT analysis
Micro-CT analysis was conducted 6 weeks post-ACLT surgery. The preparation of the right hind limb followed previously described protocols [27]. Briefly, after excising the skin and muscle tissue, the femurs and tibias were fixed in 4% formaldehyde followed by 70% ethanol. The knee joints were scanned using the Bruker SkyScan 2211 micro-CT system (Kontich, Belgium). The scans were performed over a 180° rotation with a voltage of 70 kVp, a current of 290 µA, and a 0.5 mm aluminum filter to prevent beam-hardening artifacts. Image reconstruction was performed using InstaRecon® software (Bruker MicroCT, Kontich, Belgium), and 59 reoriented slices (0.5 mm each) were selected for further analysis. Manual regions of interest (ROIs) were drawn with an irregular contour in the subchondral trabecular bone region of the medial tibial plateau.
As previously described, image analysis for thresholding, ROI delineation, and various bone morphometric parameters, including bone mineral density (BMD), bone mineral content (BMC), bone volume/total volume (BV/TV), bone surface/total volume (BS/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp), was performed using the Bruker MicroCT software (CTAn, version 1.7.1, Bruker, Kontich, Belgium) [26, 27].
Histological analysis
Histopathological changes in OA tissue were analyzed using hematoxylin and eosin (H&E) and Safranin-O/Fast Green staining under an optical microscope, as previously reported [28, 29]. Briefly, knee joint tissues fixed in 4% formaldehyde were decalcified with 10% EDTA in PBS for 14 days, followed by dehydration with increasing concentrations of ethanol (70% to 100%). The specimens were then embedded in paraffin blocks and cut into 5 µm-thick slices for histological staining. Structural changes in the cartilage of the central weight-bearing area of the medial tibial plateau were assessed using the Osteoarthritis Research Society International (OARSI) histopathology assessment system [30], which includes grading and staging scores to reflect the lesion depth and the extent of OA, respectively.
Immunohistochemistry (IHC) Staining and Quantification Scoring
Immunohistochemistry analysis was conducted using the Leica Novolink Polymer Detection System (Leica Biosystems Inc., Buffalo Grove, IL, USA), as previously described [31]. Briefly, tissue sections were pretreated with 3% hydrogen peroxide and incubated with 3% bovine serum albumin in PBS. The sections were then incubated with primary antibodies at 4°C overnight, followed by incubation with a peroxidase-conjugated secondary antibody at room temperature for 1 hour, and then stained with 3,3'-diaminobenzidine substrate.
Quantitative scoring, ranging from 0 to 7, represented both the intensity of the immunoreactive signals and the percentage of immunoreactive positive cells. The intensity of the immunoreactive signals was scored from 1 to 5 (from weak to strong). The percentage of immunoreactive cells was scored as follows: 0 = no signal; 1 = <10% positive cells; 2 = 10-29% positive cells; 3 = 30-59% positive cells; 4 = 60-100% positive cells. Scoring analysis was conducted by two independent assessors who were blinded to the treatment groups to minimize observer bias [30].
Statistical Analysis
Statistical analyses for quantified results were conducted using GraphPad Prism version 5.0 software. Data are presented as the mean ± standard deviation (SD). The paired samples t-test and one-way ANOVA followed by Bonferroni post hoc testing were used to compare results between two groups and among more than two groups, respectively. Statistical significance was determined by a p-value < 0.05 in all cases.
Results
Oral SOE Does Not Affect Body Weight Growth Curve
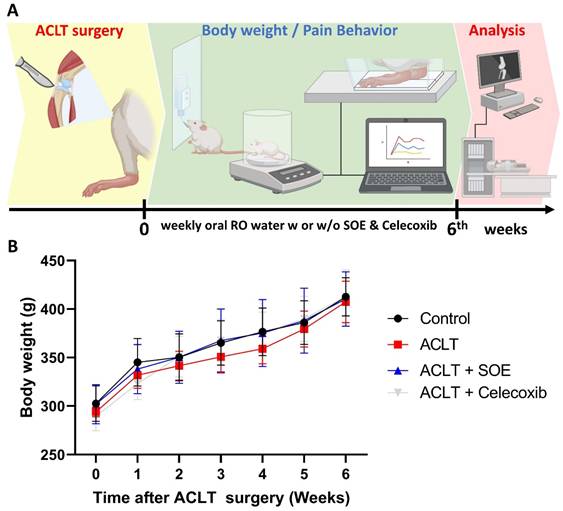
To investigate the protective effects of oral SOE, we utilized an ACLT-induced knee arthritis rat model and conducted pain behavior tests and histological analyses to explore the underlying mechanisms (Figure 1A). After a 3-5 -day habituation period in the animal center, the rats were randomly divided into four groups: arthrotomy without ACLT (Control; n=8), ACLT only (ACLT; n=8), ACLT with oral SOE (ACLT+SOE; n=8), and ACLT with celecoxib (ACLT+Celecoxib; n=8), the latter being an anti-COX-2 nonsteroidal anti-inflammatory drug that is a first-line treatment option for OA [32].
Body weights were recorded starting the day before surgery and were measured weekly until the rats were euthanized. During the experimental period, all groups showed a gradual increase in body weight, with no significant differences found among the groups (Figure 1B). This suggests that oral SOE does not have toxic side effects affecting body weight.
Oral SOE Ameliorates OA Pain
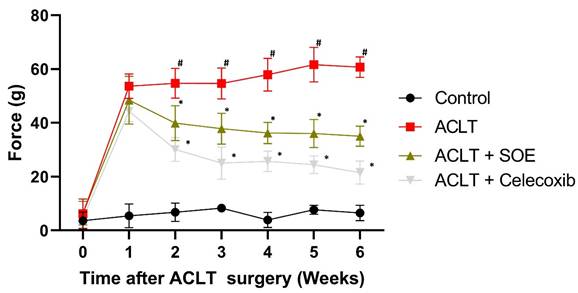
Pain behavior in the rats was evaluated using the static weight-bearing incapacitance test. In the first week post-surgery, all groups exhibited severe asymmetrical weight-bearing posture (Figure 2). This severe asymmetry worsened throughout the experimental period in the ACLT group. In contrast, the ACLT+SOE and ACLT+Celecoxib groups showed dramatic improvements in pain-associated behavior beginning in the second week post-surgery (Figure 2). These results suggest that oral SOE may effectively alleviate OA-related pain.
Oral SOE Protects Osseous Damage in ACLT-induced OA
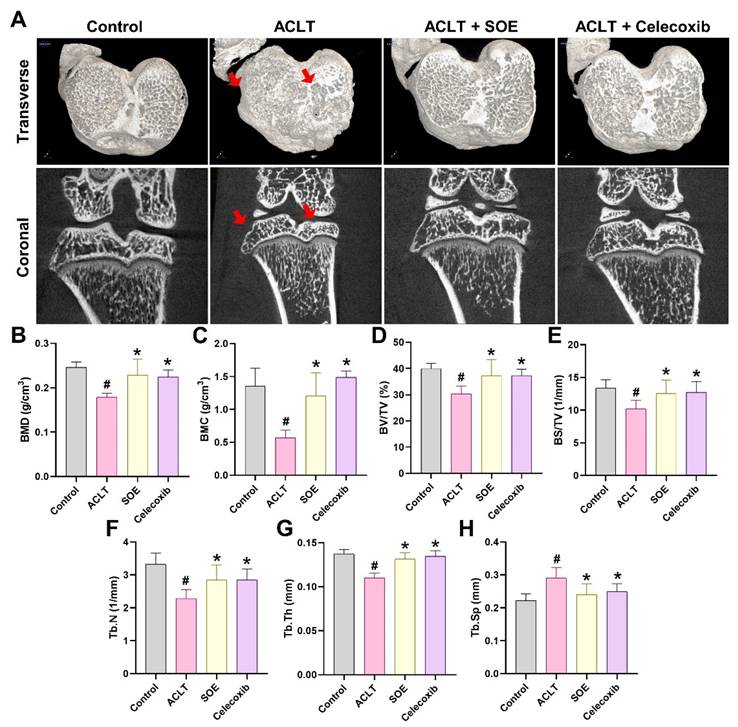
Micro-CT analysis was conducted to assess changes in trabecular microarchitecture six weeks post-ACLT surgery. Significant bone damage was observed in the ACLT group compared to the Control group, confirming the OA lesion induced by ACLT surgery (Figure 3). Quantitative analysis showed loss of BMD, BMC, BV/TV, BS/TV, Tb.N, and Tb.Th, accompanied by an increase in Tb.Sp in the ACLT group (Figure 3B-H).
In contrast, notable improvements in bone architecture were observed in rats of the ACLT+SOE and ACLT+Celecoxib groups compared to the ACLT group (Figure 3). Quantitative data showed that there was no significant bone destruction in the ACLT+SOE and ACLT+Celecoxib groups when compared to the Control group (Figure 3B-H). These results suggest that oral SOE may protect against or ameliorate osseous damage in the OA knee.
(A) Schematic diagram of experiment design. (B) Growth curve of body weight throughout the experimental period. No significant variations were observed between the study groups.
Oral SOE decelerates ACLT-induced bone pain. The pain behavior weight-bearing test suggests that rats of the ACLT+SOE and the positive control ACLT+Celecoxib groups were in less pain than ACLT group (*p < 0.05). #p < 0.05 ACLT vs. no surgery control group.
Oral SOE ameliorates osseous damage in the ACLT-induced OA knee joint. (A) Micro-CT images of rat right knee joints of control, ACLT and ACLT+SOE and ACLT+Celecoxib groups at transverse and coronal views. Red arrows indicate the bone loss. (B-H) Quantitative analyses of the BMD (B), BMC (C), (BV/TV (D), BS/TV (E), Tb.N (F), Tb.Th (G), and Tb.Sp (H) data in all study groups. # p < 0.05 vs. control group; * p < 0.05 vs. ACLT group.
Oral SOE Protects against ACLT-Promoted Articular Cartilage Damage
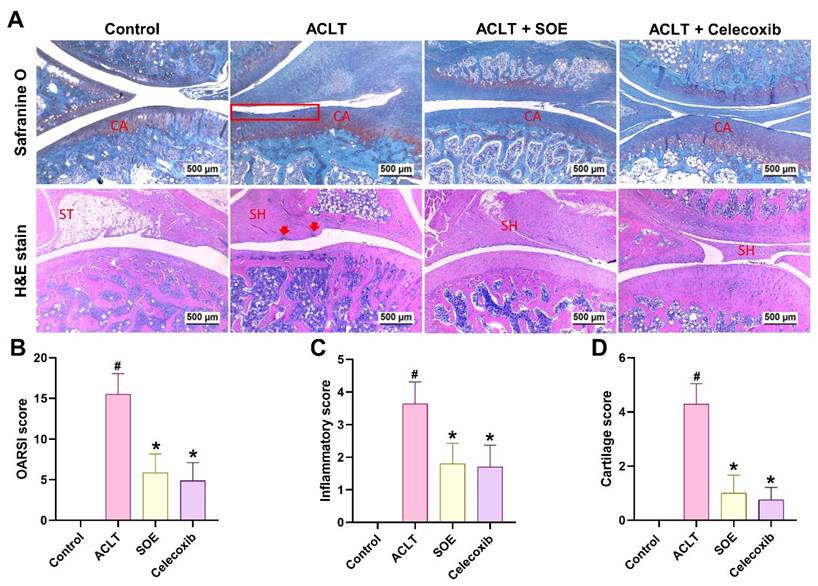
Histological analysis using H&E and Safranin-O/Fast Green staining revealed articular cartilage degradation and synovial lining hyperplasia in the ACLT group (Figure 4A). Quantification of OARSI scores, inflammation, and cartilage scores indicated that the ACLT+SOE and ACLT+Celecoxib groups exhibited fewer pathological changes in cartilage tissue and less synovial tissue hyperplasia compared to the ACLT group (Figure 4).
Oral SOE Downregulates TNF-α, IL-1β, MMP-3 and Upregulates Aggrecan and COL2 Expression in OA Cartilage and Synovium
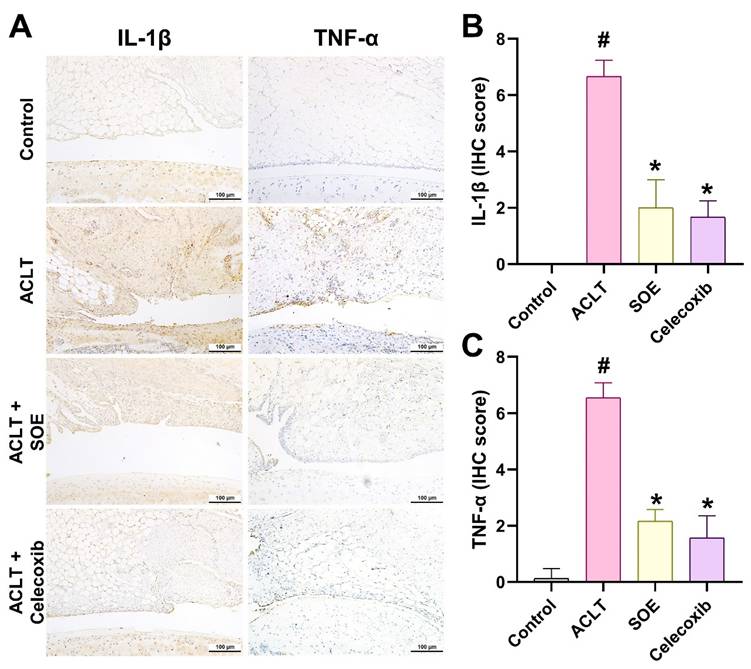
IL-1β and TNF-α are key inflammatory mediators known to drive the progression of OA [8]. IHC analysis revealed a significant elevation in the production of IL-1β and TNF-α in the synovial tissue of the ACLT group, indicating heightened inflammatory activity. This elevation was notably reduced in the ACLT+SOE and ACLT+Celecoxib groups (Figure 5).
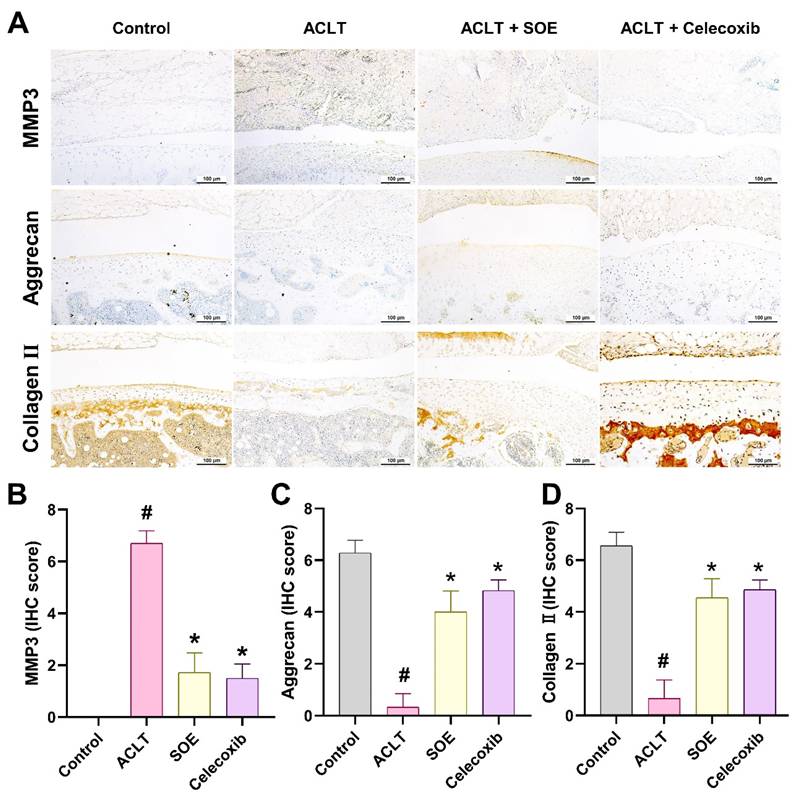
Further assessment of cartilage metabolism was conducted through IHC staining for MMP-3, aggrecan, and collagen type II (COL2), which are critical components of articular cartilage. The ACLT+SOE and ACLT+Celecoxib groups exhibited lower levels of MMP-3, accompanied by higher levels of aggrecan and COL2, compared to the ACLT group (Figure 6). These findings suggest a protective effect of SOE and celecoxib on cartilage integrity in the ACLT-induced OA model.
Oral SOE ameliorates cartilage degradation in the ACLT-induced OA knee joint. (A&B) Histopathological analysis of ACLT-induced OA knee articular cartilage damage in by Safranin-O/Fast Green (A) and H&E (B) staining. Scale bar = 500 μm. Assessment of osteoarthritic cartilage histopathology by OARSI (C), synovial membrane inflammation (D) and cartilage degeneration (E, arrow heads or red box) scores show less cartilage damage in oral SOE and celecoxib groups compared to ACLT group (* p < 0.05). # p < 0.05 vs. control group. Abbreviations: ST, synovial tissue; CA, cartilage; SH, synovial hyperplasia (arrows).
Oral SOE suppress the induction of IL-1β and TNF-α in ACLT-induced OA articular cartilage. Immuno-histochemistry analysis and scoring of IL-1β (A, B) and TNF-α (A, C) in rat right knee joint cartilage. Scale bar = 100 μm. # p<0.05 vs. control group. * p<0.05 vs. ACLT group.
Discussion
This study demonstrates the potential of soybean okara as a beneficial food supplement for protecting against OA pain and progression. The protective effects were evidenced by the reduction in key inflammatory mediators, such as IL-1β, TNF-α, and MMP-3, resulting in less damage to articular cartilage and bone in a preclinical ACLT-induced OA rat model following oral administration of SOE. Therefore, we suggest that oral supplementation with SOE may be beneficial in the prevention and management of OA. In this study, celecoxib, an anti-COX-2 nonsteroidal anti-inflammatory drug and a first-line treatment option for OA [32], was used as a positive control. COX-2 inhibition is well-established for pain relief. However, it not only provides pain relief but also prevents cartilage degradation in preclinical OA models by inhibiting the production of inflammatory cytokines, which contribute to cartilage degradation and bone erosion.
Soybeans are a rich source of essential nutrients and bioactive compounds, including proteins, lipids, dietary fiber, and isoflavones, such as genistein and daidzein, which have been extensively documented for their protective effects against various chronic diseases, including OA [11, 12, 33-35]. A clinical study comparing the effects of daily supplementation with 40 g of soy protein versus milk-based protein over three months showed that soy protein alleviated OA symptoms in men [33]. Okara, the residue left after soybean processing, retains many of these beneficial nutrients and bioactive compounds, albeit in different proportions. Despite being a byproduct, okara remains rich in isoflavones and other compounds with relevant bioactivity [16, 21, 36]. These retained components, particularly the isoflavones, likely contribute to the ability of SOE to mitigate inflammation and cartilage degradation, as demonstrated in our study [15, 16, 37]. The preservation of these bioactive compounds and their effects in okara underscores its potential as a cost-effective, functional food supplement, offering protective benefits against chronic diseases similar to those of whole soybeans.
Genistein, a major soybean-derived isoflavone, is likely a key contributor to soybeans' protective effects against bone and cartilage diseases, including OA [15, 37]. In human OA chondrocytes, genistein has been shown to reduce IL-1β-promoted production of NOS2, COX-2, and MMPs through the activation of the NRF2/HO-1 pathway. This pathway is associated with inhibition of oxidative stress, and its activation by genistein suggests a chondroprotective effect [37]. Furthermore, both in vitro and in vivo studies have shown genistein's anti-apoptotic effects on inflammatory OA chondrocytes and on rat OA articular cartilage [37, 38]. Genistein's ability to reduce IL-1β, TNF-α, and caspase-3 levels, while increasing collagen type II and aggrecan expression, aligns with the therapeutic effects observed in our study, suggesting that genistein may play a crucial role in the protective effects of oral SOE [38].
Oral SOE reserve the expression of aggrecan and collagen II accompanying with suppression of MMP3 in ACLT-induced OA articular cartilage. (A) Immuno-histochemistry analysis MMP3, aggrecan and collagen II in rat right knee joint cartilage. Scale bar = 100 μm. (B-D) Scoring of the immunosignals of MMP3 (B), aggrecan (C) and collagen II (D) in and scoring of MMP3, aggrecan and collagen II. # p<0.05 vs. control group. * p<0.05 vs. ACLT group.
The protective effects of SOE may also be linked to the estrogenic properties of soybean isoflavones [39]. Estrogen receptors are present in articular cartilage, bone, synovial tissue, and joint ligaments. Estrogen deficiency, particularly post-menopause, has been associated with an increased incidence of OA in women. In ovariectomized rats, soybean isoflavones have been shown to protect against OA [35]. However, the protective effects observed in this study were in male rats, suggesting that the estrogenic effects of SOE, if present, were minimal or not significant in this model. This observation highlights the potential of SOE as a therapeutic option for OA that may act independently of estrogenic mechanisms.
Recent research has increasingly focused on soy-derived bioactive peptides, which are generated through the enzymatic hydrolysis of soybean proteins [12, 40, 41]. These peptides exhibit a wide range of physiological functions, including hypolipidemic, immunomodulatory, anticancer, antioxidant, and anti-inflammatory effects, highlighting their significant health benefits [12, 40, 41]. Among these, lunasin, a promising soybean-derived bioactive peptide, has been found to suppress IL-1β-promoted expression of MMP-3 and MMP-13, prevent the decrease in TIMP-1 and TIMP-2, and inhibit the reduction in COL2 in chondrocytes [42]. Lunasin is a heat-stable peptide composed of 43 amino acids and is recognized for its antioxidative, anti-inflammatory, and anticancer properties [43]. Given these characteristics, it is plausible that lunasin is one of the bioactive components contributing to the chondroprotective effects observed with SOE in our study. Although bioactive components, such as isoflavones (e.g., genistein) and peptides (e.g., lunasin), contribute to the anti-OA effects of SOE, we did not perform quantitative analysis to identify the major bioactive compounds in SOE. Further investigation is needed to quantify the bioactive components in SOE.
In conclusion, our data provide the first evidence that okara, rich in soybean-derived bioactive compounds, offers protective effects against OA. These findings suggest that okara, traditionally considered a byproduct of soybean processing, retains beneficial properties similar to those of soybeans and may be effective in the prevention of chronic diseases and the promotion of overall health. Additionally, the preservation of okara using an oven dryer effectively extends its shelf life without compromising its bioactive properties. The results of this report highlight the potential health applications of okara, enhancing its economic value and offering a sustainable approach to improving human health.
Acknowledgements
Funding
This study was sponsored by grants from the Ministry of Science and Technology of Taiwan (NSTC 113-2320-B-039-049-MY3) and China Medical University (CMU104-TC-01 and CMU110-TC-03), China Medical University under the Higher Education Sprout Project, Ministry of Education, Taiwan (CMRC-CENTER-7).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-22
2. Hugle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford). 2017;56:1461-71
3. Sarhan RS, El-Hammady AM, Marei YM, Elwia SK, Ismail DM, Ahmed EAS. Plasma levels of miR-21b and miR-146a can discriminate rheumatoid arthritis diagnosis and severity. BioMedicine. 2025;15:30-41
4. Kloppenburg M. Inflammation is a relevant treatment target in osteoarthritis. Lancet. 2023;402:1725-6
5. Chang JW, Tang CH. The role of macrophage polarization in rheumatoid arthritis and osteoarthritis: Pathogenesis and therapeutic strategies. Int Immunopharmacol. 2024;142:113056
6. Plsikova Matejova J, Spakova T, Harvanova D, Lacko M, Filip V, Sepitka R. et al. A Preliminary Study of Combined Detection of COMP, TIMP-1, and MMP-3 in Synovial Fluid: Potential Indicators of Osteoarthritis Progression. Cartilage. 2021;13:1421S-30S
7. Sulastri D, Arnadi A, Afriwardi A, Desmawati D, Amir A, Irawati N. et al. Risk factor of elevated matrix metalloproteinase-3 gene expression in synovial fluid in knee osteoarthritis women. PLoS One. 2023;18:e0283831
8. Billesberger LM, Fisher KM, Qadri YJ, Boortz-Marx RL. Procedural Treatments for Knee Osteoarthritis: A Review of Current Injectable Therapies. Pain Res Manag. 2020;2020:3873098
9. Achudhan D, Li-Yun Chang S, Liu SC, Lin YY, Huang WC, Wu YC. et al. Antcin K inhibits VCAM-1-dependent monocyte adhesion in human rheumatoid arthritis synovial fibroblasts. Food Nutr Res. 2022 66
10. Hou CH, Fong YC, Tang CH. HMGB-1 induces IL-6 production in human synovial fibroblasts through c-Src, Akt and NF-kappaB pathways. J Cell Physiol. 2011;226:2006-15
11. Benkovic M, Jurinjak Tusek A, Sokac Cvetnic T, Jurina T, Valinger D, Gajdos Kljusuric J. An Overview of Ingredients Used for Plant-Based Meat Analogue Production and Their Influence on Structural and Textural Properties of the Final Product. Gels. 2023 9
12. Chatterjee C, Gleddie S, Xiao CW. Soybean Bioactive Peptides and Their Functional Properties. Nutrients. 2018 10
13. Kang JH, Sung MK, Kawada T, Yoo H, Kim YK, Kim JS. et al. Soybean saponins suppress the release of proinflammatory mediators by LPS-stimulated peritoneal macrophages. Cancer Lett. 2005;230:219-27
14. Akbari S, Akrami H, Mostafaei A, Kiani S. Bowman-Birk inhibitor modifies transcription of autophagy and apoptosis genes in an in vitro model of Alzheimer's disorder. J Cell Biochem. 2019;120:11150-7
15. Wu Z, Liu L. The protective activity of genistein against bone and cartilage diseases. Front Pharmacol. 2022;13:1016981
16. Nile SH, Venkidasamy B, Samynathan R, Nile A, Shao K, Chen T. et al. Soybean Processing Wastes: Novel Insights on Their Production, Extraction of Isoflavones, and Their Therapeutic Properties. J Agric Food Chem. 2022;70:6849-63
17. Miller IM, Yashar BM, Undiagnosed Disease N, Macnamara EF. Continuing a search for a diagnosis: the impact of adolescence and family dynamics. Orphanet J Rare Dis. 2023;18:6
18. Corpuz HM, Arimura M, Chawalitpong S, Miyazaki K, Sawaguchi M, Nakamura S. et al. Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging. Nutrients. 2019 11
19. Liu JYH, Sun MYY, Sommerville N, Ngan MP, Ponomarev ED, Lin G. et al. Soy flavonoids prevent cognitive deficits induced by intra-gastrointestinal administration of beta-amyloid. Food Chem Toxicol. 2020;141:111396
20. Lu Y, An Y, Lv C, Ma W, Xi Y, Xiao R. Dietary soybean isoflavones in Alzheimer's disease prevention. Asia Pac J Clin Nutr. 2018;27:946-54
21. Asghar A, Afzaal M, Saeed F, Ahmed A, Ateeq H, Shah YA. et al. Valorization and food applications of okara (soybean residue): A concurrent review. Food Sci Nutr. 2023;11:3631-40
22. Mukherjee A, Das B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomaterials and biosystems. 2024;13:100090
23. Chang SL, Lin YY, Liu SC, Tsai YS, Lin SW, Chen YL. et al. Oral Administration of Clostridium butyricum GKB7 Ameliorates Signs of Osteoarthritis in Rats. Cells. 2022 11
24. Liu SC, Tsai CH, Wu TY, Tsai CH, Tsai FJ, Chung JG. et al. Soya-cerebroside reduces IL-1β-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: implications for the treatment of osteoarthritis. Food Agr Immunol. 2019;30:620-32
25. Ko CY, Lin YY, Achudhan D, Chang JW, Liu SC, Lai CY. et al. Omentin-1 ameliorates the progress of osteoarthritis by promoting IL-4-dependent anti-inflammatory responses and M2 macrophage polarization. Int J Biol Sci. 2023;19:5275-89
26. Chen LC, Lin YY, Tsai YS, Chen CC, Chang TC, Chen HT. et al. Live and Dead Clostridium butyricum GKB7 Diminish Osteoarthritis Pain and Progression in Preclinical Animal Model. Environ Toxicol. 2024
27. Lin YY, Chang SL, Liu SC, Achudhan D, Tsai YS, Lin SW. et al. Therapeutic Effects of Live Lactobacillus plantarum GKD7 in a Rat Model of Knee Osteoarthritis. Nutrients. 2022 14
28. Lee HP, Chen PC, Wang SW, Fong YC, Tsai CH, Tsai FJ. et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. Journal of Functional Foods. 2019;52:537-44
29. Lee HP, Wang SW, Wu YC, Lin LW, Tsai FJ, Yang JS. et al. Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food Agr Immunol. 2020;31:193-204
30. Hou PW, Liu SC, Tsay GJ, Chang YS, Huang HC, Tang CH. et al. High-dose Tiger-Gian formula protects the knee joint from surgically induced osteoarthritis in rats. Int J Rheum Dis. 2023;26:316-26
31. Su C-H, Lin C-Y, Tsai C-H, Lee H-P, Lo L-C, Huang W-C. et al. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. Journal of Functional Foods. 2021;86:104729
32. Athow NF, Morgan PM, Brown GA. Hip and Knee Osteoarthritis, Not Nonsteroidal Anti-Inflammatory Drugs, are Linked to Cardiac Disease. J Arthroplasty. 2023;38:2455-63
33. Arjmandi BH, Khalil DA, Lucas EA, Smith BJ, Sinichi N, Hodges SB. et al. Soy protein may alleviate osteoarthritis symptoms. Phytomedicine. 2004;11:567-75
34. Hu S, Liu C, Liu X. The Beneficial Effects of Soybean Proteins and Peptides on Chronic Diseases. Nutrients. 2023 15
35. Toda T, Sugioka Y, Koike T. Soybean isoflavone can protect against osteoarthritis in ovariectomized rats. J Food Sci Technol. 2020;57:3409-14
36. Canaan JMM, Brasil GSP, de Barros NR, Mussagy CU, Guerra NB, Herculano RD. Soybean processing wastes and their potential in the generation of high value added products. Food Chem. 2022;373:131476
37. Liu FC, Wang CC, Lu JW, Lee CH, Chen SC, Ho YJ. et al. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients. 2019 11
38. Zou Y, Liu Q, Guo P, Huang Y, Ye Z, Hu J. Anti-chondrocyte apoptosis effect of genistein in treating inflammation-induced osteoarthritis. Mol Med Rep. 2020;22:2032-42
39. Belobrajdic DP, James-Martin G, Jones D, Tran CD. Soy and Gastrointestinal Health: A Review. Nutrients. 2023 15
40. Basson AR, Ahmed S, Almutairi R, Seo B, Cominelli F. Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods. 2021 10
41. Kim IS, Yang WS, Kim CH. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int J Mol Sci. 2021 22
42. Dai W, Liang Z, Liu H, Zhao G, Ju C. Lunasin abrogates the expression of matrix metalloproteinases and reduction of type II collagen. Artif Cells Nanomed Biotechnol. 2019;47:3259-64
43. Lule VK, Garg S, Pophaly SD, Hitesh, Tomar SK. "Potential health benefits of lunasin: a multifaceted soy-derived bioactive peptide". J Food Sci. 2015;80:R485-94
Author contact
![]() Corresponding author: Sunny Li-Yun Chang, Email: liyunchangcmu.edu.tw.
Corresponding author: Sunny Li-Yun Chang, Email: liyunchangcmu.edu.tw.

 Global reach, higher impact
Global reach, higher impact