3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2026; 23(1):227-242. doi:10.7150/ijms.114874 This issue Cite
Research Paper
The Sleep Connection: Exploring the Role of Composite Dietary Antioxidant Index in Sleep Quality Based on NHANES 2007-2014
1. Department of Cardiovascular Medicine, Laboratory of Chronobiology and Cardiometabolic Disease, Southwest Hospital, Army Medical University, 400038 Chongqing, China.
2. Key Laboratory of Geriatric Cardiovascular and Cerebrovascular Disease, Ministry of Education of China, 400038 Chongqing, China.
3. Department of Cardiovascular Medicine, Chongqing Hechuan Hongren Hospital, 401520 Chongqing, China.
# These authors contribute equally to this work.
Received 2025-4-1; Accepted 2025-10-10; Published 2026-1-1
Abstract
Background: Growing evidence has highlighted the critical role of oxidative stress in the pathogenesis of sleep problems. The Composite Dietary Antioxidant Index (CDAI), a holistic metric designed to assess the cumulative antioxidant capacity of dietary components, may be intricately linked to sleep quality. This study aimed to investigate the association between CDAI and sleep parameters using data from the National Health and Nutrition Examination Survey (NHANES).
Methods: Participants with complete data on CDAI, sleep parameters, and other essential covariates were included in this study. Weighted multivariable linear or logistic regression models were employed to examine the association between CDAI and sleep parameters (sleep duration, sleep trouble, and sleep disorder). Subgroup analyses, smooth curve fitting, threshold/saturation effect analyses, and sensitivity analyses were conducted to characterize the association's features.
Results: A total of 10,491 participants were finally included in the analysis. After adjusting for all potential covariates, higher CDAI levels were associated with increased sleep duration (CDAIT3 vs. CDAIT1, β=0.16, 95% CI: 0.08-0.24), while exhibiting no significant correlation with sleep trouble (CDAIT3 vs. CDAIT1, OR=1.03, 95% CI: 0.88-1.20) or sleep disorder (CDAIT3 vs. CDAIT1, OR=1.01, 95% CI: 0.79-1.28). Subgroup analyses and interaction tests indicated a more pronounced association among individuals with depression (P interaction<0.0001) and those taking sleep-modulating medications (P interaction=0.0014). Smooth curve fitting analyses identified an inverted "L"-shaped association between CDAI and sleep duration, with the inflection point of CDAI determined to be 3.2. Within this threshold, each 1-unit increase in CDAI was associated with a 0.04-hour (95% CI: 0.02-0.05) increase in sleep duration, whereas no association was observed beyond this point. Finally, sensitivity analyses, which excluded individuals with extreme energy intakes or depression, confirmed a consistent and robust association between CDAI and sleep duration.
Conclusion: The association between CDAI and sleep duration exhibits a positive, inverted “L”‑shaped pattern with a saturation effect. Further prospective or longitudinal cohort studies with larger sample sizes and longer follow‑up periods are needed to evaluate its practical value for clinical interventions.
Keywords: Composite Dietary Antioxidant Index, Sleep Duration, Sleep Trouble, Sleep Disorder, Sleep Quality
Introduction
Sleep is an innate and reversible state primarily regulated by neurobiological mechanisms, representing an essential physiological aspect of human existence crucial for maintaining optimal health [1]. The major characteristic of this state is the reduction of perception to external stimuli and cessation of motor activity, which distinguish it from wakefulness. Sleep quality can be modulated by various factors, including physical activity, dietary patterns, genetic predispositions, and environmental influences [2,4]. In fact, these determinants interact in complex ways to shape the bidirectional association between sleep and health outcomes. On the one hand, adequate sleep confers profound benefits: it reduces energy consumption, restores brain homeostasis, modulates the immune response, and consolidates memory [1]. On the other hand, however, sleep disorders are intimately linked to the pathogenesis of multiple diseases—ranging from depression [5] and arterial stiffness [6] to cardiovascular disease [7], infectious diseases [8], and even cancer [9]. Evidence from previous studies is striking: up to 50% of children [10], 27.1% of adults [11], and 50% of older Americans [12] experience varying degrees of sleep disturbances. This highlights sleep disorders as a critical public health issue, imposing a substantial burden on both individuals and healthcare systems. In the contemporary era, dramatic shifts in work schedules, lifestyle patterns, and dietary habits are likely exacerbating sleep problems across all age groups. Therefore, unraveling the key factors influencing sleep quality is not only scientifically imperative but also essential for developing targeted interventions to mitigate this growing health crisis.
In recent years, increasing evidence has revealed the close association between oxidative stress and sleep disorders [13-15]. Oxidative stress arises from the imbalanced status between production and scavenging of reactive oxygen species (ROS) [16]. Numerous dietary components with antioxidant properties have been reported to benefit humans and experimental animals through various mechanisms. A recent study using flies and mice demonstrated that sleep deprivation leads to severe ROS accumulation and oxidative stress in the gut, causing death in both species. Interestingly, oral administration of antioxidant compounds or overexpression of antioxidant enzymes in the gut enabled flies to maintain a normal lifespan even under conditions of minimal or no sleep [17]. In humans, a prospective, double-blinded randomized controlled trial showed that vitamin E supplementation can serve as an effective alternative treatment for alleviating chronic insomnia disorder [18]. Further evidence from other studies suggests that individuals with short sleep duration who consume high levels of dietary antioxidant vitamins have a significantly lower risk of obesity than those with normal sleep duration [19]. Additionally, clinical studies have shown that antioxidant dietary supplements (e.g., vitamin C, luteolin, magnesium, B6, B12, folic acid, etc.) can significantly improve sleep problems and related complications arising from various factors, such as nighttime traffic noise, sleep deprivation, and obstructive sleep apnea [20-22]. Collectively, these findings highlight the pivotal role of antioxidants in enhancing sleep quality. Therefore, a quantifiable evaluation index of antioxidant components is needed to facilitate improvements in sleep quality.
The Composite Dietary Antioxidant Index (CDAI) is a comprehensive and quantifiable indicator that integrates information on multiple dietary antioxidants (including vitamins A, C, E, zinc, selenium, and manganese) to assess an individual's overall dietary antioxidant intake profile. Numerous studies have established close associations between CDAI and various health outcomes, including hypertension [23], all-cause and cardiovascular mortality [24], sarcopenia [25], cancer risk [26], osteoporosis [27], among others. Given the intimate link between oxidative stress and sleep, a potential association may exist between CDAI and sleep quality. However, whether such a relationship actually exists remains unelucidated. Therefore, this study aimed to explore the association between CDAI and sleep quality using data from the NHANES database, with the goal of providing dietary guidance to improve sleep quality.
Materials and Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive research program conducted by the Center for Disease Control and Prevention (CDC), which aims to evaluate the health and nutritional well-being of both adults and children in the United States [28]. Started in the early 1960s, the NHANES program has conducted a series of surveys concerning different population groups, health problems, and nutrition statuses to meet the emerging public needs. The survey within NHANES program was conducted annually and a sample of about 5,000 persons each year was examined, and the demographic, socioeconomic, dietary, and health-related information was collected. The approval for protocols was already granted by the ethical review board of National Center for Health Statistics (NCHS). All researchers who have meet the requirements can utilize the database for study purposes with no need to submit additional applications [29]. The information sensitive information of all participants was anonymized and individually unrecognizable [29]. The informed consents were signed by all participants.
The sample selection flowchart is presented in Figure 1. In brief, 40,617 participants from four NHANES cycles (2007-2008, 2009-2010, 2011-2012, and 2013-2014) with available dietary CDAI component data were initially enrolled. Subsequently, 4,929 participants with missing CDAI data were excluded, followed by 12,274 participants lacking sleep parameter data. Further exclusions included participants with incomplete information on: 1) behavioral factors (smoking, alcohol consumption, physical activity); 2) comorbidities (diabetes, hypertension, cardiovascular disease, cancer, depression, anxiety); 3) pregnancy status; and 4) dietary/medication use (caffeine intake, medication use, energy, moisture). Finally, 10,491 participants were included in the analysis.
CDAI calculation and exposure definition
As previously reported [30-32], CDAI was calculated using a validated method developed by Wright with minor modifications. The core logic of the CDAI calculation is to simplify the highly correlated group of antioxidant nutrients via principal component analysis (PCA), then integrate the independent antioxidant nutrients, and finally form a comprehensive index to quantify the overall dietary intake level of antioxidant substances. This method not only retains information about the synergistic effects of different types of antioxidant nutrients but also avoids the limitations of single-nutrient analysis, making it more suitable for evaluating the association between complex dietary patterns and health outcomes (e.g., lung cancer risk [32]).
This method assesses six food-derived minerals and vitamins associated with antioxidant processes: selenium, zinc, vitamins A, C, E, and carotenoids. CDAI is computed via the following formula:
Here, Xi denotes the daily intake of antioxidant i, and μi is the mean intake of Xi across the entire cohort, and SDi represents the standard deviation for μi.
Sleep quality measures and outcome definitions
As previously described [33,34], three dimensional questions were utilized to evaluate participants' sleep quality. The information for these questions was collected, in the home, by professionally trained interviewers by utilizing the computer-assisted personal interviewing (CAPI) system, which is equipped with built-in consistency checks to minimize data entry errors and ensure data accuracy. Besides, online help screens are also available to assist interviewers in understanding and defining key terms mentioned in the questionnaire.
Specifically, the question of “(SLD010H) How much sleep do you get (hours)?” was used to get the participants' sleep duration. Besides, those participants who responded “yes” to the question of “(SLQ050) Ever told doctor had trouble sleeping?” were considered to have sleep trouble, while participants who answered “yes” to the question of “ (SLQ060) Ever told by doctor have sleep disorder?” were considered to have sleep disorder.
Flowchart of participants screening. Abbreviations: CDAI, composite dietary antioxidant index; PIR, poverty income ratio; BMI, body mass index.
Covariates
Based on previous studies on sleep, essential covariates were considered, including age (<40, 40-60, >60), gender (male, female), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), education (less than high school, high school or equivalent, college or above), marital status (married, never married, others), PIR (<1.3, 1.3-3.5, >3.5), BMI (<25, 25-30, >30), smoking status (never, former, current), alcohol consumption (mild, moderate, heavy), physical activity (PA; low, high), diabetes (no, yes), hypertension (no, yes), CVD (no, yes), cancer (no, yes), depression (no, yes), anxiety (no, yes), pregnancy (no, yes), medication use (no, yes), as well as caffeine intake, energy intake, and moisture. Age was categorized into three groups as previously described [35]: <40, 40-60, and >60. Race included five categories: Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black, and other race. Education was divided into three subgroups [35]: less than high school, high school or equivalent, and college or above. Similarly, marital status included three categories [35]: married, never married, and others. PIR was classified into three groups following previous studies [35]: <1.3, 1.3-3.5, and >3.5. Moreover, BMI was divided into three groups according to the World Health Organization (WHO) criteria [36]: normal/low body weight (<25 kg/m²), overweight (25-30 kg/m²), and obese (>30 kg/m²). Smoking status was classified into three subgroups based on self-reported questionnaire responses: never, former, and current smokers [37]. Specifically, individuals who smoked fewer than 100 cigarettes in their lifetime were defined as never smokers, those who had smoked at least 100 cigarettes but had quit smoking were classified as former smokers, and those who currently smoke and have smoked over 100 cigarettes were identified as current smokers. Alcohol consumption status was categorized into three levels based on average daily alcohol intake in the past year: mild drinking (females ≤1 drink/day; males ≤2 drinks/day), moderate drinking (females 1-3 drinks/day; males 2-4 drinks/day), and heavy drinking (females ≥4 drinks/day; males ≥5 drinks/day) [38]. Physical activity (PA) information was assessed using the Global Physical Activity Questionnaire (GPAQ), and weekly total metabolic equivalent (MET) values were used to quantify PA [39-41]. According to previous studies [42,43], a cut-off value of 500 MET-minutes/week was applied to define PA levels: low PA (<500 MET-minutes/week) and high PA (≥500 MET-minutes/week). Diabetes can be defined if participants meet any one of the following criteria [44]: 1) self-reported diabetic diagnosis by physicians, or use of medication/insulin for diabetes treatment; 2) hemoglobin A1C (HbA1C) ≥6.5% or fasting plasma glucose (FPG) ≥126 mg/dL (≥7.00 mmol/L); and 3) two-hour oral glucose tolerance test (OGTT) ≥11.10 mmol/L, random blood glucose ≥11.10 mmol/L. The diagnosis of hypertension can be established by meeting any one of the following criteria [45]: 1) self-reported hypertension diagnosis by clinicians, or the use of antihypertensive medication currently for treatment; 2) with average systolic blood pressure (SBP) more than 130 mmHg, or average diastolic blood pressure (DBP) over 80 mmHg. The diagnosis of cardiovascular disease (CVD) [46] was established if individuals responded "yes" to any of the following five clinical questions: "Have you ever been told by a physician that you had coronary heart disease (CHD), congestive heart failure (CHF), angina, a heart attack, or a stroke?". For cancer diagnosis, individuals were classified as having cancer if they responded "yes" to the questionnaire item "MCQ220 - Ever told by a doctor that you had cancer or malignancy.". A nine-item depression screening instrument [namely the Patient Health Questionnaire (PHQ-9)] was used to assess depressive symptoms. As previously described, a PHQ-9 score ≥10 was used to define clinically significant depression [47]. The anxiety state was assessed by the question asking "During the past 30 days, for about how many days have you felt worried, tense, or anxious?", and individuals were further categorized as without anxiety (felt anxious for 0-6 days per month) and with anxiety (felt anxious for 7-30 days per month) [48]. Pregnancy status was assessed by the question "RHD143 - Are you pregnant now?", and those who answered "yes" were defined as pregnant. Dietary intake data (e.g., energy, caffeine, moisture) were determined by two 24-hour dietary recall interviews: the first was collected in person at the Mobile Examination Center (MEC), and the second was conducted by telephone 3-10 days later. The status of medication use was determined by the following two questions: "RXDUSE - Taken prescription medicine in the past month" and "RXDDRUG - Generic drug name". Individuals were categorized into two groups (yes/no) based on whether medications affected sleep quality: (1) those taking medications that could impact sleep quality, and (2) those not taking any medications or taking medications without sleep-related effects.
Statistical analysis
Sample weight calculation, clustering, and stratification were taken into account for all analyses conducted in this study. The data analysis process involved utilizing the complex sampling weight calculation provided by the NHANES analysis guide. The baseline characteristics were presented based on the tertiles of CDAI, and continuous variables were presented as median and interquartile ranges (IQR) for their skewed normal distribution characteristics while categorical variables were showed as frequency (percentage). The differences in baseline characteristics across CDAI tertile groups were evaluated using the Rao-Scott chi-squared test or Kruskal-Wallis test for categorical and continuous variables, respectively.
Weighted multivariable regression models were used to explore the association between CDAI and sleep parameters, including logistic regression for categorical outcomes (sleep trouble and sleep disorder) and linear regression for the continuous outcome (sleep duration). Three models were established with increasing covariate adjustment: model 1 was unadjusted (no covariates), model 2 was adjusted for age, gender, race, education, marital status, and PIR, and model 3 further adjusted for BMI, smoking status, alcohol consumption, physical activity (PA), diabetes, hypertension, CVD, cancer, depression, anxiety, caffeine intake, medication use, energy intake, and moisture intake on the basis of model 2. Moreover, adjusted restricted cubic spline (RCS) analyses were used to explore the potential non-linear relationship between CDAI and sleep parameters, and the “segmented” package was further used to determine the inflection point once a non-linear relationship was identified as previously described [49]. Additionally, subgroup analyses and interaction tests were performed to examine the robustness and consistency of the association between CDAI and sleep quality across subgroups. When significant interaction effects were identified in the tests, subsequent stratified smooth curve fitting analyses were conducted to explore the associations between CDAI and sleep parameters in each subgroup. Finally, sensitivity analyses were conducted to further validate the association between CDAI and sleep parameters by excluding participants with extreme energy intake and those who were pregnant. R (version 3.5.3) were used to conduct statistical analyses. P-values<0.05 were considered statistically significant.
Results
Baseline characteristics of the study population
A total of 10,491 participants were finally included in this study, with baseline characteristics based on CDAI tertiles presented in Table 1. As shown in Table 1, compared with the lowest CDAI tertile group, individuals in the highest CDAI tertile group had significantly higher PIR, BMI, and intakes of caffeine, moisture, and energy. They were more frequently male, Non-Hispanic White, educated at college or above, married, never-smokers, mild drinkers, and physically active. Additionally, they were less likely to have diabetes, hypertension, CVD, depression, or anxiety, and less likely to use medications affecting sleep quality (all P < 0.05).
Baseline characteristics of the study population based on CDAI's tertile
| Characteristics | Overall | CDAI tertile | P-value | ||
|---|---|---|---|---|---|
| Low (<-1.60) | Middle (-1.60≤CDAI<1.61) | High (CDAI≥1.61) | |||
| AGE (years) | 46.00 (32.00-60.00) | 46.00 (32.00-61.00) | 46.00 (32.00-61.00) | 45.00 (32.00-60.00) | 0.245 |
| PIR | 2.52 (1.20-4.64) | 1.95 (1.03-3.77) | 2.69 (1.27-4.80) | 3.06 (1.38-5.00) | <0.001 |
| BMI (kg/m2) | 27.68 (24.15-32.07) | 28.00 (24.20-32.50) | 27.80 (24.28-32.25) | 27.23 (24.00-31.60) | <0.001 |
| Caffeine (mg) | 109.50 (37.00-215.50) | 98.00 (34.50-198.00) | 114.00 (40.00-217.50) | 118.00 (37.50-228.00) | <0.001 |
| Energy (Kcal) | 1999.00 (1553.00-2561.50) | 1488.50 (1203.00-1827.50) | 2027.50 (1705.00-2394.50) | 2645.50 (2151.00-3244.50) | <0.001 |
| Moisture (gm) | 2636.73 (2006.04-3358.13) | 2167.04 (1634.73-2887.51) | 2702.11 (2058.14-3336.64) | 3106.93 (2369.96-3994.16) | <0.001 |
| Gender | <0.001 | ||||
| Male | 5484 (52.27%) | 1366 (39.06%) | 1755 (50.19%) | 2363 (67.57%) | |
| Female | 5007 (47.73%) | 2131 (60.94%) | 1742 (49.81%) | 1134 (32.43%) | |
| Race | <0.001 | ||||
| Mexican American | 1379 (13.14%) | 466 (13.33%) | 460 (13.15%) | 453 (12.95%) | |
| Other Hispanic | 954 (9.09%) | 360 (10.29%) | 307 (8.78%) | 287 (8.21%) | |
| Non-Hispanic White | 5211 (49.67%) | 1617 (46.24%) | 1771 (50.64%) | 1823 (52.13%) | |
| Non-Hispanic Black | 2066 (19.69%) | 801 (22.91%) | 643 (18.39%) | 622 (17.79%) | |
| Other Race | 881 (8.40%) | 253 (7.23%) | 316 (9.04%) | 312 (8.92%) | |
| Education | <0.001 | ||||
| less than high school | 1957 (18.65%) | 856 (24.48%) | 616 (17.62%) | 485 (13.87%) | |
| high school or equivalent | 2275 (21.69%) | 884 (25.28%) | 720 (20.59%) | 671 (19.19%) | |
| college or above | 6259 (59.66%) | 1757 (50.24%) | 2161 (61.80%) | 2341 (66.94%) | |
| Marital status | <0.001 | ||||
| Married | 5439 (51.84%) | 1613 (46.13%) | 1863 (53.27%) | 1963 (56.13%) | |
| Never Married | 2137 (20.37%) | 751 (21.48%) | 679 (19.42%) | 707 (20.22%) | |
| Others | 2915 (27.79%) | 1133 (32.40%) | 955 (27.31%) | 827 (23.65%) | |
| Smoking | <0.001 | ||||
| Never | 5388 (51.36%) | 1672 (47.81%) | 1845 (52.76%) | 1871 (53.50%) | |
| Former | 2673 (25.48%) | 769 (21.99%) | 929 (26.57%) | 975 (27.88%) | |
| Now | 2430 (23.16%) | 1056 (30.20%) | 723 (20.67%) | 651 (18.62%) | |
| Alcohol consumption | <0.001 | ||||
| Mild drinking | 5196 (49.53%) | 1592 (45.52%) | 1756 (50.21%) | 1848 (52.85%) | |
| Moderate drinking | 3451 (32.89%) | 1250 (35.74%) | 1147 (32.80%) | 1054 (30.14%) | |
| Heavy drinking | 1844 (17.58%) | 655 (18.73%) | 594 (16.99%) | 595 (17.01%) | |
| PA group | <0.001 | ||||
| Low physical activity | 3553 (33.87%) | 1414 (40.43%) | 1213 (34.69%) | 926 (26.48%) | |
| High physical activity | 6938 (66.13%) | 2083 (59.57%) | 2284 (65.31%) | 2571 (73.52%) | |
| Diabetes | <0.001 | ||||
| No | 9022 (86.00%) | 2933 (83.87%) | 3034 (86.76%) | 3055 (87.36%) | |
| Yes | 1469 (14.00%) | 564 (16.13%) | 463 (13.24%) | 442 (12.64%) | |
| Hypertension | <0.001 | ||||
| No | 6503 (61.99%) | 2080 (59.48%) | 2175 (62.20%) | 2248 (64.28%) | |
| Yes | 3988 (38.01%) | 1417 (40.52%) | 1322 (37.80%) | 1249 (35.72%) | |
| CVD | <0.001 | ||||
| No | 9643 (91.92%) | 3167 (90.56%) | 3210 (91.79%) | 3266 (93.39%) | |
| Yes | 848 (8.08%) | 330 (9.44%) | 287 (8.21%) | 231 (6.61%) | |
| Cancer | 0.376 | ||||
| No | 9509 (90.64%) | 3186 (91.11%) | 3171 (90.68%) | 3152 (90.13%) | |
| Yes | 982 (9.36%) | 311 (8.89%) | 326 (9.32%) | 345 (9.87%) | |
| Depression | <0.001 | ||||
| No | 9614 (91.64%) | 3065 (87.65%) | 3250 (92.94%) | 3299 (94.34%) | |
| Yes | 877 (8.36%) | 432 (12.35%) | 247 (7.06%) | 198 (5.66%) | |
| Anxiety | <0.001 | ||||
| No | 5822 (55.50%) | 1844 (52.73%) | 1978 (56.56%) | 2000 (57.19%) | |
| Yes | 2038 (19.43%) | 799 (22.85%) | 653 (18.67%) | 586 (16.76%) | |
| Missing | 2631 (25.08%) | 854 (24.42%) | 866 (24.76%) | 911 (26.05%) | |
| Medication use | <0.001 | ||||
| No | 8272 (78.85%) | 2699 (77.18%) | 2735 (78.21%) | 2838 (81.16%) | |
| Yes | 2219 (21.15%) | 798 (22.82%) | 762 (21.79%) | 659 (18.84%) | |
Abbreviations: CDAI, composite dietary antioxidant index; PA, physical activity; CVD, cardiovascular disease; BMI, body mass index; PIR, ratio of family income to poverty. Note: Variables including age, PIR, BMI, caffeine, energy, moisture, and CDAI are presented as median (interquartile range, IQR) because of their skewed distribution.
Association between CDAI and sleep parameters
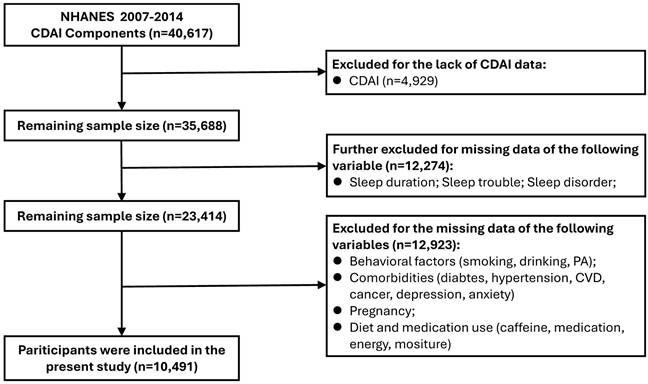
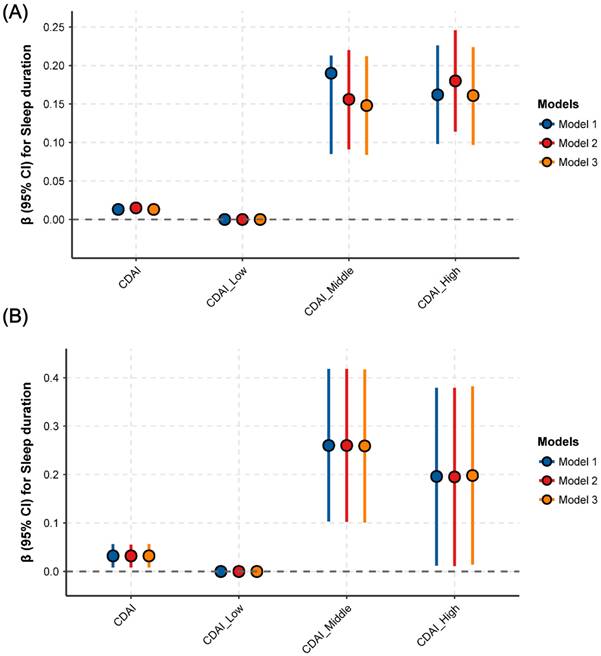
The association between CDAI and sleep parameters is presented in Fig. 2. After adjusting for all potential covariates, a positive and significant association was identified between CDAI and sleep duration: a one-unit increase in CDAI (as a continuous variable) was associated with a 0.02-hour increase in sleep duration (95% CI: 0.01-0.02, P = 0.0011) (Fig. 2A, STable 1). Similarly, compared with the lowest CDAI tertile group, the highest CDAI tertile group had a 0.16-hour longer sleep duration (95% CI: 0.08-0.24, P = 0.0001) (Fig. 2A, STable 1). However, in the fully-adjusted model (model 3), no significant associations were found between CDAI and sleep disturbance (Fig. 2B, STable 1) or sleep disorder (Fig. 2C, STable 1) (all P > 0.05).
Moreover, adjusted restricted cubic spline (RCS) analysis was used to explore the potential non-linear association between CDAI and sleep duration. As shown in Fig. 3, a significant positive non-linear association (inverse “L” shape) was found between CDAI and sleep duration (P for overall < 0.001, P for nonlinearity < 0.001). Using the "segmented" package, an inflection point of 3.2 for CDAI was identified (Fig. 3). Below this threshold, a significant positive relationship existed: each 1-unit increase in CDAI was associated with a 0.04-hour increase in sleep duration (95% CI: 0.02-0.05, P < 0.0001) (Table 2). However, no significant association was observed between CDAI and sleep duration after exceeding this point (95% CI: -0.01 to 0.01, P=0.8807) (Table 2).
Associations between CDAI and sleep duration, sleep trouble and sleep disorder. (A) sleep duration; (B) sleep trouble; (C) sleep disorder. Abbreviations: OR, odds ratio; CI, confidence intervals; CDAI, composite dietary antioxidant index; PA, physical activity; CVD, cardiovascular disease; BMI, body mass index; PIR, ratio of family income to poverty. Note: Model 1: adjusted for no covariates. Model 2: adjusted for variables of Age, Gender, Race, Education, Marital status, PIR. Model 3: adjusted for variables of age, gender, race, education, marital status, PIR, BMI, smoking, drinking, PA, diabetes, hypertension, CVD, cancer, depression, anxiety, caffeine, medication use, energy, and moisture.
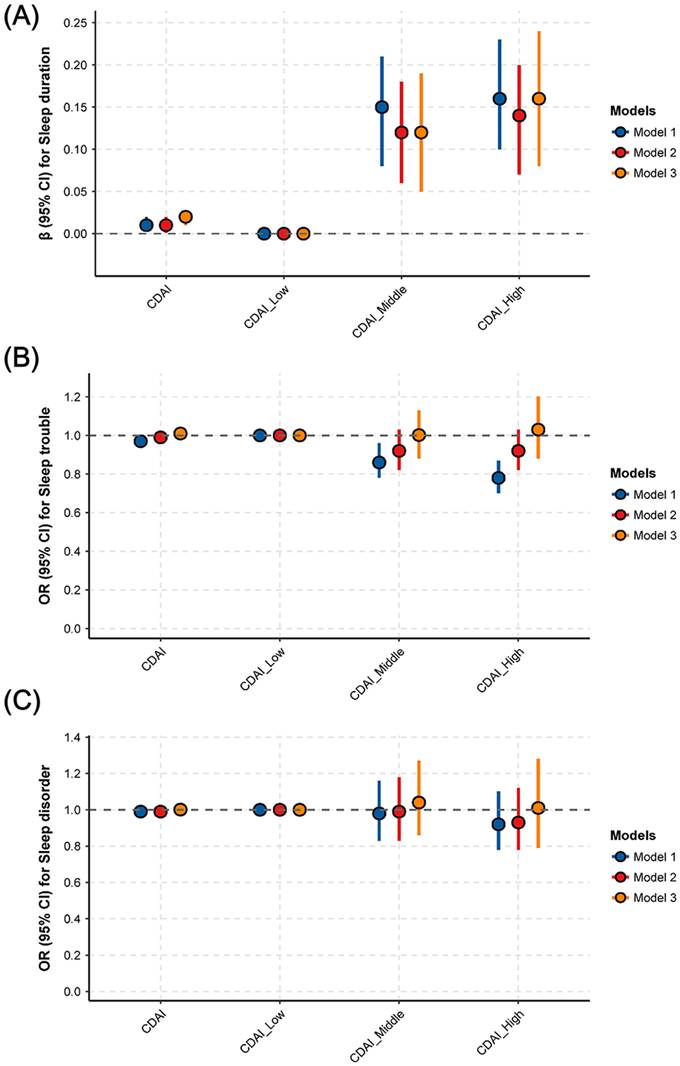
Association between CDAI and sleep duration. (A) Identifying the nonlinear association between CDAI and sleep duration using RCS analysis. (B) Smooth curve fitting showing the relationship between CDAI and predicted sleep duration (in hours). Variables of age, gender, race, education, marital status, PIR, BMI, smoking, drinking, PA, diabetes, hypertension, CVD, cancer, depression, anxiety, caffeine, medication use, energy, and moisture were adjusted during analyses.
Threshold effect and saturation effect analyses for the association between CDAI and sleep duration
| Outcome | β (95%CI) P-value |
|---|---|
| Model I | |
| A straight-line effect | 0.02 (0.01, 0.02) 0.0011 |
| Model II | |
| Fold points (K) | 3.2 |
| < K-segment effect 1 | 0.04 (0.02, 0.05) <0.0001 |
| > K-segment effect 2 | -0.00 (-0.01, 0.01) 0.8807 |
| Effect size difference of 2 vs. 1 | -0.04 (-0.06, -0.02) 0.0002 |
| Equation predicted values at break points | 6.95 (6.90, 7.00) |
| Log likelihood ratio tests | <0.001 |
Abbreviations: OR, odds ratio; CI, confidence intervals; CDAI, composite dietary antioxidant index. Adjusted for variables of Age, Gender, Race, Education, Marital status, PIR, BMI, Smoking, Drinking, PA, Diabetes, Hypertension, CVD, Cancer, Depression, Anxiety, Pregnancy, Caffeine, Medication Use, Energy, Moisture.
Subgroup analyses
To further explore the robustness of the association between CDAI and sleep duration, subgroup analyses and interaction tests were conducted. As shown in Table 3, a more pronounced association was observed in individuals aged <40 years, with higher education, never married, PIR <1.3, BMI >30 kg/m2, never smokers, heavy drinkers, low physical activity (PA) levels, without diabetes/CVD/hypertension/cancer/anxiety, and those with depression or taking medications affecting sleep quality (Table 3). Moreover, the CDAI-sleep duration association was modified by depression (P for interaction < 0.0001) and medication use (P for interaction = 0.0014), with stronger associations observed in individuals with depression or using sleep-affecting medications (Table 3). These findings were further corroborated by stratified restricted cubic spline (RCS) analyses, as illustrated in Fig. 4.
Sensitivity analyses
Given the evidence from prior studies on the effects of energy intake and pregnancy on sleep, sensitivity analyses were performed to validate the association between CDAI and sleep duration across subgroups [50-52]. In most epidemiologic studies, participants with implausible energy intakes (i.e., extreme values) are excluded, typically defined as <500 kcal/day or >3,500 kcal/day [53]. Based on this criterion, 335 participants were identified with extreme energy intakes (SFig. 1). Subsequent multivariable linear regression models were used to assess the association's robustness after excluding these outliers. The association between CDAI and sleep duration remained consistent among participants with plausible energy intakes (STable 2, Fig. 5A). Similarly, consistent results were observed after excluding participants with pregnancy (STable 3, Fig. 5B). Collectively, these findings—along with prior results—confirm a stable association between CDAI and sleep duration across diverse subgroups.
Discussion
Our study identified a significant positive nonlinear association between CDAI and sleep duration after adjusting for all potential covariates. This association was particularly pronounced in individuals with depression or taking sleep-affecting medications. Using threshold and saturation effect analysis, we identified an inflection point of 3.2 for CDAI. Below this threshold, each 1-unit increase in CDAI was associated with a 0.04-hour increase in sleep duration; however, no further improvement in sleep duration was observed above this inflection point. Collectively, our results suggest that higher CDAI levels may beneficially impact sleep duration, particularly below the identified threshold.
Association between CDAI and sleep duration in different subgroups
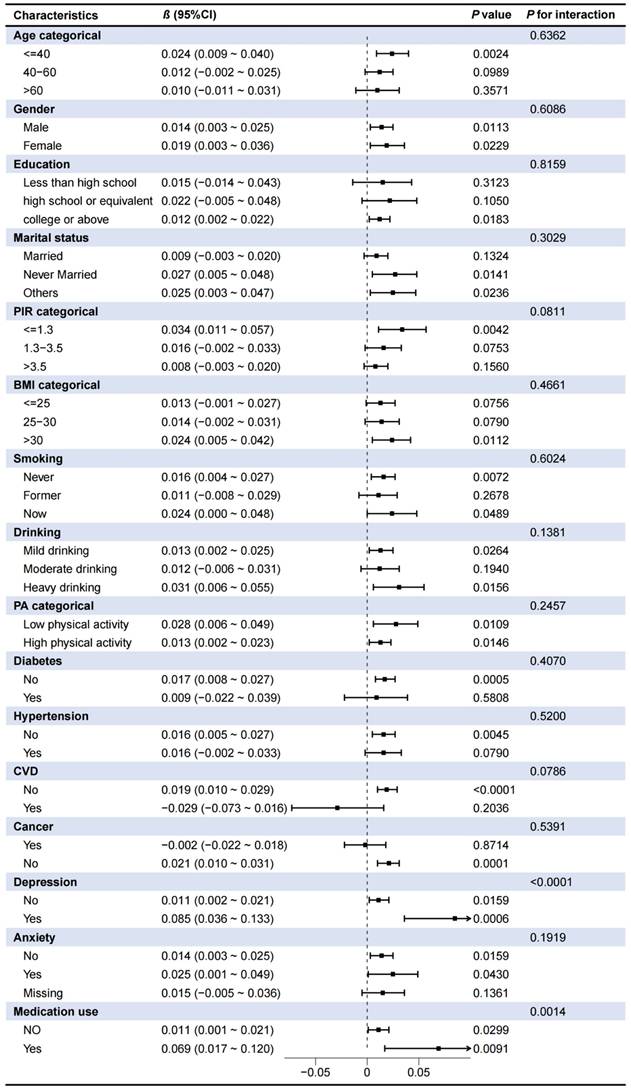
Abbreviations: CI, confidence intervals; CDAI, composite dietary antioxidant index; PA, physical activity; CVD, cardiovascular disease; BMI, body mass index; PIR, ratio of family income to poverty. The variables adjusted for subgroup analyses were consistent with Model 3 in Table 2 except the stratifying variable.
Associations between CDAI and sleep duration in different subgroups. (A) Depression; (B) Medication use.
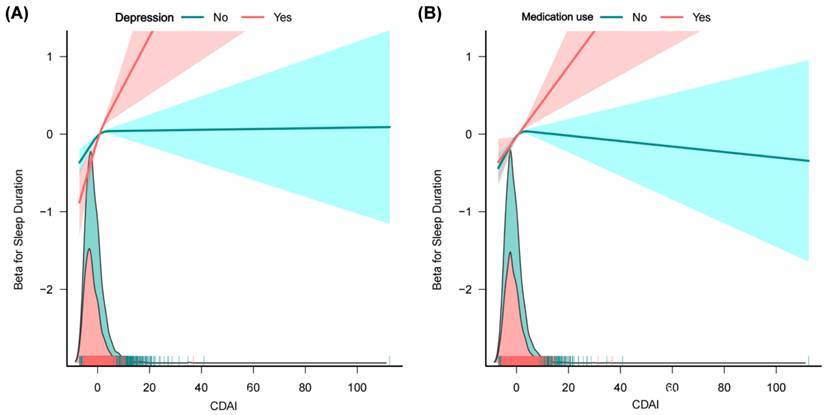
Sensitivity analyses. (A) Association between CDAI an sleep duration after excluding participants with energy extremes; (B) Association between CDAI an sleep duration after excluding participants with pregnancy. Note: variables adjusted in sensitivity analyses were consistent with Model 3 in Figure 2.
Although the specific mechanisms by which CDAI components improve sleep quality remain unclear, their antioxidant, redox modulation, and anti-inflammatory properties may play a crucial role. Previous studies have indicated that Nrf2 was a pivotal hub linking sleep to oxidative stress, inflammation, and circadian rhythms [54,55]. Insufficient sleep or sleep-disordered breathing are reported to suppress Nrf2 activity, thereby reducing antioxidant defenses and elevating inflammation—effects that further deteriorate sleep quality and perpetuate a vicious cycle [54]. In contrast, activation of Nrf2 via pharmaceutical, nutritional, or genetic modulation can partially reverse these adverse outcomes, with animal studies showing improved sleep architecture and cognitive function [54]. A growing body of evidence suggests that most key nutrients comprising the CDAI exert their neuroprotective effects primarily through activation of the Nrf2 signaling pathway. Vitamin C has been shown to upregulate Nrf2 and its downstream targets, such as HO-1 and NQO1, thereby attenuating oxidative stress [56,57]. Similarly, δ- and γ-tocopherol isoforms of vitamin E can directly promote the nuclear translocation of Nrf2 and enhance the expression of antioxidant enzymes [58]. Selenium contributes to Nrf2 activation by inducing target genes such as GPX4, which plays a critical role in protecting neural tissues against ferroptosis [59]. Zinc not only facilitates Nrf2 nuclear translocation but also suppresses ROS accumulation, consequently inhibiting activation of the NLRP3 inflammasome [60-62]. Carotenoids—including β-carotene, α-carotene, lutein, and lycopene—are also capable of inducing Nrf2 expression and boosting antioxidant enzyme levels; certain carotenoids, such as astaxanthin, may even reinforce Nrf2 activity through epigenetic regulation [63,64]. Taken together, these findings indicate that diets enriched in vitamin C, δ/γ-tocopherols, selenium, zinc, and carotenoids (namely diets with higher CDAI level) can effectively enhance antioxidant defenses and mitigate neuroinflammation via Nrf2-dependent mechanisms. This mechanistic framework may partly explain the observed association between higher CDAI levels and improvements in sleep duration and quality.
In this study, a stronger association between CDAI and sleep duration was observed in individuals with depression, indicating that participants in this subgroup were more likely to derive greater sleep benefits at higher CDAI levels. Depression is widely recognized not merely as a psychological disorder but as a physiological disease involving multisystem dysregulation, characterized principally by oxidative stress imbalance and chronic low-grade inflammation. These pathological alterations critically disrupt sleep regulatory pathways. Specifically, depressed individuals exhibit hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis, which disrupts circadian melatonin secretion and thereby disturbs sleep-wake rhythms [65,66]. Additionally, elevated serum levels of proinflammatory cytokines (e.g., CRP, IL-6, TNF-α) in depression cross the blood-brain barrier to directly inhibit sleep-regulatory functions in the prefrontal cortex and hypothalamus, leading to sleep fragmentation and reduced slow-wave sleep [67-69]. Concurrently, impaired antioxidant defenses (e.g., decreased SOD and GPx enzymatic activity) in depression enhance reactive oxygen species (ROS), inducing neuronal lipid peroxidation and DNA damage that compromise the functional integrity of sleep-wake regulatory nuclei [70-72]. Collectively, these pathological mechanisms create a "nutritional intervention window" for depressed patients: dietary intake of antioxidant-rich compounds (e.g., vitamins A, C, E) can more effectively neutralize free radicals and mitigate inflammation, thereby reconstructing a sleep-conducive molecular environment. This may explain the stronger association between CDAI and sleep duration observed in individuals with depression.
Similar enhanced associations between CDAI and sleep duration were observed among individuals using sleep-modulating medications, indicating that higher antioxidant nutrient intake may confer greater sleep benefits to this subgroup. Most sleep-modulating medications rely on multiple mechanisms to regulate neurotransmitter milieu—such as enhancing neuronal GABA receptor sensitivity or mimicking endogenous melatonin—processes that can be modulated by antioxidant nutrient levels. For example, benzodiazepines and non-benzodiazepine hypnotics (e.g., zolpidem, zopiclone) primarily reduce neuronal excitability by potentiating GABAergic neurotransmission, thereby lengthening sleep duration [73]. Notably, low oxidative stress levels enhance neuronal GABA receptor sensitivity to these drugs, further prolonging sleep. Additionally, melatonin receptor agonists like ramelteon improve sleep quality by mimicking endogenous melatonin [74,75]. When CDAI elevation alleviates oxidative damage, pinealocyte function recovers partially, increasing endogenous melatonin secretion and creating a synergistic effect with exogenous agonists. These mechanistic interactions between sleep-modulating drugs and antioxidant nutrients may underlie the stronger CDAI-sleep duration association in this population.
The study further revealed that the relationship between the Composite Dietary Antioxidant Index (CDAI) and sleep duration exhibits a clear saturation effect, with the inflection point occurring at a CDAI of 3.2. When CDAI is below 3.2, each additional unit of CDAI is associated with an increase of 2-8 minutes of sleep, gradually moving sleep duration toward the 7‑hour mark. Once CDAI reaches 3.2, sleep duration stabilizes around 7 hours, indicating that the effect has plateaued. This finding is important because a 7‑hour sleep duration has been repeatedly confirmed by epidemiological research as a “healthy threshold” that is strongly linked to lower risks of all‑cause mortality, cardiovascular disease, biological aging, metabolic syndrome, and other adverse health outcomes [76-78]. For individuals with a CDAI below 3.2—i.e., whose sleep duration has not yet reached the 7‑hour threshold—each unit increase in CDAI, although translating only into minute‑level gains in sleep, is not trivial. Instead, it represents a critical incremental step that helps them cross the “7‑hour healthy threshold.” In a context where sleep deprivation is common, this “small effect driving threshold breakthrough” holds considerable public‑health relevance. Moreover, while CDAI is formally a composite index of dietary antioxidant intake, it essentially serves as a measurable proxy for a “multidimensional healthy lifestyle.” Its association with sleep duration reflects the combined influence of “optimized diet + regular sleep schedule + moderate physical activity + low‑risk behaviors.” From this perspective, the sleep benefit linked to each unit increase in CDAI does not arise from a single dietary factor; rather, it signals the synergistic improvement of overall health‑behavior patterns. When an individual's CDAI rises and sleep duration approaches the 7‑hour threshold, this change indicates broader lifestyle optimization. Consequently, the chain of “behavior cluster - sleep threshold - health outcomes” carries far greater clinical significance than the modest minute‑level sleep gain alone. It suggests that enhancing diet (raising CDAI) can catalyze comprehensive lifestyle improvements, indirectly steering population sleep duration toward the healthy threshold and thereby reducing the risk of multi‑system diseases. This linkage has substantial public‑health implications, surpassing the impact of isolated “minute‑level sleep effects.” Briefly, although the effect size appears modest in terms of minutes, when considered alongside the saturated 7‑hour healthy threshold and the lifestyle‑proxy nature of CDAI, the findings retain clear clinical relevance and population‑health significance. They can aid in identifying high‑risk groups with insufficient sleep and in guiding holistic lifestyle interventions that begin with dietary modifications.
The sensitivity analyses in this study yielded consistent results. By excluding participants with implausible energy intake (a well-documented source of dietary reporting bias) and those with depression, the CDAI-sleep duration association persisted. This indicates that the association is not a statistical artifact caused by extreme caloric reporting errors but reflects the true biological effects of dietary antioxidants. These results, together with the identification of a nonlinear threshold and subgroup consistencies, collectively validate the fundamental role of CDAI in sleep health. Additionally, the findings reveal that optimizing CDAI through dietary strategies could complement existing sleep interventions.
Our study found a significant negative correlation between CDAI and sleep duration, while no significant association was observed between CDAI and sleep trouble or sleep disorder. This inconsistency may stem from the following aspects. First, sleep duration is a relatively objective and easily quantifiable indicator, whereas the assessment of sleep trouble/sleep disorder is more subjective and more susceptible to the influence of multiple strong confounding factors (e.g., transient emotional states, comorbid pain, and psychosocial stress). These factors may mask the relatively weak effect of dietary inflammatory potential. Second, although a comprehensive set of covariates were adjusted, residual factors such as chronic social stress were not included. These factors have been reported to be closely associated with subjective sleep quality and may have obscured CDAI's effect [79,80]. Third, CDAI is hypothesized to exert its effects through modulating inflammatory activity [81,82], and such inflammatory activity primarily impacts sleep structure by reducing deep sleep duration—with a more indirect influence on subjective sleep quality. This mechanistic pathway specifically aligns with our observation that CDAI associates only with sleep duration. The association between CDAI and sleep duration thus suggests that inflammatory activity may affect participants' total sleep duration by influencing sleep structure, but its impact on sleep quality still requires further research to verify. Future longitudinal studies should incorporate more comprehensive psychological assessments and objective sleep monitoring (e.g., actigraphy, polysomnography) to clarify the interactions among inflammation, psychological factors, and different sleep dimensions.
Our study offers several key advantages. First, it was based on a nationally representative NHANES population, which provided an adequate sample size and reliable dataset to uncover associations between CDAI and sleep quality. Second, sleep quality among enrolled participants was systematically assessed through subjective and objective measures, including self-reported sleep trouble, sleep disorders, and sleep duration. Third, adjustment for well-known potential confounding variables in sleep research enabled us to derive more accurate results. Additionally, subgroup analyses were conducted to validate the CDAI-sleep quality correlation across different populations, while interaction analyses explored how confounding variables influenced the outcomes. Finally, our findings revealed that individuals with a CDAI level ≥3.2 were more likely to have a predicted sleep duration of at least 7 hours—an insight with significant implications for informing daily lifestyle choices and clinical practices.
Several limitations of this study should be acknowledged. First, some variables were based on self-reported data (e.g., sleep parameters, alcohol consumption, physical activity, etc.), which may introduce recall and social desirability biases into the study. Especially, it is worth noting that the assessment of sleep quality in the NHANES database is not derived from objectively measured instrument data but relies on a few limited and subjective questions regarding sleep habits and sleep disorders. Consequently, this simplified evaluation method cannot fully capture key dimensions of sleep quality, such as the severity of insomnia, sleep latency (time taken to fall asleep), or sleep efficiency. The use of such subjectively evaluated key outcome variables may introduce a certain degree of bias into our findings. Therefore, future studies should employ objective sleep monitoring devices (e.g., actigraphy, polysomnography) to assess sleep parameters to further validate the relationship between CDAI and sleep quality. Second, the cross-sectional nature of the research hindered us from drawing a causal relationship between CDAI and sleep duration. Thus, future studies based on longitudinal or prospective cohort designs are warranted to further validate these findings. Third, despite adjusting for a comprehensive set of covariates, including demographic factors, lifestyle habits (e.g., smoking, alcohol use), and health indicators (e.g., BMI, comorbidity status), we cannot rule out the possibility of residual confounding. Unmeasured or imperfectly measured factors, such as chronic stress, detailed aspects of physical activity, undiagnosed sleep apnea, diet quality, socioeconomic status, smoking rates, or other unmeasured health behaviors, may influence both antioxidant intake and sleep quality and could partly account for the observed associations. Chronic social stress—defined as a sustained psychological burden arising from interpersonal relationships, work, finances, or life events—has been consistently linked to sleep quality. Prolonged stress can disrupt sleep architecture and reduce efficiency through activation of the HPA axis, sympathetic nervous system, inflammatory pathways, and emotional‑cognitive circuits. Conversely, poor sleep can heighten stress perception, creating a self‑reinforcing vicious cycle [79,80]. Although the precise expression of this bidirectional relationship may differ across populations because of variations in health status, age, and lifestyle, the overall association remains robust. Unfortunately, a key limitation of the present analysis is that the NHANES database does not contain measures of chronic social stress, preventing us from adjusting for this important confounder. Consequently, the observed link between CDAI and sleep‑related outcomes should be validated in longitudinal cohort studies or randomized controlled trials that incorporate chronic social stress (as well as other potential confounders) into the statistical models. Fourth, though we propose a plausible mechanism—CDAI influencing sleep via oxidative stress, inflammation, and the Nrf2 pathway, supported by existing literature—our study lacks relevant biomarker data (e.g., oxidative stress markers MDA/SOD, inflammatory cytokine IL-6) from NHANES. We thus cannot directly validate these mediators in our analysis, with mechanistic interpretations relying only on prior research rather than our cohort's data. Future studies incorporating these biomarkers can directly test the proposed pathway and strengthen causal inference. Fifth, while our study has revealed a statistical correlation between CDAI and sleep, the precise mechanisms by which antioxidant components impact sleep quality remain unclear. Future investigations using multi-omics techniques and specific animal models may help elucidate the specific pathways through which oxidative/antioxidant processes affect sleep quality. Sixth, this study does not adequately address the potential clustering of diet with overall health behaviors, which leads to an interpretation of the relationship between the CDAI and sleep quality that leans toward causality. In reality, higher CDAI scores often co‑occur with healthier lifestyles—such as regular sleep schedules, moderate physical activity, lower smoking and drinking frequencies, and better mental well‑being—each of which can independently improve sleep. Consequently, CDAI may serve more as a proxy for overall healthy behavior rather than as a direct, independent factor influencing sleep. Without controlling for or stratifying these concurrent health behaviors, the observed CDAI‑sleep association could be partially or entirely driven by unmeasured lifestyle differences. Therefore, future research should incorporate more comprehensive lifestyle variables (e.g., physical activity, sleep regularity, stress levels) or employ methods such as propensity‑score matching and stratified regression to disentangle CDAI's independent effect from its role as a surrogate for a healthy lifestyle, thereby providing a more nuanced and cautious interpretation. Finally, the present findings are derived exclusively from the NHANES cohort, which predominantly comprises U.S. adults of Western dietary patterns and genetic backgrounds. Consequently, the external generalizability of our results to other populations—particularly non‑Western groups with distinct eating habits and genetic profiles—remains uncertain. Future research in diverse international cohorts representing a broader range of regions, ethnicities, cultures, and dietary exposures are needed to validate and extend our results.
Conclusion
Our study identified a non-linear positive correlation between CDAI and sleep duration among US adults, with a more pronounced association observed in individuals with depression and those taking sleep-affecting medications. Additionally, an inflection point was identified at a CDAI score of 3.2. Beyond this threshold, further increases in CDAI were not associated with additional gains in sleep duration, indicating a saturation effect in which the predicted sleep duration plateaued at approximately 7 hours. To advance these findings and clarify causal mechanisms, future research should prioritize longitudinal cohort studies, mechanistic investigations leveraging multi-omics approaches (e.g., metabolomics, cytokine array techniques, proteomics), and randomized controlled trials integrating dietary antioxidant interventions with objective sleep monitoring (e.g., actigraphy, polysomnography).
Abbreviations
CDAI: composite dietary antioxidant index; BMI: body mass index; PIR: poverty income ratio; CAPI: computer-assisted personal interviewing.
Supplementary Material
Supplementary figure and tables.
Acknowledgements
The authors thank all the volunteers for the participation and personnel for their contribution to the NHANES study.
Data availability statement
The data used in this investigation was sourced from the National Health and Nutrition Examination Survey (NHANES), accessible at the website: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The program was approved by the National Center for Health Statistics Ethics Review Board. All of the participants provided written informed consent. No additional ethical review board approval was required to analyze the anonymized NHANES data.
Author contributions
All the authors have made significant contributions to the current study. XingHua Chen and Hao Gao guided this research, including design, data analysis and manuscript revision. Xiang Xu completed the main statistical analyses and wrote the main manuscript text. Xin Wei and Hong Chen performed additional statistical analyses and helped to optimizing picture layout. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sejbuk M, Mirończuk-Chodakowska I, Witkowska AM. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients [Internet]. 2022 Jan [cited. 2023 Sept 7];14(9):1912. Available from: https://www.mdpi.com/2072-6643/14/9/1912
2. Han L, Wang Q. Association between organophosphorus insecticides exposure and the prevalence of sleep problems in the US adults: An analysis based on the NHANES 2007-2018. Ecotox Environ Safe. 2023Apr15;255:114803
3. Wu X, Liu S, Wen L, Tan Y, Zeng H, Liang H, et al. Association between phthalates and sleep problems in the U.S. adult females from NHANES 2011-2014. Int J Environ Heal R. 2023 Mar 27;1-16
4. Reichert CF, Deboer T, Landolt HP. Adenosine, caffeine, and sleep-wake regulation: state of the science and perspectives. J Sleep Res. 2022Aug;31(4):e13597
5. Roberts RE, Duong HT. The prospective association between sleep deprivation and depression among adolescents. Sleep. 2014Feb1;37(2):239-44
6. Pomeroy A, Pagan Lassalle P, Kline CE, Heffernan KS, Meyer ML, Stoner L. The relationship between sleep duration and arterial stiffness: A meta-analysis. Sleep Med Rev. 2023Aug;70:101794
7. Huang BH, Duncan MJ, Cistulli PA, Nassar N, Hamer M, Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Brit J Sport Med. 2022 July;56(13):718-24
8. Besedovsky L, Lange T, Haack M. The Sleep-Immune Crosstalk in Health and Disease. Physiol Rev. 2019 July 1;99(3):1325-80
9. Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med Rev. 2021Apr;56:101409
10. Carter KA, Hathaway NE, Lettieri CF. Common sleep disorders in children. Am Fam Physician. 2014Mar1;89(5):368-77
11. Kase BE, Liu J, Wirth MD, Shivappa N, Hebert JR. Associations between dietary inflammatory index and sleep problems among adults in the United States, NHANES 2005-2016. Sleep Health. 2021Apr;7(2):273-80
12. Ancoli-Israel S, Poceta JS, Stepnowsky C, Martin J, Gehrman P. Identification and treatment of sleep problems in the elderly. Sleep Med Rev. 1997Nov;1(1):3-17
13. A V, Y KD, K N, Ea P, C L, Me G. et al. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell [Internet]. 2020 Nov 6 [cited. 2024 Sept 15];181(6). Available from: https://pubmed.ncbi.nlm.nih.gov/32502393/
14. Morris G, Stubbs B, Köhler CA, Walder K, Slyepchenko A, Berk M. et al. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev. 2018Oct;41:255-65
15. Tang L, Liu M, Mu J, Tian J. Association between circulating antioxidants and sleep disorders: comprehensive results from NHANES 2017-2018. Food Funct. 2024 June 17;15(12):6657-72
16. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012Jan;5(1):9-19
17. Vaccaro A, Kaplan Dor Y, Nambara K, Pollina EA, Lin C, Greenberg ME. et al. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 2020 June 11;181(6):1307-1328.e15
18. Thongchumnum W, Vallibhakara SAO, Sophonsritsuk A, Vallibhakara O. Effect of Vitamin E Supplementation on Chronic Insomnia Disorder in Postmenopausal Women: A Prospective, Double-Blinded Randomized Controlled Trial. Nutrients. 2023Feb27;15(5):1187
19. Doo M, Kim Y. The Consumption of Dietary Antioxidant Vitamins Modifies the Risk of Obesity among Korean Men with Short Sleep Duration. Nutrients. 2017 July 20;9(7):780
20. Baldwin CM, Bootzin RR, Schwenke DC, Quan SF. Antioxidant nutrient intake and supplements as potential moderators of cognitive decline and cardiovascular disease in obstructive sleep apnea. Sleep Med Rev [Internet]. 2005 Dec [cited. 2023 Sept 7];9(6):459-76. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1087079205000377
21. Hahad O, Herzog J, Röösli M, Schmidt FP, Daiber A, Münzel T. Acute Exposure to Simulated Nocturnal Train Noise Leads to Impaired Sleep Quality and Endothelial Dysfunction in Young Healthy Men and Women: A Sex-Specific Analysis. Int J Environ Res Public Health. 2022Oct25;19(21):13844
22. Al Wadee Z, Ooi SL, Pak SC. Serum Magnesium Levels in Patients with Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis. Biomedicines. 2022 Sept 14;10(9):2273
23. Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens [Internet]. 2023 Dec 31 [cited 2023 Sept 3];45(1):2233712. Available from: https://www.tandfonline.com/doi/full/10.1080/10641963. 2023 2233712
24. Wang L, Yi Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: A prospective cohort study. Front Cardiovasc Med [Internet]. 2022 Oct 4 [cited 2023 Sept 3];9:993930. Available from: https://www.frontiersin.org/articles/10.3389/fcvm. 2022 993930/full
25. Wu D, Wang H, Wang W, Qing C, Zhang W, Gao X. et al. Association between composite dietary antioxidant index and handgrip strength in American adults: Data from National Health and Nutrition Examination Survey (NHANES, 2011-2014). Front Nutr [Internet]. 2023 Mar 31 [cited 2023 Aug 15];10:1147869. Available from: https://www.frontiersin.org/articles/10.3389/fnut. 2023 1147869/full
26. Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR. et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004 July 1;160(1):68-76
27. Han H, Chen S, Wang X, Jin J, Li X, Li Z. Association of the composite dietary antioxidant index with bone mineral density in the United States general population: data from NHANES 2005-2010. J Bone Miner Metab. 2023 June 8
28. Liu X, Liu X, Wang Y, Zeng B, Zhu B, Dai F. Association between depression and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005-2018. J Affect Disorders. 2023 Sept 15;337:57-65
29. Zhao L, Sun Y, Cao R, Wu X, Huang T, Peng W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health [Internet]. 2022 Oct 13 [cited 2023 Sept 3];10:988727. Available from: https://www.frontiersin.org/articles/10.3389/fpubh. 2022 988727/full
30. Zheng Y, Liu W, Zhu X, Xu M, Lin B, Bai Y. Associations of dietary inflammation index and composite dietary antioxidant index with preserved ratio impaired spirometry in US adults and the mediating roles of triglyceride-glucose index: NHANES 2007-2012. Redox Biol. 2024Aug30;76:103334
31. Wang DD, Jia MQ, Xu HL, Li Y, Liu JX, Liu JC. et al. Association of pre- and post-diagnosis dietary total antioxidant capacity (TAC) and composite dietary antioxidant index (CDAI) with overall survival in patients with ovarian cancer: a prospective cohort study. J Transl Med. 2025Jan30;23:134
32. Zhang J, Shi H. Linear association between the composite dietary antioxidant index and the prevalence of sarcopenia in metabolic dysfunction-associated steatotic liver disease. Lipids Health Dis. 2025Oct14;24(1):320
33. Zhang J, Yu S, Zhao G, Jiang X, Zhu Y, Liu Z. Associations of chronic diarrheal symptoms and inflammatory bowel disease with sleep quality: A secondary analysis of NHANES 2005-2010. Front Neurol. 2022;13:858439
34. Wang L, Sun M, Guo Y, Yan S, Li X, Wang X. et al. The Role of Dietary Inflammatory Index on the Association Between Sleep Quality and Long-Term Cardiovascular Risk: A Mediation Analysis Based on NHANES (2005-2008). Nat Sci Sleep. 2022;14:483-92
35. Yang Q, Zhu X, Zhang L, Luo F. Dyslipidemia and aging: the non-linear association between atherogenic index of plasma (AIP) and aging acceleration. Cardiovascular Diabetology. 2025Apr25;24(1):181
36. Dai W, Zhang D, Wei Z, Liu P, Yang Q, Zhang L. et al. Whether weekend warriors (WWs) achieve equivalent benefits in lipid accumulation products (LAP) reduction as other leisure-time physical activity patterns? -Results from a population-based analysis of NHANES 2007-2018. BMC Public Health. 2024 June 9;24(1):1550
37. Xue H, Zou Y, Yang Q, Zhang Z, Zhang J, Wei X. et al. The association between different physical activity (PA) patterns and cardiometabolic index (CMI) in US adult population from NHANES (2007-2016). Heliyon. 2024Apr15;10(7):e28792
38. Zeng G, You D, Ye L, Wu Y, Shi H, Lin J. et al. n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not: NHANES 2007-2016. Front Nutr. 2023Apr4;10:1075877
39. Yang Z, Zhu L, Chen Z. Dose-response and isotemporal substitution analysis of domain-specific physical activity and sedentary behavior with abdominal aortic calcification risk: A cross-sectional study. PLoS One. 2025Oct7;20(10):e0332964
40. Tao X, Xu X, Xu Y, Yang Q, Yang T, Zhou X. et al. Association between physical activity and visceral adiposity index (VAI) in U.S. population with overweight or obesity: a cross-sectional study. BMC Public Health. 2024Aug26;24(1):2314
41. Wei X, Min Y, Xiang Z, Zeng Y, Wang J, Liu L. Joint association of physical activity and dietary quality with survival among US cancer survivors: a population-based cohort study. Int J Surg. Sept 1. 2024;110(9):5585-94
42. MacGregor KA, Gallagher IJ, Moran CN. Relationship Between Insulin Sensitivity and Menstrual Cycle Is Modified by BMI, Fitness, and Physical Activity in NHANES. J Clin Endocrinol Metab. 2021 June 10;106(10):2979-90
43. Leavitt MO. 2008 Physical Activity Guidelines for Americans
44. Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001-2018. Cardiovasc Diabetol. 2023Oct17;22(1):279
45. Cai Y, Chen M, Zhai W, Wang C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005-2018. BMC Public Health. 2022Mar11;22(1):481
46. Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X. et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. 2024Jan6;23(1):8
47. Zhou X, Tao XL, Zhang L, Yang QK, Li ZJ, Dai L. et al. Association between cardiometabolic index and depression: National Health and Nutrition Examination Survey (NHANES) 2011-2014. J Affect Disord. 2024Apr15;351:939-47
48. Gui J, Ding R, Huang D, Wang L, Han Z, Yang X. et al. Associations between urinary heavy metals and anxiety among adults in the National Health and Nutrition Examination Survey (NHANES), 2007-2012. Chemosphere. 2023Nov1;341:140085
49. Yang Q, Zhu S, Luo Y, Ai H, Feng L, Zhang L. et al. Cholesterol's hidden impact: the nonlinear link between remnant cholesterol (RC) and phenotypic age acceleration (PhenoAgeAccel). Biogerontology. 2025Aug;26(4):133
50. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986 July;124(1):17-27
51. Zhai L, Zhang H, Zhang D. SLEEP DURATION AND DEPRESSION AMONG ADULTS: A META-ANALYSIS OF PROSPECTIVE STUDIES. Depress Anxiety. 2015 Sept;32(9):664-70
52. Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014 July;134:86-91
53. Rhee JJ, Sampson L, Cho E, Hughes MD, Hu FB, Willett WC. Comparison of Methods to Account for Implausible Reporting of Energy Intake in Epidemiologic Studies. American Journal of Epidemiology. 2015Feb15;181(4):225-33
54. Davinelli S, Medoro A, Savino R, Scapagnini G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell Mol Neurobiol. 2024 June 25;44(1):52
55. Calabrese V, Cornelius C, Stella AMG, Calabrese EJ. Cellular Stress Responses, Mitostress and Carnitine Insufficiencies as Critical Determinants in Aging and Neurodegenerative Disorders: Role of Hormesis and Vitagenes. Neurochem Res. 2010Dec1;35(12):1880-915
56. Bsoul SA, Terezhalmy GT. Vitamin C in health and disease. J Contemp Dent Pract. 2004May15;5(2):1-13
57. Xu Y, Zheng H, Slabu I, Liehn EA, Rusu M. Vitamin C in Cardiovascular Disease: From Molecular Mechanisms to Clinical Evidence and Therapeutic Applications. Antioxidants. 2025May;14(5):506
58. Xie X, Zhao S, Fang R, Chen H, Zhang H, Wang X. et al. Vitamin E Inhibits Oxidative Stress and Inflammation in Stress-Induced Gastritis via Modulating Nrf2 and NF-κB Signalling Pathways. J Cell Mol Med. 2025May26;29(10):e70463
59. Müller M, Banning A, Brigelius-Flohé R, Kipp A. Nrf2 target genes are induced under marginal selenium-deficiency. Genes Nutr. 2010Dec;5(4):297-307
60. Lou J, Wu F, He W, Hu R, Cai Z, Chen G. et al. Hesperidin activates Nrf2 to protect cochlear hair cells from cisplatin-induced damage. Redox Report. 2024Dec31;29(1):2341470
61. Yang F, Smith MJ, Siow RCM, Aarsland D, Maret W, Mann GE. Interactions between zinc and NRF2 in vascular redox signalling. Biochem Soc Trans. 2024Feb28;52(1):269-78
62. Ge MH, Tian H, Mao L, Li DY, Lin JQ, Hu HS. et al. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther. 2021 Sept;27(9):1023-40
63. Chen P, Li L, Gao Y, Xie Z, Zhang Y, Pan Z. et al. β-carotene provides neuroprotection after experimental traumatic brain injury via the Nrf2-ARE pathway. JIN. 2019 June 30;18(2):153-61
64. Frede K, Ebert F, Kipp AP, Schwerdtle T, Baldermann S. Lutein Activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells. J Agric Food Chem. 2017 July 26;65(29):5944-52
65. Rein T, Ambrée O, Fries GR, Rappeneau V, Schmidt U, Touma C. Chapter 9 - The Hypothalamic-Pituitary-Adrenal Axis in Depression: Molecular Regulation, Pathophysiological Role, and Translational Implications. In: Quevedo J, Carvalho AF, Zarate CA, editors. Neurobiology of Depression [Internet]. Academic Press; 2019 [cited. 2025 June 30]. p. 89-96. Available from: https://www.sciencedirect.com/science/article/pii/B9780128133330000093
66. Shi L, Li N, Bo L, Xu Z. Melatonin and hypothalamic-pituitary-gonadal axis. Curr Med Chem. 2013;20(15):2017-31
67. Chang J, Jiang T, Shan X, Zhang M, Li Y, Qi X. et al. Pro-inflammatory cytokines in stress-induced depression: Novel insights into mechanisms and promising therapeutic strategies. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2024Apr20;131:110931
68. Chen R, Yin Y, Zhao Z, Huang L, Huang S, Zhuang J. et al. Elevation of serum TNF-α levels in mild and moderate Alzheimer patients with daytime sleepiness. Journal of Neuroimmunology. 2012Mar1;244(1):97-102
69. Rizavi HS, Ren X, Zhang H, Bhaumik R, Pandey GN. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Research. 2016 June 30;240:314-20
70. Lievanos-Ruiz FJ, Fenton-Navarro B. Enzymatic biomarkers of oxidative stress in patients with depressive disorders. A systematic review. Clinical Biochemistry. 2024Aug1;130:110788
71. Shichiri M. The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr. 2014May;54(3):151-60
72. Lewis S. DNA damage drives sleep. Nat Rev Neurosci. 2022Feb;23(2):69-69
73. Uygun DS, Ye Z, Zecharia AY, Harding EC, Yu X, Yustos R. et al. Bottom-Up versus Top-Down Induction of Sleep by Zolpidem Acting on Histaminergic and Neocortex Neurons. J Neurosci. 2016Nov2;36(44):11171-84
74. Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Jet lag: therapeutic use of melatonin and possible application of melatonin analogs. Travel Med Infect Dis. 2008;6(1-2):17-28
75. Pandi-Perumal SR, Srinivasan V, Spence DW, Moscovitch A, Hardeland R, Brown GM. et al. Ramelteon: a review of its therapeutic potential in sleep disorders. Adv Ther. 2009 June;26(6):613-26
76. Svensson T, Saito E, Svensson AK, Melander O, Orho-Melander M, Mimura M. et al. Association of Sleep Duration With All- and Major-Cause Mortality Among Adults in Japan, China, Singapore, and Korea. JAMA Netw Open. 2021 Sept 1;4(9):e2122837
77. You Y, Chen Y, Liu R, Zhang Y, Wang M, Yang Z. et al. Inverted U-shaped relationship between sleep duration and phenotypic age in US adults: a population-based study. Sci Rep. 2024Mar15;14(1):6247
78. Li Y, Gong W, Sahakian BJ, Huang S, Zhang W, Zhao Y. et al. Divergent biological pathways linking short and long sleep durations to mental and physical health. Nat Mental Health. 2025Mar3;3(4):429-43
79. Dubey O, Kumar DCS. A Study on the Impact of Stress on Sleep Quality Among Adults. African Journal of Biomedical Research. 2024Dec31;27(4S):12312-6
80. Lo Martire V, Caruso D, Palagini L, Zoccoli G, Bastianini S. Stress & sleep: A relationship lasting a lifetime. Neuroscience & Biobehavioral Reviews. 2020Oct1;117:65-77
81. Meng Z, Zheng W, Meng X, Xu H. The association of composite dietary antioxidant index with periodontitis in NHANES 2009-2014. Front Immunol [Internet]. 2024 June 24 [cited 2025 Oct 2];15. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu. 2024 1384272/full
82. Liu Z, Li J, Chen T, Zhao X, Chen Q, Xiao L. et al. Association between dietary antioxidant levels and chronic obstructive pulmonary disease: a mediation analysis of inflammatory factors. Front Immunol [Internet]. 2024 Jan 8 [cited 2025 Oct 2];14. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu. 2023 1310399/full
Author contact
![]() Corresponding authors: Hao Gao, haogaoedu.cn; XingHua Chen, chenxhedu.cn.
Corresponding authors: Hao Gao, haogaoedu.cn; XingHua Chen, chenxhedu.cn.

 Global reach, higher impact
Global reach, higher impact