Impact Factor
ISSN: 1449-1907
Int J Med Sci 2026; 23(1):42-52. doi:10.7150/ijms.119730 This issue Cite
Research Paper
Amelioration of contusion-induced muscle injury via regulation of the NLRP3 inflammasome
1. National Research Institute of Chinese Medicine, Ministry of Health and Welfare, Taipei 112, Taiwan.
2. Department of Exercise and Health Science, National Taipei University of Nursing and Health Sciences, Taipei 112, Taiwan.
3. National Laboratory Animal Center, National Applied Research Laboratories, Taipei 115, Taiwan.
4. Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan 333, Taiwan.
#Equally contributed first authorship.
Received 2025-6-17; Accepted 2025-10-17; Published 2026-1-1
Abstract
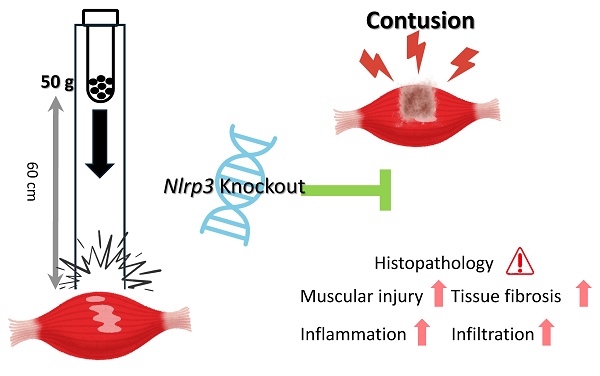
Inflammasomes, including NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing protein 3), participate in regulating immune activation and inflammation. Nonetheless, their specific roles in muscle contusion, one of the most common forms of sports injury, remain unknown. To address this, we investigated the role of NLRP3 in muscle contusion in Nlrp3-knockout (KO) mice, using a model of mass-drop injury (MDI)-induced contusion of the gastrocnemius muscle. Wild-type (WT) and Nlrp3-KO mice were assigned to four experimental groups: (1) WT-N: wild-type without MDI (control); (2) NLRP3-N: Nlrp3-KO without MDI; (3) WT-I: WT with MDI; and (4) NLRP3-I: Nlrp3-KO with MDI. Following MDI, tissue and serum samples were collected at 0, 48, 96, and 192 h. Muscle injury, recovery, and the extent of inflammation were evaluated by measuring body and muscle weight, serum biochemical markers, complete blood counts, and via histopathological immunohistochemical analysis. In the early phase following contusion, muscle weight was lower in the NLRP3-I group than in the WT-I group; however, in the later stages, it was significantly greater in the NLRP3-I group. Serum aspartate aminotransferase, lactate dehydrogenase, and creatine kinase were significantly lower in the NLRP3-I group than in the WT-I group, indicating reduced muscle damage following Nlrp3 knockout. The white blood cell and neutrophil counts were markedly higher in the WT-I group than in the NLRP3-I group. Based on histopathology, the NLRP3-I group exhibited less severe muscle injury, reduced tumor necrosis factor (TNF)-α, interleukin (IL)-6, CD68, CD206 and Caspase-3 expression, and reduced fibrosis, indicating a diminished contusion-induced inflammatory response and improved regeneration relative to the WT-I group. Nlrp3 knockout thus ameliorated contusion-induced muscle injury and inflammation. We hypothesize that NLRP3 regulates TNF-α and IL-6, with Nlrp3 downregulation reducing contusion-related muscle damage and fibrosis. NLRP3 thus represents a promising therapeutic target for treating sports injuries and for rehabilitation.
Keywords: inflammasome, cytokine, muscle contusion, inflammation, sport injury
Introduction
Contusions and strains account for approximately 60-70% of all sports-related injuries, with muscle contusions comprising one-third of all sports injuries. These soft-tissue injuries, most often affecting the quadriceps and gastrocnemius muscle, typically result from blunt force trauma, such as a kick, fall, or blow, and are common in contact sports as well as from accidental contact in non-contact sports. Among soccer players, rotator cuff contusions represent nearly half of all shoulder injuries, with a minority of cases progressing to more severe injuries, such as rotator cuff tears [1]. In a survey on the prevalence of exercise-related injuries at fitness centers [2], exercise duration and class type were associated with different types of injuries; while muscle strains and bruises were the most common injuries, muscle cramps, spasms, and contusions (including hematomas and bruises) were more frequent in classes shorter than an hour. In the different trial, contusions were most common in the knee, followed by the shoulder [3].
The immediate symptoms of contusion typically include pain, swelling, and discoloration. Injuries to skeletal muscle not only damage muscle cells but may also lead to capillary rupture, infiltrative bleeding, inflammation, oxidative stress, and fibrosis, with the severity depending on the extent of the injury [4]. Within the complex inflammatory response to contusion, neutrophils and macrophages may exert different functions at different stages of pathogenesis and recovery. A standardized non-invasive contusion injury model has been proposed as being ideal for investigating immune responses to mechanical injury of skeletal muscle [5]. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and free radicals play important roles in repairing muscle damage [6, 7]. In the inflammatory phase, neutrophils and macrophages exhibit chemotactic movement toward the injured lesions for phagocytosis. M1 macrophages, especially in the early stages, participate in the inflammatory response and contribute to damage. M2 macrophages, in contrast, release various growth factors that directly accelerate myocyte replication and regeneration [8]. M2 macrophages also stimulate regulatory T cells to secrete cytokines such as IL-4 and IL-10 to activate and differentiate satellite cells, thereby improving the capacity for muscle regeneration [9].
Pattern-recognition receptors (PRRs), expressed on immune cells, are categorized as toll-like receptors (TLRs) when expressed on the cell membrane and as nucleotide-binding and oligomerization domain-like receptors (NLRs) when expressed in the cytoplasm. NLRs function as inflammasomes, with NLRPs (nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing proteins) representing the largest NLR family. Four distinct inflammasomes (NLRP1, NLRP3, NLRC4, and AIM2), associated with caspase activation and pro-inflammatory cytokine secretion, have been identified [10]. Inflammasomes play a pivotal role in the host's innate defense system, and inflammasome dysregulation has been reported in several inflammatory, immune, and metabolic disorders [11]. The NLRP3 inflammasome, occurring primarily in monocytes and macrophages, participates in cardiomyocyte injury and immune disorders, based on animal model studies [12-14]. In inclusion body myositis, Nlrp3 and its associated genes related to inflammasome assembly, T-cell migration, and MHC-I expression are highly co-upregulated [15]. In Nlrp3-knockout (KO) mice with sepsis, serum interleukin-1β (IL-1β) was reduced, mitigating muscular atrophy; the same authors proposed that inhibiting IL-1β activation might prevent inflammation-induced muscle failure [16].
Nonetheless, the precise role of NLRP3 in skeletal muscle injury, including the specific cell types and cytokines involved in modulating damage severity and regeneration following contusion, remains insufficiently understood. We hypothesized that downregulating NLRP3 will attenuate muscle injury and promote regeneration, potentially by modulating the key regulatory factors involved in muscle repair. To test this, we used a skeletal-muscle contusion NLRP3-KO mouse model to investigate the role of NLRP3 in inflammation and regeneration following sports-related muscle injury.
Material and Methods
Animals and treatments
The nine-week-old male C57BL/6Narl mice, raised under specific pathogen-free conditions, were obtained from the National Laboratory Animal Center (NLAC, Taiwan). Nlrp3-KO mice (B6.129S6-Nlrp3tm1Bhk/J) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The animals were fed a standard laboratory chow diet (No. 5001, PMI Nutrition International, Brentwood, MO, USA), provided with distilled water ad libitum, and appropriately housed with a 12:12 h light-dark cycle at 21 ± 1 °C and 55%-65% humidity. After one week of acclimation, the animals were randomly divided into four groups (n = 24 per group for each indicator tested): (1) WT-N: wild-type without injury (control); (2) NLRP3-N: Nlrp3-KO without injury; (3) WT-I: WT with mass-drop injury (MDI); and (4) NLRP3-I: Nlrp3-KO with MDI.
Contusion induction and sample collection time-points
The hindlimbs of the mice were subjected to MDI-induced contusion as previously described [17], with slight modifications. Briefly, a 50 g weight was dropped from a height of 60 cm onto the medial surface of the left gastrocnemius muscle after the mouse was anesthetized with 3-4% isoflurane. Six mice from each group were then sacrificed via CO2 asphyxiation followed by cardiac puncture, at 0, 48, 96, and 192 h after injury. Blood samples were collected into tubes with or without anticoagulant for clinical chemistry and complete blood count (CBC) analysis. Gastrocnemius muscle tissue was preserved in 10% formalin and paraffin-embedded for histopathological examination and immunohistochemical (IHC) staining.
Clinical chemistry and CBC
Serum samples were centrifuged at 2700 ×g for 10 min. Muscle injury was assessed by measuring serum aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase (CK) levels using an automatic analyzer (HITACHI 7080, Hitachi, Tokyo, Japan). The anticoagulated blood for complete CBC assay was treated with ethylenediaminetetraacetic acid. Total blood cells, differential leukocytes, erythrocytes, and platelets were quantified using the Bayer Hematology System (ADVIA 2010 Autoanalyzer, Bayer Diagnostics, Leverkusen, Germany).
Histological examination and Masson's trichrome staining
Muscle fixation, processing, and embedding were performed as previously described [18]. Transverse sections of the gastrocnemius were obtained and stained with hematoxylin and eosin for histological observation and with Masson's trichrome stain to examine fibrosis severity. Paraffin sections were subjected to deparaffinization by placing the sections in xylene for 5 min (three times), followed by immersion in ethanol (absolute) for 5 min (twice), then at 95% for 5 min, 90% for 5 min, and 80% for 5 min, before washing with water. Sections were then stained for 5 min using Weigert's hematoxylin from the Masson trichrome staining kit (HT15, Merk, Rahway, NJ, USA), then washed with water. The sections were then stained with Ponceau S solution for 10 min, washed with water, and counterstained with Aniline blue solution for 5 min. Finally, the sections were treated with 1% acetic acid for 2 min, dehydrated, mounted, and observed under a microscope.
IHC staining
Paraffin-embedded muscle sections were deparaffinized, rehydrated, and subjected to antigen retrieval, followed by incubation with 3% H2O2 to eliminate endogenous peroxidase activity. The sections were incubated at 4 °C overnight with rabbit anti-mouse TNF-α (1:100) polyclonal antibodies (AF-410, Novus Biologicals, Centennial, CO, USA), goat anti-mouse IL-6 (1:100) polyclonal antibody (AF-406, Novus Biologicals), rabbit anti-mouse CD206 (1:500) antibody (#24595, Cell Signaling, Danvers, MA, USA), rabbit anti-mouse CD68 (1:200) antibody (ab125212, Abcam, Waltham, MA, USA), and rabbit anti-mouse cleaved Caspase-3 (1:400) antibody (#9661, Cell Signaling, Danvers, MA, USA). They were then incubated with horseradish peroxidase-conjugated anti-rabbit (87-9963, Invitrogen, Waltham, MA, USA) or anti-goat polymer (MP-7405, VectorLabs, Newark, CA, USA), and signals were detected after adding a chromogenic substrate (3-amino-9-ethylcarbazole [AEC] or 3,3′-diaminobenzidine [DAB]). The sections were counterstained with hematoxylin and mounted for histological analysis.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA). Results are presented as the mean ± SD. Differences between groups were analyzed using two-way ANOVA with Fisher's protected least significant difference (LSD) for multiple comparisons. Differences were considered significant at p <0.05.
Results
Body and muscle weight
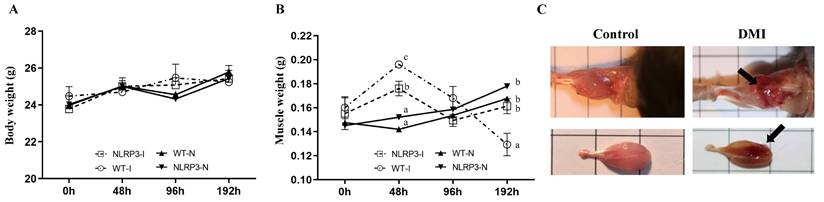
Body weight did not vary significantly between the WT-N, NLRP3-N, WT-I, and NLRP3-I groups at any time-point (Figure 1A). However, gastrocnemius muscle weight varied significantly among the groups at 48 and 192 h, being higher in the contusion groups (WT-I and NLRP3-I) than in the non-injury controls (WT-N and NLRP3-N). At 48 h, gastrocnemius muscle weight was significantly higher in the WT-I group than in the NLRP3-I group, and at 96 h, it was lowest (by a significant margin) in the NLRP3-I group (Figure 1B). In terms of gross structure, the gastrocnemius muscle exhibited a spindle shape, smooth surface, and normal muscle appearance, whereas the impacted surface of the injured muscle exhibited a large area of red hemorrhagic lesions (indicated by arrows) (Figure 1C).
Clinical chemistry
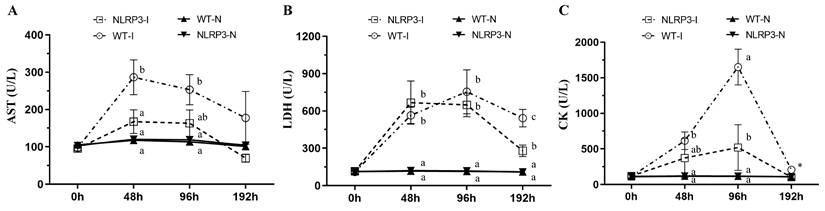
Muscle injury caused by MDI may be associated with several biochemical variables, including AST, LDH, and CK. At 48 and 96 h post-contusion, AST concentration was significantly higher in the WT-I group than in the NLRP3-I and non-contusion groups (WT-N and NLRP3-N) (Figure 2A); at 192 h, however, no significant differences in AST were observed among the groups. At 48 and 96 h, LDH was significantly higher in the WT-I and NLRP3-I groups than in the non-contusion groups, with no significant difference between the WT-I and NLRP3-I groups; at 192 h, it was significantly lower in the NLRP3-I group than in the WT-I group, but remained significantly higher than in the WT-N and NLRP3-N groups (Figure 2B). At 48 h, CK was significantly elevated, being significantly higher in the WT-I group than in the WT-N and NLRP3-N groups; at 96 h, it was significantly lower in the NLRP3-I group than in the WT-I group but remained significantly higher than in the WT-N and NLRP3-N groups (Figure 2C). Even at 192 h, CK was highest (by a significant margin) in the WT-I group (Figure 2C).
Complete blood count
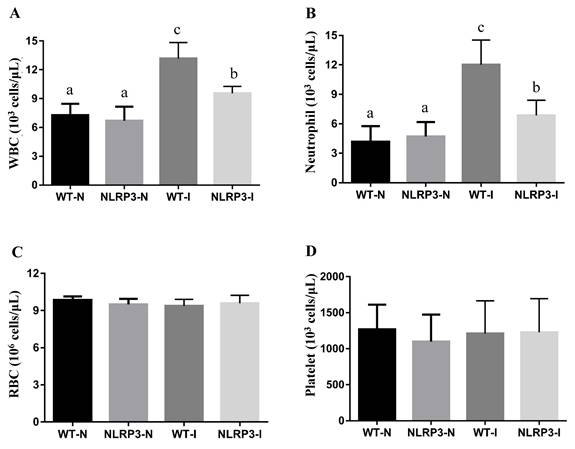
CBC analysis at 192 h, carried out to assess the hematological impact of MDI, revealed significant differences in white blood cell (WBC) and neutrophil counts among the groups. WBC count was markedly higher in the WT-I group than in the NLRP3-I and non-MDI (WT-N and NLRP3-N) groups; while it was significantly lower in the NLRP3-I group than in the WT-I group, it was notably higher than in the WT-N and NLRP3-N groups (Figure 3A). Similarly, the neutrophil count was significantly elevated in both the WT-I and NLRP3-I groups relative to their respective controls, although it was significantly lower in the NLRP3-I group than in the WT-I group (Figure 3B). No statistically significant differences were found in red blood cell or platelet counts among the groups (Figure 3C, D).
Histopathology
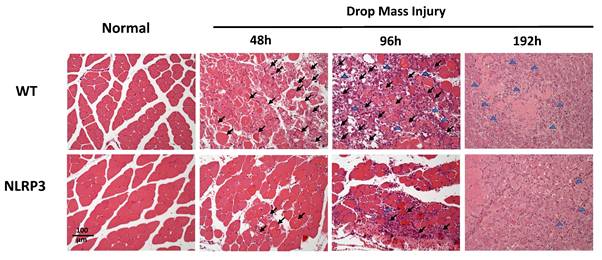
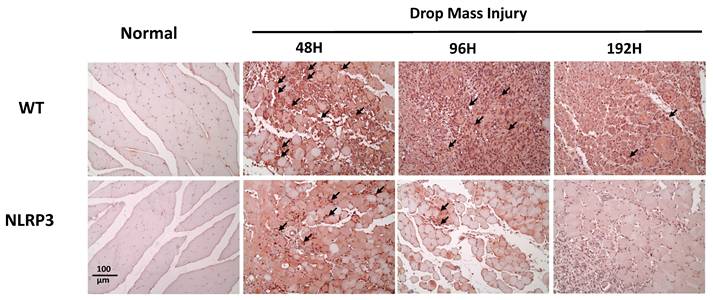
Histological examination of HE-stained tissue samples from the WT-I group at 48 h revealed large areas of muscle fiber necrosis and degeneration, accompanied by swelling, fragmentation, loss of striation, and glassy degeneration (Zenker's necrosis) in the affected regions (arrows, Figure 4). In contrast, the NLRP3-I group exhibited only mild to moderate pathological changes consistent with muscle injury at 48 h. At 96 h, the WT-I group showed extensive infiltration of inflammatory cells (primarily mononuclear macrophages, with a few polymorphonuclear neutrophils indicated by arrows) in the lesion, which also exhibited numerous myoblasts, characterized by scant cytoplasm and hyperchromatic nuclei. In contrast, the NLRP3-I group exhibited less severe inflammatory cell infiltration and fewer myoblasts than the WT-I group. At 192 h, in the WT-I group, most of the necrotic striated muscle fibers had been replaced by newly formed muscle fibers containing nuclei, although a few elongated fibroblasts (indicated by triangles) were present in the lesion; in the NLRP3-I group, in contrast, only new muscle fibers were present, without no evidence of fibroblasts. These findings suggest that muscle injury was less severe in the NLRP3-I group than in the WT-I group. No pathological changes were observed in the muscle tissue of the WT-N or NLRP3-N groups.
Mouse growth (A) and gastrocnemius muscle weight (B) at different time-points after mass-drop injury (MDI) in Nlrp3-knockout (KO) and C57BL/6Narl mice. (C) Muscle injury gross morphology. '-I', with injury; '-N', non-injured control. Different superscript lowercase letters identify groups with significant differences (p < 0.05). An arrow indicates the MDI-induced hemorrhage.
Injury-associated biochemical marker levels following mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl mice. (A) Aspartate aminotransferase (AST); (B) lactate dehydrogenase (LDH); (C) creatine kinase (CK). '-I', with injury; '-N', non-injured control. Different lowercase superscript letters identify groups with significant differences (p < 0.05). *, highest in the WT-I group, by a significant margin.
The effects of mass-drop injury (MDI) on complete blood count on Nlrp3-knockout and C57BL/6Narl mice. (A) White blood cell (WBC), (B) neutrophil, (C) red blood cell (RBC), and (D) platelet, at 192 h post-contusion. '-I', with injury; '-N', non-injured control. Different lowercase superscript letters identify groups with significant differences (p < 0.05).
Muscle histopathogenesis following mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with hematoxylin and eosin. Arrows, large areas of muscle fiber necrosis and degeneration, with swelling, fragmentation, loss of striation, and glassy degeneration. Blue triangles, sparse elongated fibroblasts in the lesion (×200 magnification).
IHC staining of TNF-α, IL-6, CD68, and CD206, and Caspase-3
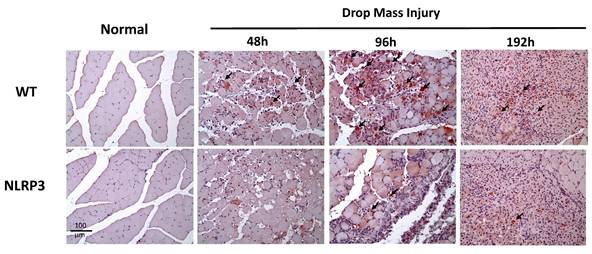
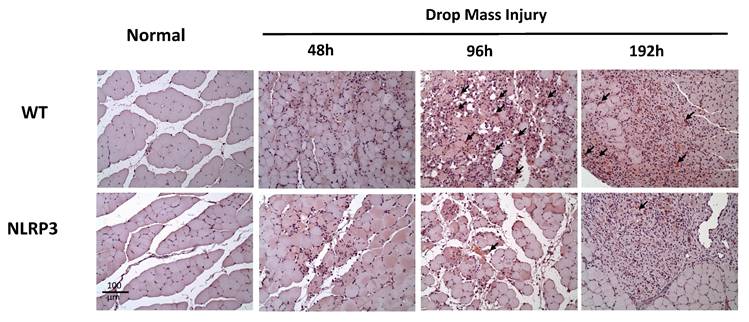
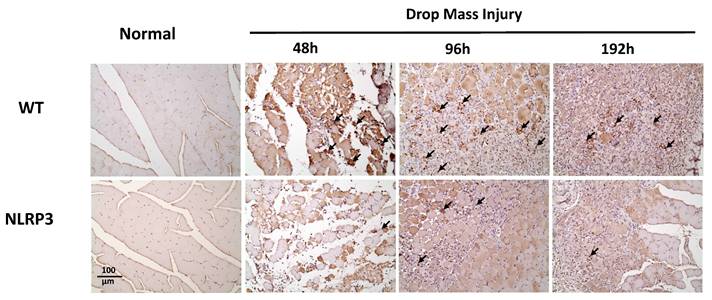
The injured muscle tissue exhibits positive TNF-α staining (red-brown, indicated by arrows), primarily in the nucleated muscle fibers and certain inflammatory cells. In the WT-I group, TNF-α expression was detectable as early as 48 h post-injury, with a significant increase in TNF-α-positive cells observed at 96 h and persisting through 192 h. In contrast, in the NLRP3-I group, only a small number of TNF-α-positive cells were detected at 96 and 192 h (Figure 5). IL-6 expression was observed primarily in nucleated muscle fibers and some inflammatory cells. In the WT-I group, a significant number of IL-6-positive cells were detected at 96 h, with this expression persisting through 192 h. In contrast, the NLRP3-I group exhibited only a small number of IL-6-positive cells at 96 and 192 h (Figure 6). CD68 is widely utilized as a histochemical and cytochemical inflammatory marker, specifically in relation to the presence and activity of monocytes and macrophages. CD68-positive cells, appearing brown-black and predominantly mononuclear macrophages (indicated by arrows), were detectable in the WT-I group as early as 48 h post-injury. A significant increase in CD68-positive cells was observed at 96 h, with the signal persisting through 192 h. In contrast, the NLRP3-I group exhibited only a small number of CD68-positive cells at the 96 and 192 h, with less pronounced staining than in the WT-I group (Figure 7). Levels of CD206-positive cells were significantly higher at 96 and 192 h in the WT-I group than in the NLRP3-I group (Figure 8). Active Caspase-3 expression exhibited a pronounced elevation in the WT-I group, peaking at 48 hours post-injury and subsequently declining progressively from 96 to 192 hours. A comparable temporal pattern was detected in the NLRP3-I group; however, the magnitude of expression was markedly attenuated relative to the corresponding time points in the WT-I group (Figure 9).
Masson's trichrome staining for fibrosis
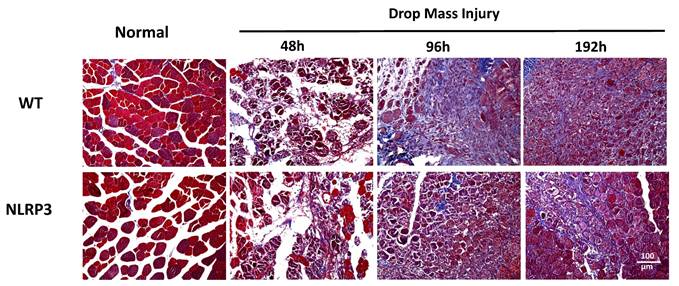
Histopathological evaluation through Masson's trichrome staining demonstrated distinct tissue-specific chromatic differentiation: normal muscle fibers exhibited characteristic reddish-brown coloration, while collagen-rich connective tissue and fibrotic lesions consistently manifested light blue chromatic properties. Blue-stained connective tissue first appeared in the WT-I group at 96 h, with the severity of fibrosis progressively increasing until 192 h. In contrast, NLRP3-I group exhibited only moderate fibrosis at 192 h (Figure 10).
TNF-α expression after mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type (WT) mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with TNF-α. Arrows, TNF-α-positive cells (×200 magnification).
IL-6 expression after mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type (WT) mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with IL-6. Arrows, IL-6-positive cells (×200 magnification).
CD68 expression after mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type (WT) mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with CD68, to reveal the M1 macrophage distribution. Arrows, CD68-positive cells (×200 magnification).
CD206 expression after mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with CD206, to reveal the M2 macrophage distribution. Arrows, CD206-positive cells (×200 magnification).
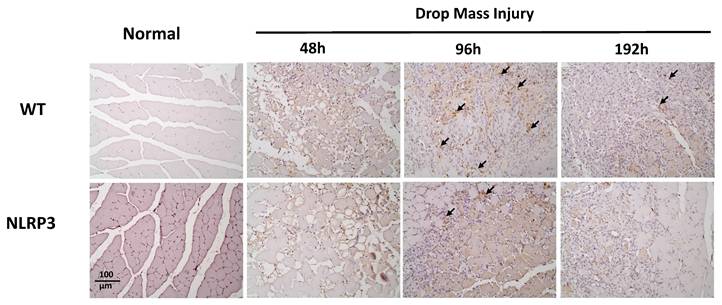
The Caspase-3 (cleavage type) expression after mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with Caspase-3. Arrows, Caspase-3 positive cells (×200 magnification).
Detection of collagen and fibrosis following mass-drop injury (MDI) in Nlrp3-knockout and C57BL/6Narl wild-type mice. Following MDI, gastrocnemius muscle tissue was collected at 0, 48, 96, and 192 h and transverse sections stained with Masson's trichrome stain for differential staining of collagen and muscle fiber. Connective tissue and fibrotic tissue are stained light blue (×200 magnification).
Discussion
MDI animal models effectively replicate the contusion injuries commonly encountered in sports, reproducing both their physiological responses and histopathological characteristics. The NLRP3 inflammasome plays a pivotal role in modulating inflammatory and immune responses by activating caspase-1, which in turn promotes the maturation and release of proinflammatory cytokines such as IL-1β and IL-18. These mediators participate in both protective immune responses and tissue repair. It has been suggested that cold water immersion after eccentric muscle contraction may regulate NLRP3 activation by upregulating ubiquitin-proteasome system-related proteins [19]. Nonetheless, the role of NLRP3 in structural muscle damage during physical injury remains unclear. In the present contusion model, Nlrp3-KO individuals exhibited reduced muscle injury and inflammation, based on histopathological and physiological assessments. NLRP3 plays a key role not only in traumatic brain and spinal cord injury but also in sports-related musculoskeletal injury.
Skeletal muscle injuries are extremely common, accounting for approximately 10%-35% of sports injuries [20]. In a prior study, relative to non-injured controls, MDI-induced contusion models exhibited significantly elevated serum CK and LDH at 192 h post-contusion, whereas AST levels were not significantly affected [17]. In the present study, CK and LDH levels remained significantly elevated from 48 to 192 h post-injury; AST levels, in contrast, were significantly elevated at 48 and 96 h, but returned to baseline by 192 h. In muscle injury resulting from completing a full marathon, serum CK and AST peaked on day 1 after the race, while serum LDH peaked immediately post-race [21]. Levels of muscle injury-related markers change over time not only after intensive exercise but also after substantive muscle damage. For instance, in an isoprenaline-induced cardiac infarction model, tiron, an antioxidant and iron chelator, significantly reduced serum CK-MB, LDH, and AST and downregulated immune responses involving IL-1β, NF-κB, TLR4, and iNOS in infarcted cardiac tissues; it also effectively mitigated NLRP3 inflammasome activation and inhibited the TLR4/NF-κB/iNOS signaling cascade, thereby alleviating oxidative and inflammatory stress [22]. As muscle serum markers, LDH, CK, myoglobin, and AST serve as valuable indicators for monitoring metabolic adaptations to physical exercise; such monitoring provides critical insights into muscular workload and the potential for exercise-induced muscle damage [23]. Here, at 96 and 192 h post-contusion, the NLRP3-I group exhibited significantly lower serum CK levels than the WT-I group, while its LDH levels were lower only at 192 h, suggesting that the myofibril damage is attenuated through Nlrp3 knockout.
The NLRP3 inflammasome regulated the maturation and secretion of the cytokine IL-1β. This signaling pathway functions by binding IL-1β to the IL-1 receptor, thereby activating the IL-1 receptor-associated kinase 1 and subsequently the transcription factor NF-κB. A study of sepsis-induced muscle atrophy in a mouse model revealed that the NLRP3 inflammasome participates in regulating muscle atrophy, as NLRP3-KO mice exhibited less severe muscle atrophy and lower serum IL-1β levels than WT mice. Based on those findings, inhibiting the NLRP3 inflammasome and its activation of the IL-1β pathway may help prevent inflammation-induced muscle atrophy in septic patients [24]. Klotho, which mitigates diabetes-induced elevation of serum creatine kinase-muscle/brain (CK-MB) and LDH, cardiac fibrosis, cardiomyocyte apoptosis, and cardiac dysfunction, also inhibits NLRP3 activation and TNF-α, IL-1β, and IL-18 expression. Its protective effects in diabetes-induced cardiac injury are therefore associated with suppression of the NLRP3 inflammasome pathway [25]. In a study comparing inflammatory cytokine expression 24, 72, 168, and 336 h after muscle contusion by heavy object impact, analysis of qPCR data revealed significantly elevated TNF-α expression in the contusion group at 24 and 72 h post-injury, persisting through 168 and 336 h, relative to the non-injured controls [26]. This is consistent with our finding of elevated TNF-α expression in the wild-type mice after impact injury.
The role of macrophages in skeletal muscle regeneration has been demonstrated [8, 27]. While M1 macrophages are associated with muscle necrosis, M2 macrophages are associated with regenerative myofibers and participate in inhibiting fibro-adipogenic progenitors and promoting muscle regeneration. Here, we examined changes in the levels of CD68, an M1 marker, after muscle contusion in Nlrp3-KO and WT mice. In a previous study, the phytocompound berberine significantly downregulated the expression of the NLRP3 inflammasome and its associated molecules in macrophages, thereby attenuating NLRP3 inflammasome activation-induced M1 macrophage polarization and subsequent inflammation. The neuroprotective effects of berberine were closely associated with suppression of inflammation following peripheral nerve injury; berberine achieved these effects by inhibiting NLRP3 activation-induced M1 macrophage polarization [28]. Imbalance in macrophage phenotypes and dysregulation of the M1-M2 transition contribute substantially to the persistence of inflammation and impaired tissue regeneration. Biomaterials and biomaterial-based delivery systems offer promising strategies to regulate macrophage polarization [29]. Notably, M2 macrophages play a pivotal role in facilitating tendon tissue regeneration and remodeling by promoting cellular proliferation, adipocyte differentiation, and extracellular matrix component regulation [30]. In the present study, M1 macrophages responded first to contusion, reaching the injury site 48-96 h post-injury in the WT-I group. In Nlrp3-KO mice, the number of M1 macrophages decreased in the early inflammatory phase after skeletal muscle injury. At 96 and 192 h post-injury, the WT-I group exhibited significantly more CD206-positive cells than the NLRP3-I group. These findings suggest a mechanism whereby inflammatory balance contributes to tissue adaptation and repair following injury induction.
The sequence of immune cell invasion after muscle contusion is similar to that observed in other injury models; it involves expression of inflammatory cytokines such as TNF-α and IL-6, which can activate macrophages and mediate inflammatory responses [31]. Both damaged myocytes and activated macrophages exhibit substantial expression of IL-6 and TNF-α in the early stages of muscle injury, suggesting that M1 macrophages might be related to the cytokines secreted after injury [32]. Here, IL-6-positive cells were observed in nucleated muscle fibers and inflammatory cells in lesions. In the WT-I group, many IL-6-positive cells were observed from 96 h after injury, persisting until 192 h. In contrast, in the NLRP3-I group, few IL-6 positive cells were present at 96 h and 192 h. These results are consistent with previous finding that inhibiting IL-6 expression reduces the inflammatory response of muscle tissue and fibrosis severity. Signaling pathways downstream of IL-6 (the IL-6/Stat3 and IL-1β/Egr1 pathways) are closely associated with fibrosis [33]. In synthesis, we propose that downregulation of Nlrp3 expression might reduce muscle fibrosis by modulating the IL-6 fibrosis-related pathway.
Exercise interventions can regulate the NLRP3 inflammasome. In osteoarthritis, moderate-intensity exercise reduces inflammation and pyroptosis by increasing the expression of Metrnl, an adipomyokine that inhibits the PI3K/Akt/NF-κB signaling pathway and subsequently the NLRP3/caspase-1/GSDMD pathway [34]. Exercise training is widely recognized as a physiological stressor that elicits beneficial cellular adaptations, ultimately inducing a cardioprotective phenotype. Accordingly, regular exercise confers protection against myocardial injury. The mechanisms underlying exercise-induced cardiac preconditioning involve activation of the anti-radical defense system, enhanced nitric oxide production, and the modulation of opioids, myokines, and adenosine-5'-triphosphate (ATP)-dependent potassium channels [35]. Notably, in myocardial ischemia-reperfusion injury, regular exercise has been shown to downregulate NLRP3 expression in cardiac tissue, thereby mitigating NLRP3-mediated pyroptosis and contributing to myocardial protection [36]. Aerobic exercise alleviates pyroptosis in endothelial cells, adipocytes, and hippocampal cells, thereby mitigating pyroptosis-related diseases by inhibiting NLRP3 [37]. In an animal model of ventilator-induced lung injury (VILI), PKC activation increased NLRP3 levels, leading to lung injury; exercise was found to reduce lung injury by inhibiting PKC and NLRP3 activation, suggesting it as a potential clinical strategy for preventing VILI [38]. Exercise interventions thus yield positive physiological benefits by downregulating NLRP3. Consistent with this, we found that Nlrp3 knockdown reduced inflammation and accelerated recovery following MDI-induced muscle injury. Nonetheless, the downregulation of NLRP3 should be further investigated in relation to exercise performance under conditions of injury. At 192 h, the NLRP3-I group exhibited significantly less fibrotic tissue, based on Masson's trichrome staining, than the WT-I group (Figure 10), indicating that NLRP3 knockout might improve fibrotic gene expression in mice with contusion-induced muscle injury. We therefore hypothesize that reduced NLRP3 expression might improve muscle recovery by increasing the expression of myogenic genes such as Myod and Pax-7 in muscle cells and reducing fibrogenic gene expression during muscle repair after contusion-induced muscle injury [39]. The molecular basis of the function of NLRP3 thus requires further investigation, potentially using an Nrlp3-knockout animal model of muscular injury.
Mouse models of MDI-induced muscle contusion provide a valuable tool for investigating the mechanisms of muscle recovery and evaluating the efficacy of therapeutic interventions. Here, in Nrlp3-KO mice, the inflammatory response was inhibited, reducing contusion-induced muscle damage and fibrosis. This mechanism may involve regulation of downstream cytokines such as TNF-α and IL-6, leading to reduced inflammation and increased muscle regeneration. Nlrp3 thus provides a potential target for pharmaceutical development to improve treatment outcomes and recovery in muscle injury in sports. Novel screening techniques and functional assays are required to test the new anti-NLRP3 compounds being developed in the rapidly expanding field of inflammasome biology in musculoskeletal health [40]. Moreover, the potential for developing ergogenic aids that alter Nlrp3 regulation warrants further investigation. However, the possibility that such drugs or supplements would be prohibited by the World Anti-Doping Agency requires consideration.
Acknowledgements
We would like to thank Uni-edit (www.uni-edit.net) for editing and proofreading this manuscript.
Funding
This work was financially supported by National Science and Technology Council (NSTC 113-2423-H-227-001-MY3).
Ethics approval
All experimental procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan Sport University, and the experimental protocol guidelines #10806 were approved by the ethics committee of the IACUC. The study was conducted in compliance with the ARRIVE guidelines.
Availability of data and materials
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
Author contributions
Yen-Peng Lee and Chien-Chao Chiu contributed to the conceptualization, investigation, methodology, data curation and drafting the original manuscript. Liang-Ju Chiu, Chien-Chao Chiu, and Yi-Hsun Chen contributed to the investigation and data curation. Wen-Ching Huang was responsible for the conceptualization, methodology, supervision, validation, visualization, manuscript review and editing, as well as funding acquisition. All authors have read and approved the submitted version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cohen SB, Towers JD, Bradley JP. Rotator cuff contusions of the shoulder in professional football players: epidemiology and magnetic resonance imaging findings. Am J Sports Med. 2007;35(3):442-7 doi: 10.1177/0363546506295082
2. Hetaimish B, Ahmed H, Otayn A, Alzahrani AM, Almasoudi E, Elaiw M. et al. Prevalence, and types of overuse injuries in gym centers: A cross-sectional study in Saudi Arabia. Medicine (Baltimore). 2024;103(28):e38830 doi: 10.1097/MD.0000000000038830
3. Alrushud AS. A cross-sectional study of musculoskeletal injuries related to exercise among gym members in Saudi Arabia in 2022: prevalence, common types, and predictor factors. BMC Musculoskelet Disord. 2024;25(1):621 doi: 10.1186/s12891-024-07733-2
4. Baghdadi MB, Tajbakhsh S. Regulation and phylogeny of skeletal muscle regeneration. Dev Biol. 2018;433(2):200-209 doi: 10.1016/j.ydbio.2017.07.026
5. Smith C, Kruger MJ, Smith RM, Myburgh KH. The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med. 2008;38(11):947-69 doi: 10.2165/00007256-200838110-00005
6. Zuo Q, Qu F, Li N, Wang S, Liu J, Xu C. et al. Eccentric exercise results in a prolonged increase in interleukin-6 and tumor necrosis factor-α levels in rat skeletal muscle. J Muscle Res Cell Motil. 2019;40(3-4):379-387 doi: 10.1007/s10974-019-09554-6
7. Bian AL, Hu HY, Rong YD, Wang J, Wang JX, Zhou XZ. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017;22(1):25 doi: 10.1186/s40001-017-0266-9
8. Liu X, Liu Y, Zhao L, Zeng Z, Xiao W, Chen P. Macrophage depletion impairs skeletal muscle regeneration: The roles of regulatory factors for muscle regeneration. Cell Biol Int. 2017;41(3):228-238 doi: 10.1002/cbin.10705
9. Raimondo TM, Mooney DJ. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc Natl Acad Sci USA. 2018;115(42):10648-10653 doi: 10.1073/pnas.1806908115
10. Wicherska-Pawłowska K, Wróbel T, Rybka J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int J Mol Sci. 2021;22(24):13397 doi: 10.3390/ijms222413397
11. Rathinam VA, Fitzgerald KA. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell. 2016;165(4):792-800 doi: 10.1016/j.cell.2016.03.046
12. Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H. et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp Physiol. 2013;98(2):462-72 doi: 10.1113/expphysiol.2012.068338
13. Guo C, Fu R, Wang S, Huang Y, Li X, Zhou M. et al. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 2018;194(2):231-243 doi: 10.1111/cei.13167
14. Low LY, Harrison PF, Gould J, Powell DR, Choo JM, Forster SC. et al. Concurrent Host-Pathogen Transcriptional Responses in a Clostridium perfringens Murine Myonecrosis Infection. mBio. 2018;9(2):e00473-18 doi: 10.1128/mBio.00473-18
15. Kummer K, Bertram I, Zechel S, Hoffmann DB, Schmidt J. Inflammasome in Skeletal Muscle: NLRP3 Is an Inflammatory Cell Stress Component in Inclusion Body Myositis. Int J Mol Sci. 2023;24(13):10675 doi: 10.3390/ijms241310675
16. Busch K, Kny M, Huang N, Klassert TE, Stock M, Hahn A. et al. Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy. J Cachexia Sarcopenia Muscle. 2021;12(6):1653-1668 doi: 10.1002/jcsm.12763
17. Tsai SW, Hsu YJ, Lee MC, Huang HE, Huang CC, Tung YT. Effects of dextrose prolotherapy on contusion-induced muscle injuries in mice. Int J Med Sci. 2018;15(11):1251-1259 doi: 10.7150/ijms.24170
18. Hsu YJ, Ho CS, Lee MC, Ho CS, Huang CC, Kan NW. Protective Effects of Resveratrol Supplementation on Contusion Induced Muscle Injury. Int J Med Sci. 2020;17(1):53-62 doi: 10.7150/ijms.35977
19. Abolfathi F, Ranjbar R, Tabandeh MR, Habibi A. Cold water immersion regulates NLRP3 inflammasome pathway in the rat skeletal muscle after eccentric exercise by regulating the ubiquitin proteasome related proteins. Cytokine. 2024;184:156793
20. Thomas RE, Thomas BC. A systematic review of injuries in gymnastics. Phys Sportsmed. 2019;47(1):96-121 doi: 10.1080/00913847.2018.1527646
21. Tokinoya K, Ishikura K, Ra SG, Ebina K, Miyakawa S, Ohmori H. Relationship between early-onset muscle soreness and indirect muscle damage markers and their dynamics after a full marathon. J Exerc Sci Fit. 2020;18(3):115-121 doi: 10.1016/j.jesf.2020.03.001
22. Abdelrahaman D, Habotta OA, Taher ES, El-Ashry ES, Ibrahim I, Abdeen A. et al. Suppression of NLRP3 inflammasome orchestrates the protective efficacy of tiron against isoprenaline-induced myocardial injury. Front Pharmacol. 2024;15:1379908 doi: 10.3389/fphar.2024.1379908
23. Delsmann MM, Stürznickel J, Amling M, Ueblacker P, Rolvien T. Musculoskeletal laboratory diagnostics in competitive sport. Orthopade. 2021;50(9):700-712
24. Huang N, Kny M, Riediger F, Busch K, Schmidt S, Luft FC, Slevogt H. et al. Deletion of Nlrp3 protects from inflammation-induced skeletal muscle atrophy. Intensive Care Med Exp. 2017;5(1):3 doi:10.1186/s40635-016-0115-0
25. Li X, Li Z, Li B, Zhu X, Lai X. (2019). Klotho improves diabetic cardiomyopathy by suppressing the NLRP3 inflammasome pathway. Life Sci. 2019;234:116773 doi:10.1016/j.lfs.2019.116773
26. Liu X, Zeng Z, Zhao L, Xiao W, Chen P. Changes in inflammatory and oxidative stress factors and the protein synthesis pathway in injured skeletal muscle after contusion. Exp Ther Med. 2018;15(2):2196-2202 doi:10.3892/etm.2017.5625
27. Shang M, Cappellesso F, Amorim R, Serneels J, Virga F, Eelen G. et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature. 2020;587(7835):626-631 doi: 10.1038/s41586-020-2857-9
28. Sun J, Zeng Q, Wu Z, Huang L, Sun T, Ling C. et al. Berberine inhibits NLRP3 inflammasome activation and proinflammatory macrophage M1 polarization to accelerate peripheral nerve regeneration. Neurotherapeutics. 2024;21(4):e00347 doi: 10.1016/j.neurot.2024.e00347
29. Ye J, Xie C, Wang C, Huang J, Yin Z, Heng BC. et al. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact Mater. 2021;6(11):4096-4109 doi: 10.1016/j.bioactmat.2021.04.017
30. Wang Y, Lu X, Lu J, Hernigou P, Jin F. The role of macrophage polarization in tendon healing and therapeutic strategies: Insights from animal models. Front Bioeng Biotechnol. 2024;12:1366398
31. Kawanishi N, Mizokami T, Niihara H, Yada K, Suzuki K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem Biophys Rep. 2015;5:146-151 doi: 10.1016/j.bbrep.2015.11.022
32. Fernández-Lázaro D, Mielgo-Ayuso J, Seco Calvo J, Córdova Martínez A, Caballero García A, Fernandez-Lazaro CI. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients. 2020;12(2):501 doi: 10.3390/nu12020501
33. Voss JG, Shagal AG, Tsuji JM, MacDonald JW, Bammler TK, Farin FM. et al. Time Course of Inflammatory Gene Expression Following Crush Injury in Murine Skeletal Muscle. Nurs Res. 2017;66(2):63-74 doi: 10.1097/NNR.0000000000000209
34. Liu J, Jia S, Yang Y, Piao L, Wang Z, Jin Z. et al. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-κB and NLRP3/caspase-1/GSDMD signaling. Biomed Pharmacother. 2023;158:114118 doi: 10.1016/j.biopha.2022.114118
35. Penna C, Alloatti G, Crisafulli A. Mechanisms Involved in Cardioprotection Induced by Physical Exercise. Antioxid Redox Signal. 2020;32(15):1115-1134 doi: 10.1089/ars.2019.8009
36. Wang Y, Li Y, Chen C, Zhang H, Liu W, Wu C, Chen H, Li R, Wang J, Shi Y, Wang S, Gao C. Moderate-intensity aerobic exercise inhibits cell pyroptosis to improve myocardial ischemia-reperfusion injury. Mol Biol Rep. 2024;52(1):5 doi: 10.1007/s11033-024-10065-y
37. Hu S, Wan X, Li X, Wang X. Aerobic exercise alleviates pyroptosis-related diseases by regulating NLRP3 inflammasome. Front Physiol. 2022;13:965366 doi: 10.3389/fphys.2022.965366
38. Liu M, Zhang Y, Yan J, Wang Y. Aerobic exercise alleviates ventilator-induced lung injury by inhibiting NLRP3 inflammasome activation. BMC Anesthesiol. 2022;22(1):369 doi: 10.1186/s12871-022-01874-4
39. Tavi P, Korhonen T, Hänninen SL, Bruton JD, Lööf S, Simon A. et al. Myogenic skeletal muscle satellite cells communicate by tunnelling nanotubes. J Cell Physiol. 2010;223(2):376-83 doi: 10.1002/jcp.22044
40. Tengesdal IW, Banks M, Dinarello CA, Marchetti C. Screening NLRP3 drug candidates in clinical development: lessons from existing and emerging technologies. Front Immunol. 2024;15:1422249 doi: 10.3389/fimmu.2024.1422249
Author contact
![]() Corresponding author: Wen-Ching Huang, Address: No.365, Ming-te Road, Peitou District, Taipei, 112, Taiwan. Email: wenchingedu.tw; Tel.: +886-2-2822-7107#7721.
Corresponding author: Wen-Ching Huang, Address: No.365, Ming-te Road, Peitou District, Taipei, 112, Taiwan. Email: wenchingedu.tw; Tel.: +886-2-2822-7107#7721.

 Global reach, higher impact
Global reach, higher impact