3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3429-3438. doi:10.7150/ijms.96371 This issue Cite
Research Paper
Alpha-bisabolol protects against neonatal asthma by suppressing airway inflammatory signaling
1. Centre for Global Health Research-Helix Research Lab, Department of Neonatology, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, 600077, India.
2. Department of Integrative Bioscience & Biotechnology, Institute of Bioscience, Sejong University, Seoul 05006, Republic of Korea.
3. Institute of Forest Science, Kangwon National University, Chuncheon 24341, Republic of Korea.
4. Department of Botany and Microbiology, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia.
5. Department of Preventive Medicine, College of Medicine, Dong-A University, Busan 49201, Republic of Korea.
6. Environmental Health Center, Dong-A University, Busan 49201, Republic of Korea.
Received 2025-3-18; Accepted 2025-7-5; Published 2025-7-24
Abstract
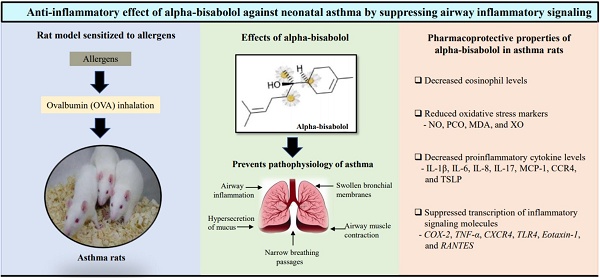
Objective: This study aimed to evaluate the anti-inflammatory effects of alpha-bisabolol (AB) in allergic airway inflammation-induced rat pups.
Methods: We evaluated the anti-adverse effects of AB against allergic airway inflammation-induced male Wistar rat pups, with four categorized groups including vehicle-controls (group 1), controls treated with 25 mg/kg of AB (group 2), allergic airway inflammation-induced cases (group 3), and cases treated with 25 mg/kg of AB before allergic airway inflammation induction (group 4). Lung histopathology, bronchoalveolar lavage fluid eosinophils, and several inflammatory markers were also examined in each group.
Results: AB significantly decreased mucous gland hypertrophy, eosinophil infiltration, and oxidative stress marker levels in the allergic airway inflammation-induced AB-pretreated rats. Moreover, AB pretreatment significantly reduced the levels of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8, IL-17, monocyte chemoattractant protein-1, C-X-C chemokine receptor type 4 (CXCR4), and thymic stromal lymphopoietin, which were increased in allergic airway inflammation-induced cases. Furthermore, transcription of cyclooxygenase-2, tumor necrosis factor-α, CXCR4, toll-like receptor 4, Eotaxin-1, and regulated upon activation normal T cell expressed and secreted were significantly suppressed in allergic airway inflammation-induced AB-pretreated rats.
Conclusions: These results indicate that AB can protect against neonatal asthma by inhibiting acute or chronic inflammation induced during disease onset.
Keywords: Alpha-bisabolol, Neonatal asthma, Inflammation
Introduction
Asthma, a chronic respiratory disease, poses a major health problem in industrialized societies [1,2]. Individuals with asthma experience mild to severe breathlessness, chest tightness, and prominent wheezing, which are common symptoms of asthma due to mucus accumulation in the airway passage [3]. This causes airway hyperresponsiveness, which narrows the airway passage and smooth muscle cell contraction [4]. Inflammation, a major pathological event in asthma, causes airway wall thickening [4]. Further, airway smooth muscle cell hypertrophy, increased subepithelial fibrosis, epithelial goblet cells, mucous gland hyperplasia, extracellular matrix degradation, and inflammatory cell infiltration narrow the airways in asthma cases [5-8].
Various studies have experimentally revealed lung inflammation in animal models and developed airway remodeling in the lungs, similar to the effects of asthma in humans [9]. The detailed mechanism of airway remodeling through airway inflammation remains unclear, but T-cell and eosinophil recruitment in the lungs [10,11] and lung parenchyma [12] has been accepted as the determining event in the development of asthma.
The importance of T-helper type 2 (Th2) cytokines, such as interleukin (IL)-4, IL-5, IL-9, and IL-13, and chemokines in the pathogenesis of asthma is well known [13]. Corticosteroid administration in the form of inhalers has been regularly used by patients with asthma to relieve lung or chest discomfort, release accumulated mucus, and partially decrease airway wall thickness [14]. Continuous corticosteroid administration causes side effects [15]; hence, herbal medicine-based therapy is advocated [16].
Alpha-bisabolol (AB) is a natural monocyclic sesquiterpene alcohol found in many aromatic plants including black pepper (Piper nigrum) and ylang ylang (Cananga odorata) with well known anti-inflammatory, antibiotic, analgesic, and anticancer properties [17,18]. AB is considered safe because of its low toxicity, as recommended by the Food and Drug Administration, and thus has been used in various commercial products, including cosmetic formulations, as a skin conditioning agent [17]. Therefore, the current study evaluated the anti-adverse effects of AB in allergic airway inflammation-induced rat pups developed via allergen exposure, such as ovalbumin (OVA), which mimics the process of allergen exposure observed in human patients with asthma.
Materials and Methods
Allergic airway inflammation induction and AB treatment
Twenty-four male Wistar strain rat pups (10 days-old) were used in animal experiments for allergic airway inflammation. All pups were maintained at 20-25 °C with 50-70% relative humidity, fed commercial rat chow, and had free access to clean tap water. Wistar rat pups were categorized into four groups (n=6 for each group): vehicle controls administered normal saline (0.9% NaCl) (group 1), controls treated with 25 mg/kg of AB (group 2), allergic airway inflammation-induced cases (group 3), and cases treated with 25 mg/kg of AB before allergic airway inflammation induction (group 4). Sensitization and OVA treatment were conducted according to the previous procedures with slight modifications [19,20]. Briefly, rat pups were sensitized by 0.75% (w/w) OVA inhalation using an ultrasonic nebulizer for 10 min daily for 45 days. Group 4 rats with allergic airway inflammation were orally co-administered AB (25 mg/kg) daily for same 45 days. Group 4 rats with allergic airway inflammation were orally co-administered AB (25 mg/kg). Following all treatments, all rat pups were euthanized via cervical vertebral decapitation. Lung tissue and blood samples were collected for histological and biochemical analyses.
Histological analyses
Lung tissues were isolated from each rat and fixed in 10% formalin solution for 24 h. Paraffin wax-embedded tissues were cut into 5-µm slices using a microtome and stained with Hematoxylin and Eosin Staining Kit (Abcam Inc., Boston, MA, USA).
Assessment of eosinophil infiltration and serum Immunoglobulin E levels
Lung fluid was collected to estimate eosinophil infiltration in the bronchoalveolar lavage fluid (BALF), and bronchoalveolar lavage cells were counted using a hemocytometer after staining. IgE levels in the serum were determined using a commercial rat IgE enzyme-linked immunosorbent assay (ELISA) kit (Elabscience, Texas, USA) following the manufacturer's instructions.
Estimation of oxidative stress markers
Rat lung weights were measured, and the lungs were homogenized in a glass homogenizer using 10 mL/g ice-cold phosphate-buffered saline (PBS, pH 7.5). After centrifuging all samples for 10 min at 4 °C and 12,000 rpm, the supernatants were stored at -80 °C for consecutive assays. Colorimetric assay kits (Nitric oxide assay kit, Protein carbonyl content assay kit, Lipid peroxidation (MDA) assay kit, and Xanthin oxidase activity assay kit (Abcam Inc, Boston, USA)) were used to estimate oxidative stress markers, such as nitric oxide (NO), protein carbonyl content (PCO), malondialdehyde (MDA), and xanthine oxidase (XO) in serum of lung tissue following manufacturers' recommendation [21].
Detection of inflammation-related signaling molecules
Inflammation-related lipids, such as cysteinyl leukotriene, prostaglandin E2, thromboxane B2, and inflammation-related proteins, including proteoglycan 4 (PRG4) and glycosaminoglycan, were elucidated using commercial lipid assay kits, including Cysteinyl leukotriene ELISA kit (Biomol, Hamburg, Germany), Prostaglandin E2 ELISA kit (Abcam Inc., Boston, MA, USA), Thromboxane B2 ELISA kit (Abcam Inc., Boston, MA, USA), ELISA kit for Proteoglycan 4 (Biozol Diagnostica, Eching, Germany), and total glycosaminoglycan assay kit (Abcam Inc., Boston, MA, USA).
Serum cytokine level estimation
Proinflammatory cytokines, such as IL-1β, IL-6, IL-8, IL-17, monocyte chemoattractant protein-1 (MCP-1), C-motif chemokine receptor-4 (CCR4), and thymic stromal lymphopoietin (TSLP), and anti-inflammatory cytokines, such as IL-2, IL-9, and IL-13, in serum samples were evaluated using ELISA commercial kits (rat interleukin 8 receptor beta ELISA kit (Biomatik, Delaware, USA), CCR4 ELISA kit (Aviva Systems Biology Corp., California, USA), Rat IL-9 ELISA kit (Abcam Inc., Boston, USA), Rat IL-1β ELISA kit, Rat IL-6 ELISA kit, Rat IL-17 ELISA kit, Rat MCP-1 ELISA kit, mouse thymic stromal lymphopoietin ELISA kit, Rat IL-2 ELISA kit, and Rat IL-13 ELISA kit (Elabscience, Texas, USA)) according to the manufacturer's instructions.
Reverse transcription-polymerase chain reaction (RT-PCR)
mRNA was extracted from neonatal lung tissues using RNeasy Pure mRNA Bead kit (Qiagen, Hilden, Germany) to elucidate inflammatory signaling activated in allergic airway. A high-capacity cDNA Reverse Transcription kit (Bio-Rad, Hercules, CA, USA) was used to convert 20 µL of mRNA into cDNA. Real-time RT-PCR for specific genes was performed with reverse transcribed cDNA using the SYBR® Green PCR kit (Bio-Rad, Hercules, CA, USA). The Ct values obtained were compared with the control value, and the comparative Ct method (ΔΔCT) was used to determine the fold transcription. GAPDH, a housekeeping gene, was used as the internal gene expression control. Primer sequences used are listed in Table 1.
Statistical evaluation
All data obtained from the experiments were expressed as mean ± standard error of the mean. Comparisons among groups were conducted using analysis of variance (ANOVA) with GraphPad Prism software (Dotmatics, Boston, USA). Significant differences among the groups were determined based on a p-value of < 0.05.
Results
Histological assessment
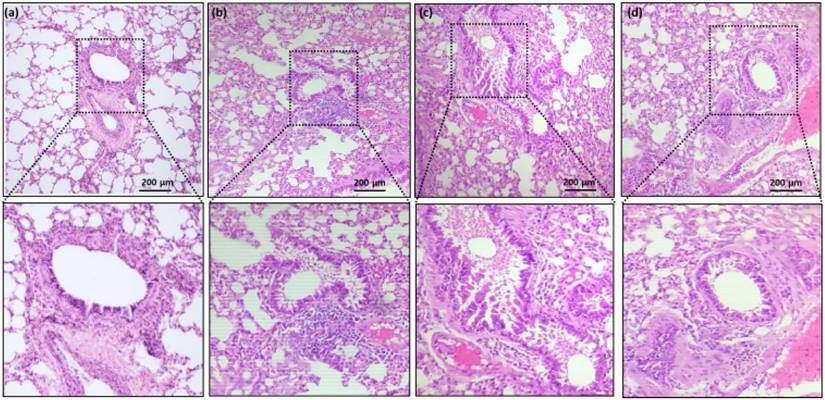
Compared to the controls (Fig. 1a and 1b), histological assessment of the lung tissues revealed significantly increased vascular congestion, mucous gland hypertrophy, and smooth muscle mass (Fig. 1c). Rats coadministered with AB (Fig. 1d) demonstrated significantly reduced soft muscle mass and hypertrophy development compared to allergic airway inflammation-induced rats (Fig. 1c). OVA sensitization and challenge increased airway reactivity, whereas AB treatment group displayed reduced airway inflammation compared with OVA-sensitized and challenged group or control group.
Eosinophil infiltration and serum Immunoglobulin E levels
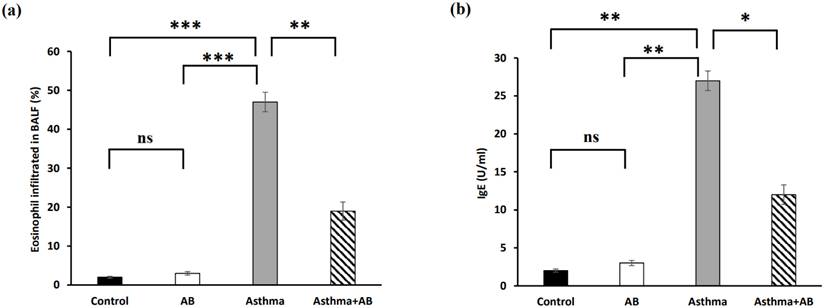
Eosinophil infiltration in BALF significantly increased in allergic airway inflammation-induced rat pups compared to controls, with eosinophils constituting 1.96% of the total leukocytes in normal rat pups and 47% in allergic airway inflammation-induced rat pups (Fig. 2a). This trend was also observed for IgE, with the lowest IgE level (2 U/ml) in normal rat pups and the highest IgE level (27 U/ml) in allergic airway inflammation-induced rat pups (Fig. 2b). Conversely, AB treatment substantially decreased both eosinophil infiltration (19%) and IgE levels (12 U/ml) in allergic airway inflammation-induced rat pups, indicating a protective effect of AB against allergic airway inflammation (Fig. 2).
Primers used in polymerase chain reaction.
| Gene | Forward primer (5ʹ - 3ʹ) | Reverse primer (5ʹ - 3ʹ) | Annealing temperature (°C) |
|---|---|---|---|
| COX-2 | CTCAGCCATGCAGCAAATCC | GGGTGGGCTTCAGCAGTAAT | 58 |
| TNF-α | AAGCTGTCTTCAGGCCAACA | CCCGTAGGGCGATTACAGTC | 59 |
| CXCR4 | GCCATGGCTGACTGGTACTT | CACCCACATAGACGGCCTTT | 58 |
| TLR4 | CCTCGAGTGGGAGGACAAT | TGAGGTTAGAAGCCTCGTGC | 59 |
| Eotaxin-1 | TTTCTTGCACCCCAGCTTTG | AAGGTCACGCAGCAAGATGA | 59 |
| RANTES | TGCCCACGTGAAGGAGTATT | GGAGTAGGGGGTTGCTCAGT | 58 |
| GAPDH | GAGCGAGATCCCGTCAAG | ATTTCTCGTGGTTCACACCCA | 58 |
Hematoxylin and eosin (H&E stain) histopathology of rat lung tissue. (a) Group 1 (Control) shows the normal architecture of lung with moderate size of inflammatory cell infiltrates. (b) Group 2 (control treated with AB) shows reduced size of inflammatory cell infiltrates compared with group 1. (c) Group 3 (allergic airway inflammation-induced cases) shows a largest size of inflammatory cell infiltrates. (d) Group 4 (allergic airway inflammation-induced, but treated with AB) shows reduced size of inflammatory cell infiltrates compared with group 3. Black scale bar represents 200 µm.
The percent of eosinophils infiltrated in rat BALF and serum IgE level. (a) Eosinophil infiltration in BALF (n = 6 rats/group) and (b) serum IgE (n = 6 rats/group) were calculated. Values were expressed as mean ± SD. Statistical significance was expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. ns, non-significance.
Oxidative stress markers
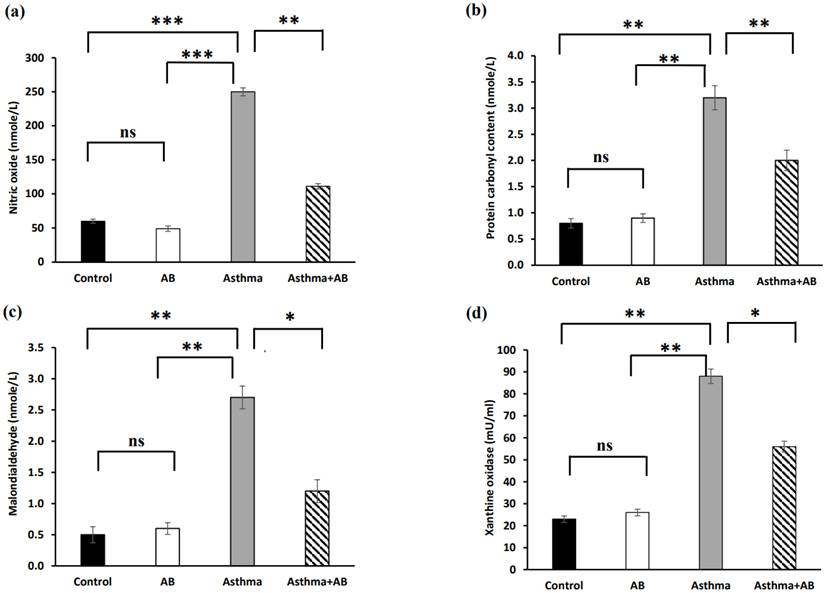
Oxidative stress indicators, such as NO, PCO, MDA, and XO, were estimated in serum (Fig. 3), and significantly increased levels of all oxidative stress indicators were exhibited in allergic airway inflammation-induced rat pups (250 nmole/L, 3.2 nmole/L, 2.7 nmole/L, and 88 mU/mL, respectively) compared to the controls (60 nmole/L, 0.8 nmole/L, 0.5 nmole/L, and 23 mU/mL, respectively). However, all of these oxidative stress parameters were significantly attenuated in AB-treated allergic airway inflammation-induced rat pups (111 nmole/L, 2 nmole/L, 1.2 nmole/L, and 56 mU/mL, respectively) compared with allergic airway inflammation-induced rat pups.
Inflammation-related signaling molecules
Inflammation-related lipids (cysteinyl leukotriene, prostaglandin E2, and thromboxane B2) and inflammation-related proteins (PRG4 and glycosaminoglycan) were assessed to evaluate the allergic airway inflammation-induced conditions. The results revealed significant increases in all three inflammation-related lipids in the allergic airway inflammation-induced pups (323, 177, and 15 ng/mL, respectively) compared to the control group (90, 45, and 2 ng/mL, respectively) (Fig. 4). However, AB-treated allergic airway inflammation-induced pups displayed an obvious decrease in inflammation-related lipid levels (189, 111, and 9 ng/mL, respectively) compared to allergic airway inflammation-induced rat pups not treated with AB (Fig. 4), indicating the significant protective effects of AB. Furthermore, both inflammation-related protein levels (PRG4 and glycosaminoglycan) were complemented in AB-treated allergic airway inflammation-induced pups (8 and 89 ng/mL, respectively) compared to those in allergic airway inflammation-induced pups (5 and 55 ng/mL, respectively) (Fig. 5). AB supplementation decreased inflammation-related protein levels in allergic airway inflammation-induced pups.
Oxidative stress marker levels in serums of rat lung tissues. (a) Nitric oxide levels, (b) protein carbonyl content, (c) malondialdehyde levels, and (d) xanthine oxidase levels were measured. Values were expressed as mean ± SD. Statistical significance was expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. ns, non-significance.
Inflammation-related lipid levels in serums of rat lung tissues. (a) Cysteinyl leukotriene levels, (b) prostaglandin levels, and (c) thromboxane B2 levels were measured. Values were expressed as mean ± SD. Statistical significance was expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. ns, non-significance.
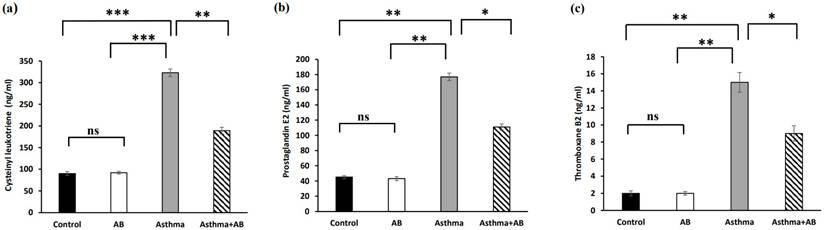
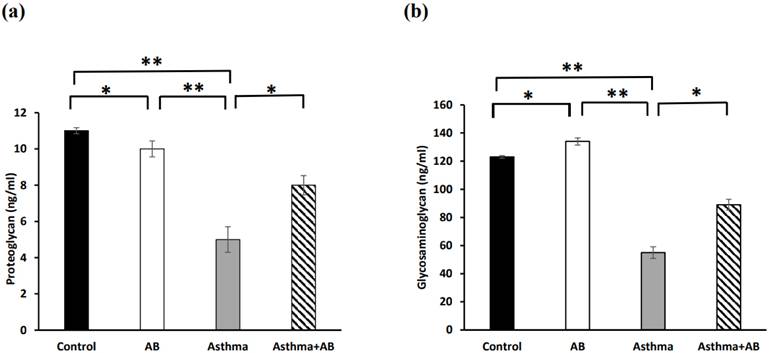
Inflammation-related protein levels in serums of rat lung tissues. (a) Proteoglycan levels and (b) glycosaminoglycan levels were measured. Values were expressed as mean ± SD. Statistical significance was expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. ns, non-significance.
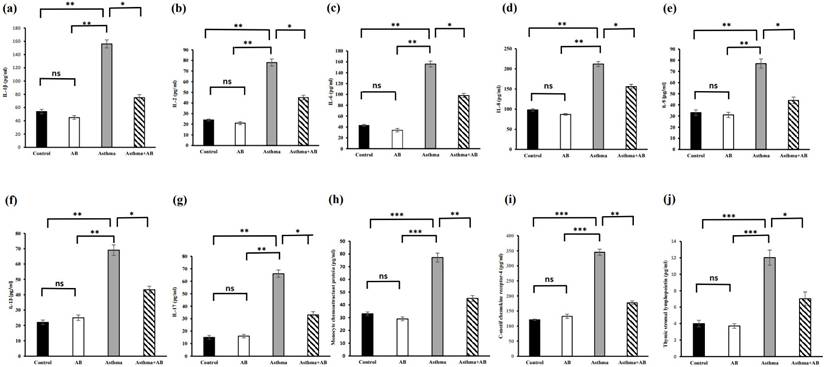
Cytokine levels in serums of rat lung tissues. (a) IL-1β level, (b) IL-2 level, (c) IL-6 levels, (d) IL-8 levels, (e) IL-9 levels, (f) IL-13 levels, (g) IL-17 levels, (h) monocyte chemoattractant protein levels, (i) C Motif Chemokine Receptor 2 levels, and (j) thymic stromal lymphopoietin levels were measured. Values were expressed as mean ± SD. Statistical significance was expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. ns, non-significance.
Estimation of cytokine levels
The serum cytokine levels were also assessed (Fig. 6). Allergic airway inflammation-induced pups showed substantial increases in all proinflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, MCP-1, CCR4, and TSLP at 156, 156, 212, 66, 77.2, 345, and 12 pg/mL, respectively) and anti-inflammatory cytokine levels (IL-2, IL-9, and IL-13 at 78, 77, and 69 pg/mL, respectively) compared to the control (54, 43, 98, 15, 33.1, 121, and 4 pg/mL; and 24, 33, and 22 pg/mL, respectively). However, these cytokine levels were reduced in AB-treated allergic airway inflammation-induced pups (75, 98, 156, 33, 45.1, 177, and 7 pg/mL; and 45, 44, and 43.3 pg/mL, respectively) (Fig. 6), indicating that AB treatment actively suppressed asthma progression, probably by inhibiting the action of inflammatory molecules.
Fold changes in mRNA levels of inflammation-related genes
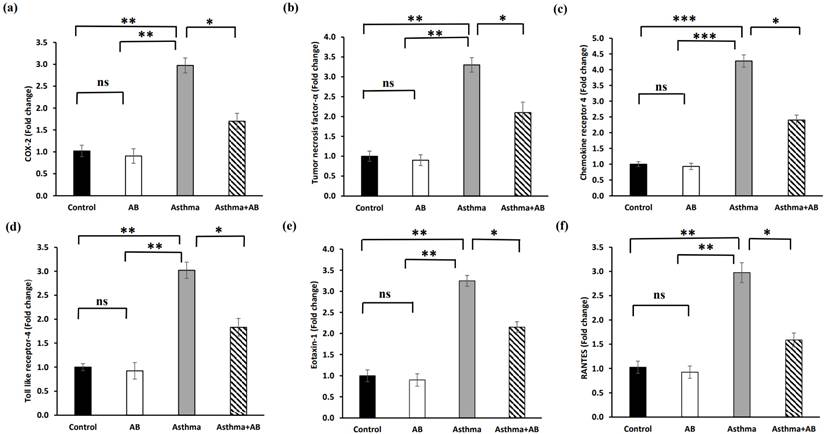
The transcription levels of inflammation-related genes were determined to substantiate the role of AB in inflammatory signal interruption (Fig. 7). The mRNA expression of cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α), C-X-C chemokine receptor type 4 (CXCR4), toll-like receptor 4 (TLR4), Eotaxin-1, and regulated upon activation of normal T-cell expressed and secreted (RANTES) were significantly increased in allergic airway inflammation-induced pups by 2.9-fold, 3.0-fold, 4.2-fold, 3.0-fold, 3.2-fold, and 2.9-fold, respectively, compared to the controls (Fig. 7). However, AB treatment suppressed these transcriptional inflammatory signalings, indicating that AB exerts anti-inflammatory effects against allergic airway inflammation-induced conditions.
Fold changes in mRNA levels of inflammation-related genes. (a) COX-2 (b) tumor necrosis factor-α, (c) chemokine receptor 4, (d) toll like receptor-4, (e) eotaxin-1, and (f) RANTES were calculated. Values were expressed as mean ± SD. Statistical significance was expressed as *p < 0.05, **p < 0.01 and ***p < 0.001. ns, non-significance.
Discussion
Asthma is a chronic inflammatory disease related with airway hyperresponsiveness to various irritants like smoke, dust, pollen, or other allergens [3]. This leads to wheezing, due to mucus accumulation in the airway passage, resulting in breathing discomfort [22]. The infiltration of immune cells like eosinophils, mast cells, and other leukocytes has been associated with disease pathophysiology, causing inflammation owing to their activation by allergens [22]. In our study, OVA sensitization and challenge increased alveolar reactivity in histological assessment and AB reversed it to normal condition. The mucous overproduction, goblet cell hyperplasia, and increased eosinophil infiltration in the peribronchial epithelium were observed in the bronchi, when compared the OVA group to the control group. OVA caused an increase in mucus production, which is driven by the Th2 cytokines IL-4 and IL-13. In particular, IL-13 stimulated mucus-secreting goblet cells in the airway epithelium, while IL-4 promoted mucin gene expression, resulting in mucus hypersecretion [23]. The degeneration of alveolar cells, collagen deposition, and goblet cells that secrete mucus around the airway, on the other hand, resulted from the increased inflammation caused by neutrophils, eosinophils, and activated macrophages during the airway inflammation [24]. Histological lung sections in our study showed that the AB treatment reduced the amount of mucus in respiratory epithelial cells, goblet cells, and eosinophils, resulting in the degree of inflammation surrounding the bronchus to the levels similar to those in the control group. Previous studies have shown increased infiltration of these cells in the BALF of asthmatic individuals, indicating disease severity that cause subsequent mucus secretion and airway inflammation [25]. Significant airway structural remodeling, airway hyperresponsiveness, and airway inflammation can all be exacerbated by early postpartum hyperoxia exposure [25]. Airway epithelial barrier integrity can be compromised by reactive oxygen species (ROS), which can also impair cellular function and damage the airway epithelium [26]. Ultimately, this can result in an increase in airway smooth muscle, an increase in extracellular interstitial deposition surrounding the airway, and cellular senescence, which can cause airway remodeling [24]. Li et al. [27] reported that there was a significant increase in the expression of type 2 cytokines, IL-5 and IL-13, in the BALF of the group treated with O2 and OVA. An increased infiltration of immune cells are also associated with OVA-specific IgE and Th2 cytokine production [28]. An increase in airway smooth muscle proliferation has been observed in OVA-exposed animals [29]. Treatment with chamomile oil has shown promising effect in reducing the hyperresponsive reaction by decreasing eosinophilic infiltration and IgE levels [30]. Our histological analysis indicated that AB treatment could reduce eosinophil infiltration and airway inflammation. Airway inflammation due to immune cell infiltration prompts the production of ROS, leading to oxidative damage to tissues [31]. The ROS accumulation induces toxic proteins and lipid peroxidation products [32] and increases endogenous oxidants such as NO, thereby producing NO-derived reactive nitrogen species and XO [33]. Superoxide anions produced by eosinophils react with NO to produce reactive nitrogen species, leading to oxidative stress and lung inflammation in OVA-induced asthmatic animals [34,35]. Our results revealed significant reductions in stress indicator levels, such as NO, MDA, PCO, and XO, following AB treatment in allergic airway inflammation-induced rats.
Eosinophilia, a characteristic feature of allergic airway inflammation, is associated with increased expression of Th2 cytokines and IgE against OVA [36]. Allergens can activate immune cells, such as airway epithelial cells, dendritic cells, alveolar macrophages, smooth muscle, and goblet cells [36]. These cells produce the Th2 cytokine IL-13, which is involved in IgE production [37]. The current study revealed that AB treatment reduced the inflammatory cells associated with serum IgE expression.
Leukotrienes, produced by activated mast cells and eosinophils [38] are responsible for airway smooth muscle contraction and hyperresponsiveness [39]. Cysteinyl leukotrienes, which are potent bronchoconstrictors, are synthesized de novo in patients with asthma during an allergic attack, increased in OVA-induced animals, and acted as chemoattractants for eosinophils into the airway mucosa to increase inflammatory process [40]. They reduce ciliary motility, hinder mucus clearance, and extrapolate the asthmatic symptoms of wheezing and breathing discomfort by accumulating in the lungs [40]. Thromboxane A2, another airway inflammation indicator, contributes to inflammation by thickening and remodeling the airway wall [41]. Prostaglandin E2, which is produced by the airway smooth muscles, enhances leukotriene-induced inflammation [42,43], and acts in several ways to increase respiratory inflammation [44]. AB treatment reduces eosinophils, effectively reduces leukotriene expression and airway hyperresponsiveness, and inhibits the release of thromboxane B2 and prostaglandin E2 [45].
The IgE immune complex (IgE cx) in the acute smooth muscles of asthma triggers an altered airway response, and Th2 cytokines play pivotal roles in asthma pathophysiology [46]. IgE immune complexes trigger IL-1β production, a proinflammatory cytokine that mediates airway smooth muscle changes [47]. Furthermore, inflammatory cytokines (TNF-α and IL-1β) and Th2 cytokines (IL-5 and IL-13) change the contractile and relaxant responses of airway smooth muscle [48]. AB treatment inhibited these responses, decreasing IL-1β expression [49]. IL-2 induces calcium release by activating its receptors, resulting in airway smooth muscle contraction [50].
Lin et al. [51] indicated that oxidative stress, various genetic transcriptional regulations, and NF-κB, a proinflammatory transcription factor, induce IL-8, MCP-1, RANTES, eotaxin1, and various proinflammatory cytokines (TNF-α, IL-1β, IL-2, and IL-6) during lung airway inflammation. Another study supported this phenomenon, where the above cytokines were regulated during airway inflammation [52]. Our findings showed increased levels of IL-9, IL-13, and IL-17 in allergic airway inflammation-induced rat pups. These cytokines induce Th2 polarization [53]. This polarization triggers the degranulation of eosinophils and mast cells, thereby increasing airway hypersensitivity [54]. The increased airway hypersensitivity observed in allergic airway inflammation-induced rats was decreased by reducing these inflammatory mediators (IL-9, IL-13, and IL-17) in AB-treated rats, which is associated with the anti-inflammatory effect of AB [55].
Based on our results with previously published knowledge, we summarize that AB exerts its anti-inflammatory effects in neonatal asthma through various mechanisms. (i) AB downregulates the expression and release of proinflammatory cytokines thereby attenuating the inflammatory cascade and reducing airway inflammation [16]. (ii) AB inhibits the recruitment and activation of inflammatory cells like eosinophils and T cells, reducing their infiltration into the airways and mitigating airway inflammation and remodeling. (iii) AB interferes with the activity of transcription factors such as NF-κB and AP-1, which control the expression of inflammatory genes, leading to suppression of proinflammatory mediator production and dampening of the inflammatory response [16,17].
Conclusions
The present study revealed that our animal model was successful in inducing asthmatic symptom, allergic airway inflammation in rats, presenting allergen hyperresponsiveness and airway inflammation in lungs of rat pops. OVA-mediated inflammation in our model was confirmed by increased eosinophil infiltration in the airway smooth muscles. Furthermore, a spike in inflammatory cytokine and chemokine expression activates eosinophils to increase airway hyperresponsiveness in airway inflammation-induced models. Thus, the amelioration of asthma symptoms with reduced airway inflammation is associated with the anti-inflammatory activity of AB. Our results indicate that AB can balance bronchial hematosis. The present in vivo study could be a potential approach for using AB as an alternative treatment for asthma. However, further investigation is required to fully understand the pathway and their mechanisms of action. Future research should aim to deepen our understanding of AB's mechanisms of action in neonatal asthma and explore its clinical application as a promising adjunct or alternative treatment option. By advancing our knowledge in this area, we can potentially improve asthma management and enhance the quality of life of pediatric patients with asthma.
Abbreviations
AB: Alpha-bisabolol; BALF: bronchoalveolar lavage fluid; CCR4: C-motif chemokine receptor-4; COX-2: cyclooxygenase-2; CXCR4: C-X-C chemokine receptor type 4; IL: interleukin; MCP-1: monocyte chemoattractant protein-1; MDA: malondialdehyde; NO: nitric oxide; OVA: ovalbumin; PCO: protein carbonyl content; PRG4: proteoglycan 4; RANTES: regulated upon activation of normal T-cell expressed and secreted; ROS: reactive oxygen species; RT-PCR: Reverse transcription-polymerase chain reaction; Th2: T-helper type 2; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor-α; TSLP: thymic stromal lymphopoietin; XO: xanthine oxidase.
Acknowledgements
Funding
The authors express their sincere appreciation to the Ongoing Research Funding program (ORF-2025-679), King Saud University, Riyadh, Saudi Arabia.
Data availability statement
Data will be made available on reasonable request.
Ethical approval
The Institutional Ethics Committee at Periyar University, India, approved the experimental protocol (approval number: 1085/ac/07/CPCSEA/PUIAEC/March-2014/06), and the procedures were performed according to the guidelines. All pup treatments were strictly performed under the approval and regulations of the institutional committee.
Author contribution
Rekha Thiruvengadam: Conceptualiation, Methodology, Data curation, Formal analysis, Validation, Writing the original draft. Mydhili Govindarasu: Methodology, Investigation, Data curation, and Formal analysis. Jamal Mohammed Ali Khaled: Resources, Funding acquisition, Writing - Review and Editing. Seungho Lee: Formal analysis, Writing - review and editing. Jin Hee Kim: Conceptualization, Validation, Writing - review and editing, Funding acquisition, and Supervision.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
References
1. Syamlal G, Dodd KE, Mazurek JM. Asthma, chronic obstructive pulmonary disease, and asthma-COPD overlap among US working adults. J Asthma. 2023;60(4):718-726
2. Al Okla SM, Al Rasbi FAZK, Al Marhubi HS, Al Mataani SS, Al Sawai YM, Mohammed HI, Al Mamari MAS, Al Balushi SAA, Abbady AQ. The impact of air pollution on asthma severity among residents living near the main industrial complex in oman: A cross-sectional study. Int J Env Res Public Health. 2024;21(5):553
3. He Z, Feng J, Xia J, Wu Q, Yang H, Ma Q. Frequency of signs and symptoms in persons with asthma. Respir Care. 2020;65(2):252-264
4. Xiong DJP, Martin JG, Lauzon AM. Airway smooth muscle function in asthma. Front Physiol. 2022;13:993406
5. Carroll N, Lehmann E, Barret J, Morton A, Cooke C, James A. Variability of airway structure and inflammation in normal subjects and in cases of nonfatal and fatal asthma. Pathol Res Pract. 1996;192(3):238-248
6. James A, Maxwell PS, Pearce-Pinto G, Elliot JG, Carroll NG. The relationship of reticular basement membrane thickness to airway wall remodeling in asthma. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1590-1595
7. James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR. et al. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185(10):1058-1064
8. Joseph C, Tatler AL. Pathobiology of airway remodeling in asthma: The emerging role of integrins. J Asthma Allergy. 2022;15:595-610
9. Woodrow JS, Sheats MK, Cooper B, Bayless R. Asthma: The use of animal models and their translational utility. Cells. 2023;12(7):1091
10. Wardzynska A, Pawelczyk M, Rywaniak J, Kurowski M, Makowska JS, Kowalski ML. Circulating microRNAs and T-cell cytokine expression are associated with the characteristics of asthma exacerbation. Allergy Asthma Immunol Res. 2020;12(1):125-136
11. Zinellu E, Piras B, Ruzittu GGM, Fois SS, Fois AG, Pirina P. Recent advances in inflammation and treatment of small airways in asthma. Int J Mol Sci. 2019;20(11):2617
12. Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996;154(5):1505-1510
13. Yao X, Sun Y. The sounds of small airways: emerging role in pathogenesis and clinical expression of asthma. China Med J. 2014;127(1):173-179
14. Rogers L, Reibman J. Pharmacologic approaches to life-threatening asthma. Ther Adv Respir Dis. 2011;5(6):397-408
15. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703
16. Usmani K, Jain SK, Yadav S. Mechanism of action of certain medicinal plants for the treatment of asthma. J Ethnopharmacol. 2023;317:116828
17. Eddin LB, Jha NK, Goyal SN, Agrawal YO, Subramanya SB, Bastaki SMA. et al. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol. Nutrients. 2022;14(7):1370
18. Li G, Wu H, Sun L, Cheng K, Lv Z, Chen K, Qian F, Li Y. (-)-α-Bisabolol alleviates atopic dermatitis by inhibiting MAPK and NF-κB signaling in mast cell. Molecules. 2022;27(13):3985
19. Dong F, Wang C, Duan J, Zhang W, Xiang D, Li M, Puerarin attenuates ovalbumin-induced lung inflammation, hemostatic unbalance in rat asthma model. Evid. Based Complement. Alternat. Med. 2014;2014:726740
20. Liu S, Shudou M, Maeyama K. Early activation of mucosal mast cells during the primary immune response in a rodent model of neonatal asthma. Immunol Cell Biol. 2011;89(2):239-245
21. Saadat S, Beheshti F, Askari VR, Mahmoud H, Nema MR, Boskabady MH. Aminoguanidine affects systemic and lung inflammation induced by lipopolysaccharide in rats. Respir Res. 2019;20:96
22. MY Lee, Seo CS, Ha H, Jung D, Lee H, Lee NH. et al. Protective effects of Ulmus davidiana var. japonica against OVA-induced murine asthma model via upregulation of heme oxygenase-1. J Ethnopharmacol. 2010;130(1):61-69
23. Ullah I, Choi HS, Choi C, Chung K, Jung JW, Yun G. et al. Targeted siRNA delivery to lung epithelia reduces airway inflammation in a mouse model of allergic asthma. Biotechnol Bioproc E. 2024;29:97-108
24. Yang W-K, Kim S-W, Youn SH, Hyun SH, Han C-K, Park Y-C, Lee Y-C, Kim S-H. Respiratory protective effects of Korean Red Ginseng in a mouse model of particulate matter 4-induced airway inflammation. J Ginseng Res. 2023;47(1):81-88
25. Du Y, Luan J, Jiang RP, Liu J, Ma Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int J Immunopathol Pharmacol. 2020;34:2058738420954948
26. Zhang D, Yang J, Zhao Y, Shan J, Wang L, Yang G, He S, Li E. RSV infection in neonatal mice induces pulmonary eosinophilia responsible for asthmatic reaction. Front Immunol. 2022;13:817113
27. Li J, Bao T, Cao L, Ma M, Yu B, Zhang Y, Wu R, Zhu H, Tian Z. Establishment of a juvenile mouse asthma model induced by postnatal hyperoxia exposure combined with early OVA sensitization. Heliyon. 2024;10:e23291
28. Hylkema MN, Hoekstra MO, Luinge M, Timens W. The strength of the OVA-induced airway inflammation in rats is strain dependent. Clin Exp Immunol. 2002;129(3):390-396
29. Takayama S, Tamaoka M, Takayama K, Okayasu K, Tsuchiya K, Miyazaki Y, Sumi Y, Martin JG, Inase N. Synthetic double-stranded RNA enhances airway inflammation and remodelling in a rat model of asthma. Immunology. 2011;134(2):140-50
30. Lee SH, Heo Y, Kim YC. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J Vet Sci. 2010;11(1):35-41
31. Laxmi V, Gupta R, Bhattacharya SK, Ray A, Gulati K. Inhibitory effects of sildenafil and tadalafil on inflammation, oxidative stress and nitrosative stress in animal model of bronchial asthma. Pharmacol Rep. 2019;71(3):517-521
32. Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45(4):367-376
33. Qu J, Li Y, Zhong W, Gao P, Hu C. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis. 2017;9(1):E32-E43
34. Kharitonov SA, Donnelly LE, Montuschi P, Corradi M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action of inhaled budesonide on exhaled nitric oxide and symptoms in mild asthma. THORAX. 2002;57(10):889-896
35. Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533(1-3):222-239
36. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014;7:53-65
37. Van der Pouw Kraan TC, Van der Zee JS, Boeije LC, De Groot ER, Stapel SO, Aarden LA. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin Exp Immunol. 1998;111(1):129-135
38. Sverrild A, Bergqvist A, Baines KJ, Porsbjerg C, Andersson CK, Thomsen SF. et al. Airway responsiveness to mannitol in asthma is associated with chymase-positive mast cells and eosinophilic airway inflammation. Clin Exp Allergy. 2016;46(2):288-297
39. Huang CQ, Li W, Wu B, Chen WM, Chen LH, Mo GW. et al. Pheretima aspergillum decoction suppresses inflammation and relieves asthma in a mouse model of bronchial asthma by NF-κB inhibition. J Ethnopharmacol. 2016;189:22-30
40. Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy. 2001;56(Suppl 66):7-11
41. Peebles Jr RS. Prostaglandins in asthma and allergic diseases. Pharmacol Ther. 2019;193:1-19
42. Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2(5):603-630
43. Sastre B, del Pozo V. Role of PGE2 in asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm. 2012;2012:645383
44. Montaño LM, Flores-Soto E, Sommer B, Solís-Chagoyán H, Perusquía M. Androgens are effective bronchodilators with anti-inflammatory properties: A potential alternative for asthma therapy. Steroids. 2020;153:108509
45. Maurya AK, Singh M, Dubey V, Srivastava S, Luqman S, Bawankule DU. α-(-)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr Pharm Biotechnol. 2014;15(2):173-181
46. Whelan R, Kim C, Chen M, Leiter J, Grunstein MM, Hakonarson H. Role and regulation of interleukin-1 molecules in pro-asthmatic sensitised airway smooth muscle. Eur Respir J. 2004;24(4):559-567
47. Hakonarson H, Maskeri N, Carter C, Chuang S, Grunstein MM. Autocrine interaction between IL-5 and IL-1beta mediates altered responsiveness of atopic asthmatic sensitized airway smooth muscle. J Clin Invest. 1999;104(5):657-667
48. Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol. 2002;29(10):859-866
49. Muñoz-Pérez VM, Ortiz MI, Ponce-Monter HA, Monter-Pérez V, Barragán-Ramírez G. Anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human uterus. Kor J Physiol Pharmacol. 2018;22(4):391-398
50. Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ. et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol. 1999;56(3):657-663
51. Lin SC, Shi LS, Ye YL. Advanced molecular knowledge of therapeutic drugs and natural products focusing on inflammatory cytokines in asthma. Cells. 2019;8(7):685
52. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023
53. Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res. 2014;3:127
54. Wang J, Zhou Y, Zhang H, Hu L, Liu J, Wang L. et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target Ther. 2023;8(1):138
55. Barreto RSS, Quintans JSS, Amarante RKL, Nascimento TS, Amarante RS, Barreto AS. et al. Evidence for the involvement of TNF-alpha and IL-1beta in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (-)-alpha-bisabolol, its main compound, in mice. J Ethnopharmacol. 2016;191:9-18
Author contact
![]() Corresponding author: Jin Hee Kim, Department of Integrative Bioscience & Biotechnology, Institute of Bioscience, Sejong University. 209 Neungdong-ro, Gwangjin-gu, Seoul 05006, Republic of Korea. Tel.: +82 2 3408 3655; Fax.: +82 2 3408 4334; E-mail: jhkim777ac.kr.
Corresponding author: Jin Hee Kim, Department of Integrative Bioscience & Biotechnology, Institute of Bioscience, Sejong University. 209 Neungdong-ro, Gwangjin-gu, Seoul 05006, Republic of Korea. Tel.: +82 2 3408 3655; Fax.: +82 2 3408 4334; E-mail: jhkim777ac.kr.

 Global reach, higher impact
Global reach, higher impact