3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3422-3428. doi:10.7150/ijms.116551 This issue Cite
Research Paper
Psoriasis Patients are Associated with Increased Risk of New-Onset Irritable Bowel Syndrome: A Multicenter, Retrospective Cohort Study
1. Department of Neurosurgery, MacKay Memorial Hospital, Taipei, Taiwan.
2. Department of Medicine, MacKay Medical College, New Taipei City, Taiwan.
3. Evidence-based Medicine Center, Chung Shan Medical University Hospital, Taichung, Taiwan.
4. Library, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
6. Department and Graduate Institute of Business Administration, National Taiwan University, Taipei, Taiwan.
7. Department of Pharmacology, Chung Shan Medical University, Taichung, Taiwan.
8. Orthopedics Department, Chi-Mei Medical Center, Tainan, Taiwan.
* Contributed equally and shared the corresponding authorship.
Received 2025-4-27; Accepted 2025-7-8; Published 2025-7-24
Abstract
Background: Psoriasis is a chronic systemic inflammatory disease linked to multiple comorbidities. The association between psoriasis and irritable bowel syndrome (IBS) remains insufficiently characterized.
Methods: We conducted a retrospective cohort study using the TriNetX US Collaborative Network. Adults with psoriasis (ICD-10-CM L40) diagnosed between 2005 and 2023 were matched 1:1 with non-psoriasis controls based on demographics, socioeconomic status, comorbidities, and healthcare utilization. IBS (ICD-10-CM K58) diagnoses within 90 days of index were excluded. Cox regression models estimated hazard ratios (HRs) with 95% confidence intervals (CIs). Sensitivity analyses varied wash-out periods, follow-up durations, and exposure definitions.
Results: After matching (n=256,550 per group), people with psoriasis were associated with a higher risk of IBS (HR 1.244, 95% CI 1.168-1.325) in the 15-year follow-up model while comparing with non-psoriasis controls. Subgroup analyses validated elevated risks among both age groups (e.g., age ≥65 years: HR 1.325, 95% CI 1.167-1.505) and sexes (female: HR 1.291, 95% CI 1.197-1.393). All sensitivity models yielded consistent results.
Conclusion: Psoriasis is independently associated with an increased risk of subsequent IBS. Routine gastrointestinal symptom screening in psoriasis patients may improve comprehensive care.
Introduction
Psoriasis is a chronic immune-mediated dermatologic condition affecting approximately 2-3% of the global population [1]. Beyond cutaneous involvement, psoriasis is associated with systemic comorbidities, including increased risks of inflammatory, psychiatric, and gastrointestinal diseases [2-5]. In particular, psoriasis has been significantly linked to inflammatory bowel diseases (IBD), with meta-analyses indicating a 1.7-fold increased odds of ulcerative colitis and a 2.5-fold increased odds of Crohn's disease, supporting the concept of a gut-skin axis driven by chronic systemic inflammation [6].
Irritable bowel syndrome (IBS), a prevalent disorder of gut-brain interaction affecting approximately 11% of the global population, is characterized by recurrent abdominal pain and altered bowel habits [7]. IBS frequently coexists with psychological distress, with about one-third of patients experiencing anxiety or depression [8]. This association highlights the role of stress and systemic factors in the manifestation of the disorder. Although IBS patients typically do not exhibit the overt intestinal inflammation seen in IBD, the condition has been linked to subtle immune dysregulation, alterations in the gut microbiota and increased intestinal permeability [9, 10].
The co-occurrence of psoriasis with gastrointestinal diseases other than IBD has received comparatively less attention. It is biologically plausible that psoriasis and IBS could be linked via shared immunological pathways or risk factors. Both conditions have been associated with alterations in the gut microbiome and immune profiles. A small case-control study suggested a high prevalence of IBS in psoriasis patients [11]; however, large scale studies in real-world setting are lacking.
Given the systemic pro-inflammatory milieu of psoriasis and its overlap with pathways implicated in functional bowel disorders, we hypothesized that psoriasis is associated with an increased risk of developing IBS. We therefore conducted a large multicenter retrospective cohort study using a federated electronic health record network to investigate the risk of IBS among patients with psoriasis compared to matched controls. This study aimed to elucidate the association between psoriasis and IBS, explore subgroup differences, and advance understanding of the gut-skin axis in immune-mediated disease.
Methods
Study design and data source
We conducted a retrospective cohort study using the TriNetX US Collaborative Network, a federated database of de-identified electronic health records from 69 healthcare organizations across the United States. The research network has been widely applied in the field of health economics and outcomes research [12, 13]. The study followed STROBE guidelines, and the Institutional Review Board of Chung Shan Medical University Hospital waived the need of informed consent of this study (CS1-25002).
Population and outcome definition
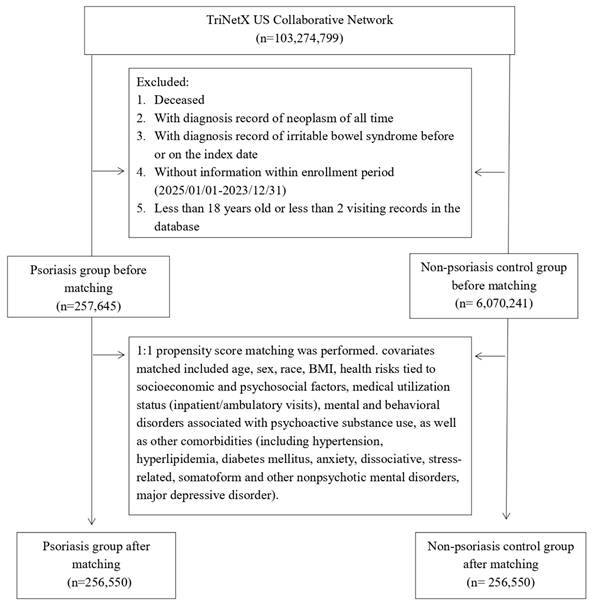
The psoriasis cohort included adult patients (≥ 18 years) with at least one recorded diagnosis of psoriasis (ICD-10-CM code L40) between January 1, 2005, and December 31, 2023. The index date was defined as the first psoriasis diagnosis. The primary outcome was incident IBS occurring at least 90 days after the index date to reduce reverse causation. We excluded individuals with a prior diagnosis of IBS (ICD-10-CM code K58), any malignant neoplasm, or those who died on or before the index date. The control cohort was composed of patients without psoriasis, selected from the same network (Figure 1). Controls were required to have a general medical examination (ICD-10-CM code Z00) to establish an index date and were matched 1:1 to psoriasis patients based on similar inclusion criteria. In this study, a diagnosis of IBS (ICD-10-CM code K58) was used to define the outcome of interest. Follow-up for each patient ended at the time of this diagnosis, which served as the censoring point. Detailed information of used ICD-10-CM codes were presented in Table S1.
Matching and covariates
Propensity score matching was performed to control for baseline confounders. Covariates included age, sex, race, BMI (≥ 25 vs < 25 kg/m²), socioeconomic and psychosocial risk factors (ICD-10 Z55-Z65), healthcare utilization, substance use disorders (ICD-10 F10-F19), and comorbidities such as hypertension, hyperlipidemia, diabetes, depression, and anxiety. The variables used for matching were selected because previous research indicates they may be linked to IBS, making them likely confounding factors that could influence the study's results. The 1:1 nearest-neighbor matching used a caliper of 0.1 standard deviations. Balance was assessed using standardized mean differences (SMD, with the value of < 0.1 indicated adequate matching).
Statistical analysis
The Analytic tool of TriNetX Research Network, Python (version 3.12.7) and Microsoft Excel 2019 was used for main analysis and figure generation. Descriptive statistics compared baseline characteristics pre- and post-matching. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated in each analysis. Cox proportional hazards regression was performed using the TriNetX built-in analytics system, which computes HRs and CIs based on time-to-event data aggregated from electronic health records. A cumulative incidence plot was presented, with the x-axis representing time since the index date (in months), and the y-axis showing the cumulative incidence of IBS. Differences between curves were tested using the log-rank test, with statistical significance defined as p < 0.05. Subgroup analyses stratified by age (< 65 vs ≥ 65 years) and sex, and multiple sensitivity analyses were performed to test robustness across washout periods, follow-up lengths, matching strategies, and stricter psoriasis definitions (Details reported in Table S2).
Patient selection flowchart.
Results
Baseline characteristics
Before matching, 257,645 psoriasis patients and 6,070,241 non-psoriasis controls met inclusion criteria. After 1:1 propensity score matching, 256,550 patients remained in each group (Table 1). Baseline characteristics were notably imbalanced pre-matching, with psoriasis patients showing higher age, greater proportions of White individuals, and a higher burden of cardiometabolic and psychiatric comorbidities. After matching, covariate balance was achieved (all standardized mean differences < 0.1).
Risk of IBS and sensitivity analysis
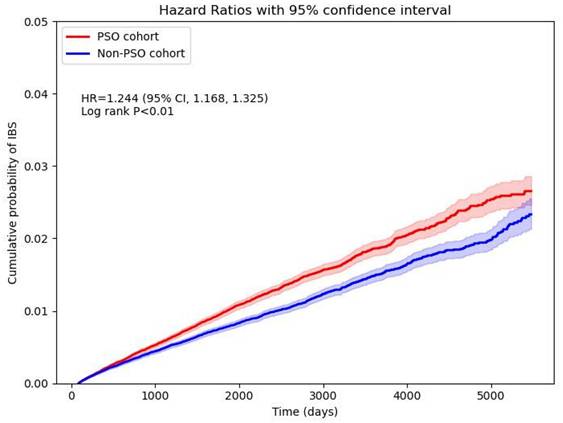
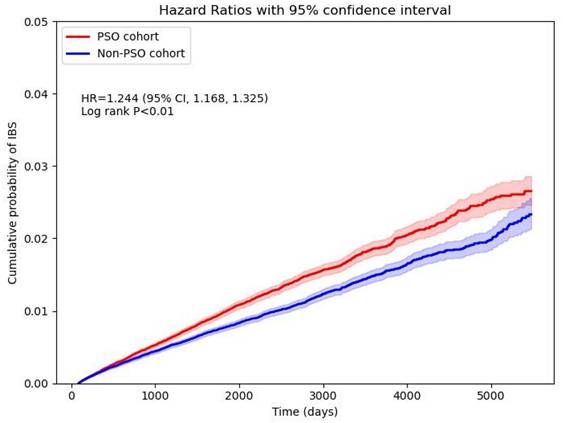
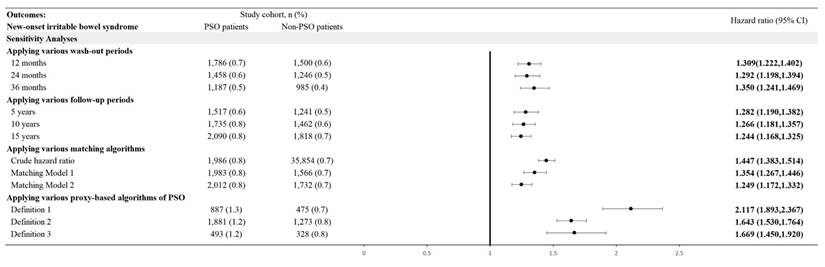
In the 15-year follow-up, the risk for psoriasis patients developing new onset IBS, while comparing with non-psoriasis controls, was 1.244-fold (95% CI, 1.168-1.325) (Figure 2). Robustness of the primary finding was confirmed in multiple sensitivity analyses (Figure 3). We varied the IBS wash-out period to 12, 24, and 36 months to address potential misclassification of pre-existing IBS. HRs remained statistically significant and consistent across all time frames (e.g., HR at 12 months = 1.309, 95% CI 1.222-1.402; at 36 months = 1.350, 95% CI 1.241-1.469), suggesting that reverse causation or latent cases did not explain the observed association. When altering the maximum follow-up duration yielded consistent results. With follow-up truncated at 5 years, the HR for IBS in the psoriasis group was 1.282 (95% CI, 1.190-1.382); with 10 years, HR was 1.266 (95% CI, 1.181-1.357)—supporting the presence of short- and intermediate-term risk elevations. Alternative analytic approaches of matching covariates were also tested. In the unmatched crude cohort, the HR for IBS was 1.447 (95% CI, 1.383-1.514). Additionally, we applied strict definitions of psoriasis to address potential misclassification bias. When psoriasis exposure was defined as ≥ 2 visiting records plus an inpatient encounter (Algorithm 1), the HR for IBS was 2.117 (95% CI, 1.893-2.367). Among those receiving systemic corticosteroids (Algorithm 2), the HR was 1.643 (95% CI, 1.530-1.764); for those treated with biologics (Algorithm 3), the HR was 1.669 (95% CI, 1.450-1.920). Since IBD and IBS exhibit overlapping symptoms, and IBD has a well-established link to psoriasis, there's a risk that the observed relationship between psoriasis and IBS could be influenced by the presence of IBD. After exclusion of IBD patients, the association between psoriasis and IBS remained statistically significant, with a hazard ratio of 1.312 (95% CI, 1.242-1.386).
Stratified analyses
Subgroup analyses confirmed that the association was consistent across age and sex strata (Table 2). Among individuals under 65 years, the adjusted HR for IBS was 1.254 (95% CI, 1.165-1.351), while in those aged ≥ 65, the HR was 1.325 (95% CI, 1.167-1.505). The HR among female patients was 1.291 (95% CI 1.197-1.393), and 1.155 (95% CI 1.012-1.318) among males.
Discussion
In this large, multicenter retrospective cohort study using an electronic health record derived real-world database, we found that patients with psoriasis had a significantly increased risk of developing IBS compared to matched controls, with a hazard ratio of 1.244 (95% CI, 1.168-1.325) in 15-year follow-up period. To our knowledge, this is the first large-scale study to quantify this association longitudinally, using a rigorously matched population and real-world data from a diverse US patient cohort.
Baseline characteristics
| Before matching | After matchinga | |||||
|---|---|---|---|---|---|---|
| PSO cohort (n=257,645) | Non-PSO control cohort (n=6,070,241) | SMD | PSO cohort (n=256,550) | Non-PSO control cohort (n=256,550) | SMD | |
| Age at index | ||||||
| Mean±SD | 46.3 ± 17.9 | 38.2 ± 20.2 | 0.43 | 46.3 ± 17.9 | 46.3 ± 19.0 | 0.00 |
| Sex | ||||||
| Male | 112007 (43.7) | 2565805 (42.5) | 0.02 | 112007 (43.7) | 109968 (42.9) | 0.02 |
| Female | 131997 (51.5) | 3125056 (51.7) | 0.01 | 131997 (51.5) | 131897 (51.4) | 0.00 |
| Unknown | 12546 (4.9) | 352472 (5.8) | 0.04 | 12546 (4.9) | 14685 (5.7) | 0.04 |
| Race, n (%) | ||||||
| White | 177766 (69.3) | 3480142 (57.6) | 0.24 | 177766 (69.3) | 177724 (69.3) | 0.00 |
| Black or African American | 16471 (6.4) | 892867 (14.8) | 0.27 | 16471 (6.4) | 16604 (6.5) | 0.00 |
| Asian | 10383 (4.0) | 295999 (4.9) | 0.04 | 10383 (4.0) | 11121 (4.3) | 0.01 |
| Native Hawaiian or Other Pacific Islander | 1924 (0.8) | 38613 (0.6) | 0.01 | 1924 (0.8) | 1475 (0.6) | 0.02 |
| American Indian or Alaska Native | 865 (0.3) | 19756 (0.3) | 0.00 | 865 (0.3) | 731 (0.3) | 0.01 |
| Unknown Race | 36880 (14.4) | 962041 (15.9) | 0.04 | 36880 (14.4) | 36185 (14.1) | 0.01 |
| Other Race | 12261 (4.8) | 353915 (5.9) | 0.05 | 12261 (4.8) | 12710 (5.0) | 0.01 |
| BMI, n (%) | ||||||
| ≥ 25 (kg/m2) | 73013 (28.5) | 1498134 (24.8) | 0.08 | 73013 (28.5) | 73301 (28.6) | 0.00 |
| Medical Utilization Status, n (%) | ||||||
| Visit: Ambulatory | 160824 (62.7) | 3555817 (58.8) | 0.08 | 160824 (62.7) | 161066 (62.8) | 0.00 |
| Visit: Inpatient Encounter | 37478 (14.6) | 739908 (12.2) | 0.07 | 37478 (14.6) | 37298 (14.5) | 0.00 |
| Socioeconomic status, n (%) | ||||||
| Persons with potential health hazards related to socioeconomic and psychosocial circumstances | 3088 (1.2) | 87661 (1.5) | 0.02 | 3088 (1.2) | 2818 (1.1) | 0.01 |
| Lifestyle, n (%) | ||||||
| Mental and behavioral disorders due to psychoactive substance use | 22171 (8.6) | 356705 (5.9) | 0.11 | 22171 (8.6) | 22058 (8.6) | 0.00 |
| Comorbidities, n (%) | ||||||
| Major depressive disorder | 5123 (2.0) | 91264 (1.5) | 0.04 | 5123 (2.0) | 4796 (1.9) | 0.01 |
| Anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders | 29268 (11.4) | 536049 (8.9) | 0.08 | 29268 (11.4) | 28911 (11.3) | 0.00 |
| Hyperlipidemia | 26737 (10.4) | 476675 (7.9) | 0.09 | 26737 (10.4) | 26472 (10.3) | 0.00 |
| Essential hypertension | 43810 (17.1) | 803107 (13.3) | 0.11 | 43810 (17.1) | 43607 (17.0) | 0.00 |
| Diabetes mellitus | 19670 (7.7) | 330822 (5.5) | 0.09 | 19670 (7.7) | 19345 (7.5) | 0.00 |
PSO, psoriasis; SMD, standardized mean difference; SD, standardized difference
a The covariates matched included age, sex, race, BMI, health risks tied to socioeconomic and psychosocial factors, medical utilization status (inpatient/ambulatory visits), mental and behavioral disorders associated with psychoactive substance use, as well as other comorbidities (including hypertension, hyperlipidemia, diabetes mellitus, anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders, major depressive disorder).
Stratification analysis of IBS risk in PSO patients in 15-year follow-up
| Cases occurring new-onset IBS | |||
|---|---|---|---|
| Subgroups | PSO cohort No. of outcome events (%) | Control cohort No. of outcome events (%) | HR (95% CI)a |
| Age at index date | |||
| 18-64 years old | 1,527 (0.9) | 1,295 (0.7) | 1.254 (1.165,1.351) |
| ≥ 65 years old | 521 (0.7) | 438 (0.6) | 1.325 (1.167,1.505) |
| Sex | |||
| Male | 463 (0.4) | 418 (0.4) | 1.155 (1.012,1.318) |
| Female | 1,443 (1.1) | 1,244 (1.0) | 1.291 (1.197,1.393) |
a The covariates matched included age, sex, race, BMI, health risks tied to socioeconomic and psychosocial factors, medical utilization status (inpatient/ambulatory visits), mental and behavioral disorders associated with psychoactive substance use, as well as other comorbidities (including hypertension, hyperlipidemia, diabetes mellitus, anxiety, dissociative, stress-related, somatoform and other nonpsychotic mental disorders, major depressive disorder).
Cumulative probability curve of irritable bowel syndrome risk in psoriasis and non-psoriasis cohorts.
Risk of irritable bowel syndrome in various sensitivity mode.
Our findings align with prior smaller-scale studies, including the case-control study by Unal et al., which reported a substantially higher prevalence of IBS in psoriasis patients (36.9%) compared to controls (12.6%) [11]. Although their effect size was larger, differences in study design likely explain the disparity: their study used direct symptom screening (Rome III criteria), whereas ours relied on diagnostic coding from clinical encounters. Our design likely captured moderate-to-severe IBS that came to clinical attention, possibly underestimating subclinical or mild cases. Nevertheless, both studies support the concept of a psoriasis-IBS link. Importantly, our study demonstrated temporal sequencing, with psoriasis preceding IBS onset in all cases. The association persisted after rigorous propensity score matching and covariate adjustment, including control for psychiatric comorbidities such as depression and anxiety, which are independently associated with IBS. This suggests that shared risk factors alone are insufficient to account for the observed relationship, pointing toward a potential pathophysiological connection between the two conditions.
The concept of a gut-skin axis has gained considerable traction, particularly in the context of immune-mediated diseases. Psoriasis shares immunological pathways with IBD, including Crohn's disease and ulcerative colitis, notably through dysregulation of the IL-23/Th17 axis [14]. While IBS lacks the overt mucosal inflammation of IBD, evidence suggests that low-grade immune activation may underlie a subset of IBS cases [10, 15]. Systemic inflammation is a hallmark of psoriasis, driven by pro-inflammatory cytokines such as TNF-α, IL-17, and IL-23 [16], which have known effects on gut permeability and neuroenteric signaling [17, 18]. These cytokines may “prime” the gut environment for IBS-like symptoms by disrupting mucosal homeostasis or altering the enteric nervous system. Our findings extend the gut-skin axis model by implicating not only classical inflammatory bowel diseases but also functional disorders like IBS in the comorbidity spectrum of psoriasis.
Another potential mechanistic link between psoriasis and IBS involves the gut microbiome. Psoriasis has been associated with alterations in gut microbiota composition, particularly a reduction in anti-inflammatory bacterial genera [19]. For instance, a recent case-control study noted significantly elevated gut interleukin-1α levels and altered gut microbial composition in psoriasis patients compared to controls [20]. Such dysbiosis may lead to changes in microbial metabolites—such as imbalanced short-chain fatty acids—which can affect gut motility and visceral sensitivity [21]. Similarly, patients with IBS often exhibit microbial imbalance, and in some cases, fungal overgrowth [10]. The "leaky gut" phenomenon—characterized by increased intestinal permeability noted in IBS—may permit the translocation of microbial products into systemic circulation, fueling chronic inflammation and potentially exacerbating psoriasis, forming a self-perpetuating inflammatory loop [22]. An altered microbiome in individuals with psoriasis may predispose them to IBS-like symptoms by promoting gas production, disrupting bile acid metabolism, or activating toll-like receptors, thereby contributing to visceral hypersensitivity [23, 24].
Moreover, the brain-gut-skin axis provides an integrative framework, wherein psychological stress can concurrently trigger neuroimmune responses in both the gastrointestinal tract and the skin [25, 26]. Stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis, sympathetic nervous system, and subsequent cortisol release can impair skin barrier function, alter gut motility, and provoke IBS flare-ups and psoriasis exacerbations, linking both conditions through a neuro-immuno-endocrine pathway [27, 28].
Several limitations warrant consideration. First, although the longitudinal design supports temporal inference, this remains an observational study and cannot establish causality. Despite comprehensive matching and adjustment, residual confounding by unmeasured variables (e.g., stress levels, dietary habits, physical activity) may exist [29]. Second, our reliance on ICD-10 codes for psoriasis and IBS introduces the potential for misclassification. Some patients may have been miscoded, and we lacked data on standardized IBS diagnostic criteria (e.g., Rome IV). However, such misclassification would likely be non-differential between groups, biasing estimates toward the null. Third, the cohort was drawn from healthcare-engaged individuals within the US, and findings may not generalize to those with limited healthcare access or in other countries. The sample was predominantly White, which mirrors psoriasis epidemiology in the US, but limits extrapolation to other racial/ethnic groups. Fourth, psoriasis severity could not be precisely assessed. Although we used proxy markers such as inpatient encounters and systemic therapy, we lacked standardized measures like the Psoriasis Area and Severity Index (PASI). It remains possible that IBS risk is higher in more severe cases, and future studies should explore this gradient.
Conclusion
From a clinical standpoint, our findings underscore the importance of recognizing IBS as a potential comorbidity in patients with psoriasis. Clinicians should maintain a high index of suspicion for IBS in psoriasis patients who report chronic abdominal pain, bloating, or altered bowel habits—particularly when IBD has been ruled out. Dermatologists and gastroenterologists should consider interdisciplinary collaboration in such cases to optimize patient care. Early diagnosis and management of IBS may significantly improve quality of life in this population.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Funding
This study was partially funded by Chung Shan Medical University Hospital (CSH-2025-C-007).
Ethics statement
This study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (CS1-25002).
Data sharing statement
Data in this study were retrieved from TriNetX Research Network. All data available in the database were administrated by the TriNetX platform. Detailed information can be retrieved at the official website of the research network (https://trinetx.com).
Author contributions
All the authors involved in drafting or revising the article and approved of the submitted version.
Study conception and design: Chang HC, Chen SJ, Gau SY.
Data acquisition: Chang HC, Gau SY.
Data analysis and demonstration: Chang HC, Chen SJ, Gau SY.
Original draft preparation: Chang HC, Chen SJ, Gau SY.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jin L, Yang J, Wan P, Cheng Y. Incidence trends of pediatric psoriasis in 1990-2021: findings from the Global Burden of Disease Study 2021. Clinical and experimental dermatology. 2025;50(6):1171-1179
2. Li CP, Lo SW, Tsai RY, Chang HC, Gau SY. New-Onset Hidradenitis Suppurativa in Psoriasis Patients: A Multi-Center, Retrospective Cohort Study. Life (Basel, Switzerland). 2024;14:730
3. Gau SY, Huang KH, Lee CH, Kuan YH, Tsai TH, Lee CY. Bidirectional Association Between Psoriasis and Nonalcoholic Fatty Liver Disease: Real-World Evidence From Two Longitudinal Cohort Studies. Front Immunol. 2022;13:840106
4. Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. The Journal of investigative dermatology. 2014;134:1542-51
5. Gau SY, Preclaro IAC, Wei JC, Lee CY, Kuan YH, Hsiao YP, Juang SE, Ma KS. Risk of psoriasis in people with hidradenitis suppurativa: A systematic review and meta-analysis. Front Immunol. 2022;13:1033844
6. Fu Y, Lee CH, Chi CC. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA dermatology. 2018;154:1417-23
7. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2012;10:712-21.e4
8. Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2019;50:132-43
9. Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L. Irritable bowel syndrome and mental health comorbidity - approach to multidisciplinary management. Nature reviews. Gastroenterology & hepatology. 2023;20:582-96
10. Inczefi O, Bacsur P, Resál T, Keresztes C, Molnár T. The Influence of Nutrition on Intestinal Permeability and the Microbiome in Health and Disease. Frontiers in Nutrition. 2022;9:718710
11. Gulbahar Urun Unal KM, Mehmet Ali Eryilmaz, Mehmet Unal, Orhan Kulahci. Skin and Gut: Psoriasis and irritable bowel syndrome. Is there an association? Annals of Medical Research. 2021;27:1611-5
12. Chang HC, Lu HY, Guo YC, Lin CY, Chen SJ, Gau SY. Depression risk in chronic tonsillitis patients underwent tonsillectomy: a global federated health network analysis. International journal of medical sciences. 2024;21:949-57
13. Chang H-C, Lin C-Y, Guo Y-C, Lu H-Y, Lee C-Y, Wu M-C, Gau S-Y. Association between hidradenitis suppurativa and atopic diseases: a multi-center, propensity-score-matched cohort study. International Journal of Medical Sciences. 2024;21:299-305
14. Krueger JG, Eyerich K, Kuchroo VK, Ritchlin CT, Abreu MT, Elloso MM. et al. IL-23 past, present, and future: a roadmap to advancing IL-23 science and therapy. Frontiers in Immunology. 2024 15
15. Yuan Y, Xiyang W, Shun H, Hao W, Shen G. Low-level inflammation, immunity, and brain-gut axis in IBS: unraveling the complex relationships. Gut Microbes. 2023;15:2263209
16. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397:1301-15
17. Lee Jacob S, Tato Cristina M, Joyce-Shaikh B, Gulen Muhammet F, Cayatte C, Chen Y. et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727-38
18. Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH. et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021;109:2691-706.e5
19. Jun YK, Yoon HT, Kwon SH, Jo UH, Kim JE, Han YM. et al. Regulation of psoriasis, colitis, and the intestinal microbiota by clusterin. Scientific Reports. 2023;13:15405
20. Yegorov S, Babenko D, Kozhakhmetov S, Akhmaltdinova L, Kadyrova I, Nurgozhina A. et al. Psoriasis Is Associated With Elevated Gut IL-1α and Intestinal Microbiome Alterations. Frontiers in Immunology. 2020;11:571319
21. Vargas A, Robinson BL, Houston K, Vilela Sangay AR, Saadeh M, D'Souza S, Johnson DA. Gut microbiota-derived metabolites and chronic inflammatory diseases. Exploration of Medicine. 2025;6:1001275
22. Azarfarin M, Moradikor N, Matin S, Dadkhah M. Association Between Stress, Neuroinflammation, and Irritable Bowel Syndrome: The Positive Effects of Probiotic Therapy. Cell biochemistry and function. 2024;42:e70009
23. Zhu Q, Wu K, Yang Q, Meng B, Niu Y, Zhao F. Advances in psoriasis and gut microorganisms with co-metabolites. Frontiers in Microbiology. 2023;14:1192543
24. Hao Y, Zhou P, Zhu YJ, Zou S, Zhao Q, Yu J, Hu Y, Li J. Gut Microbiota Dysbiosis and Altered Bile Acid Catabolism Lead to Metabolic Disorder in Psoriasis Mice. Front Microbiol. 2022;13:853566
25. Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L. et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186:2823-38.e20
26. Rousset L, Halioua B. Stress and psoriasis. Int J Dermatol. 2018;57:1165-72
27. Chen G, Chen ZM, Fan XY, Jin YL, Li X, Wu SR, Ge WW, Lv CH, Wang YK, Chen JG. Gut-Brain-Skin Axis in Psoriasis: A Review. Dermatol Ther (Heidelb). 2021;11:25-38
28. Mbiydzenyuy NE, Qulu L-A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metabolic Brain Disease. 2024;39:1613-36
29. Gau SY. Methotrexate use and liver outcomes in psoriasis and rheumatoid arthritis patients: A commentary on "Risk of liver disease in patients with psoriasis, psoriatic arthritis and rheumatoid arthritis receiving methotrexate: A population-based study". J Am Acad Dermatol. 2021;85:e399-e400
Author contact
![]() Corresponding author: Dr. Shuo-Yan Gau, MD, FRSPH, Department and Graduate Institute of Business Administration, National Taiwan University, Taipei, Taiwan, No. 1, Sec. 4, Roosevelt Rd., Taipei 106319, Taiwan, email: sixsamurai.shien15com.
Corresponding author: Dr. Shuo-Yan Gau, MD, FRSPH, Department and Graduate Institute of Business Administration, National Taiwan University, Taipei, Taiwan, No. 1, Sec. 4, Roosevelt Rd., Taipei 106319, Taiwan, email: sixsamurai.shien15com.

 Global reach, higher impact
Global reach, higher impact