3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3393-3411. doi:10.7150/ijms.111082 This issue Cite
Review
Exploring the Multifactorial Regulation of PIEZO1 in Chondrocytes: Mechanisms and Implications
1. Department of Orthopedic Surgery, Hubei Provincial Hospital of Integrated Chinese & Western Medicine, Wuhan 430015, China.
2. Department of Orthopedics, General Hospital of Central Theater Command, Wuhan 430070, China.
3. College of Acupuncture and Orthopedics, Hubei University of Chinese Medicine, Wuhan 430065, China
4. School of Medicine, Wuhan University of Science and Technology, Wuhan 430081, China.
*These authors contributed equally in this manuscript.
Received 2025-1-25; Accepted 2025-7-8; Published 2025-7-24
Abstract
The mechanosensitive PIEZO1 ion channel plays a pivotal role in the regulation of chondrocyte function and is involved in various physiological and pathological processes, including cartilage degradation and osteoarthritis (OA). This review explores the regulatory mechanisms governing PIEZO1 activation and its interactions with mechanical stress, extracellular matrix (ECM) stiffness, inflammatory factors, and other ion channels. We discuss the role of PIEZO1 in calcium signaling, its modulation by ECM stiffness, and the implications for cartilage health, particularly under high mechanical load or inflammatory conditions. Additionally, the review highlights pharmacological modulators, including PIEZO1 activators such as Yoda1 and Jedi1/2, and inhibitors like GsMTx4, as potential therapeutic targets for OA treatment. The synergistic interactions of PIEZO1 with other mechanosensitive channels, such as TRPV4 and PIEZO2, are also examined, providing insights into their collective role in mechanotransduction. Further research is essential to clarify the spatiotemporal activation patterns of PIEZO1, its downstream effects, and the potential for targeting this channel in clinical interventions for degenerative cartilage diseases.
Keywords: chondrocyte, mechanotransduction, mechanosensitive ion channels, PIEZO1
1. Introduction
Chondrocytes, the only cell type in articular cartilage, play a pivotal role in regulating joint mechanical stress distribution, sensing external stimuli, and maintaining the metabolic homeostasis of the extracellular matrix (ECM) under mechanical load [1-3]. These cells are able to perceive mechanical load through mechanosensitive ion channels, including PIEZO1, PIEZO2, and transient receptor potential vanilloid 4 (TRPV4) [4]. The mechanical stress that is exerted on the cells is a primary physical regulatory factor that influences a number of cellular processes, including proliferation, differentiation, apoptosis, and matrix synthesis or degradation. Under normal physiological conditions, moderate mechanical stress promotes ECM synthesis, such as type II collagen and aggrecan, thereby maintaining cartilage function [5,6]. However, excessive or abnormal mechanical loads (e.g., high pressure or shear force) have been shown to induce apoptosis of the cartilage cells, as well as inflammatory responses and matrix degradation, thus leading to cartilage degeneration and the subsequent development of osteoarthritis (OA) and post-traumatic osteoarthritis (PTOA) [7-8]. Conversely, a reduction in mechanical load has been shown to alleviate cartilage destruction, subchondral bone changes, and secondary inflammatory alterations in OA animal models [9].
In recent years, the critical role of mechanosensitive ion channels (MSCs) in chondrocyte mechanotransduction has become increasingly evident. The influx of extracellular calcium is a central mechanism underlying cellular responses to mechanical load [10]. Variations in the concentration of calcium ions within the cell are primarily mediated by calcium channels located on the cell membrane. Chondrocytes express a number of different calcium channels, including TRPV4, T/L-type voltage-gated Ca²⁺ channels (VGCCs), PIEZO1/2 channels, transient receptor potential (TRP) family channels, and calcium release-activated Ca²⁺ channels (CRACs) [11]. Among these, PIEZO1—a large mechanosensitive calcium channel—responds directly to membrane tension changes, triggering calcium ion (Ca²⁺) influx and initiating a cascade of intracellular signaling pathways [12]. This process regulates the proliferation, apoptosis, and autophagy of cells, as well as the metabolic balance of the ECM [13].
PIEZO1, the largest plasma membrane ion channel complex identified to date, exhibits unique structural and functional characteristics that are central to cellular responses to mechanical stimuli. Unlike other ion channels or proteins, PIEZO1 is a high-molecular-weight protein that lacks significant sequence similarity with known ion channels or proteins, thus marking it as a distinct mechanosensitive channel [14]. Structurally, PIEZO1 shares similarities with PIEZO2, being large conserved transmembrane proteins composed of homotrimeric structures resembling a triskelion or three-bladed propeller [14-16]. This distinctive architecture enables PIEZO1 to sense mechanical forces by responding to membrane tension changes, opening ion channels for calcium influx, and subsequently activating intracellular signaling cascades.
The structure of PIEZO1 can be divided into two primary modules: a peripheral mechanotransduction module and a central ion-conduction pore module [15]. The peripheral mechanotransduction module, comprising multiple subunits, transduces mechanical stimuli into chemical signals through transmembrane structures. Meanwhile, the central ion-conduction pore module forms a functional ion channel, facilitating transmembrane ion flux, particularly that of calcium ions, which are essential for channel activation [17]. The trimeric structure of PIEZO1 features three peripheral blades, each consisting of 14 distinct transmembrane regions, which are critical for its mechanosensing capabilities [18]. These blades enable PIEZO1 to detect external mechanical stimuli via membrane deformation, resulting in ion flux modulation. Furthermore, the C-terminal region of PIEZO1 contains two transmembrane structures, which are thought to form the ion-conduction pore [19]. While the structural details of PIEZO1 have been extensively elucidated in murine models, further studies are required to clarify its configuration in humans. The complexity and uniqueness of PIEZO1 make it a vital model for studying mechanosensitive ion channels, providing essential insights into its role in cellular mechanotransduction.
Significant progress has been made in elucidating the physiological and pathological roles of PIEZO1 in chondrocytes, thereby highlighting its growing importance in cartilage biology and the progression of related diseases. Studies have revealed that PIEZO1 plays critical roles in OA and PTOA [20], as well as in bone fracture healing, skeletal development [21], cartilage regeneration, and other mechanically related diseases [22-27]. However, most current research has focused on PIEZO1 activation under single mechanical stress modalities, with limited exploration of its differential regulation under diverse mechanical stimuli, such as tension, shear, and compression [1,28]. Moreover, the intricate interactions and feedback mechanisms between PIEZO1 and inflammatory factors (e.g., IL-1β) remain poorly understood. For instance, IL-1β upregulates PIEZO1 expression through the NF-κB signaling pathway, while abnormal activation of PIEZO1 has been found to exacerbate inflammatory responses [29]. Furthermore, research on pharmacological and chemical regulation of PIEZO1 remains in its infancy, with only a few activators (e.g., Yoda1) and inhibitors (e.g., GsMTx4) identified, whose specificity and translational potential require further investigation [30-32].
This review aims to systematically summarize the factors influencing PIEZO1 channels in chondrocytes, including mechanical stress, matrix environment, inflammatory factors, chemical substances, and other mechanosensitive channels. Additionally, it explores their regulatory roles and molecular mechanisms in related diseases, thus providing both theoretical support and practical insights for the advancement of PIEZO1 research and the development of therapeutic targets.
2. Mechanical Stress and Piezo1 in Chondrocytes
PIEZO1 is one of the key ion channels in chondrocytes responsible for responding to mechanical stress. Its activation leads to changes in intracellular calcium concentrations, thereby influencing the physiological functions of chondrocytes. PIEZO1 is able to sense and respond to diverse forms of mechanical stimuli, including externally applied poke, stretch, and shear stress, as well as endogenously derived local membrane tension and myosin II-mediated traction forces [12,33]. The role of mechanical stress in maintaining the function of these cells is twofold. Moderate mechanical stimulation activates PIEZO1, contributing to calcium homeostasis, sustaining chondrocyte function, and promoting ECM synthesis. Conversely, abnormal or excessive mechanical stress has been shown to trigger excessive calcium influx, resulting in cellular dysfunction, damage, and apoptosis [1,7,28]. Therefore, the type, intensity, and mode of mechanical stress significantly influence PIEZO1 activation and chondrocyte physiological responses.
2.1 Physiological Mechanical Stress and Piezo1 Activation
Under physiological conditions, chondrocytes are exposed to moderate mechanical stress, such as during natural joint movement and weight-bearing. These stimuli appropriately activate PIEZO1, leading to calcium signaling that maintains cellular homeostasis. Physiological mechanical stress supports the synthesis of ECM proteins, including type II collagen and aggrecan, which are essential for preserving cartilage structure and function [5,6].
Moderate mechanical stress promotes ECM synthesis in chondrocytes via PIEZO1 activation, in addition to regulating various functions of the cells, including proliferation, differentiation, and metabolic activities [34]. It has been demonstrated that appropriate mechanical loading upregulates PIEZO1 expression, thereby enhancing the cell's ability to respond to mechanical signals. Conversely, insufficient mechanical loading significantly reduces PIEZO1 expression in chondrocytes, impairing their capacity to sense and respond to mechanical stimuli. The study further elucidates that the downregulation of PIEZO1, induced by reduced mechanical loading, activates the ApoE signaling pathway, which in turn induces cellular senescence in the cells. The upregulation of senescence markers such as p16 and p21 is a hallmark of these conditions, further exacerbating cellular senescence and functional decline. In animal models, reduced mechanical loading diminishes PIEZO1 expression, disrupts cartilage proliferation and differentiation, impedes callus formation, and hinders endochondral ossification, ultimately delaying fracture healing [35].
The loss of PIEZO1 has been demonstrated lead to functional disorders in chondrocytes. In healthy joints, the mechanosensing function of these cells is characterised by high levels of organisation and regulation, which ensure the production of adaptive cellular responses to varying stress environments [2]. PIEZO1 deficiency has been shown to disrupt calcium signaling pathways, leading to functional abnormalities in these cells, characterised by increased expression of matrix-degrading genes such as matrix metalloproteinases (MMPs). This, in turn, has been demonstrated to exacerbate cellular aging and dysfunction [21]. Studies indicate that moderate mechanical stress not only activates PIEZO1 but also works synergistically with other mechanosensitive ion channels, such as TRPV4, to promote proliferation of the cells responsible for producing the cartilage in the joint [20]. This interplay between multiple ion channels plays a crucial role in physiological adaptation and mitigating OA progression.
2.2 Pathological Stress and Piezo1 Activation
Pathological mechanical stress, such as excessive high-intensity loads or traumatic stresses, leads to overactivation of PIEZO1 channels, resulting in excessive intracellular calcium accumulation and promoting chondrocyte apoptosis [11,36,37]. This process activates multiple intracellular signaling pathways, including calcium/calmodulin-dependent protein kinase II (CaMKII) and mitogen-activated protein kinase (MAPK), which culminate in apoptosis or senescence [38,39]. Overactivation has been observed to upregulate proteins associated with cell cycle arrest, including Kif18A and β-tubulin, which ultimately impedes chondrocyte proliferation [40]. Additionally, excessive mechanical loading has been demonstrated to accelerate the process of chondrocyte senescence via the activation of PIEZO1. In vivo studies using chondrocyte-specific Piezo1 knockout mice (Col2a1-Cre; Piezo1^fl/fl^) demonstrated that excessive mechanical loading activates PIEZO1 in chondrocytes, leading to increased intracellular calcium accumulation, which in turn induces senescence and accelerates cartilage degeneration [41]. In spinal growth plate chondrocytes, PIEZO1 activation triggers calcium overload and apoptosis under compression [42]. Whether this mechanism operates in articular chondrocytes requires further validation. Notably, pathological mechanical stress not only elevates intracellular calcium levels but also induces oxidative stress, further damaging chondrocytes [43]. PIEZO1 activation is a critical trigger in this process [38]. Consequently, the inhibition of aberrant PIEZO1 activation may represent a potential strategy for mitigating OA progression caused by pathological mechanical stress.
2.3 Types and Intensities of Stress on Piezo1
The effects of different types and intensities of mechanical stress on PIEZO1 activation and its biological outcomes are distinct. For instance, stretch stress, compressive stress, and shear stress all activate PIEZO1, but the mechanisms and effects of these different stresses vary [44,45].
Stretch stress activates PIEZO1 by inducing membrane deformation, promoting calcium influx, and regulating ECM metabolism and cytoskeletal remodeling. It is particularly critical for type II collagen synthesis, enhancing cartilage resilience and function [11]. However, excessive stretch stress, such as high-frequency cyclic stretching, significantly increases intracellular calcium levels, exacerbates ECM degradation through F-actin polymerization, and elevates reactive oxygen species (ROS), leading to MMP-13 expression and subsequent ECM breakdown and apoptosis [46].
Chondrocytes experience compressive stress during weight-bearing activities. Moderate compressive stress activates PIEZO1, promoting calcium influx and enhancing ECM synthesis (e.g., type II collagen and aggrecan) whilst suppressing MMP expression, thereby maintaining homeostasis within the cell [47]. However, it has been demonstrated that sustained or excessive compressive stress can lead to overactivation of PIEZO1, resulting in excessive calcium influx, cellular senescence, apoptosis, and matrix degradation, disrupting adaptive responses and contributing to cartilage degeneration and OA progression [13,32,48,49].
Excessive shear stress has been demonstrated to potentially compromise the functionality of chondrocytes, particularly under pathological conditions such as arthritis. Research indicates that mechanical stress conditions (e.g., pressure, duration) significantly influence PIEZO1 activation. For example, Li et al. (2017) reported that mechanical loading at 150 kPa promoted survival of cartilage cells, while loading at 200 kPa significantly increased cell death and cartilage degeneration [50]. Similarly, Chen et al. (2007) observed that chondrocyte proliferation remained unaffected by a 90 kPa load applied for 1 hour had no effect on cartilage cell proliferation, but that a 6-hour stimulus significantly reduced proliferation [51]. In addition, studies on PIEZO1 activation thresholds provide insights into its biophysical responses. Savadipour et al. found that PIEZO1 responds to membrane strain only when membrane tension reaches a specific threshold (apparent strain ~1.31), triggering calcium signaling [47] These findings reveal that the biophysical response of chondrocytes to mechanical stress exhibits a strong threshold dependence, suggesting that the impact of mechanical stress of varying intensities on PIEZO1 activation may demonstrate pronounced nonlinear characteristics.
The dual role of PIEZO1 activation in chondrocytes under physiological and pathological mechanical stress. Under physiological conditions (green box), moderate mechanical stress activates PIEZO1, leading to calcium influx, which supports cellular functions such as ECM synthesis (e.g., type II collagen and aggrecan) and chondrocyte proliferation and differentiation. However, under pathological conditions (red box), excessive mechanical stress over activates PIEZO1, resulting in excessive calcium influx, apoptosis, and cellular senescence. This process involves activation of key intracellular signaling pathways, including CaMKII and MAPK, which exacerbate cartilage degeneration.
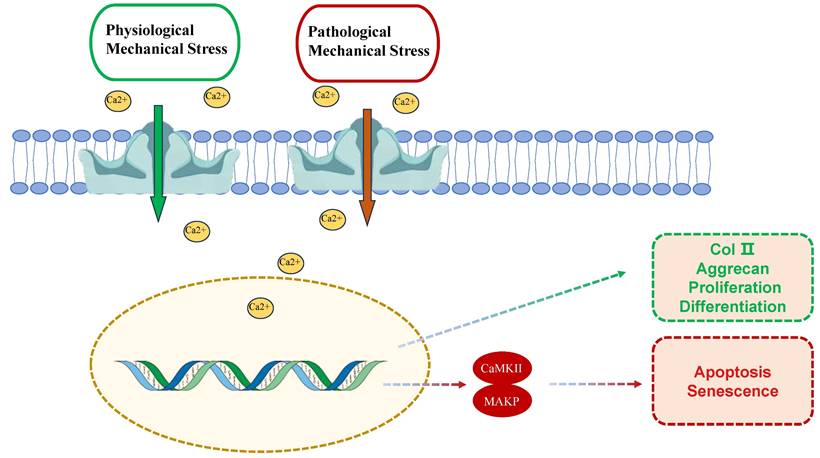
PIEZO1's mechanosensitivity stems from its unique trimeric propeller-like structure, which directly senses membrane tension changes. Structural studies reveal that mechanical forces induce conformational rearrangements in PIEZO1's peripheral blades, leading to pore dilation and Ca²⁺ influx [18]. Physiological mechanical stress (e.g., 10-15% strain) promotes transient Ca²⁺ oscillations that activate Ca²⁺/calmodulin-dependent kinase II (CaMKII), enhancing ECM synthesis via SOX9 and COL2A1 upregulation [47,48]. Conversely, pathological overload (>20% strain) triggers sustained Ca²⁺ overload, activating MAPK/p38 and NF-κB pathways to drive MMP-13 expression and apoptosis [13,38]. Notably, shear stress amplifies PIEZO1-TRPV4 crosstalk via cytoskeletal remodeling, exacerbating IL-6/IL-8 release through NLRP3 inflammasome activation [34,48] These findings highlight the strain threshold-dependent dual roles of PIEZO1 in cartilage homeostasis and degeneration.
Despite the considerable progress that has been made in comprehending the function of PIEZO1 in chondrocytes, the majority of studies have been confined to examining a single type of mechanical stress (e.g., stretch or compression). Comparative analyses of multiple mechanical stimuli, such as shear and cyclic stress, remain scarce. Furthermore, while physiological and pathological mechanical stresses differentially regulate PIEZO1 activation, precise modulation of stress types, intensities, and activation levels remains a key challenge. Consequently, future research should emphasize the dynamic spatiotemporal regulation of PIEZO1 under diverse stress conditions to enable precise control of its activity and avoid cell damage due to overactivation.
3. Matrix Environment and Piezo1 Regulation
Chondrocytes adjust their metabolism, differentiation, and phenotype maintenance by sensing ECM stiffness. Research indicates that ECM stiffness influences PIEZO1 activation, modulating intracellular calcium signaling and regulating the physiological and pathological responses of chondrocytes [52].
3.1 Matrix Stiffness and Piezo1 Activation
ECM stiffness is a crucial physical factor affecting chondrocyte function and mechanical adaptability. In low-stiffness ECM environments, the phenotype of these cells is maintained at a relatively stable level, as evidenced by the high expression levels of type II collagen and aggrecan, which are essential for the normal structure and function of cartilage [53]. Conversely, in conditions of high ECM stiffness, PIEZO1 activation is repressed, thereby supporting homeostasis and physiological functions in these cells. In contrast, high ECM stiffness has been shown to suppress phenotype maintenance in these cells, promote cytoskeletal remodeling, and enhance focal adhesion formation, thus resulting in heightened PIEZO1 activation. This, in turn, causes significant increases in intracellular calcium concentration [37]. Calcium, a key secondary messenger, regulates vital biological processes such as metabolism, differentiation, and autophagy. However, excessive calcium signaling in high-stiffness ECM environments may drive ECM degradation and induce disc chondrocyte senescence and apoptosis through oxidative and endoplasmic reticulum stress pathways [54].
The present study sought to investigate the impact of ECM stiffness on PIEZO1 activation and calcium signaling. While low ECM stiffness permits PIEZO1 to primarily mediate calcium signaling for maintaining normal chondrocyte functions and phenotypes, high ECM stiffness amplifies calcium oscillations, intensifying chondrocyte responses to mechanical stress [55]. It has been demonstrated that high ECM stiffness not only increases calcium influx but also exacerbates oxidative stress and endoplasmic reticulum stress, accelerating cellular senescence and apoptosis. Conversely, softer ECM environments have been shown to inhibit PIEZO1 activation, thereby maintaining intracellular calcium homeostasis, reducing ECM degradation, and preventing cartilage damage and aging [54].
However, the extent to which ECM stiffness affects PIEZO1 localization and function in chondrocytes remains to be elucidated, beyond the regulation of PIEZO1 activity. As with other cell types, changes in ECM properties may influence PIEZO1 redistribution on the chondrocyte membrane, though this remains to be confirmed. For instance, studies suggest that cytoskeletal remodeling and increased focal adhesion formation in response to high ECM stiffness may drive PIEZO1 accumulation on the cell surface, enhancing its activation levels.
3.2 Primary Cilia and PIEZO1 in chondrocytes
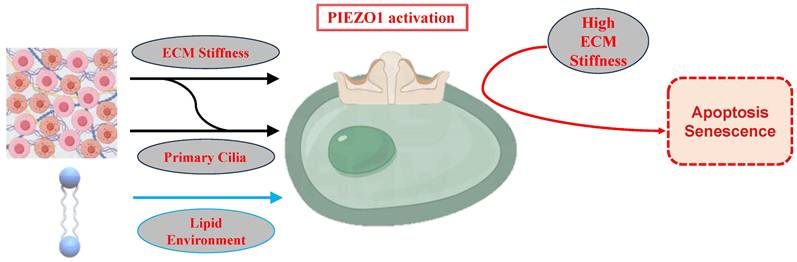
Primary cilia, organelles located on the surface of chondrocytes, have been shown to play a vital role in sensing mechanical signals and modulating cellular responses [56]. It has been demonstrated that primary cilia are capable of regulating PIEZO1 sensitivity, thereby altering intracellular calcium levels [57]. Furthermore, the ECM stiffness has been observed to modulate PIEZO1 activation, and also to influence primary cilia morphology by altering the activity of histone deacetylase 6 (HDAC6) [58]. The loss of primary cilia has been associated with significant changes in cartilage structure and mechanical properties [59], as well as abnormalities in weight-bearing cartilage. This emphasises the pivotal interplay between ECM stiffness, PIEZO1 channels, and primary cilia in chondrocyte adaptive responses to varying mechanical environments. Primary cilia act as a bridge between ECM stiffness and PIEZO1 activity, further modulating cellular responses to changes in the mechanical environment. These findings reveal a complex interaction between ECM stiffness, PIEZO1, and primary cilia, contributing to the molecular mechanisms underlying chondrocyte adaptation to ECM hardness.
In environments where the ECM has low stiffness, homeostasis of the chondrocyte is supported by the inhibition of excessive PIEZO1 activation, with a resultant reduction of ECM degradation and cellular damage. Conversely, elevated ECM stiffness has been demonstrated to result in overactivation of PIEZO1, enhanced calcium signaling, and accelerated cellular senescence and apoptosis, which may contribute to the progression of degenerative cartilage diseases. While current studies have elucidated the relationship between ECM stiffness and PIEZO1, further research is required to determine PIEZO1 activation thresholds under varying stiffness levels and its cooperative interaction with other ion channels, such as TRPV4. For example, evidence suggests that PIEZO1 dominates calcium signaling in low-stiffness ECM, whereas TRPV4 plays a more significant role in high-stiffness ECM [37] Additionally, ECM stiffness appears to regulate PIEZO1 localization and cytoskeletal interactions, offering novel molecular mechanisms for how chondrocytes sense and respond to ECM hardness. Further research is needed to clarify the spatiotemporal characteristics of PIEZO1 activation, its interplay with the cytoskeleton and primary cilia, and its precise regulatory mechanisms under different mechanical environments.
3.3 Lipid Regulation of PIEZO1 Channels
The lipid bilayer actively modulates PIEZO1 activity through composition and mechanical properties. Cholesterol-rich lipid rafts enhance PIEZO1 clustering and stabilize its open state under mechanical stress, while cholesterol depletion reduces channel activation [60,61]. Phosphatidylserine (PS) externalization in stressed membranes amplifies PIEZO1 sensitivity, particularly in inflammatory cartilage, exacerbating calcium overload and ECM degradation [62]. Additionally, phosphatidylinositol 4,5-bisphosphate (PIP2) binds PIEZO1's C-terminal domain, facilitating channel clustering; PIP2 depletion disrupts mechanotransduction even under high load [19]. These lipid-protein interactions highlight the membrane's dynamic role in PIEZO1 regulation [63,64].
In osteoarthritic cartilage, altered lipid metabolism—elevated cholesterol oxides and ceramides—disrupts membrane fluidity, driving aberrant PIEZO1 activation and calcium dysregulation [65,66]. This lipid imbalance correlates with cartilage degradation and oxidative stress, suggesting therapeutic targeting of lipid-PIEZO1 interactions [54]. Strategies such as cholesterol modulation or PIP2 stabilization may mitigate OA progression by restoring mechanotransduction homeostasis.
High ECM stiffness (> 20 kPa) enhances integrin-β1 clustering and RhoA/ROCK-mediated actomyosin contractility, increasing membrane tension to sensitize PIEZO1 [37,67]. This primes chondrocytes for exaggerated Ca²⁺ responses to mechanical stimuli, accelerating senescence via oxidative stress [43]. Conversely, soft ECM (< 5 kPa) suppresses PIEZO1 by reducing focal adhesion kinase (FAK) signaling, favoring YAP/TAZ nuclear retention to maintain chondrogenic phenotypes [53]. Primary cilia act as mechanosensory hubs: HDAC6 deacetylates α-tubulin under high stiffness, shortening cilia length and disrupting PIEZO1-TRPV4 colocalization, thereby dysregulating Ca²⁺ homeostasis [20,58]. This ECM-cytoskeleton-cilia axis critically determines PIEZO1's mechanoadaptive responses.
4. Inflammatory Factors and Piezo1 Regulation
Chondrocytes have been shown to express functional interleukin-1 (IL-1) receptors and respond to both isoforms of IL-1 (α and β) [68]. These cells are able to sense and react to inflammatory factors, particularly interleukin-1 (IL-1) and tumour necrosis factor-alpha (TNF-α). The secretion of matrix-degrading enzymes by these cells has been shown to be induced by inflammatory factors, including IL-1β, IL-1α, and TNF-α, thereby exacerbating cartilage degeneration. This process is further facilitated by the modulation of PIEZO1 channel expression and function. Recent studies reveal that mechanical stress-induced PIEZO1 activation triggers mitochondrial Ca²⁺ overload and mitochondrial DNA (mtDNA) release, which activates the cGAS-STING pathway, amplifying inflammatory responses through type I interferon signaling [69]. This process plays a crucial role in the progression of diseases like OA.
Regulation of PIEZO1 Activation by ECM Stiffness, Primary Cilia, and Lipid Environment. Low ECM stiffness inhibits PIEZO1, preserving chondrocyte phenotype and calcium homeostasis. High ECM stiffness activates PIEZO1, enhancing calcium influx and stress responses, contributing to senescence and apoptosis. Primary cilia modulate PIEZO1 sensitivity, while the lipid bilayer influences PIEZO1 activity.
4.1 Inflammatory Modulation of Piezo1 Expression
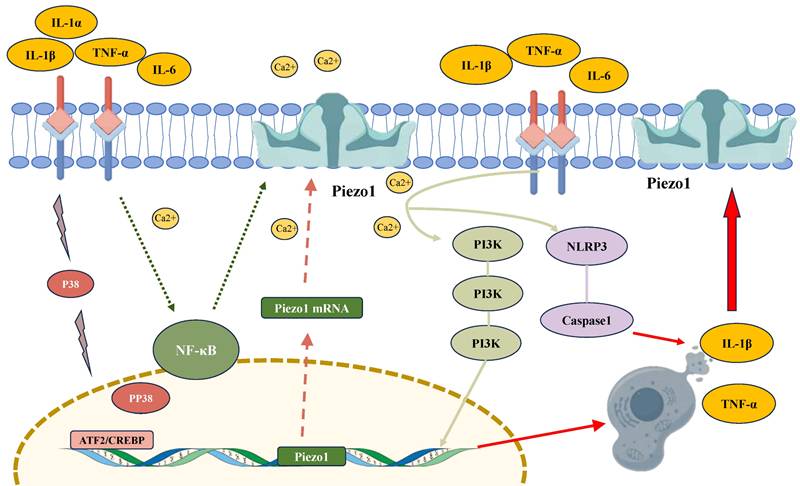
IL-1β, a primary inflammatory mediator, has been demonstrated to upregulate PIEZO1 expression in chondrocytes [29]. Furthermore, PIEZO1 activation has been shown to be positively correlated with the release of inflammatory cytokines such as IL-1β in the nucleus [70]. The influence of IL-1β has been found to result in a significant increase in both the mRNA and protein levels of PIEZO1. This upregulation may occur through the activation of the nuclear factor-kappa B (NF-κB) signaling pathway, as inhibiting the Ca²⁺/NF-κB pathway substantially reduces PIEZO1-dependent inflammatory responses [70]. Mechanistically, mechanical stress amplifies this regulation by inducing Piezo1-mediated Ca²⁺ influx, which activates NF-κB and synergizes with IL-1β to promote mtDNA release and cGAS-STING signaling [69].
IL-1β-induced PIEZO1 activation partially suppresses autophagy in chondrocytes while promoting apoptosis. This is evidenced by increased expression of apoptosis-related proteins such as cleaved caspase-3 and Bax, and decreased levels of autophagy-related proteins like Bcl2, LC3, and p62. Inhibition of the PI3K/AKT/mTOR pathway has been shown to mitigate the suppression of autophagy and activation of apoptosis caused by PIEZO1, suggesting it as a potential therapeutic target for PIEZO1 regulation [29]. Furthermore, IL-1α has been observed to enhance PIEZO1 expression through pathways involving p38 MAP kinase, hepatocyte nuclear factor 4 (HNF4), and ATF2/CREBP1. CREBP1 binds directly to the proximal promoter of the PIEZO1 gene, promoting protein synthesis and enhancing cellular sensitivity to mechanical stress [48]. The structural and functional similarities between IL-1β and IL-1α further support IL-1β's regulatory role in the activity of PIEZO1 in chondrocyte. PIEZO1 activation correlated closely with the expression of apoptosis-related proteins such as Bax and caspase-3, suggesting that the inhibition of PIEZO1 expression may effectively alleviate IL-1β-induced damage to chondrocytes [29].
4.2 Feedback Between Inflammation and Piezo1 Activation
Inflammatory factors have been demonstrated to exacerbate damage to chondrocytes through the activation of PIEZO1 channels. Furthermore, these factors have been shown to form positive feedback loops that worsen the progression of OA. For instance, the PIEZO1 agonist Yoda1 significantly increases the expression of inflammatory cytokines such as IL-6 and IL-8, while PIEZO1 knockdown partially reverses this effect [1] Notably, mechanical stress-induced PIEZO1 activation promotes mitochondrial permeability transition pore (mPTP) opening via Ca²⁺ overload, leading to mtDNA leakage into the cytoplasm. Cytosolic mtDNA activates the cGAS-STING pathway, which drives the production of pro-inflammatory cytokines (e.g., IL-6, TNF-α, IFN-β) and exacerbates cartilage degradation [69]. This pathway synergizes with NLRP3 inflammasome activation, as PIEZO1 activation also promotes caspase-1-dependent IL-1β maturation [30], creating a dual inflammatory cascade. The activation of PIEZO1 has been shown to be closely associated with the inflammatory response, with IL-1β, IL-6, and IL-8 levels having been found to be positively correlated with its activity. Moreover, PIEZO1 activation has been shown to promote the assembly of the NLRP3 inflammasome, significantly upregulate caspase-1 activity, and facilitate the maturation of IL-1β [30]. This process is further amplified by cGAS-STING activation, which induces TBK1/IRF3 phosphorylation and type I interferon production, establishing a feedforward loop between mechanical stress and inflammation [69].
Additionally, PIEZO1 activity can be indirectly regulated by inflammatory factors. For instance, elevated joint osmotic pressure has been observed to activate PIEZO1 in chondrocytes, inducing calcium overload, endoplasmic reticulum, and mitochondrial stress, and leading to apoptosis. Chondrocyte damage has the effect of triggering interstitial fluid leakage and inflammatory infiltration (e.g., TNF-α, prostaglandin E2), which in turn damage newly formed cartilage tissue. This exudate increases intra-articular pressure, which in turn activates PIEZO1, thus creating a cycle of injury [71]. The interplay between inflammatory factors and mechanical stress exacerbates cartilage degeneration and OA progression, thus providing new insights for therapeutic strategies.
While the role of PIEZO1 activation in inflammatory cartilage injury has been preliminarily elucidated, the specific mechanisms by which different inflammatory factors (e.g., TNF-α, IL-1β, IL-6) regulate PIEZO1 remain incompletely understood, particularly in various physiological and pathological contexts. Moreover, the interaction between inflammatory factors and mechanical stress in modulating PIEZO1 activity and its effects on apoptosis and matrix degradation warrants further investigation. A more complete understanding of the precise role of PIEZO1 in inflammatory joint diseases, such as OA, and the refinement of its regulation, could offer novel therapeutic approaches for clinical applications.
4.3 Inflammatory Signaling Converges on PIEZO1 via Transcriptional and Post-Translational Regulation
IL-1β binds IL-1R to activate NF-κB, which directly binds the PIEZO1 promoter to upregulate its expression [29]. Concurrently, TNF-α induces mitochondrial ROS, oxidizing PIEZO1's cysteine residues (Cys1563/Cys1707) to enhance channel open probability [48]. This ROS-NF-κB-PIEZO1 feedforward loop sustains inflammatory ECM degradation. Furthermore, IL-1β suppresses autophagy via PI3K/AKT/mTOR, impairing PIEZO1 turnover and prolonging Ca²⁺ signaling [29]. Pharmacologically, GsMTx4 disrupts this cycle by inhibiting PIEZO1/calcineurin/NFAT1 signaling, restoring autophagy flux and reducing apoptosis [32].
The regulatory role of inflammatory factors in modulating PIEZO1 activity in chondrocytes. Inflammatory cytokines, such as IL-1β and TNF-α, activate PIEZO1 channels, leading to increased intracellular calcium (Ca²⁺) influx. This activation upregulates PIEZO1 expression via signaling pathways involving NF-κB, PI3K, and ATF2/CREBP1. The enhanced PIEZO1 activity promotes inflammation by facilitating the release of pro-inflammatory cytokines (IL-1β, TNF-α), forming a feedback loop that exacerbates cellular damage. Furthermore, PIEZO1 activation triggers the NLRP3 inflammasome and caspase-1, contributing to the maturation of IL-1β and further promoting chondrocyte apoptosis.
5. Chemical and Pharmacological Modulation of Piezo1
The recent discovery of PIEZO1 channels has prompted a surge in research interest, driven by their critical roles across multiple systems and tissues. Consequently, there has been a proliferation of studies investigating the development of PIEZO1 activators and inhibitors. These substances have emerged as valuable tools for modulating PIEZO1 activity and exploring their therapeutic potential.
5.1 Activators of Piezo1
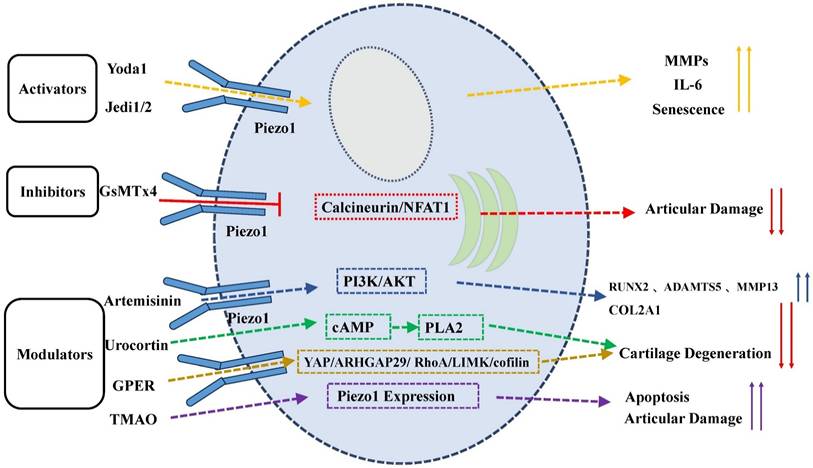
Yoda1, first identified in 2015 by the Coste team, is one of the most well-known PIEZO1 activators in heterologous systems [30]. It has been demonstrated that Yoda1 can promote conformational changes in PIEZO1, thereby stabilizing its open state and lowering the mechanical threshold required for channel activation [72]. Acting as a molecular wedge, Yoda1 enhances calcium influx by altering PIEZO1's configuration. In addition, studies have shown that Yoda1 treatment significantly reduces the proliferation and colony formation capabilities of primary chondrocytes while increasing cellular senescence. This effect is attributed to the acceleration of intracellular calcium accumulation, which in turn promotes the process of cellular senescence [73]. Furthermore, Yoda1 has been observed to upregulate the expression of MMPs and inflammatory cytokines such as IL-6 and IL-8, exacerbating ECM degradation [1,34]. In another study, Yoda1 was observed to reduce the expression of Col10a1 in ATDC5 cells, thus implicating its role in ECM disruption [24]. Despite these effects, Yoda1 has shown potential in promoting chondrocyte differentiation and improving fracture healing under conditions of mechanical unloading, thus highlighting its dual roles [35]. Its ability to activate PIEZO1 without the need for external mechanical stimuli makes it a valuable tool for exploring PIEZO1 regulation.
Jedi1/2, a class of PIEZO1 activators identified in 2018 by Xiao and He, represents a novel category of pharmacological agents. In contrast to Yoda1, Jedi1/2 exerts its effect by targeting PIEZO1's peripheral blades, thereby modulating the activity of its lever-like structures composed of blades and beams [74]. In a manner analogous to Yoda1, Jedi1/2 has the capacity to activate PIEZO1 in the absence of mechanical stimuli, and further derivatives, such as Yoda2, have been synthesised to enhance PIEZO1 efficacy [75]. However, the application of Jedi1/2 and Yoda2 in chondrocytes has not yet been explored, providing future directions for research on novel PIEZO1 activators.
5.2 Inhibitors of Piezo1
While PIEZO1 channels have been shown to play critical roles in various physiological and pathological processes. However, overactivation of these channels has been implicated in diseases such as cartilage degeneration and OA. Consequently, PIEZO1 inhibitors have gained attention as a means of regulating channel activity. Existing PIEZO1 inhibitors, including GsMTx4, gadolinium ions (Gd³⁺), ruthenium red, and drugs like benzbromarone, are primarily nonspecific and act through diverse mechanisms [31,76].
GsMTx4, a 34-amino acid peptide derived from tarantula venom, is one of the most commonly used PIEZO1 inhibitors. It has been demonstrated that GsMTx4 exerts its inhibitory effect by reducing PIEZO1 activation, rather than by direct binding to the channel itself [31]. By penetrating deeper into the lipid bilayer under increased membrane tension, GsMTx4 decreases lateral pressure on the membrane, ultimately inhibiting PIEZO1 activity [77]. Experimental evidence has demonstrated that GsMTx4 significantly attenuates calcium influx induced by mechanical stress, thereby mitigating the responses of articular cells to damaging strains [78]. In cartilage explants, GsMTx4 pre-treatment has been shown to reduce mechanical stress-induced damage [36]. In vivo, GsMTx4 has been observed to alleviate cartilage degeneration and pain by inhibiting the PIEZO1/Calcineurin/NFAT1 signaling axis, reducing calcium influx, and preserving proteoglycan integrity [7,32,42,79]. These findings position GsMTx4 as a promising tool for treating degenerative diseases like OA, though its nonspecific action may limit its therapeutic precision.
As nonspecific cation channel blockers, Gd³⁺ and ruthenium red have been shown to effectively inhibit PIEZO1 activity by disrupting membrane mechanosensing capabilities [12,80,81] These inhibitors reduce calcium influx by impairing PIEZO1 activation. However, their lack of specificity limits their clinical utility, as they may interfere with other ion channels. A similar inhibitory effect has been demonstrated for Streptomycin, a PIEZO1 open conformation inhibitor, but this approach faces similar challenges [31,82].
Although GsMTx4 and other PIEZO1 inhibitors show potential in mitigating mechanical stress-induced damage to chondrocytes, inflammation, and ECM degradation, their nonspecific effects on other mechanosensitive ion channels may impact therapeutic outcomes. It is therefore recommended that future research prioritize the development of more specific PIEZO1 inhibitors, with a view to minimising off-target effects. Furthermore, there is a necessity for additional studies to be conducted on the safety and efficacy of PIEZO1 inhibitors in clinical applications.
The employment of PIEZO1 activators and inhibitors has resulted in the development of novel molecular instruments, facilitating the exploration of this channel's functions in both physiological and pathological contexts. Activators such as Yoda1 and Jedi1/2 facilitate the study of PIEZO1's activation mechanisms, while inhibitors such as GsMTx4 and Dooku1 hold therapeutic promise for mitigating conditions associated with PIEZO1 overactivation. However, further investigation is required into the specificity, safety, and clinical utility of these substances, particularly in the context of cartilage degeneration and OA treatment.
5.3 Natural Compounds and Piezo1 Regulation
In recent years, natural compounds and TCM monomers have gained attention for their potential in regulating PIEZO1 channels. Compounds such as artemisinin and urocortin have demonstrated efficacy in modulating PIEZO1 activation, cartilage cell function, and associated diseases, thus providing a novel therapeutic approach for degenerative conditions such as OA.
Artemisinin, a naturally occurring compound derived from Artemisia annua, has been identified as a modulator of PIEZO1 activity. In chondrocytes, Artemisinin was found to inhibit mechanical stress-induced PIEZO1 activation, thereby reducing intracellular calcium accumulation, and alleviating cellular stress responses and ECM degradation [83]. Its mechanism of action involves suppressing PIEZO1 activity to protect against damage related to OA. Studies have shown that artemisinin reduces calcium influx triggered by PIEZO1 activation and modulates downstream PI3K/AKT signaling pathways, improving cartilage erosion, proteoglycan loss, synovial hyperplasia, osteophyte formation, and pain in OA mouse models. Additionally, artemisinin treatment has been shown to reduce the expression of RUNX2, ADAMTS5, and MMP13 while increasing COL2A1 expression, indicating a protective effect against PIEZO1-mediated cartilage damage [83]. Collectively, these findings suggest artemisinin's potential in controlling excessive PIEZO1 activation.
Urocortin exerts an indirect inhibitory effect on PIEZO1 activity by binding to corticotropin-releasing factor receptor 1 (CRF-R1). This interaction activates adenylyl cyclase, increasing cyclic AMP (cAMP) production, and inactivates phospholipase A2 (PLA2), thereby reducing membrane phospholipid degradation and maintaining PIEZO1 in a closed state [84]. Research on cartilage explants subjected to excessive impact loading has shown that urocortin effectively blocks PIEZO1 activation, significantly reducing chondrocyte damage and cartilage degeneration [85]. The ability of urocortin to regulate membrane phospholipid metabolism provides a valuable foundation for the development of new cartilage-protective agents.
TMAO, a gut microbial metabolite derived from diets rich in quaternary amines and meat, has been linked to the progression of OA [86]. Higher concentrations of TMAO have been observed to correlate with increased synovial inflammation scores in patients with OA [87]. Zhuang et al. have demonstrated that TMAO significantly upregulates PIEZO1 mRNA and protein expression in vitro and in vivo, increasing intracellular calcium levels and sensitizing chondrocytes to mechanical loads [88]. This heightened sensitivity exacerbates mechanical strain-induced chondrocyte apoptosis and OA-related joint damage. While TMAO has been shown to enhance PIEZO1 expression, it does not appear to directly activate the channel. Consequently, dietary modifications aimed at reducing TMAO production may offer a potential strategy for mitigating PIEZO1 overactivation.
The G-protein-coupled estrogen receptor (GPER) has been demonstrated to play a key role in regulating PIEZO1 function in chondrocytes. GPER activation has been shown to promote the translocation of the transcription factor Yes-associated protein (YAP) from the cytoplasm to the nucleus, thereby modulating apoptosis through the interaction with the Hippo signaling pathway, involving the proteins Yes-associated protein 29 (YAP), Rho-associated protein kinase (ROCK), LIMK and cofilin. In mechanical stress-induced PIEZO1 activation, GPER has been identified as a crucial regulator. Its expression has been shown to be significantly reduced in human OA cartilage, and intra-articular injection of a GPER-selective agonist has been observed to alleviate cartilage degeneration in OA animal models [38]. The protective effects of GPER may be particularly relevant in female OA patients due to its association with oestrogen levels [89]. Furthermore, the absence of PIEZO1 has been demonstrated to enhance GPER expression at both the mRNA and protein levels, thereby underscoring their interplay in response to mechanical loads.
Yoda1 binds PIEZO1's C-terminal extracellular cap, stabilizing the open state by reducing the mechanical activation threshold [72]. Jedi1/2, in contrast, targets the peripheral blades to allosterically gate the channel [74]. Conversely, GsMTx4 embeds into lipid bilayers under high membrane tension, counteracting lateral pressure to mechanically “clamp” PIEZO1 [77]. Natural compounds like artemisinin block PIEZO1 via direct pore occlusion, while urocortin indirectly inhibits it via CRF-R1/cAMP/PLA2 signaling to reduce membrane phospholipid degradation [83,84]. These structure-activity relationships underscore the potential for designing domain-specific therapeutics.
Natural compounds like artemisinin, urocortin, TMAO, and GPER significantly regulate PIEZO1 channels, especially in cartilage degeneration and OA. Artemisinin inhibits calcium influx, reducing cellular stress and ECM degradation; urocortin stabilizes membrane tension via CRF-R1, keeping PIEZO1 closed; TMAO upregulates PIEZO1 expression, sensitizing chondrocytes to mechanical stress and promoting cartilage degradation. However, their low bioavailability and metabolic instability, particularly for artemisinin, limit clinical applications. Given the complex regulatory mechanisms of PIEZO1, combining these compounds with established therapies like anti-inflammatory medications may offer more effective OA treatment by addressing multiple aspects of PIEZO1 modulation. Future research should explore these interactions to enhance therapeutic outcomes in OA and other PIEZO1-related conditions.
Drugs that can regulate piezo1 in chondrocytes.
| Category | Drug | Property | Selectivity | Experimental Model | Experiment | Mechanism of Action | References |
|---|---|---|---|---|---|---|---|
| Activator | Yoda1 | Compound | Specific | Human chondrocytes | In vitro | Lowers the mechanical threshold for channel activation; increases the expression of pro-inflammatory cytokines IL-6 and IL-8, MMP1, MMP3, and MMP13, while decreasing BMP2 expression in healthy and OA cartilage cells. | Syeda et al., 2015 [30] |
| Rat chondrocytes | In vitro | Ca²⁺ accumulation decreases chondrocyte proliferation, induces cellular senescence, increases markers p16 and p21, and raises SASP markers IL-6 and MMP3. | Ren et al., 2022 [72] | ||||
| Mice | In vivo | Increases intracellular calcium influx, influences PI3K/AKT phosphorylation; intra-articular injection worsens OA pathology (cartilage erosion, proteoglycan loss, osteophyte growth, and pain); OA-related genes RUNX2, MMP13 are upregulated, COL2A1 is downregulated. | Gan et al., 2024 [83] | ||||
| Human OA chondrocytes | In vitro | ||||||
| ATDC5 cells | In vitro | Yoda1 induces a twofold increase in Sox9 and enhances the expression of Ccn2/Ctgf genes. | Brylka et al., 2024 [21] | ||||
| Inhibitor | GsMTx4 | Polypeptide toxin | Non-specific | Rat and chondrocytes | In vivo and in vitro | Intra-articular injection improves OA progression by reducing MMP3 and MMP13 expression and increasing COL2 and Aggrecan expression; blocks Piezo1/CaN/NFAT1 signaling to inhibit apoptosis and promote cartilage matrix production. | Ren et al., 2023 [32] |
| Mice | In vivo | Intra-articular injection increases Gpx4 expression, alleviates ferroptosis, and reduces OA severity in surgery-induced models. | Wang et al., 2022 [79] | ||||
| Pig joint organ culture | In vitro | Preventive use reduces mechanically induced damage regions. | Lee et al., 2014 [78] | ||||
| Pig chondrocytes | In vitro | GsMTx4 prevents inflammation-induced F-actin disassembly or relaxation, maintaining membrane elasticity under IL-1α. | Lee et al., 2017 [36] | ||||
| Modulators | Urocortin-1 | Neuropeptide | Non-specific | Human chondrocytes | In vitro | Binds to CRF-R1, activates adenylyl cyclase to generate cAMP, leading to PLA2 inactivation, reducing membrane phospholipid degradation, and keeping Piezo1 closed. Stabilizes membrane phospholipids | Lawrence et al., 2017 [84]; Jones et al., 2022 [85]; |
| Pig joint organ culture | In vitro | ||||||
| GPER | Membrane receptor protein | Non-specific | Rat chondrocytes | In vivo and in vitro | Leads to actin depolymerization via YAP/ARHGAP29/RhoA/LIMK/cofilin pathway; blocks Piezo1 activation by mechanical stress, improving chondrocyte survival. | Sun Y et al., 2021 [38] | |
| TMAO | Organic compound | Non-specific | Rats | In vivo and in vitro | Intra-articular injection and in vitro studies show TMAO increases Piezo1 expression, raises baseline Ca²⁺ levels, reduces F-actin, and increases apoptosis under mechanical strain. | Zhuang et al., 2023 [88] | |
| Artemisinin | Traditional medicine extract | Non-specific | Mice | In vivo | Inhibits calcium influx induced by Piezo1 activation, modulates PI3K/AKT phosphorylation, reverses OA-related gene expression, and mitigates Piezo1 activation-induced OA damage. | Gan et al., 2024 [83] |
Modulation of PIEZO1 Activity by Activators, Inhibitors, and Modulators. Activators like Yoda1 and Jedi1/2 enhance PIEZO1 activity, promoting calcium influx, senescence, ECM degradation, and inflammation. Inhibitors like GsMTx4 prevent activation, preserving cartilage integrity. Natural compounds and modulators (e.g., artemisinin, urocortin, TMAO, GPER) regulate PIEZO1, affecting cartilage degeneration and apoptosis.
6. Interactions Between Piezo1 and Other Mechanosensitive Channels
6.1 TRPV4 and Piezo1 Interaction
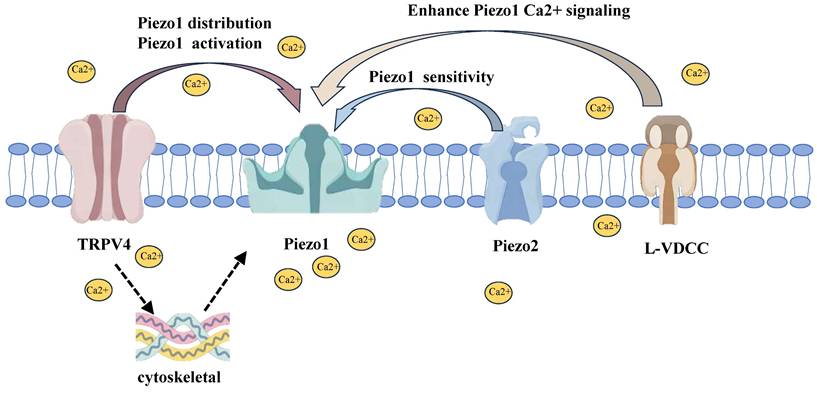
TRPV4 channel is another key mechanosensitive ion channel in chondrocytes that responds to extracellular mechanical stimuli by promoting calcium influx [90]. TRPV4 and PIEZO1 channels exhibit synergistic activity in regulating mechanical signal transduction, mediating compression-induced membrane strain, and resulting in intracellular calcium oscillations [20,91]. Furthermore, they cooperate in responding to harmful mechanical loads, contributing to the mechanotransduction in chondrocytes [78,90].
However, TRPV4 and PIEZO1 differ in their mechanogating mechanisms. Unlike PIEZO1, TRPV4 cannot be efficiently gated by pressure-induced membrane stretching and is not activated by cell indentation [20]. While TRPV4 inhibition has shown protective effects against aging-related OA, it does not prevent trauma-induced OA, underscoring its specific role in mechanotransduction [92]. TRPV4 is known to respond primarily to low-intensity mechanical stress, initiating calcium signalling during physiological strains of between 3% and 8%. In contrast, PIEZO1 exhibits higher sensitivity to high-intensity strains, such as those caused by traumatic mechanical stress of up to 18% [78]. This divergence in response thresholds gives rise to a complementary signaling network between TRPV4 and PIEZO1 [20]. Furthermore, Studies have revealed that physiological mechanical loading transduced by TRPV4 and supraphysiological loading transduced by PIEZO1 elicit distinct transcriptomic responses [93]. TRPV4 modulates cytoskeletal tension, indirectly affecting PIEZO1 activation and mechanical adaptability [20].
The interaction between TRPV4 and PIEZO1 extends to their regulation by exogenous stimuli, with both channels being targeted by their respective activators (e.g., Yoda1 for PIEZO1 and GSK1016790A for TRPV4). The effects of the first agonist applied are suppressed by the second, highlighting a regulatory interplay [34]. In addition, the dual silencing of TRPV4 and PIEZO1 genes has been shown to enhance the expression of critical ECM components like aggrecan and type II collagen, suggesting that their combined activity plays an essential role in cartilage repair [94].
A central question in understanding TRPV4-PIEZO1 synergy is whether TRPV4 directly modulates PIEZO1 activity through interactions on the cell membrane. Evidence suggests that TRPV4 activation can alter the open state of PIEZO1 channels, thereby modifying calcium signaling characteristics within chondrocytes. This interplay has significant implications for how these cells adapt to complex mechanical environments and provides a foundation for developing therapies that simultaneously target TRPV4 and PIEZO1 to optimise mechanotransduction and cartilage protection. The coordinated activity of TRPV4 and PIEZO1 channels plays a vital role in the mechanotransduction process within chondrocytes. While TRPV4 responds to low-intensity stress and PIEZO1 to high-intensity stress, their interactions create a dynamic signaling network that is essential for adapting to varying mechanical environments. Further research into the mutual modulation mechanisms between these channels could pave the way for innovative therapeutic strategies targeting both TRPV4 and PIEZO1, especially in the treatment of OA and other cartilage-related diseases.
6.2 Piezo2 Synergy with Piezo1
PIEZO1 and PIEZO2 are two critical mechanosensitive ion channels expressed across various tissues and cells, each with distinct physiological and pathological roles. PIEZO1 is ubiquitously expressed, while PIEZO2 is predominantly found in peripheral sensory neurons, where it is involved in tactile sensation, pain perception, and proprioception [95,96]. Interestingly, PIEZO2 has also been identified in chondrocytes, suggesting a functional role in cartilage physiology [20,78]. Both channels exhibit voltage-dependent inactivation [97], with PIEZO2 inactivating more rapidly than PIEZO1. For instance, under a -80 mV holding potential, PIEZO2 has an inactivation time constant of approximately 5 ms, compared to PIEZO1's 15 ms [12,98].
The synergy between PIEZO1 and PIEZO2 in chondrocytes plays a crucial role in the perception and transduction of mechanical signals. Collectively, their activation regulates the chondrocyte response to mechanical stress, particularly in bone and cartilage tissues. The channels facilitate calcium signaling, which in turn modulates downstream transcription factors such as NFAT, YAP1 and β-catenin [78,99]. PIEZO2 primarily mediates calcium signaling under low-intensity stress, thereby maintaining mechanical sensitivity within a physiological range, whereas PIEZO1 takes on a more dominant role under high-intensity stress [100]. he functional interdependence of these channels is further highlighted by the observation that single knockouts of PIEZO1 or PIEZO2 nearly abolish mechanically induced calcium influx in chondrocytes [78]. This finding suggests that their roles in mechanotransduction are complementary, with both channels jointly mediating the response to harmful mechanical stimuli [36]. However, under certain conditions, PIEZO1 can operate independently of PIEZO2, particularly during high-intensity strain where PIEZO1 alone can initiate calcium signaling [47].
Despite the demonstrated synergy between PIEZO1 and PIEZO2, recent studies have indicated that the dual knockout of these channels does not significantly affect joint development or normal function. In a Gdf5-Cre transgenic mouse model, PIEZO1 and PIEZO2 double-knockout mice exhibited OA progression similar to that of the control group, suggesting that the direct influence of Piezo channels on OA progression may be limited [101]. This finding implies a potential functional redundancy between PIEZO1 and PIEZO2 in chondrocytes, with less pronounced synergistic effects under physiological and pathological conditions than had previously been anticipate.
While PIEZO2 enhances PIEZO1 sensitivity and the two channels jointly regulate chondrocyte responses to mechanical stimuli, the molecular mechanisms underlying their synergy remain unclear. It is therefore recommended that future research focus on elucidating the pathways through which PIEZO1 and PIEZO2 interact at the molecular level, particularly in response to varying intensities of mechanical stress. A more profound comprehension of these mechanisms would facilitate our understanding of how these cells adapt to complex mechanical environments and could inform therapeutic strategies targeting Piezo channels in degenerative cartilage diseases.
6.3 L-Type Calcium Channels and Piezo1
L-type voltage-gated calcium channels (L-VDCCs) have been shown to play a critical role in mediating calcium influx during membrane depolarization in chondrocytes [102]. These channels have been observed to interact with PIEZO1 to regulate calcium influx and exhibit synergistic effects in pathological responses of chondrocytes. Following the activation of PIEZO1 by abnormal mechanical stress, L-VDCCs have been shown to amplify calcium signaling, further increasing intracellular calcium concentrations [36,103].
In OA, PIEZO1 and L-VDCCs work collaboratively to enhance intracellular calcium signaling, a process which is associated with excessive mechanical stress and prolonged joint loading, both of which can exacerbate the progression of OA. L-VDCCs amplify calcium influx through PIEZO1, intensifying calcium signalling triggered by mechanical stimuli and worsening disease outcomes [104] Experiments using L-VDCC inhibitors, such as verapamil, demonstrate that blocking L-VDCCs significantly reduces high-strain-induced calcium influx influx, thus highlighting the supportive role of L-VDCCs in amplifying PIEZO1-mediated calcium signaling [105]. Such synergistic effects are likely to magnify the response of chondrocytes to mechanical stress, influencing both physiological and pathological functions. In spinal growth plate chondrocytes, PIEZO1 and L-VDCCs synergistically amplify calcium signaling under compressive stress [42]. However, whether this synergy exists in articular chondrocytes requires further validation. PIEZO1 and TRPV4 exhibit complementary roles: TRPV4 initiates low-intensity Ca²⁺ transients that prime PIEZO1 for high-strain responses via cytoskeletal prestress [20]. Co-activation amplites ERK1/2 and YAP signaling, driving hypertrophic differentiation in OA [78]. L-type Ca²⁺ channels further exacerbate Ca²⁺ overload by depolarizing the membrane, creating a voltage-PIEZO1 positive feedback loop [42]. Dual inhibition of PIEZO1 and TRPV4/L-type channels may thus offer synergistic cartilage protection. However, the precise molecular mechanisms underlying this interaction remain to be elucidated. The following key questions require elucidation:
• Do PIEZO1 and L-VDCCs exhibit feedback mechanisms? PIEZO1-induced calcium influx might influence L-VDCC activity, creating a positive feedback loop that further elevates intracellular calcium levels. This could exacerbate stress-induced damage in OA.
• PIEZO1 and L-VDCCs share regulatory pathways: The investigation of shared signaling cascades has the potential to reveal new targets for the modulation of their activity in disease contexts.
• Differential roles in mechanical sensitivity exist: L-VDCCs might contribute more significantly to sustained calcium signaling, while PIEZO1 primarily mediates the initial mechanical response.
7. Conclusions and Perspectives
PIEZO1, as validated in chondrocyte-specific knockout models, is a critical regulator of articular chondrocyte mechanotransduction in OA, playing a central role in mechanical responses, matrix metabolism regulation, and inflammatory processes. As a mechanosensitive channel, PIEZO1 is essential for maintaining chondrocyte health and protecting articular cartilage. This review focuses on PIEZO1's roles explicitly validated in chondrocyte models. The regulation of PIEZO1 activation has significant clinical potential for the prevention of cartilage degeneration, the promotion of tissue repair, and the mitigation of the progression of joint diseases such as OA. Despite significant advancements in our understanding of PIEZO1's functions and mechanisms in chondrocytes, there are still substantial knowledge gaps, particularly concerning its activation dynamics, interactions with other channels, and therapeutic applications.
The interactions between PIEZO1 and other mechanosensitive channels. TRPV4 modulates PIEZO1 distribution and activation by responding to low-intensity mechanical stress, enhancing PIEZO1 activity and calcium signaling. PIEZO2, primarily involved in low-intensity stress, enhances PIEZO1 sensitivity, thereby facilitating more efficient responses to mechanical stimuli. L-VDCCs amplify PIEZO1-mediated calcium influx, intensifying calcium signaling, especially during high mechanical stress. Together, these channels form a coordinated network that regulates chondrocyte mechanotransduction.
Evidence hierarchy for piezo1 functions in chondrocytes.
| Tier | Study Type | Example References | Experimental Model | Support Strength for Chondrocyte Conclusions |
|---|---|---|---|---|
| Tier 1: Chondrocyte-Specific Models | Primary chondrocytes, chondrocyte-targeted genetically modified mice | Brylka et al., 2024 (Ref 21) | Col2a1-Cre; Piezo1ᶠˡ/ᶠˡ mice | ★★★★★ |
| Sun L et al., 2025 (Ref 69) | Col2a1CreERT; Piezo1flox/flox mice | ★★★★★ | ||
| Sun Y et al., 2021 (Ref 38) | Human OA chondrocytes + murine joint injury model | ★★★★★ | ||
| Savadipour et al., 2023 (Ref 47) | Primary chondrocyte compression assays | ★★★★☆ | ||
| Tier 2: Non-Specific In vivo Models | Systemic knockout mice, pharmacological intervention models | Lee et al., 2014 (Ref 78) | Global Piezo1⁻/⁻ mice | ★★☆☆☆ |
| Gan et al., 2024 (Ref 83) | DMM model mice + artemisinin intervention | ★★★☆☆ | ||
| Tier 3: Non-Chondrocyte Models | Endothelial cells, neurons, other cell lines | Coste et al., 2010 (Ref 12) | Mechanosensitivity studies in neuronal cells | ★☆☆☆☆ |
| Syeda et al., 2015 (Ref 30) | HEK293 cells + Yoda1 activation mechanism | ★☆☆☆☆ |
Key Notes:
Tier 1: Direct evidence from chondrocyte-specific models (highest reliability).
Tier 2: Systemic or non-specific models (caution required for cartilage-specific conclusions).
Tier 3: Findings from non-chondrocyte systems (exploratory only, not generalizable to chondrocytes).
Star Rating: ★★★★★ = Strongest evidence; ★☆☆☆☆ = Weakest evidence.
Summary of mouse model data in piezo1 regulation studies.
| Category | Mouse Model Type | Experiment (In vivo/In vitro) | Effect | Limitations | References |
|---|---|---|---|---|---|
| Mechanical Stress | Global Piezo1 knockout (Piezo1⁻/⁻) | In vivo | High mechanical stress induces chondrocyte apoptosis and accelerates OA progression. | Systemic knockout affects other tissues; cartilage-specific effects cannot be isolated. | Lee et al., 2014 [78] |
| Chondrocyte-specific Piezo1 knockout (Col2a1-Cre; Piezo1ᶠˡ/ᶠˡ) | In vivo | Loss of Piezo1 in chondrocytes impairs endochondral ossification and reduces OA severity. | Limited to developmental stages; adult OA-specific effects require further validation. | Brylka et al., 2024 [21] | |
| Primary chondrocytes under compression (15% strain) | In vitro | PIEZO1 activation triggers Ca²⁺ influx and ECM degradation. | In vitro models lack systemic inflammatory or biomechanical context. | Savadipour et al., 2023 [47] | |
| ECM Environment | 3D hydrogel culture (stiffness gradient) | In vitro | High stiffness (>20 kPa) upregulates PIEZO1 expression and promotes senescence. | Hydrogel stiffness may not fully replicate native cartilage ECM complexity. | Du G et al., 2022 [37] |
| Primary cilia-deficient mice (Ift88⁻/⁻) | In vivo | Impaired ECM stiffness sensing and altered PIEZO1 activation thresholds. | Global cilia dysfunction affects multiple cell types beyond chondrocytes. | Guo et al., 2024 [63] | |
| Inflammatory Factors | IL-1β intra-articular injection (C57BL/6 mice) | In vivo | IL-1β upregulates PIEZO1 via NF-κB, exacerbating cartilage degradation. | Acute inflammation model may not mimic chronic OA progression. | Lee et al., 2021 [48] |
| TNF-α stimulation in primary chondrocytes | In vitro | TNF-α enhances PIEZO1 open probability via ROS-mediated oxidation. | In vitro ROS levels may exceed physiological relevance. | Lee et al., 2021 [48] | |
| Chondrocyte-specific Piezo1 activation under stress | In vivo + In vitro | Mechanical overload → Piezo1-mediated mtDNA release → activates cGAS-STING → inflammation via IFN-β, IL-6, TNF-α. | Model focuses on acute inflammatory amplification; long-term OA outcomes need validation. | Sun L et al., 2025 [69] | |
| Agonists | Intra-articular Yoda1 (wild-type mice) | In vivo | Yoda1 activates PIEZO1, increasing senescence and MMP-13 expression. | Off-target effects of Yoda1 on other ion channels are not ruled out. | Gan et al., 2024 [83] |
| Inhibitors | Intra-articular GsMTx4 (surgery-induced OA mice) | In vivo | GsMTx4 inhibits PIEZO1, reducing cartilage erosion and pain. | Peptide-based inhibitors face challenges in clinical translation (e.g., stability, delivery). | Ren et al., 2023 [32] |
| Modulators | Artemisinin dietary intervention (DMM model mice) | In vivo | Suppresses PIEZO1 activity, improving cartilage integrity. | Low bioavailability of oral artemisinin in joints may limit efficacy. | Gan et al., 2024 [83 |
| Urocortin-treated cartilage explants | In vitro | Inhibits PIEZO1 via CRF-R1/cAMP signaling, reducing mechanical injury. | Explant models lack dynamic mechanical loading and systemic feedback. | Lawrence et al., 2017 [84] | |
| Other Ion Channels | TRPV4/PIEZO1 double knockout (Col2a1-Cre mice) | In vivo | Dual knockout suppresses mechano-induced Ca²⁺ signaling, protecting cartilage. | Compensatory upregulation of other mechanosensitive channels (e.g., TRPA1) not assessed. | Servin-Vences et al., 2017 [20] |
| L-type Ca²⁺ channel inhibitor (Verapamil in OA mice) | In vivo | Reduces PIEZO1-mediated Ca²⁺ overload, alleviating OA. | Verapamil's cardiovascular side effects complicate systemic use for OA. | Takamatsu et al., 2023 [104] |
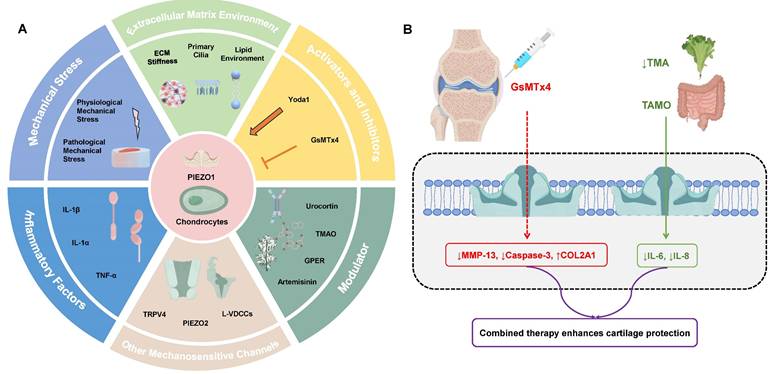
A: Overview of PIEZO1 Regulation in Chondrocytes. Mechanical Stress: Both physiological and pathological mechanical stress regulate PIEZO1 activation, impacting cartilage function and health. Physiological stress supports ECM synthesis, while pathological stress leads to excessive calcium influx, contributing to cartilage degradation. Extracellular Matrix (ECM) Environment: ECM stiffness and primary cilia significantly affect PIEZO1 activity. High ECM stiffness enhances PIEZO1 activation, while primary cilia modulate PIEZO1 sensitivity. Inflammatory Factors: Cytokines like IL-1β and TNF-α upregulate PIEZO1 expression and its activation, exacerbating cartilage degradation. Other Mechanosensitive Channels: PIEZO1 interacts with channels like TRPV4 and PIEZO2, enhancing calcium signaling and regulating cellular responses to mechanical stimuli. Activators and Inhibitors: Chemical agents such as Yoda1 and Jedi1/2 activate PIEZO1, while GsMTx4 inhibit its activity, offering potential therapeutic avenues for modulating PIEZO1 in OA. Modulators: Natural compounds like urocortin, TMAO, and artemisinin, along with the GPER receptor, also modulate PIEZO1 activation and its effects on chondrocyte functions. B: Clinical strategies targeting PIEZO1 in OA management: Intra-articular injection of the PIEZO1 inhibitor GsMTx4 blocks pathological Ca²⁺ influx, reducing MMP-13 expression and apoptosis while preserving ECM integrity. Dietary reduction of TMAO (e.g., low-meat/high-fiber diets) suppresses gut microbiota-derived TMAO, thereby downregulating PIEZO1 expression and mitigating cartilage degradation. Combined approaches may offer enhanced therapeutic efficacy by addressing both mechanical and inflammatory drivers of OA.
PIEZO1 activity correlates with OA severity, serving as a potential diagnostic biomarker via synovial fluid analysis. Pharmacologically, inhibitors like GsMTx4 reduce cartilage degradation in preclinical models, while activators (e.g., Yoda1) aid fracture repair under mechanical unloading. Natural compounds (e.g., artemisinin) suppress PIEZO1 overactivation, offering therapeutic potential. The spatiotemporal dynamics of PIEZO1 activation under various mechanical stresses, such as compression and stretching, and its behaviour in different cellular states, are not yet fully understood. Furthermore, further investigation is required into PIEZO1's interactions with other mechanosensitive channels, such as TRPV4 and PIEZO2. These channels appear to cooperate in regulating mechanical signaling, but the precise mechanisms of their synergy, particularly in pathological contexts such as joint injuries and OA, remain unclear. Furthermore, while PIEZO1 modulators, including activators and inhibitors, have demonstrated efficacy in preclinical studies, concerns regarding their specificity, selectivity, and potential adverse effects persist. The majority of available modulators are still in the preclinical stage and require extensive clinical validation.
The potential of PIEZO1 as a therapeutic target for the treatment of degenerative joint disease is a subject that offers considerable promise. Nevertheless, the successful translation of this research into clinical practice is predicated on the successful resolution of several significant challenges:
1. Molecular regulation: Future studies should focus on elucidating the molecular mechanisms underlying PIEZO1 activation, its regulatory networks and its interactions with other channels.
2. Channel synergy: Further research is required into the cooperative functions of PIEZO1 with TRPV4, PIEZO2 and other mechanosensitive channels under different mechanical stimuli. This will provide insights into their collective roles in cartilage physiology and pathology.
3. Finally, the development of new drugs should be a priority. Efforts should be directed towards the development of highly selective and potent PIEZO1 modulators (activators and inhibitors) and the evaluation of their safety and efficacy in both preclinical and clinical settings.
4. Combination therapies: Exploration of the synergistic potential of PIEZO1 inhibitors in combination with anti-inflammatory drugs or other agents could enhance therapeutic efficacy and minimise cartilage degeneration.
The present study investigates the role of PIEZO1 in cartilage biology and disease progression, thereby highlighting its potential as a transformative target for understanding and treating cartilage degeneration, repair, and development. The multifactorial regulation of PIEZO1 not only deepens our understanding of OA pathophysiology but also opens avenues for clinical innovation. Targeting PIEZO1 hyperactivity with inhibitors like GsMTx4 or natural compounds (e.g., artemisinin) offers potential disease-modifying therapies, while its context-dependent activation may aid cartilage repair. Advancing research into its molecular regulation, synergistic interactions, and pharmacological modulation will provide valuable insights and therapeutic strategies, ultimately benefiting patients suffering from OA and other degenerative joint disorders.
Acknowledgements
Funding
This study was supported by the Wuhan Knowledge Innovation Project 2023 (2023020201010179) and the Hubei Provincial Health Commission Translational Medicine Project (WJ2021ZH0010).
Author Contributions
GWY and ZRX wrote the draft of the manuscript. GWY, KX and GDW were involved in discussing, drafting, and editing the manuscript. All authors contributed to the article and approved the submitted version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Han J, Zhan L nan, Huang Y, Guo S, Zhou X, Kapilevich L. et al. Moderate mechanical stress suppresses chondrocyte ferroptosis in osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway. Sci Rep. 2024;14(1):5078
2. Paggi CA, Hendriks J, Karperien M, Gac SL. Emulating the chondrocyte microenvironment using multi-directional mechanical stimulation in a cartilage-on-chip. Lab Chip. 2022;22(9):1815-1828
3. Statham P, Jones E, Jennings LM, Fermor HL. Reproducing the biomechanical environment of the chondrocyte for cartilage tissue engineering. Tissue Engineering Part B: Reviews. 2022;28(2):405-420
4. Savadipour A, Palmer D, Ely EV, Collins KH, Garcia-Castorena JM, Harissa Z. et al. The role of PIEZO ion channels in the musculoskeletal system. American Journal of Physiology-Cell Physiology. 2023;324(3):C728-740
5. Uzieliene I, Bironaite D, Bagdonas E, Pachaleva J, Sobolev A, Tsai WB. et al. The Effects of Mechanical Load on Chondrogenic Responses of Bone Marrow Mesenchymal Stem Cells and Chondrocytes Encapsulated in Chondroitin Sulfate-Based Hydrogel. Int J Mol Sci. 2023;24(3):2915
6. Gilbert SJ, Bonnet CS, Blain EJ. Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage. Int J Mol Sci. 2021;22(24):13595
7. Feng X, Li S, Wang S, Meng Y, Zheng S, Liu C. et al. Piezo1 mediates the degradation of cartilage extracellular matrix in malocclusion-induced TMJOA. Oral Diseases. 2024;30(4):2425-2438
8. Porter A, Wang L, Han L, Lu XL. Bio-orthogonal Click Chemistry Methods to Evaluate the Metabolism of Inflammatory Challenged Cartilage after Traumatic Overloading. ACS Biomater Sci Eng. 2022;8(6):2564-2573
9. He Z, Nie P, Lu J, Ling Y, Guo J, Zhang B. et al. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Joint Res. 2020;9(10):731-741
10. Jones TJ, Nauli SM. Mechanosensory calcium signaling. Adv Exp Med Biol. 2012;740:1001-1015
11. Zhang K, Wang L, Liu Z, Geng B, Teng Y, Liu X. et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels. 2021;15(1):339-359
12. Coste B, Mathur J, Schmidt M. et al. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science. 2010;330(6000):55-60
13. Liu Y, Zhang Z, Li J, Chang B, Lin Q, Wang F. et al. Piezo1 transforms mechanical stress into pro senescence signals and promotes osteoarthritis severity. Mech Ageing Dev. 2023;216:111880
14. Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q. et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573(7773):225-229
15. Jiang Y, Yang X, Jiang J, Xiao B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends in Biochemical Sciences. 2021;46(6):472-488
16. Zhao Q, Zhou H, Li X, Xiao B. The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism. The FEBS Journal. 2019;286(13):2461-2470
17. Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554(7693):481-486
18. Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P. et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527(7576):64-69
19. Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P. et al. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron. 2016;89(6):1248-1263
20. Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife. 2017;6:e21074
21. Brylka LJ, Alimy AR, Tschaffon-Müller MEA, Jiang S, Ballhause TM, Baranowsky A. et al. Piezo1 expression in chondrocytes controls endochondral ossification and osteoarthritis development. Bone Res. 2024;12:12
22. Chen P, Zhang G, Jiang S, Ning Y, Deng B, Pan X. et al. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium. 2021;97:102431
23. Liu Y, Tian H, Hu Y, Cao Y, Song H, Lan S. et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int J Biol Sci. 2022;18(10):3961-3980
24. Hendrickx G, Fischer V, Liedert A, Von Kroge S, Haffner-Luntzer M, Brylka L. et al. Piezo1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. Journal of Bone and Mineral Research. 2020;36(2):369-384
25. Wang J, Sun YX, Li J. The role of mechanosensor Piezo1 in bone homeostasis and mechanobiology. Developmental Biology. 2023;493:80-88
26. Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 2020;11:282
27. Chen S, Li Z, Chen D, Cui H, Wang J, Li Z. et al. Piezo1-mediated mechanotransduction promotes entheseal pathological new bone formation in ankylosing spondylitis. Annals of the Rheumatic Diseases. 2023;82(4):533-545
28. Huang W, Warner M, Sasaki H, Furukawa KS, Ushida T. Layer dependence in strain distribution and chondrocyte damage in porcine articular cartilage exposed to excessive compressive stress loading. Journal of the Mechanical Behavior of Biomedical Materials. 2020;112:104088
29. Chen W, Zhang H. Elucidating the mechanism of IL-1β-Mediated Piezo1 expression regulation of chondrocyte autophagy and apoptosis via the PI3K/AKT/mTOR signaling Pathway. Tissue and Cell. 2024;86:102291
30. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T. et al. Chemical activation of the mechanotransduction channel Piezo1. eLife. 2015;4:e07369
31. Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50(29):6295-300
32. Ren X, Zhuang H, Li B, Jiang F, Zhang Y, Zhou P. Gsmtx4 Alleviated Osteoarthritis through Piezo1/Calcineurin/NFAT1 Signaling Axis under Excessive Mechanical Strain. Int J Mol Sci. 2023;24(4):4022
33. Ellefsen KL, Holt JR, Chang AC, Nourse JL, Arulmoli J, Mekhdjian AH. et al. Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers. Commun Biol. 2019;2:298
34. Steinecker-Frohnwieser B, Lohberger B, Toegel S, Windhager R, Glanz V, Kratschmann C. et al. Activation of the Mechanosensitive Ion Channels Piezo1 and TRPV4 in Primary Human Healthy and Osteoarthritic Chondrocytes Exhibits Ion Channel Crosstalk and Modulates Gene Expression. Int J Mol Sci. 2023;24(9):7868
35. Jia S, Liu W, Zhang M, Wang L, Ren C, Feng C. et al. Insufficient Mechanical Loading Downregulates Piezo1 in Chondrocytes and Impairs Fracture Healing Through ApoE-Induced Senescence. Adv Sci (Weinh). 2024;11(46):e2400502
36. Lee W, Guilak F, Liedtke W. Role of Piezo Channels in Joint Health and Injury. Curr Top Membr. 2017;79:263-273
37. Du G, Chen W, Li L, Zhang Q. The potential role of mechanosensitive ion channels in substrate stiffness-regulated Ca2+ response in chondrocytes. Connect Tissue Res. 2022;63(5):453-462
38. Sun Y, Leng P, Guo P, Gao H, Liu Y, Li C. et al. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol Med. 2021;27(1):96
39. Zhang Q, Godfred KT, Zhang Y, Wei X, Chen W, Zhang Q. [Research progress of chondrocyte mechanotransduction mediated by TRPV4 and PIEZOs]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2023;40(4):638-44
40. Sun Y, Leng P, Li D, Gao H, Li Z, Li C. et al. Mechanism of Abnormal Chondrocyte Proliferation Induced by Piezo1-siRNA Exposed to Mechanical Stretch. Biomed Res Int. 2020;2020:8538463
41. Li J, Wang X, Li X, Liu D, Zhai L, Wang X. et al. Mechanical Loading Promotes the Migration of Endogenous Stem Cells and Chondrogenic Differentiation in a Mouse Model of Osteoarthritis. Calcif Tissue Int. 2023;112(3):363-376
42. Chen F, Sun M, Peng F, Lai Y, Jiang Z, Zhang W. et al. Compressive stress induces spinal vertebral growth plate chondrocytes apoptosis via Piezo1. J Orthop Res. 2023;41(8):1792-1802
43. Wang B, Shi Y, Chen J, Shao Z, Ni L, Lin Y. et al. High glucose suppresses autophagy through the AMPK pathway while it induces autophagy via oxidative stress in chondrocytes. Cell Death Dis. 2021;12(6):506
44. Song J, Liu L, Lv L, Hu S, Tariq A, Wang W. et al. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol Int. 2020;44(7):1491-1502
45. De Vecchis D, Beech DJ, Kalli AC. Molecular dynamics simulations of Piezo1 channel opening by increases in membrane tension. Biophys J. 2021;120(8):1510-1521
46. Li Y, Yang J, Feng Q, Li SQ, Lang Y, Zhang XF. et al. High cyclic tensile stress disrupts the extracellular matrix in human chondrocyte by F-actin cytoskeletal polymerization and reactive oxygen species production. J Biol Regul Homeost Agents. 2021;35(3):965-974
47. Savadipour A, Nims RJ, Rashidi N. et al. Membrane stretch as the mechanism of activation of PIEZO1 ion channels in chondrocytes. Proc Natl Acad Sci USA. 2023;120(30):e2221958120
48. Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F. et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci USA. 2021;118(13):e2001611118
49. Liu Y, Zhang Z, Lu X, Liu C, Zhang H. Senescence-responsive miR-33-5p promotes chondrocyte senescence and osteoarthritis progression by targeting SIRT6. Int Immunopharmacol. 2023;121:110506
50. Li H, Huang L, Xie Q, Cai X, Yang C, Wang S. et al. Study on the effects of gradient mechanical pressures on the proliferation, apoptosis, chondrogenesis and hypertrophy of mandibular condylar chondrocytes in vitro. Arch Oral Biol. 2017;73:186-192
51. Chen YJ, Zhang M, Wang JJ. Study on the effects of mechanical pressure to the ultrastructure and secretion ability of mandibular condylar chondrocytes. Arch Oral Biol. 2007;52(2):173-181
52. Zhang M, Meng N, Wang X, Chen W, Zhang Q. TRPV4 and PIEZO Channels Mediate the Mechanosensing of Chondrocytes to the Biomechanical Microenvironment. Membranes (Basel). 2022;12(2):237
53. Zhou C, Yang Y, Duan M, Chen C, Pi C, Zhang D. et al. Biomimetic Fibers Based on Equidistant Micropillar Arrays Determines Chondrocyte Fate via Mechanoadaptability. Adv Healthc Mater. 2023;12(30):e2301685
54. Wang B, Ke W, Wang K, Li G, Ma L, Lu S. et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2021;2021:8884922
55. Zhang M, Wu X, Du G, Chen W, Zhang Q. Substrate stiffness-dependent regulatory volume decrease and calcium signaling in chondrocytes. Acta Biochim Biophys Sin (Shanghai). 2022;54(1):113-125
56. Williantarra I, Leung S, Choi YS, Chhana A, McGlashan SR. Chondrocyte-specific response to stiffness-mediated primary cilia formation and centriole positioning. Am J Physiol Cell Physiol. 2022;323(1):C236-247
57. Zhang Y, Tawiah GK, Wu X, Zhang Y, Wang X, Wei X. et al. Primary cilium-mediated mechanotransduction in cartilage chondrocytes. Exp Biol Med (Maywood). 2023;248(15):1279-1287
58. Guo H, Lan M, Zhang Q, Liu Y, Zhang Y, Zhang Q. et al. [Piezo1 Mediates the Regulation of Substrate Stiffness on Primary Cilia in Chondrocytes]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024;55(1):67-73
59. Sheffield ID, McGee MA, Glenn SJ, Baek DY, Coleman JM, Dorius BK. et al. Osteoarthritis-Like Changes in Bardet-Biedl Syndrome Mutant Ciliopathy Mice (Bbs1M390R/M390R): Evidence for a Role of Primary Cilia in Cartilage Homeostasis and Regulation of Inflammation. Front Physiol. 2018;9:708
60. Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF. et al. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun. 2019;10(1):1200
61. Dubin AE, Murthy S, Lewis AH, Brosse L, Cahalan SM, Grandl J. et al. Endogenous Piezo1 Can Confound Mechanically Activated Channel Identification and Characterization. Neuron. 2017;94(2):266-270.e3
62. Nourse JL, Pathak MM. How cells channel their stress: Interplay between Piezo1 and the cytoskeleton. Seminars in Cell & Developmental Biology. 2017;71:3-12
63. Shi J, Hyman AJ, De Vecchis D, Chong J, Lichtenstein L, Futers TS. et al. Sphingomyelinase Disables Inactivation in Endogenous PIEZO1 Channels. Cell Reports. 2020;33(1):108225
64. Chong J, De Vecchis D, Hyman AJ, Povstyan OV, Ludlow MJ, Shi J. et al. Modeling of full-length Piezo1 suggests importance of the proximal N-terminus for dome structure. Biophysical Journal. 2021;120(8):1343-1356
65. Villalvilla A, Gómez R, Largo R, Herrero-Beaumont G. Lipid Transport and Metabolism in Healthy and Osteoarthritic Cartilage. IJMS. 2013;14(10):20793-20808
66. Kosinska MK, Mastbergen SC, Liebisch G, Wilhelm J, Dettmeyer RB, Ishaque B. et al. Comparative lipidomic analysis of synovial fluid in human and canine osteoarthritis. Osteoarthritis and Cartilage. 2016;24(8):1470-1478
67. Wu Y, Xu X, Liu F, Jing Z, Shen D, He P. et al. Three-Dimensional Matrix Stiffness Activates the Piezo1-AMPK-Autophagy Axis to Regulate the Cellular Osteogenic Differentiation. ACS Biomater Sci Eng. 2023;9(8):4735-4746
68. McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31(7):1039-1045
69. Sun L, Wang Y, Kan T, Wang H, Cui J, Wang L. et al. Elevated expression of Piezo1 activates the cGAS-STING pathway in chondrocytes by releasing mitochondrial DNA. Osteoarthritis Cartilage. 2025;33(5):601-615
70. Sun Y, Leng P, Song M, Li D, Guo P, Xu X. et al. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-κB pathway. International Immunopharmacology. 2020;85:106681
71. Liu H, Hu J, Zheng Q, Feng X, Zhan F, Wang X. et al. Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front Immunol. 2022;13:816149
72. Botello-Smith WM, Jiang W, Zhang H, Ozkan AD, Lin YC, Pham CN. et al. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun. 2019;10(1):4503
73. Ren X, Li B, Xu C, Zhuang H, Lei T, Jiang F. et al. High expression of Piezo1 induces senescence in chondrocytes through calcium ions accumulation. Biochem Biophys Res Commun. 2022;607:138-145
74. Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q. et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun. 2018;9(1):1300
75. Parsonage G, Cuthbertson K, Endesh N, Murciano N, Hyman AJ, Revill CH. et al. Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1. Br J Pharmacol. 2023;180(16):2039-2063
76. Xiao B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu Rev Pharmacol Toxicol. 2020;60:195-218
77. Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS. et al. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys J. 2017;112(1):31-45
78. Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL. et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111(47):E5114-5122
79. Wang S, Li W, Zhang P, Wang Z, Ma X, Liu C. et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J Adv Res. 2022;41:63-75
80. Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243(4894 Pt 1):1068-1071
81. Shahidullah M, Rosales JL, Delamere N. Activation of Piezo1 Increases Na,K-ATPase-Mediated Ion Transport in Mouse Lens. Int J Mol Sci. 2022;23(21):12870
82. Hamill OP, McBride DW. The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48(2):231-252
83. Gan D, Tao C, Jin X, Wu X, Yan Q, Zhong Y. et al. Piezo1 activation accelerates osteoarthritis progression and the targeted therapy effect of artemisinin. J Adv Res. 2024;62:105-117
84. Lawrence KM, Jones RC, Jackson TR, Baylie RL, Abbott B, Bruhn-Olszewska B. et al. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci Rep. 2017;7(1):5147
85. Jones RC, Lawrence KM, Higgins SM, Richardson SM, Townsend PA. Urocortin-1 Is Chondroprotective in Response to Acute Cartilage Injury via Modulation of Piezo1. Int J Mol Sci. 2022;23(9):5119
86. Lian WS, Wang FS, Chen YS, Tsai MH, Chao HR, Jahr H. et al. Gut Microbiota Ecosystem Governance of Host Inflammation, Mitochondrial Respiration and Skeletal Homeostasis. Biomedicines. 2022;10(4):860
87. Murillo-Saich JD, Coras R, Meyer R, Llorente C, Lane NE, Guma M. Synovial tissue metabolomic profiling reveal biomarkers of synovial inflammation in patients with osteoarthritis. Osteoarthr Cartil Open. 2022;4(3):100295
88. Zhuang H, Ren X, Zhang Y, Jiang F, Zhou P. Trimethylamine-N-oxide sensitizes chondrocytes to mechanical loading through the upregulation of Piezo1. Food Chem Toxicol. 2023;175:113726
89. Xu F, Ma J, Wang X, Wang X, Fang W, Sun J. et al. The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology. Biomolecules. 2023;13(9):1410
90. Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB. et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60(10):3028-3037
91. Lv M, Zhou Y, Chen X, Han L, Wang L, Lu XL. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J Orthop Res. 2018;36(2):730-738
92. O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111(4):1316-1321
93. Nims R, Palmer DR, Kassab J, Zhang B, Guilak F. The chondrocyte “mechanome”: Activation of the mechanosensitive ion channels TRPV4 and PIEZO1 drives unique transcriptional signatures. FASEB J. 2024;38(13):e23778
94. Jia Z, Wang J, Li X, Yang Q, Han J. Repair Effect of siRNA Double Silencing of the Novel Mechanically Sensitive Ion Channels Piezo1 and TRPV4 on an Osteoarthritis Rat Model. Curr Mol Pharmacol. 2024;17:e18761429317745
95. Zhou Z, Martinac B. Mechanisms of PIEZO Channel Inactivation. Int J Mol Sci. 2023;24(18):14113
96. Xiao B. Mechanisms of mechanotransduction and physiological roles of PIEZO channels. Nat Rev Mol Cell Biol. 2024;25(11):886-903
97. Sánchez-Carranza O, Chakrabarti S, Kühnemund J, Schwaller F, Bégay V, García-Contreras JA. et al. Piezo2 voltage-block regulates mechanical pain sensitivity. Brain. 2024;147(10):3487-500
98. Glogowska E, Arhatte M, Chatelain FC, Lesage F, Xu A, Grashoff C. et al. Piezo1 and Piezo2 foster mechanical gating of K2P channels. Cell Rep. 2021;37(9):110070
99. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q. et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife. 2020;9:e52779
100. Gao W, Hasan H, Anderson DE, Lee W. The Role of Mechanically-Activated Ion Channels Piezo1, Piezo2, and TRPV4 in Chondrocyte Mechanotransduction and Mechano-Therapeutics for Osteoarthritis. Front Cell Dev Biol. 2022;10:885224
101. Young C, Kobayashi T. Limited roles of Piezo mechanosensing channels in articular cartilage development and osteoarthritis progression. Osteoarthritis Cartilage. 2023;31(6):775-779
102. Xu L, Sun L, Xie L, Mou S, Zhang D, Zhu J. et al. Advances in L-Type Calcium Channel Structures, Functions and Molecular Modeling. Curr Med Chem. 2021;28(3):514-524
103. Irianto J, Swift J, Martins RP, McPhail GD, Knight MM, Discher DE. et al. Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys J. 2013;104(4):759-769
104. Takamatsu A, Ohkawara B, Ito M, Masuda A, Sakai T, Ishiguro N. et al. Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling. PLoS One. 2014;9(3):e92699
105. Xu BY, Jin Y, Ma XH, Wang CY, Guo Y, Zhou D. The potential role of mechanically sensitive ion channels in the physiology, injury, and repair of articular cartilage. J Orthop Surg (Hong Kong). 2020;28(3):2309499020950262
Author contact
![]() Corresponding authors: Ximing Liu, Department of Orthopedics, General Hospital of Central Theater Command, Wuhan, China. Email: gklxmcom; Zhigang Li, Department of Orthopedic Surgery, Hubei Provincial Hospital of Integrated Chinese & Western Medicine, Wuhan, China.Email: dortorli100com.
Corresponding authors: Ximing Liu, Department of Orthopedics, General Hospital of Central Theater Command, Wuhan, China. Email: gklxmcom; Zhigang Li, Department of Orthopedic Surgery, Hubei Provincial Hospital of Integrated Chinese & Western Medicine, Wuhan, China.Email: dortorli100com.

 Global reach, higher impact
Global reach, higher impact