Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3268-3276. doi:10.7150/ijms.117603 This issue Cite
Review
Single-Gene Mutations in Hepatocellular Carcinoma: Applications and Challenges in Precision Medicine
1. Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
2. Department of Radiology, Lianyungang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Lianyungang, 222004, China.
3. Department of gynecology, Fudan University Shanghai Cancer Center Minhang District, Shanghai 200240, China.
*These authors contributed equally.
Received 2025-5-15; Accepted 2025-6-28; Published 2025-7-10
Abstract
Hepatocellular carcinoma (HCC) is a genetically heterogeneous malignancy in which single-gene mutations serve as critical drivers of tumor initiation, progression, and therapeutic resistance. Advances in high-throughput genomics and liquid biopsy technologies have highlighted the clinical utility of mutations in genes such as TP53, CTNNB1, and TERT as diagnostic, prognostic, and predictive biomarkers. These mutations disrupt key oncogenic pathways, modulate the tumor immune microenvironment, and contribute to intratumoral heterogeneity, complicating disease management. Mutation-guided precision medicine, including telomerase inhibitors, Wnt/β-catenin pathway modulators, and immune checkpoint blockade, offers promising avenues for individualized treatment in HCC. However, challenges persist in translating these findings into clinical practice due to mutation complexity, resistance mechanisms, and limitations in biomarker standardization. Emerging strategies such as multi-omics integration, artificial intelligence, and gene editing technologies hold potential to overcome these barriers and facilitate the development of personalized therapeutic regimens. This review summarizes the molecular mechanisms, clinical applications, and translational challenges of single-gene mutations in HCC, with the aim of guiding future research and precision oncology.
Keywords: Hepatocellular carcinoma, HCC, Single-gene mutations, Precision medicine, Targeted therapy, Immunotherapy.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and has been witnessing a steadily increasing incidence worldwide. Major etiological factors include chronic viral hepatitis (particularly hepatitis B and C), liver cirrhosis, and metabolic syndrome [1]. In recent years, advances in molecular biology and genomics have elucidated the critical roles that single-gene mutations play in the initiation and progression of HCC. These mutations not only alter key biological properties of tumor cells but also represent potential therapeutic targets, offering new avenues for precision medicine [2,3].
Among the genetic alterations associated with HCC, mutations in TP53 are the most frequently observed and are closely linked to tumor initiation, progression, and prognosis [4]. TP53 mutations lead to dysregulation of the cell cycle and may impair antitumor immune responses within the tumor microenvironment, thereby influencing patient outcomes [5]. Other key mutations, such as those in CTNNB1 and AXIN1, contribute to hepatocarcinogenesis by modulating key signaling pathways [6]. The advent of single-cell sequencing technologies has enabled high-resolution analysis of the genomic, transcriptomic, and epigenomic landscapes of HCC. This approach facilitates the identification of intratumoral heterogeneity and rare cellular subpopulations, offering insights into their roles in disease progression and revealing novel therapeutic targets [2,7,8].
In the context of precision medicine, single-gene mutation profiling provides promising biomarkers for early diagnosis and prognostic assessment of HCC, thereby laying the foundation for individualized treatment strategies. For instance, specific mutations may predict patient response to immune checkpoint inhibitors and guide clinical decision-making [9,10]. Additionally, liquid biopsy technologies enable the detection of circulating tumor DNA (ctDNA) mutations, allowing for real-time monitoring of tumor dynamics and the optimization of personalized therapeutic regimens [11,12].
In summary, research on single-gene mutations in HCC has significantly enhanced our understanding of its pathogenesis and propelled the development of precision oncology. With continued advancements in genomic profiling and personalized therapeutic approaches, there is great potential to improve long-term outcomes and quality of life for patients with HCC.
Pathogenic Mechanisms of Single-Gene Mutations in HCC
Single-gene mutations serve as critical pathogenic drivers in the initiation and progression of HCC. These mutations not only play a direct role in tumorigenesis but also contribute to disease progression by modulating key signaling pathways and exacerbating tumor heterogeneity [4-6]. This section will provide a focused discussion on three major aspects: (i) the most common types of driver gene mutations in HCC, (ii) the mechanisms by which these mutations lead to aberrant activation of signaling pathways, and (iii) the potential impact of single-gene mutations on intratumoral heterogeneity. These insights aim to deepen our understanding of the molecular pathogenesis of HCC and support the development of targeted precision therapies.
Common Driver Gene Mutations in HCC
Driver gene mutations involved in the initiation and progression of HCC can be broadly categorized into three groups: tumor suppressor gene mutations, oncogene mutations, and mutations in epigenetic regulatory genes. Among tumor suppressors, TP53 is the most frequently mutated gene in HCC. The p53 protein encoded by TP53 plays a pivotal role in cell cycle regulation and apoptosis. Approximately 30% of HCC patients harbor TP53 mutations, which impair the cellular response to DNA damage and thereby promote tumorigenesis [4,13]. Mutations in other tumor suppressor genes such as AXIN1 and RB1 are also closely associated with HCC. AXIN1 mutations activate the Wnt/β-catenin signaling pathway, enhancing cellular proliferation, while RB1 regulates the G1/S cell cycle checkpoint. Loss of RB1 function disrupts cell cycle control and contributes to malignant transformation [14-16].
In terms of oncogenes, mutations in CTNNB1 are frequently observed in HCC and similarly lead to aberrant activation of the Wnt/β-catenin pathway, driving tumor development. Additionally, mutations in the TERT promoter region activate telomerase, extending cellular lifespan and facilitating malignant transformation of hepatocytes [5,14,17,18].
Mutations in epigenetic regulatory genes also play significant roles in HCC pathogenesis. Alterations in ARID1A and ARID2 disrupt chromatin remodeling, affecting gene expression and cellular proliferation. Mutations in MLL3 and KMT2D (also known as MLL2) lead to aberrant histone methylation patterns, contributing to epigenetic dysregulation and tumorigenesis [9,18,19].
Aberrant Activation of Key Signaling Pathways
The pathogenesis of HCC is strongly associated with the dysregulation of several key signaling pathways. Among these, the Wnt/β-catenin signaling pathway is one of the most frequently altered. Its activation is often driven by mutations in CTNNB1, resulting in enhanced cell proliferation and tumor formation [14]. Another critical pathway is the p53/MDM2 axis; overexpression of MDM2 promotes the degradation of p53, thereby inhibiting its tumor-suppressive function and facilitating carcinogenesis [4].
In addition, aberrant activation of the Mitogen-Activated Protein Kinase (MAPK) and PI3K/Protein kinase B (AKT)/mTOR pathways is closely linked to HCC progression. These pathways promote cell growth, survival, and proliferation, contributing significantly to the malignant behavior of HCC cells [5,20].
Impact of Single-Gene Mutations on HCC Heterogeneity
Single-gene mutations not only contribute to the initiation of HCC but also play a crucial role in shaping tumor heterogeneity. Spatial and temporal heterogeneity of genetic mutations results in distinct biological behaviors among different tumor cell subpopulations, which may underlie therapeutic resistance and disease recurrence [2,22]. Studies have shown that HCCs harboring different mutation profiles exhibit marked differences in clinicopathological characteristics. For instance, HCCs with CTNNB1 mutations are often associated with higher tumor grades and poorer prognoses [5,23]. A deeper understanding of how single-gene mutations influence tumor heterogeneity is essential for the development of effective, personalized treatment strategies.
Applications of Single-Gene Mutations in Precision Medicine for HCC
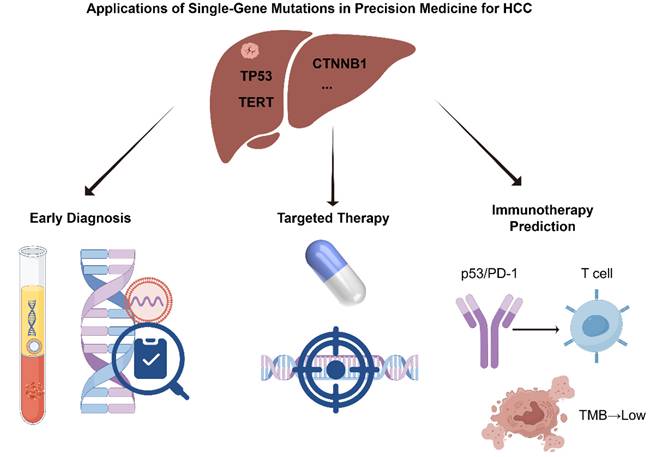
With the growing understanding of the molecular mechanisms underlying HCC, the clinical value of single-gene mutations in precision oncology has become increasingly evident [24,25]. Specific genetic alterations not only play critical roles in HCC initiation and progression but also serve as potential biomarkers for early diagnosis and provide theoretical and practical guidance for targeted and immunotherapeutic strategies [26,27]. In recent years, significant advances have been made in mutation-informed liquid biopsy technologies, the development of targeted therapies, and the prediction of immunotherapy responses, offering more precise and individualized treatment options for HCC patients [28,29]. This section provides a comprehensive overview of the multifaceted applications of single-gene mutations in HCC precision medicine, with a focus on early diagnosis, targeted therapy, and immunotherapy (Figure 1).
Application of Single-Gene Mutations in Early Diagnosis of HCC
Applications of Single-Gene Mutations in Precision Medicine for HCC (by Figdraw).
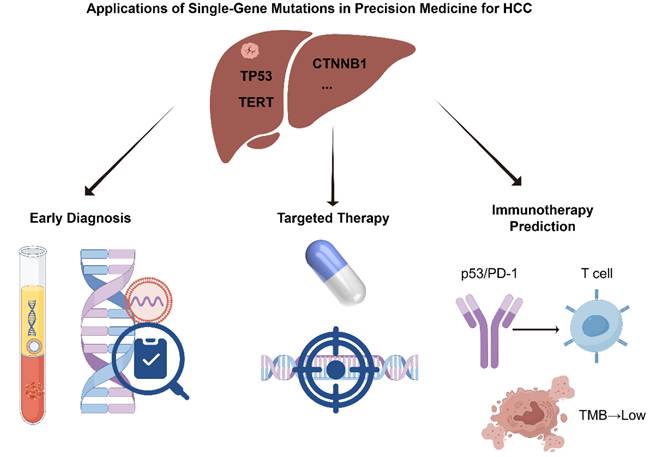
Early diagnosis of HCC is crucial for improving therapeutic outcomes and patient prognosis. However, due to the asymptomatic nature of early-stage disease, traditional imaging and serological markers often lack sufficient sensitivity and specificity. As a result, molecular diagnostic approaches based on single-gene mutations have emerged as promising tools for early HCC detection [30]. In particular, mutations in driver genes such as TERT and CTNNB1 have become focal points of recent research. Concurrently, advances in liquid biopsy technologies have greatly enhanced the feasibility and accuracy of early HCC detection [31].
Studies have shown that TERT promoter mutations occur at a high frequency in HCC and are closely associated with tumorigenesis, making them valuable molecular markers for early diagnosis and prognostic assessment [32]. Mutations in CTNNB1 are often linked to aberrant activation of the Wnt/β-catenin signaling pathway and are useful for identifying molecular subtypes and tracking disease progression in HCC [33]. The detection of these key mutations in blood samples enables the identification of high-risk individuals prior to the onset of clinical symptoms, providing a foundation for early intervention and precision disease management [34].
The development of liquid biopsy has introduced new opportunities for the non-invasive diagnosis of HCC. Circulating tumor DNA (ctDNA) reflects the mutational landscape of tumor tissues and has been shown to reliably detect various HCC-related mutations, including those in TERT and CTNNB1 [35,36]. In addition, extracellular vesicles (EVs) containing microRNAs (miRNAs) have emerged as promising biomarkers. For instance, miR-122 levels are significantly reduced in the EVs of HCC patients, and restoration of its expression has been shown to suppress tumor progression, highlighting its potential value in early HCC detection [37,38]. Liquid biopsy technologies offer the advantages of being minimally invasive and clinically accessible, providing an effective means of capturing tumor-specific genetic information. These approaches hold great promise for the early detection and individualized treatment of HCC.
Targeted Therapy Guided by Single-Gene Mutations
As research into the molecular mechanisms of HCC has progressed, the identification of specific driver gene mutations has opened new avenues for personalized therapy. Therapeutic strategies targeting these mutations aim to improve treatment efficacy and enhance patient outcomes.
TERT Promoter Mutations and Telomerase Inhibitors
Mutations in the promoter region of the TERT (telomerase reverse transcriptase) gene are among the most common genetic alterations in HCC. These mutations lead to increased telomerase activity, enabling unlimited proliferation of tumor cells [39]. Consequently, the development of telomerase inhibitors has become a key area of research. Preclinical studies have demonstrated that these inhibitors can effectively suppress HCC cell proliferation and delay tumor progression in animal models [40,41]. However, such agents remain in the preclinical stage, and their efficacy and safety in humans require validation through large-scale clinical trials.
CTNNB1 Mutations and Wnt/β-Catenin Pathway Inhibitors
The CTNNB1 gene encodes β-catenin, a central component of the Wnt signaling pathway. Mutations in CTNNB1 result in aberrant activation of Wnt/β-catenin signaling, thereby driving HCC development and progression [14]. Inhibitors targeting this pathway—such as small molecules and monoclonal antibodies—have shown tumor-suppressive potential in preclinical studies. However, since the Wnt pathway also plays essential roles in normal physiological processes, off-target effects and toxicity have limited their clinical application. Several of these inhibitors are currently undergoing early-phase clinical trials to evaluate their safety and therapeutic potential [42,43].
FGFR4 Mutations and FGFR Inhibitors
Fibroblast growth factor receptor 4 (FGFR4) is frequently overexpressed or mutated in HCC, contributing to increased tumor invasiveness and metastatic potential [44]. FGFR inhibitors, particularly tyrosine kinase inhibitors (TKIs), have been evaluated in both preclinical models and clinical trials. For HCC patients with aberrant FGFR4 signaling, these inhibitors exhibit promising antitumor activity and may improve clinical outcomes [44-46].
Other Potential Targeted Therapies
In addition to the above mutations, other molecular targets are actively being investigated for their therapeutic potential in HCC. For instance, vascular endothelial growth factor (VEGF) and its receptors play critical roles in angiogenesis. Anti-VEGF agents such as sorafenib and lenvatinib have already been approved for first-line treatment of advanced HCC [47].
Genetic Mutation Biomarkers in Immunotherapy
With the expanding use of immune checkpoint inhibitors (ICIs) in HCC treatment, identifying biomarkers that predict immunotherapeutic efficacy has become a key focus in precision immuno-oncology. Recent studies have highlighted the role of specific gene mutations in modulating the tumor immune microenvironment and influencing response to ICIs. Among these, mutations in TP53, CTNNB1, and tumor mutation burden (TMB) are the most representative and well-studied [48-50].
TP53 is one of the most frequently mutated genes in HCC. The protein it encodes, p53, plays a critical role in maintaining genomic stability and regulating apoptosis [48,51]. Loss-of-function TP53 mutations not only drive tumorigenesis but also contribute to immune evasion by upregulating PD-L1 expression and impairing antitumor immune responses. These mutations are associated with decreased T cell activity and poor response to immunotherapy, suggesting that TP53 status may serve as a potential biomarker for predicting ICI sensitivity [48,51].
Mutations in CTNNB1, on the other hand, impact the immune microenvironment primarily through activation of the Wnt/β-catenin signaling pathway [14]. Mutant CTNNB1 leads to a significant reduction in T cell infiltration, resulting in a so-called “cold tumor” phenotype that is inherently resistant to ICIs. HCC patients harboring CTNNB1 mutations typically exhibit poor responses to immunotherapy, further underscoring the negative predictive value of this alteration [52-54].
Additionally, tumor mutation burden (TMB) has emerged as a pan-cancer biomarker for assessing immunotherapy efficacy and is increasingly being investigated in the context of HCC [55,56]. Although the overall TMB in HCC is relatively low compared to other cancer types, subsets of patients with high TMB tend to respond better to PD-1/PD-L1 blockade and have more favorable prognoses [55,57]. Thus, TMB may also hold promise as a predictive biomarker for immunotherapy in HCC.
Despite these promising developments, translating mutation-informed insights into clinical practice remains fraught with challenges.
Challenges of Single-Gene Mutations in Precision Medicine
Despite significant advancements in the molecular characterization of HCC and the development of precision medicine strategies, translating these findings into clinical practice remains challenging. On one hand, the genetic landscape of HCC is highly complex, involving not only cooperative interactions among multiple genes but also numerous rare single-gene mutations, which limit the broad applicability of targeted therapies [3,4,58]. On the other hand, clinical implementation of precision medicine is hindered by factors such as drug resistance, inter-individual genetic heterogeneity, and the limited stability of biomarkers [3,48,59,60]. Furthermore, although emerging technologies such as liquid biopsy show considerable promise, their diagnostic performance and clinical validation require further refinement. While targeted and immunotherapeutic interventions have shown favorable outcomes in selected clinical cohorts, their real-world efficacy and consistency with mutational profiles remain to be fully elucidated. This section discusses key challenges currently facing HCC research, focusing on the complexity of genetic mutations, barriers to clinical translation, and the application of molecular biomarkers.
Complexity of Single-Gene Mutations
HCC is a genetically heterogeneous malignancy characterized by intricate networks of gene-gene interactions and mutation combinations. Common driver mutations, such as those in TP53 and CTNNB1, often co-occur and may synergistically affect cellular proliferation, apoptosis, and metabolic pathways, thereby accelerating tumor progression [4,61]. These combinatorial mutations can also influence therapeutic efficacy; for instance, certain mutations may confer resistance to targeted therapies, complicating treatment planning and response prediction [3].
In addition, a substantial number of rare genetic mutations have been identified in HCC, the functional characterization of which remains a significant challenge. Due to their low prevalence and limited clinical data, it is often difficult to obtain sufficient cases for large-scale functional validation [10]. Although such rare mutations may play critical roles in specific subsets of patients, their biological relevance and clinical significance require further investigation and verification.
Challenges in the Clinical Translation of Single-Gene Mutations in Precision Medicine
The clinical application of precision medicine in HCC faces several translational hurdles. One of the most critical limitations is the development of resistance to targeted therapies. Although initial responses to treatment are often favorable, tumor cells frequently acquire resistance through mechanisms such as secondary mutations, signaling pathway reprogramming, or alterations in the tumor microenvironment, ultimately leading to therapeutic failure [62]. In addition, pronounced inter-population heterogeneity in mutation profiles significantly limits the universal applicability of precision treatment strategies [63,64]. Genetic mutations can vary in type and frequency depending on factors such as ethnicity, geographic origin, and environmental exposure, all of which influence drug response and prognosis [63,64]. These issues underscore the need for deeper investigation into resistance mechanisms and the establishment of population-specific mutation databases to enable truly individualized therapeutic approaches.
Reliability and Standardization of Single-Gene Mutations as Biomarkers
As a non-invasive approach, liquid biopsy has shown promise in the early detection and dynamic monitoring of HCC. However, it still faces technical limitations in sensitivity and specificity, particularly in early-stage tumors where false-negative rates remain high, compromising diagnostic accuracy [65,66]. Moreover, most existing studies are based on single-center cohorts with small sample sizes, lacking large-scale, multi-center clinical validation. This hampers the broad adoption and clinical integration of liquid biopsy in routine practice [65-68]. Therefore, improving the analytical performance of liquid biopsy platforms and establishing standardized clinical workflows through multi-center trials are crucial next steps to enhance the reliability and translational impact of mutation-based biomarkers in HCC.
Future Directions in Single-Gene Mutation Research
As understanding of the molecular mechanisms underlying HCC continues to deepen, future research must not only adopt more sophisticated technologies and multidimensional data integration but also focus on bridging therapeutic strategies with clinical translation [69,70]. This section explores the latest advances and prospective directions in HCC research—from multi-omics integration and AI-assisted precision medicine to novel therapeutic strategies and clinical trial design—providing a theoretical foundation and strategic insights for advancing personalized medical practice (Figure 2).
Integrated Multi-Omics Analysis
Integrated multi-omics analysis is becoming increasingly vital in HCC research. By systematically combining genomic, transcriptomic, proteomic, and metabolomic data, researchers can comprehensively elucidate the molecular mechanisms, tumor heterogeneity, and progression dynamics of HCC. This approach facilitates the identification of key driver genes, signaling pathways, and potential therapeutic targets, enabling the construction of regulatory networks centered on single-gene mutations. Such networks help uncover core oncogenic drivers and regulatory axes that may guide therapeutic interventions [71-73].
The rapid development of single-cell omics technologies has made it possible to perform high-resolution genomic, transcriptomic, and epigenetic analyses of HCC tissues at the single-cell level [74,75]. This has greatly advanced our understanding of intratumoral heterogeneity, dynamic changes in the immune microenvironment, and the roles of rare cellular subpopulations. These insights provide a robust theoretical basis for precision subtyping and individualized treatment strategies.
Artificial Intelligence
With the widespread application of artificial intelligence (AI) and big data analytics in biomedical research, HCC studies are entering a new era of innovation. AI technologies—particularly deep learning and graph neural networks—are capable of efficiently processing large-scale omics datasets, clinical variables, and imaging data to uncover latent biomarkers and predictive models [76]. In recent years, AI has been increasingly employed in critical aspects of HCC research, including drug response prediction, immune response profiling, and prognostic modeling. For instance, by training models on multi-omics datasets correlated with treatment outcomes, AI can identify patient subgroups that are more likely to benefit from immune checkpoint inhibitors or targeted therapies, thereby enhancing the precision of therapeutic decision-making [77]. Additionally, AI can support intelligent patient stratification and adaptive design in clinical trials, improving their efficiency and scientific rigor [78]. Looking ahead, the deep integration of multi-omics data with AI-driven algorithms is expected to yield high-throughput, high-precision, and interpretable disease models for HCC, substantially accelerating the translation of basic research into clinical applications and advancing the implementation of precision medicine in HCC management.
Future Directions in Single-Gene Mutation Research (by Figdraw).
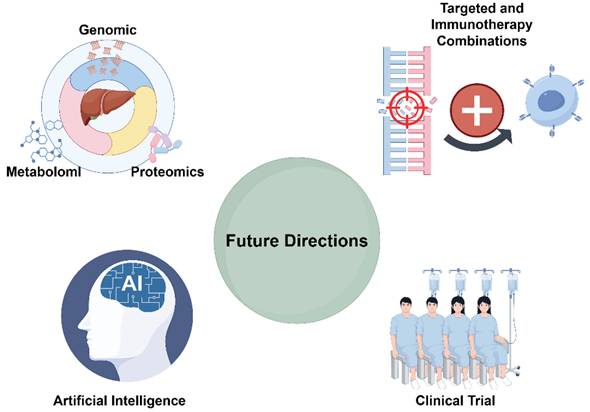
Emerging Strategies in Targeted and Immunotherapy Combinations
Innovative combinations of targeted therapy and immunotherapy are becoming a central focus in HCC treatment research. The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) / Cas9 gene-editing system, with its high efficiency and specificity, offers a novel avenue for molecular therapy in HCC [79]. This technology allows for precise targeting and modification of oncogenes that are critically involved in HCC progression, thereby effectively suppressing tumor cell proliferation, invasion, and metastasis [79]. Recent studies have shown that CRISPR not only serves as a powerful tool for gene function validation and elucidation of oncogenic mechanisms but also holds promise in combination with immunotherapies—such as immune checkpoint inhibitors—to modulate the tumor immune microenvironment and enhance the antitumor efficacy of immune cells [79,80].
Moreover, patient responses to immune checkpoint blockade in HCC are highly heterogeneous, with a subset of individuals exhibiting immune tolerance or evasion [81]. Prior studies suggest that mutations in key driver genes such as TP53 and CTNNB1 can influence tumor immune phenotypes and therapeutic sensitivity [4]. Therefore, stratifying patients based on their mutational profiles and designing personalized immunotherapeutic regimens may improve clinical outcomes and reduce immune-related adverse events. The integration of gene-editing technologies with immune checkpoint inhibitors not only expands the therapeutic arsenal against HCC but also provides a robust theoretical and technical foundation for precision and individualized interventions.
Clinical Trial Design and Implementation
In designing clinical trials for HCC, accounting for patient heterogeneity and tumor complexity is essential [81]. Flexible trial frameworks, such as adaptive trial designs, allow for real-time modifications to treatment strategies based on interim results, thereby enhancing trial efficiency and relevance [82]. Moreover, integrating multi-omics data and AI-driven predictive modeling at the design phase can optimize patient selection and tailor treatment regimens accordingly [83]. Ensuring patient engagement and adherence during trial implementation is also critical for success. Researchers must design protocols that are patient-friendly and minimize barriers to participation, thus improving enrollment and compliance [84]. Collectively, these strategies can significantly advance clinical research in HCC and broaden access to effective, personalized treatment options.
Conclusion
Research on single-gene mutations in HCC plays a pivotal role in elucidating disease mechanisms, identifying biomarkers, and advancing personalized therapeutic strategies. The pathogenic effects of different mutations vary across populations, shaped by the interplay between genetic background and environmental factors. Integrated multi-omics analysis offers a powerful approach to dissect the complex biology of HCC and to uncover novel therapeutic targets. Future efforts should focus on bridging basic scientific discoveries with clinical application, thereby accelerating the implementation of precision medicine in the diagnosis and treatment of HCC.
To achieve this goal, greater emphasis should be placed on multidisciplinary collaboration, innovative clinical trial designs, and international data sharing frameworks. These strategies will be essential for overcoming translational barriers and ensuring the real-world applicability of mutation-informed precision oncology in HCC.
Acknowledgements
Funding
This study was supported by National Science Foundation of China (Grant No.82202973, 2023YFC2411404).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yang X, Wang H, Yu C. The Mechanism of APOBEC3B in Hepatitis B Virus Infection and HBV Related Hepatocellular Carcinoma Progression, Therapeutic and Prognostic Potential. Infect Drug Resist. 2024;17:4477-4486
2. Aliya S, Lee H, Alhammadi M. et al. An Overview on Single-Cell Technology for Hepatocellular Carcinoma Diagnosis. Int J Mol Sci. 2022;23(3):1402
3. Cucarull B, Tutusaus A, Rider P. et al. Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances. Cancers (Basel). 2022;14(3):621
4. Yang Y, Qu Y, Li Z. et al. Identification of Novel Characteristics in TP53-Mutant Hepatocellular Carcinoma Using Bioinformatics. Front Genet. 2022;13:874805
5. Shen J, Sun W, Liu J. et al. Metabolism-related signatures is correlated with poor prognosis and immune infiltration in hepatocellular carcinoma via multi-omics analysis and basic experiments. Front Oncol. 2023;13:1130094
6. Lehrich BM, Delgado ER, Yasaka TM. et al. Precision targeting of β-catenin induces tumor reprogramming and immunity in hepatocellular cancers. Nat Commun. 2025;16(1):5009
7. Hu P, Xu L, Liu Y. et al. Identification of molecular pattern and prognostic risk model based on ligand-receptor pairs in liver cancer. Front Immunol. 2023;14:1187108
8. He Y, Qi W, Xie X. et al. Identification and validation of a novel predictive signature based on hepatocyte-specific genes in hepatocellular carcinoma by integrated analysis of single-cell and bulk RNA sequencing. BMC Med Genomics. 2024;17(1):103
9. Cheng Y, Tang R, Li X. et al. LRP1B is a Potential Biomarker for Tumor Immunogenicity and Prognosis of HCC Patients Receiving ICI Treatment. J Hepatocell Carcinoma. 2022;9:203-220
10. Chen YY, Chen CS, Huang JF. et al. The obesity-related mutation gene on nonalcoholic fatty liver disease. Hum Genet. 2025;144(1):1-14
11. Liu M, Wen Y. Point-of-care testing for early-stage liver cancer diagnosis and personalized medicine: Biomarkers, current technologies and perspectives. Heliyon. 2024;10(19):e38444
12. von Felden J, Garcia-Lezana T, Schulze K. et al. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut. 2020;69(11):2025-2034
13. Li J, Bai L, Xin Z. et al. TERT-TP53 mutations: a novel biomarker pair for hepatocellular carcinoma recurrence and prognosis. Sci Rep. 2025;15(1):3620
14. Xu C, Xu Z, Zhang Y. et al. β-Catenin signaling in hepatocellular carcinoma. J Clin Invest. 2022;132(4):e154515
15. Cecchini MJ, Thwaites MJ, Talluri S. et al. A retinoblastoma allele that is mutated at its common E2F interaction site inhibits cell proliferation in gene-targeted mice. Mol Cell Biol. 2014;34(11):2029-45
16. Erdur E, Guzel Y, Yildirim OA. et al. Roles of metabolic tumor volume and total lesion glycolysis on 18F-FDG PET/CT, CA19-9 levels, and complete blood count parameters in predicting survival in patients with unresectable or metastatic pancreatic cancer. Ann Ital Chir. 2022;93:85-95
17. Nault JC, Ningarhari M, Rebouissou S. et al. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat Rev Gastroenterol Hepatol. 2019;16(9):544-558
18. Bala P, Singh AK, Kavadipula P. et al. Exome sequencing identifies ARID2 as a novel tumor suppressor in early-onset sporadic rectal cancer. Oncogene. 2021;40(4):863-874
19. Zhu C, Soto-Feliciano YM, Morris JP. et al. MLL3 regulates the CDKN2A tumor suppressor locus in liver cancer. Elife. 2023;12:e80854
20. Xu J, Lin H, Wu G. et al. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front Oncol. 2021;11:760971
21. Liu D, Li Q, Zang Y. et al. USP1 modulates hepatocellular carcinoma progression via the Hippo/TAZ axis. Cell Death Dis. 2023;14(4):264
22. Li A, Kibby D, Foo J. A comparison of mutation and amplification-driven resistance mechanisms and their impacts on tumor recurrence. J Math Biol. 2023;87(4):59
23. Hwang YJ, Lee Y, Yu SJ. et al. Correlation between CTNNB1 mutation status and tumour phenotype in hepatitis B virus-related hepatocellular carcinoma. Histopathology. 2025;86(4):547-558
24. Gorji L, Brown ZJ, Pawlik TM. Mutational Landscape and Precision Medicine in Hepatocellular Carcinoma. Cancers (Basel). 2023;15(17):4221
25. Elmas A, Lujambio A, Huang KL. Proteomic Analyses Identify Therapeutic Targets in Hepatocellular Carcinoma. Front Oncol. 2022;12:814120
26. Yang F, Deng K, Zheng H. et al. Progress of targeted and immunotherapy for hepatocellular carcinoma and the application of next-generation sequencing. Ann Hepatol. 2022;27(2):100677
27. Testa U. Recent developments in molecular targeted therapies for hepatocellular carcinoma in the genomic era. Expert Rev Mol Diagn. 2024;24(9):803-827
28. Yan T, Yu L, Zhang N. et al. The advanced development of molecular targeted therapy for hepatocellular carcinoma. Cancer Biol Med. 2022;19(6):802-17
29. Chan YT, Zhang C, Wu J. et al. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol Cancer. 2024;23(1):189
30. Pezzuto F, Buonaguro L, Buonaguro FM. et al. The Role of Circulating Free DNA and MicroRNA in Non-Invasive Diagnosis of HBV- and HCV-Related Hepatocellular Carcinoma. Int J Mol Sci. 2018;19(4):1007
31. Lee HW, Kim E, Cho KJ. et al. Applications of molecular barcode sequencing for the detection of low-frequency variants in circulating tumour DNA from hepatocellular carcinoma. Liver Int. 2022;42(10):2317-2326
32. Tang Q, Hu G, Sang Y. et al. Therapeutic targeting of PLK1 in TERT promoter-mutant hepatocellular carcinoma. Clin Transl Med. 2024;14(5):e1703
33. Liu LJ, Xie SX, Chen YT. et al. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J Gastroenterol. 2016;22(33):7486-99
34. Ding Y, Yao J, Wen M. et al. The potential, analysis and prospect of ctDNA sequencing in hepatocellular carcinoma. PeerJ. 2022;10:e13473
35. Ahmed E, El-Dien A N, Sabet S. et al. Detection of MET gene somatic mutations in hepatocellular carcinoma of Egyptian patients using next-generation sequencing. Biomarkers. 2023;28(4):379-386
36. Sun Y, Kong X, Yu J. et al. Characterization of genomic clones by targeted deep sequencing of ctDNA to monitor liver cancer. Transl Cancer Res. 2021;10(10):4387-4402
37. Wang HC, Yin WX, Jiang M. et al. Function and biomedical implications of exosomal microRNAs delivered by parenchymal and nonparenchymal cells in hepatocellular carcinoma. World J Gastroenterol. 2023;29(39):5435-5451
38. Luo X, Jiao L, Guo Q. et al. Diagnostic model for hepatocellular carcinoma using small extracellular vesicle-propagated miRNA signatures. Front Mol Biosci. 2024;11:1419093
39. Nault JC, Zucman-Rossi J. TERT promoter mutations in primary liver tumors. Clin Res Hepatol Gastroenterol. 2016;40(1):9-14
40. Ko E, Seo HW, Jung G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology. 2018;67(4):1378-1391
41. Ko E, Seo HW, Jung ES. et al. PI3Kδ Is a Therapeutic Target in Hepatocellular Carcinoma. Hepatology. 2018;68(6):2285-2300
42. Manni W, Min W. Signaling pathways in the regulation of cancer stem cells and associated targeted therapy. MedComm (2020). 2022;3(4):e176
43. Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51(5):1357-1369
44. Hagel M, Miduturu C, Sheets M. et al. First Selective Small Molecule Inhibitor of FGFR4 for the Treatment of Hepatocellular Carcinomas with an Activated FGFR4 Signaling Pathway. Cancer Discov. 2015;5(4):424-37
45. Tao Z, Cui Y, Xu X. et al. FGFR redundancy limits the efficacy of FGFR4-selective inhibitors in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2022;119(40):e2208844119
46. Lu X, Chen H, Patterson AV. et al. Fibroblast Growth Factor Receptor 4 (FGFR4) Selective Inhibitors as Hepatocellular Carcinoma Therapy: Advances and Prospects. J Med Chem. 2019;62(6):2905-2915
47. Ferrarese A, Sciarrone SS, Pellone M. et al. Current and future perspective on targeted agents and immunotherapies in hepatocellular carcinoma. Minerva Gastroenterol (Torino). 2021;67(1):4-10
48. Li L, Rao X, Wen Z. et al. Implications of driver genes associated with a high tumor mutation burden identified using next-generation sequencing on immunotherapy in hepatocellular carcinoma. Oncol Lett. 2020;19(4):2739-2748
49. Yin L, Zhou L, Xu R. Identification of Tumor Mutation Burden and Immune Infiltrates in Hepatocellular Carcinoma Based on Multi-Omics Analysis. Front Mol Biosci. 2021;7:599142
50. Zhang P, An Z, Sun C. et al. FLG Gene Mutation Up-regulates the Abnormal Tumor Immune Response and Promotes the Progression of Prostate Cancer. Curr Pharm Biotechnol. 2022;23(14):1658-1670
51. Yu J, Ling S, Hong J. et al. TP53/mTORC1-mediated bidirectional regulation of PD-L1 modulates immune evasion in hepatocellular carcinoma. J Immunother Cancer. 2023;11(11):e007479
52. Hong T, Su W, Pan Y. et al. Aging-related features predict prognosis and immunotherapy efficacy in hepatocellular carcinoma. Front Immunol. 2022;13:951459
53. Murai H, Kodama T, Maesaka K. et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology. 2023;77(1):77-91
54. Guo Y, Yang J, Ren K. et al. The Heterogeneity of Immune Cell Infiltration Landscape and Its Immunotherapeutic Implications in Hepatocellular Carcinoma. Front Immunol. 2022;13:861525
55. Zheng J, Shao M, Yang W. et al. Benefits of combination therapy with immune checkpoint inhibitors and predictive role of tumour mutation burden in hepatocellular carcinoma: A systematic review and meta-analysis. Int Immunopharmacol. 2022;112:109244
56. Gabbia D, De Martin S. Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. Int J Mol Sci. 2023;24(4):3441
57. Zeng Z, Yang B, Liao Z. Biomarkers in Immunotherapy-Based Precision Treatments of Digestive System Tumors. Front Oncol. 2021;11:650481
58. Cavalli M, Diamanti K, Pan G. et al. A Multi-Omics Approach to Liver Diseases: Integration of Single Nuclei Transcriptomics with Proteomics and HiCap Bulk Data in Human Liver. OMICS. 2020;24(4):180-194
59. Deng K, Xing J, Xu G. et al. Urinary biomarkers for hepatocellular carcinoma: current knowledge for clinicians. Cancer Cell Int. 2023;23(1):239
60. Carneiro CFD, Drude N, Hülsemann M. et al. Mapping strategies towards improved external validity in preclinical translational research. Expert Opin Drug Discov. 2023;18(11):1273-1285
61. Torbenson M, McCabe CE, O'Brien DR. et al. Morphological heterogeneity in beta-catenin-mutated hepatocellular carcinomas: implications for tumor molecular classification. Hum Pathol. 2022;119:15-27
62. Yang H, Lin H, Sun X. Multiscale modeling of drug resistance in glioblastoma with gene mutations and angiogenesis. Comput Struct Biotechnol J. 2023;21:5285-5295
63. Fujimoto A, Furuta M, Totoki Y. et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016;48(5):500-9
64. Rao CV, Asch AS, Yamada HY. Frequently mutated genes/pathways and genomic instability as prevention targets in liver cancer. Carcinogenesis. 2017;38(1):2-11
65. Hu S, Liu Y, Yang Q. et al. Liquid biopsy using cell-free DNA in the early diagnosis of hepatocellular carcinoma. Invest New Drugs. 2023;41(3):532-538
66. Manea I, Iacob R, Iacob S. et al. Liquid biopsy for early detection of hepatocellular carcinoma. Front Med (Lausanne). 2023;10:1218705
67. Kopystecka A, Patryn R, Leśniewska M. et al. The Use of ctDNA in the Diagnosis and Monitoring of Hepatocellular Carcinoma-Literature Review. Int J Mol Sci. 2023;24(11):9342
68. Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut. 2018;67(12):2204-2212
69. Korhan P, Tercan Avcı S, Yılmaz Y. et al. Role of Biobanks for Cancer Research and Precision Medicine in Hepatocellular Carcinoma. J Gastrointest Cancer. 2021;52(4):1232-1247
70. Papadakos SP, Stergiou IE, Gkolemi N. et al. Unraveling the Significance of EPH/Ephrin Signaling in Liver Cancer: Insights into Tumor Progression and Therapeutic Implications. Cancers (Basel). 2023;15(13):3434
71. Liu XN, Cui DN, Li YF. et al. Multiple "Omics" data-based biomarker screening for hepatocellular carcinoma diagnosis. World J Gastroenterol. 2019;25(30):4199-4212
72. Ye J, Lin Y, Liao Z. et al. Single cell-spatial transcriptomics and bulk multi-omics analysis of heterogeneity and ecosystems in hepatocellular carcinoma. NPJ Precis Oncol. 2024;8(1):262
73. Bourganou MV, Chondrogianni ME, Kyrou I. et al. Unraveling Metabolic Dysfunction-Associated Steatotic Liver Disease Through the Use of Omics Technologies. Int J Mol Sci. 2025;26(4):1589
74. Ahn HR, Kim S, Baek GO. et al. Effect of Sortilin1 on promoting angiogenesis and systemic metastasis in hepatocellular carcinoma via the Notch signaling pathway and CD133. Cell Death Dis. 2024;15(8):634
75. Liu D, Li H, Dong H. et al. Spatial Multiomics Analysis Reveals Only Minor Genetic and Epigenetic Changes in Human Liver Cancer Stem-Like Cells Compared With Other Tumor Parenchymal Cells. Front Cell Dev Biol. 2022;10:810687
76. Varga P, Obeidat M, Máté V. et al. From simple factors to artificial intelligence: evolution of prognosis prediction in childhood cancer: a systematic review and meta-analysis. EClinicalMedicine. 2024;78:102902
77. Gschwind A, Ossowski S. AI Model for Predicting Anti-PD1 Response in Melanoma Using Multi-Omics Biomarkers. Cancers (Basel). 2025;17(5):714
78. Zhao C, Zhang Z, Tao J. A Novel Ferroptosis-Related Signature for Prediction of Prognosis, Immune Profiles and Drug Sensitivity in Hepatocellular Carcinoma Patients. Curr Oncol. 2022;29(10):6992-7011
79. Palaz F, Ozsoz M, Zarrinpar A. et al. CRISPR in Targeted Therapy and Adoptive T Cell Immunotherapy for Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2024;11:975-995
80. Zhen S, Qiang R, Lu J. et al. CRISPR/Cas9-HPV-liposome enhances antitumor immunity and treatment of HPV infection-associated cervical cancer. J Med Virol. 2023;95(1):e28144
81. Chen X, Wu S, He H. et al. G2M-checkpoint related immune barrier structure and signature for prognosis and immunotherapy response in hepatocellular carcinoma: insights from spatial transcriptome and machine learning. J Transl Med. 2025;23(1):202
82. Cervantes L, Jolles MP, Rizzolo K. et al. Contributions from the Implementation Science Field to Clinical Trial Design for Kidney Research: Hybrid Effectiveness-Implementation Studies. J Am Soc Nephrol. 2024;36(7):1442-5
83. Theodros E, Nagaraju GP. Artificial Intelligence and Precision Medicine: Outcome of Immunotherapy in Hepatocellular Carcinoma. Crit Rev Immunol. 2022;42(6):1-8
84. Samper-Ternent R, Silveira SL, Stevens A. et al. Considerations When Designing and Implementing Pragmatic Clinical Trials That Include Older Hispanics. Ethn Dis. 2024;33(2-3):76-83
Author contact
![]() Corresponding authors: Congling Xin, E-mail: Congling0828com; Yu Zhou, E-mail: zy12534com.cn; Xiaoyi Ding, E-mail: dxy10456com.cn.
Corresponding authors: Congling Xin, E-mail: Congling0828com; Yu Zhou, E-mail: zy12534com.cn; Xiaoyi Ding, E-mail: dxy10456com.cn.

 Global reach, higher impact
Global reach, higher impact