3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(13):3242-3249. doi:10.7150/ijms.112883 This issue Cite
Research Paper
Association of long noncoding RNA MEG3 genetic variants with the risk of diabetic neuropathy
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
2. Department of Neurology, Chung Shan Medical University Hospital, Taichung, Taiwan
3. School of Medicine, Chung Shan Medical University, Taichung, Taiwan
4. Department of Family and Community Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
5. Department of Biomedical Sciences, Chung Shan Medical University, Taichung, Taiwan
6. Department of Mathematics and Statistics, Florida Atlantic University, Boca Raton, Florida, USA
7. Whole-Genome Research Core Laboratory of Human Diseases, Chang Gung Memorial Hospital, Keelung, Taiwan
8. Department of Medical Biotechnology and Laboratory Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan
9. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
Received 2025-2-26; Accepted 2025-6-27; Published 2025-7-10
Abstract
Diabetic neuropathy (DN), known to result from an interplay of acquired and genetic factors, is a common comorbidity of diabetes characterized by various forms of nerve damage. Maternally expressed gene 3 (MEG3) is an imprinted, non-coding RNA gene originally identified as a tumor suppressor. Recently, dysregulation of MEG3 levels was also observed in various neurodegenerative diseases. In this study, we aimed to investigate the potential association of MEG3 gene polymorphisms with the risk for DN through genotyping five single-nucleotide polymorphisms (SNPs) of MEG3 gene (rs4081134, rs10144253, rs7158663, rs3087918, and rs11160608) between 712 DN patients and 820 controls (diabetic individuals without neuropathic conditions). Our survey revealed a gender-specific association of rs7158663 with DN. We found that rs7158663 of MEG3 gene was associated with an increased risk for DN in diabetic women (GA vs GG, AOR=1.604, p=0.005; GA+AA vs GG, AOR=1.547, p=0.007). Nevertheless, such genetic association was particularly seen in women but not detected in diabetic males. Moreover, a higher level of LDL-cholesterol was noted in female DN patients who carry homozygous major allele of rs7158663 (GG) than in those bearing at least one minor allele (GA+AA) (p=0.016), suggesting an effect of rs7158663 on modulating lipoprotein levels. Taken together, our results demonstrate a link of MEG3 gene variants with dyslipidemia and neuropathic conditions in diabetic patients in a gender-specific manner.
Keywords: Maternally expressed gene 3, single-nucleotide polymorphism, diabetic neuropathy, LDL-cholesterol
Introduction
Diabetic neuropathy (DN), the most common comorbidity of diabetic individuals, is a debilitating illness that substantially affects patients by causing recurrent falls and severe pain, thereby significantly reducing quality of life [1]. Damages to both central and peripheral nervous system in DN patients lead to various clinical manifestations. Among them, distal symmetric polyneuropathy (DSP) is the most frequently occurring type of DN, mainly presenting with symptoms like distal sensory loss, pain, tingling on the skin, and foot ulceration that could likely require amputation [2]. To date, the sole disease-modifying treatment of DN is improved glycemic management, as pain control is commonly used as a supplementary therapy to considerably alleviate the neuropathic conditions of patients [3]. Extensive research has shown that the pathogenic mechanisms of DN comprise an intricacy of dysregulated metabolism, inflammation, microvascularization and neurodegeneration [4]. This to a great degree accounts for the lack of promising therapeutic choices against DN. Numerous etiological factors of DN have been identified, with high levels of blood sugar being the most crucial contributor to this devastating disorder [5]. Besides several non-modifiable causes of disease (such as gender, chronological age, body height, and genetic background), additional likely-modifiable risks consist of hyperlipidemia, obesity, hypertension, and habitual use of cigarette and alcohol. Such complex interplay of disease etiologies elevates the heterogeneity in treatment outcomes, thus prompting us to discover novel therapeutic targets to improve DN prevention and management.
Currently, a definitive link between genetic factors and the risk of developing both diabetes and its comorbidities has been proposed [6]. A list of genetic variations has been assessed as susceptibility factors for DN, with the majority of these factors rendering a direct impact on pathological mechanisms such as inflammatory dysfunction, immune regulation, impaired neurovascularization, production of reactive oxygen species, modulation of glycosylated peptides, and functional activity of noncoding RNA [7]. Thus far, association of DN with MTHFR (methylenetetrahydrofolate reductase), ACE (angiotensin I converting enzyme), and VEGF (vascular endothelial growth factor) gene polymorphisms has been replicated with large sample sizes in diverse populations [8]. Other than protein coding genes, a role of microRNAs (miRNAs) [9] and long noncoding RNAs (lncRNAs) [10] in DN pathogenesis has been noted. Nevertheless, the highly heterogeneous nature of DN genetics merely provides a partial explanation on why some subjects are susceptible to neuropathic conditions and others not [11]. Therefore, a better understanding on the genetic architecture of DN may offer insight into its diagnostic and therapeutic progress.
Maternally expressed gene 3 (MEG3), originally discovered as a tumor suppressor [12], is a maternally expressed, imprinted long noncoding RNA gene. It acts as a scaffold, sponge, or signal hub to mediate cancer hallmarks, and its dysfunction has been linked to poor prognosis and drug resistance in malignant diseases [13]. In addition to tumorigenesis, fluctuations in MEG3 expression levels have been correlated with the development of many diabetes-related complications [14]. Moreover, Zheng et al. reported that lncRNA MEG3 levels are abnormally upregulated in gestational diabetes mellitus [15]. It was demonstrated that MEG3 expression was essential for insulin production and secretion in pancreatic β-cells, and downregulation of MEG3 was observed in mouse models of diabetes [16]. Additionally, MEG3 has been reported to modulate lipid metabolism by interacting with key signaling pathways such as AMP-activated protein kinase (AMPK) and sterol regulatory element-binding proteins (SREBPs), both of which play critical roles in regulating lipid biosynthesis and cholesterol homeostasis [17]. Dysregulation of these pathways can result in elevated LDL-C levels and increased lipid accumulation, thereby contributing to vascular and neural damage in diabetes. Not only associated with hyperglycemic conditions, MEG3 levels are also dysregulated in various neurodegenerative diseases [18]. Specifically, MEG3 was found to be highly expressed in neurons in the cortex of the brain [19] and plays a crucial role in learning, memory, and motion functions [20]. These findings collectively suggest a connection of MEG3 to neuropathic symptoms of diabetic individuals. As yet, the impact of MEG3 gene polymorphisms on the development of DN is largely unclear, although associations between MEG3 gene variations and cancer risks have been extensively studied [21]. Here, we attempted to assess the potential effect of MEG3 gene polymorphisms on the risk of developing DN.
Materials and Methods
Study cohorts
A total of 712 DN patients were enrolled to evaluate the influence of MEG3 gene polymorphisms on the development of DN. Definition of neuropathy was set as a score of ≥ 4 based on the Michigan Neuropathy Screening Instrument (MNSI) [22]. Sensory functions of median and peroneal nerves were evaluated based on a current perception threshold (CPT) via the Neurometer® instrument (Neurotron, Baltimore, MD, USA), with a CPT of < 6 or > 13 being considered abnormal [23]. Besides, 820 diabetic subjects without neuropathy were recruited as the control group. Demographic and laboratory data concerning age, gender, diabetic condition, HbA1c, serum creatinine, GFR, and lipid profiles (including total cholesterol, HDL cholesterol, LDL cholesterol triglycerides, and TC/HDL ratio) were obtained by from the Department of Clinical Laboratory at Chung Shan Medical University Hospital. This study was approved by the institutional review board (CSMUH No: CS2-22190) in Chung Shan Medical University Hospital, Taichung, Taiwan. Clinical data concerning kidney function, and glycemic and lipidemic status, as well as informed consent were obtained from each participant.
Genotype determination
Five loci of MEG3 gene (rs4081134, rs10144253, rs7158663, rs3087918, and rs11160608) selected according to their putative configuration of disease susceptibility [24-27] were genotyped in this survey. Moreover, MEG3 rs7158663 has been shown to alter the RNA secondary structure of MEG3, thereby influencing its interactions with miRNAs and ultimately affecting the expression of its target miRNAs and/or MEG3 itself [28]. Isolation of genomic DNA was conducted by using QIAamp DNA Blood Mini kit (Qiagen, Valencia, CA, USA), and biallelic discrimination of five loci was performed via the TaqMan assay (Applied Biosystems, Foster City, CA, USA). Each assay included non-template and known genotype controls in every run to detect reagent contamination and maintain quality control. Determination of genotypes was carried out by using SDS version 3.0 software.
Statistical analysis
Comparisons of demographic and clinical parameters between two study groups were conducted with the Mann-Whitney U test. Correlation of gene polymorphisms with the development of DN was assessed by multiple logistic regression analyses, jointed with the adjustment for possible confounding factors. Moreover, in this study, five MEG3 SNPs were evaluated, and the Bonferroni correction was applied to account for multiple comparisons. The significance threshold was adjusted accordingly by dividing the conventional alpha level (0.05) by the number of SNPs tested (n = 5), resulting in an adjusted p-value threshold of 0.01. Levels of low-density lipoprotein (LDL)-cholesterol between genotypic groups were compared by using t-test. Variations in MEG3 expression among genotypic groups from the Genotype-Tissue Expression (GTEx) database [29] were calculated with one-way ANOVA.
Results
Subject characteristics
To explore the association of MEG3 gene polymorphisms with the development of DN, 712 patients were enrolled to compare with 820 controls (diabetic individuals without neuropathic conditions). Demographic and clinical features of two study cohorts were assessed (Table 1). The age at enrollment and the age at diabetes onset in the DN group (63.08 ± 11.34 and 52.23 ± 10.83 years, respectively) were higher than those in the control group (59.84 ± 12.72 and 50.55 ± 12.08 years, respectively), while no significant difference in gender distribution was observed between the two groups. DN cases had a longer duration of diabetes than did the controls. Moreover, as compared with the controls, higher levels of renal function loss (impaired glomerular filtration rate) were detected in the DN group. Two measurements for the risk of cardiovascular disorders, LDL-cholesterol and ratio of total cholesterol to HDL-cholesterol, were significantly lower in the DN group.
Clinical and laboratory characteristics of diabetic patients with neuropathy and with normal neurologic function.
| Variable | Non-Diabetic Neuropathy (N=820) | Diabetic Neuropathy (N=712) | p value |
|---|---|---|---|
| Age at enrollment (years) | 59.84 ± 12.72 | 63.08 ± 11.34 | <0.001 |
| Onset of Age (years) | 50.55 ± 12.08 | 52.23 ± 10.83 | 0.005 |
| Male gender [n (%)] | 451 (55.0%) | 370 (52.0%) | 0.235 |
| Duration of diabetes (years) | 9.29 ± 7.93 | 10.84 ± 7.44 | <0.001 |
| Body mass index [kg/m2] | 26.12 ± 4.40 | 25.98 ± 4.60 | 0.526 |
| HbA1c [% (mmol/mol)] | 7.24 ± 1.37 | 7.22 ± 1.37 | 0.728 |
| Serum creatinine [mg/dL] | 1.11 ± 1.18 | 1.11 ± 0.94 | 0.900 |
| Glomerular filtration rate [ml/min] | 80.87 ± 36.26 | 76.12 ± 30.41 | 0.006 |
| Total cholesterol [mmol/L] | 163.31 ± 47.11 | 158.86 ± 41.38 | 0.052 |
| HDL cholesterol [μmol/L] | 45.03 ± 12.74 | 46.17 ± 13.42 | 0.093 |
| LDL cholesterol [μmol/L] | 87.98 ± 32.60 | 83.14 ± 28.96 | 0.002 |
| Triglycerides, [μmol/L] | 152.36 ± 214.81 | 135.20 ± 116.69 | 0.058 |
| TC/HDL ratio | 3.88 ± 2.05 | 3.67 ± 1.51 | 0.028 |
Gender-specific association between MEG3 gene polymorphism and DN risk
To examine the connection between MEG3 gene variations and the risk for DN, genotypes of five single-nucleotide polymorphisms (SNPs) of the MEG3 gene (rs4081134, rs10144253, rs7158663, rs3087918, and rs11160608) were analyzed in our participants. Nevertheless, we did not observe any significant association of these SNPs with the risk for DN from our cohorts (Table 2). Notably, further stratification revealed a correlation of rs7158663 with DN in women (Table 3). We found that rs7158663 of MEG3 gene was associated with an increased risk for DN in diabetic females (GA vs GG, AOR=1.604, p=0.005; GA+AA vs GG, AOR=1.547, p=0.007). Yet, this genetic association was particularly seen in female subjects with diabetes but not observed in diabetic males (Table 4). These findings suggest a gender-specific interaction of MEG3 gene polymorphisms with the occurrence of neuropathic conditions in diabetic individuals.
Association of MEG3 rs7158663 genotypes with LDL-cholesterol levels in DN patients
We subsequently tested whether rs7158663 genotypes influence the clinical parameters of DN cases to gain extra relevance of DN-associated MEG3 SNPs in DN.
Association of MEG3 genotypic frequencies with the risk of diabetic neuropathy.
| Variable | Non-Diabetic Neuropathy (N=820) | Diabetic Neuropathy (N=712) | AOR (95% CI) | p value |
|---|---|---|---|---|
| rs4081134 | ||||
| GG | 476 (58.0%) | 397 (55.8%) | 1.000 (reference) | |
| GA | 277 (33.8%) | 266 (37.4%) | 1.157 (0.929-1.441) | p=0.194 |
| AA | 67 (8.2%) | 49 (6.8%) | 0.898 (0.602-1.341) | p=0.599 |
| GA+AA | 344 (42.0%) | 315 (44.2%) | 1.107 (0.899-1.362) | p=0.338 |
| rs10144253 | ||||
| TT | 226 (27.6%) | 209 (29.4%) | 1.000 (reference) | |
| TC | 399 (48.7%) | 348 (48.9%) | 0.956 (0.750-1.219) | p=0.717 |
| CC | 195 (23.7%) | 155 (21.7%) | 0.878 (0.657-1.174) | p=0.381 |
| TC+CC | 594 (72.4%) | 503 (70.6%) | 0.931 (0.741-1.169) | p=0.583 |
| rs7158663 | ||||
| GG | 488 (59.5%) | 401 (56.3%) | 1.000 (reference) | |
| GA | 292 (35.6%) | 275 (38.6%) | 1.146 (0.923-1.423) | p=0.218 |
| AA | 40 (4.9%) | 36 (5.1%) | 1.047 (0.644-1.703) | p=0.853 |
| GA+AA | 332 (40.5%) | 311 (43.7%) | 1.134 (0.920-1.397) | p=0.238 |
| rs3087918 | ||||
| TT | 264 (32.2%) | 213 (29.9%) | 1.000 (reference) | |
| TG | 401 (48.9%) | 357 (50.1%) | 1.095 (0.865-1.386) | p=0.449 |
| GG | 155 (18.9%) | 142 (20.0%) | 1.208 (0.895-1.630) | p=0.216 |
| TG+GG | 556 (67.8%) | 499 (70.1%) | 1.125 (0.901-1.406) | p=0.298 |
| rs11160608 | ||||
| AA | 228 (27.8%) | 194 (27.2%) | 1.000 (reference) | |
| AC | 401 (48.9%) | 356 (50.0%) | 1.029 (0.806-1.313) | p=0.820 |
| CC | 191 (23.3%) | 162 (22.8%) | 1.036 (0.774-1.388) | p=0.810 |
| AC+CC | 592 (72.2%) | 518 (72.8%) | 1.031 (0.819-1.298) | p=0.794 |
The adjusted odds ratio (AOR) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for age, duration of diabetes, glomerular filtration rate, LDL cholesterol, and TC/HDL ratio.
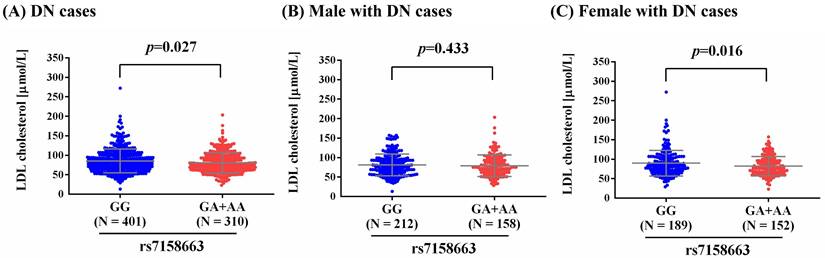
As metabolic imbalance of lipid exacerbated neuropathic conditions at the whole-organism scale, and management of such dysregulation led to prevention of distinct modalities of DN in murine models [30], we observed a higher level of LDL-cholesterol in total DN patients and female DN patients who carry homozygous major allele of rs7158663 (GG) than in those bearing at least one minor allele (GA+AA) (p=0.027; p=0.016) (Figure 1). Such association of rs7158663 on LDL-cholesterol levels was exclusively seen in total DN patients and female DN cases but not in male DN patients. Furthermore, we demonstrated variations of MEG3 expression in multiple portions of brain tissues among distinct genotypic groups of rs7158663 in the Genotype-Tissue Expression (GTEx) database (Figure 2). These data indicate a gender-specific effect of rs7158663 on modulating lipoprotein levels in diabetic individuals with neuropathic symptoms.
Association of MEG3 genotypic frequencies with the risk of diabetic neuropathy in female group.
| Variable | Non-Diabetic Neuropathy (N=369) | Diabetic Neuropathy (N=342) | AOR (95% CI) | p value |
|---|---|---|---|---|
| rs4081134 | ||||
| GG | 209 (56.6%) | 195 (57.0%) | 1.000 (reference) | |
| GA | 128 (34.7%) | 116 (33.9%) | 0.973 (0.701-1.351) | p=0.870 |
| AA | 32 (8.7%) | 31 (9.1%) | 1.025 (0.596-1.765) | p=0.929 |
| GA+AA | 160 (43.4%) | 147 (43.0%) | 0.984 (0.724-1.336) | p=0.916 |
| rs10144253 | ||||
| TT | 105 (28.5%) | 99 (28.9%) | 1.000 (reference) | |
| TC | 174 (47.2%) | 173 (50.6%) | 1.069 (0.748-1.528) | p=0.713 |
| CC | 90 (24.3%) | 70 (20.5%) | 0.859 (0.558-1.324) | p=0.491 |
| TC+CC | 264 (71.5%) | 243 (71.1%) | 0.989 (0.741-1.400) | p=0.968 |
| rs7158663 | ||||
| GG | 236 (64.0%) | 189 (55.3%) | 1.000 (reference) | |
| GA | 114 (30.9%) | 136 (39.8%) | 1.604 (1.156-2.226) | p=0.005* |
| AA | 19 (5.1%) | 17 (4.9%) | 1.191 (0.579-2.451) | p=0.634 |
| GA+AA | 133 (36.0%) | 153 (44.7%) | 1.547 (1.129-2.118) | p=0.007* |
| rs3087918 | ||||
| TT | 116 (31.4%) | 109 (31.9%) | 1.000 (reference) | |
| TG | 189 (51.2%) | 155 (45.3%) | 1.395 (0.901-2.161) | p=0.135 |
| GG | 64 (17.4%) | 78 (22.8%) | 0.887 (0.627-1.254) | p=0.496 |
| TG+GG | 253 (68.6%) | 233 (68.1%) | 1.011 (0.730-1.400) | p=0.948 |
| rs11160608 | ||||
| AA | 105 (28.5%) | 97 (28.4%) | 1.000 (reference) | |
| AC | 181 (49.1%) | 155 (45.3%) | 0.947 (0.661-1.358) | p=0.768 |
| CC | 83 (22.4%) | 90 (26.3%) | 1.258 (0.825-1.918) | p=0.286 |
| AC+CC | 264 (71.5%) | 245 (71.6%) | 1.042 (0.744-1.459) | p=0.810 |
The adjusted odds ratio (AOR) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for age, duration of diabetes, glomerular filtration rate, LDL cholesterol, and TC/HDL ratio.
Discussion
A growing body of evidence has exhibited that the intricate etiologies of DN are affected by a combination of acquired and inherited risk factors [31]. In this work, we showed a link of MEG3 rs7158663 to the risk for DN in diabetic females. Moreover, association between rs7158663 genotypes and LDL-cholesterol levels was observed in female DN patients, revealing a correlation of MEG3 gene polymorphisms with dyslipidemia and neuropathic conditions in diabetic patients in a gender-specific manner.
Association of MEG3 genotypic frequencies with the risk of diabetic neuropathy in male group.
| Variable | Non-Diabetic Neuropathy (N=451) | Diabetic Neuropathy (N=370) | AOR (95% CI) | p value |
|---|---|---|---|---|
| rs4081134 | ||||
| GG | 267 (59.2%) | 202 (54.6%) | 1.000 (reference) | |
| GA | 149 (33.0%) | 150 (40.5%) | 1.345 (0.998-1.811) | p=0.051 |
| AA | 35 (7.8%) | 18 (4.9%) | 0.696 (0.378-1.284) | p=0.247 |
| GA+AA | 184 (40.8%) | 168 (45.4%) | 1.224 (0.921-1.626) | p=0.164 |
| rs10144253 | ||||
| TT | 121 (26.8%) | 110 (29.7%) | 1.000 (reference) | |
| TC | 225 (49.9%) | 175 (47.3%) | 0.867 (0.622-1.209) | p=0.401 |
| CC | 105 (23.3%) | 85 (23.0%) | 0.907 (0.612-1.345) | p=0.628 |
| TC+CC | 330 (73.2%) | 260 (70.3%) | 0.880 (0.6474-1.202) | p=0.422 |
| rs7158663 | ||||
| GG | 252 (55.9%) | 212 (57.3%) | 1.000 (reference) | |
| GA | 178 (39.5%) | 139 (37.6%) | 0.894 (0.666-1.200) | p=0.456 |
| AA | 21 (4.7%) | 19 (5.1%) | 0.936 (0.480-1.823) | p=0.846 |
| GA+AA | 199 (44.1%) | 158 (42.7%) | 0.899 (0.676-1.194) | p=0.461 |
| rs3087918 | ||||
| TT | 148 (32.8%) | 104 (28.1%) | 1.000 (reference) | |
| TG | 212 (47.0%) | 202 (54.6%) | 1.299 (0.940-1.795) | p=0.113 |
| GG | 91 (20.2%) | 64 (17.3%) | 1.045 (0.688-1.589) | p=0.835 |
| TG+GG | 303 (67.2%) | 266 (71.9%) | 1.227 (0.902-1.669) | p=0.192 |
| rs11160608 | ||||
| AA | 123 (27.3%) | 97 (26.2%) | 1.000 (reference) | |
| AC | 220 (48.8%) | 201 (54.3%) | 1.103 (0.789-1.542) | p=0.567 |
| CC | 108 (23.9%) | 72 (19.5%) | 0.853 (0.565-1.287) | p=0.448 |
| AC+CC | 328 (72.7%) | 273 (73.8%) | 1.024 (0.745-1.408) | p=0.885 |
The adjusted odds ratio (AOR) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for age, duration of diabetes, glomerular filtration rate, LDL cholesterol, and TC/HDL ratio.
In addition to conferring the susceptibility to various types of cancer [32-34], MEG3 rs7158663 was recently shown to be associated with renal [35] and ocular complications [36] of diabetes. It has been demonstrated that MEG3 expression is essential for insulin production in pancreatic β-cells, and its downregulation has been observed in both mouse models of diabetes [16] and human islets from diabetic patients [37], highlighting a regulatory role of the long noncoding RNA MEG3 in glucose metabolism. In addition to its association with hyperglycemic conditions, dysregulation of MEG3 expression has been implicated in the pathogenesis of various cardio-cerebrovascular and neurodegenerative diseases, largely through its involvement in apoptosis, inflammation, and oxidative stress pathways [38]. Emerging evidence suggests that these mechanisms—particularly oxidative stress and chronic low-grade inflammation—are central to the development and progression of diabetic neuropathy DN [39]. In neuropathic conditions, MEG3 has been shown to regulate the expression of several pro-apoptotic and inflammatory genes by acting as a competing endogenous RNA (ceRNA), modulating microRNA availability and thereby affecting downstream signaling pathways [40]. For example, MEG3 can act as a molecular sponge for miR-34a and miR-181a, both of which have established roles in neuronal injury and survival [41]. Through this mechanism, MEG3 may contribute to Schwann cell dysfunction, axonal degeneration, and impaired neuronal repair observed in DN. In a bioinformatics analysis of lncRNA structures, MEG3 rs7158663 was shown to change the MEG3 RNA folding conformation and influence miRNA-MEG3 interactions, ultimately affecting the expression of its target miRNAs and/or expression of MEG3 [28]. Consistent with our finding that MEG3 expression was fluctuated among distinct genotypic groups of rs7158663 in multiple brain parts, MEG3 rs7158663 likely acts as an expression quantitative trait locus (eQTL) to manage its putative configuration of disease susceptibility. In addition, another bioinformatic prediction reported that the polymorphic allele (A) of MEG3 rs7158663 has the potential to mediate the binding of miR-4307 and miR-1265 to MEG3 [32]. As miRNAs are functionally involved in the initiation, progression, and treatment of DN [42], it is plausible that alterations in MEG3's capacity to sponge miRNAs by virtue of gene polymorphisms contribute to the neuropathic disease state of diabetic patients. Hence, a tissue-specific transcriptional profile due to MEG3 gene polymorphisms can be created, leading to long-term damages to peripheral nerves of diabetic individuals.
Furthermore, we found that rs7158663 was associated with LDL-cholesterol levels and the development of DN in a gender-specific manner. This sex-specific genetic architecture is commonly present in numerous human illnesses, such as autoimmune, hypertensive heart, and allergic disorders [43]. It is recognized that the cellular environments differ substantially in men and women, given known variations in their hormonal milieu and transcriptional profiles [44]. Besides morphological differences and neurobiological circuits, sex has measurable effects on a variety of quantitative traits [45]. Among these sexually dimorphic traits, raised blood pressure, lipoprotein levels, and body height are considered potential risks of DN. Thus, the gender, as an environmental factor, might combine with MEG3 gene polymorphisms, resulting in differences of allelic effects between men and women. The effect of MEG3 gene variations on the susceptibility to DN could be another instance of genotype-gender interactions in human diseases, just as observations on variants of RELN gene with schizophrenia [46] and polymorphic alleles of ACE gene with hypertension [47]. Our findings reflect an interactive effect of sex and MEG3 alleles on eliciting neuropathic conditions in diabetic subjects.
Effect of rs7158663 genotypes on LDL-cholesterol levels in different DN groups. Comparisons of LDL-cholesterol levels between two rs7158663 genotypic groups among different DN groups (all DN cases, male DN cases, and female DN cases). p values were calculated between two groups by Student's t-test.
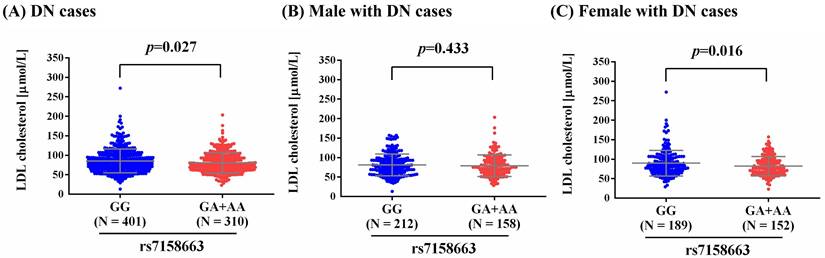
Effect of rs7158663 genotypes on MEG3 expression. Comparisons of MEG3 expression among different genotypic groups in representative brain parts based on data from the GTEx portal. p values were calculated among groups by one-way ANOVA.
Other than high levels of blood sugar, dyslipidemia represents an active contributor to neuropathic conditions in patients with metabolic syndromes [2]. Noteworthily, our results revealed an association between MEG3 rs7158663 and LDL-cholesterol levels in diabetic women. Abnormal levels of plasma LDL-cholesterol and triglyceride have been connected to disease deterioration of DN [48, 49]. Insulin resistance has been shown to accelerate lipid mobilization, leading to an excessive influx of free fatty acids into neurons [50]. This disturbance elicits changes in the physical and chemical characteristics of the cell membrane as well as drives mitochondrial bioenergetics from fatty acid synthesis towards massive oxidation, depleting important myelin lipid components [51]. Such dysregulated substrate utilization may enhance mitochondrial generation of reactive oxygen species, release of cytochrome C, and activation of proapoptotic pathways leading to neuronal damages [30, 52]. Apart from these metabolic mechanisms, balance of cholesterol levels is central to inflammatory events [53] and innate immune system [54]. Formation of cholesterol crystals can trigger a maladaptive immune response to hamper myelin repair [55], and aberrant cholesterol efflux is known to activate NLRP3 inflammasome, which induces neuronal pyroptosis [56]. These observations, therefore, implicate lipids as a pharmacological target in DN. Collectively, our data concerning the correlation between MEG3 rs7158663 and LDL-cholesterol levels in diabetic women offer insights into the potential role of MEG3 gene variations in neurotoxicity.
In this study, an effect of MEG3 gene polymorphisms on the risk for DN was demonstrated. Nevertheless, additional efforts are needed to address several study limitations. Firstly, there are numerous complications associated with diabetes (e.g. ocular, renal, cutaneous, and cardiovascular conditions), and the genetic architecture of each comorbidity could potentially influence our result regarding the association of MEG3 gene variations with DN. Secondly, we did not perform functional analyses of MEG3 rs7158663 on its capacity to sponge miRNAs. Also unavailable are the results to highlight a role of rs7158663 in modifying the binding of MEG3 gene to its enhancers or cognate transcription factors. Moreover, the genetic association detected here may be restricted to certain ethnic groups unless replication experiments using additional populations are conducted in the future.
Taken together, our results revealed an association of MEG3 gene polymorphisms with the risk of developing neuropathy in patients with diabetes. This genetic effect presumably links gender- and genotype-specific expression of MEG3 to dysregulated lipoprotein levels, causing nerve damages in diabetic individuals.
Acknowledgements
We are grateful to the Human Biobank of Chung Shan Medical University Hospital, Taichung, Taiwan for sample preparation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA. et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:136-54
2. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL. et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41
3. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521-34
4. Ratan Y, Rajput A, Pareek A, Pareek A, Kaur R, Sonia S. et al. Recent Advances in Biomolecular Patho-Mechanistic Pathways behind the Development and Progression of Diabetic Neuropathy. Biomedicines. 2024 12
5. Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr Opin Endocrinol Diabetes Obes. 2017;24:103-11
6. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-90
7. Jankovic M, Novakovic I, Nikolic D, Mitrovic Maksic J, Brankovic S, Petronic I. et al. Genetic and Epigenomic Modifiers of Diabetic Neuropathy. Int J Mol Sci. 2021 22
8. Politi C, Ciccacci C, D'Amato C, Novelli G, Borgiani P, Spallone V. Recent advances in exploring the genetic susceptibility to diabetic neuropathy. Diabetes Res Clin Pract. 2016;120:198-208
9. Fan B, Chopp M, Zhang ZG, Liu XS. Emerging Roles of microRNAs as Biomarkers and Therapeutic Targets for Diabetic Neuropathy. Front Neurol. 2020;11:558758
10. Wu M, Feng Y, Shi X. Advances with Long Non-Coding RNAs in Diabetic Peripheral Neuropathy. Diabetes Metab Syndr Obes. 2020;13:1429-34
11. Hajdu N, Racz R, Tordai DZ, Bekeffy M, Vagi OE, Istenes I. et al. Genetic Variants Influence the Development of Diabetic Neuropathy. Int J Mol Sci. 2024 25
12. Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR. et al. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab. 2003;88:5119-26
13. Zhang L, Zhao F, Li W, Song G, Kasim V, Wu S. The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer. Cancers (Basel). 2022 14
14. Hussein RM. Long non-coding RNAs: The hidden players in diabetes mellitus-related complications. Diabetes Metab Syndr. 2023;17:102872
15. Zhang H. Mechanism associated with aberrant lncRNA MEG3 expression in gestational diabetes mellitus. Exp Ther Med. 2019;18:3699-706
16. You L, Wang N, Yin D, Wang L, Jin F, Zhu Y. et al. Downregulation of Long Noncoding RNA Meg3 Affects Insulin Synthesis and Secretion in Mouse Pancreatic Beta Cells. J Cell Physiol. 2016;231:852-62
17. Tello-Flores VA, Beltrán-Anaya FO, Ramírez-Vargas MA, Esteban-Casales BE, Navarro-Tito N, Alarcón-Romero LDC. et al. Role of Long Non-Coding RNAs and the Molecular Mechanisms Involved in Insulin Resistance. Int J Mol Sci. 2021 22
18. Baazaoui N, M YA, Ben Saad R, Garzoli S. Potential role of long noncoding RNA maternally expressed gene 3 (MEG3) in the process of neurodegeneration. Neuroscience. 2025;565:487-98
19. Reddy AS, O'Brien D, Pisat N, Weichselbaum CT, Sakers K, Lisci M. et al. A Comprehensive Analysis of Cell Type-Specific Nuclear RNA From Neurons and Glia of the Brain. Biol Psychiatry. 2017;81:252-64
20. Yan H, Rao J, Yuan J, Gao L, Huang W, Zhao L. et al. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway. Cell Death Dis. 2017;8:3211
21. Ghafouri-Fard S, Taheri M. Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed Pharmacother. 2019;118:109129
22. Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW. et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937-44
23. Nather A, Keng Lin W, Aziz Z, Hj Ong C, Mc Feng B, C BL. Assessment of sensory neuropathy in patients with diabetic foot problems. Diabet Foot Ankle. 2011 2
24. Yang Z, Li H, Li J, Lv X, Gao M, Bi Y. et al. Association Between Long Noncoding RNA MEG3 Polymorphisms and Lung Cancer Susceptibility in Chinese Northeast Population. DNA Cell Biol. 2018;37:812-20
25. Pei JS, Chang WS, Chen CC, Mong MC, Hsu SW, Hsu PC. et al. Novel Contribution of Long Non-coding RNA MEG3 Genotype to Prediction of Childhood Leukemia Risk. Cancer Genomics Proteomics. 2022;19:27-34
26. Zheng Y, Wang M, Wang S, Xu P, Deng Y, Lin S. et al. LncRNA MEG3 rs3087918 was associated with a decreased breast cancer risk in a Chinese population: a case-control study. BMC Cancer. 2020;20:659
27. Hou Y, Zhang B, Miao L, Ji Y, Yu Y, Zhu L. et al. Association of long non-coding RNA MEG3 polymorphisms with oral squamous cell carcinoma risk. Oral Dis. 2019;25:1318-24
28. Gong J, Liu W, Zhang J, Miao X, Guo AY. lncRNASNP: a database of SNPs in lncRNAs and their potential functions in human and mouse. Nucleic Acids Res. 2015;43:D181-6
29. Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nature genetics. 2013;45:580-5
30. Perez-Matos MC, Morales-Alvarez MC, Mendivil CO. Lipids: A Suitable Therapeutic Target in Diabetic Neuropathy? J Diabetes Res. 2017;2017:6943851
31. Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG. et al. New perspectives in diabetic neuropathy. Neuron. 2023;111:2623-41
32. Gao X, Li X, Zhang S, Wang X. The Association of MEG3 Gene rs7158663 Polymorphism with Cancer Susceptibility. Front Oncol. 2021;11:796774
33. Wang Y, Gao F, Lu J. MEG3 rs7158663 genetic polymorphism is associated with the risk of hepatocellular carcinoma. Nucleosides Nucleotides Nucleic Acids. 2024:1-11
34. Lao X, Wang Y, Huang R, He Y, Lu H, Liang D. Genetic variants of LncRNAs HOTTIP and MEG3 influence nasopharyngeal carcinoma susceptibility and clinicopathologic characteristics in the Southern Chinese population. Infect Agent Cancer. 2024;19:32
35. Dieter C, Lemos NE, Girardi E, Ramos DT, Pellenz FM, Canani LH. et al. The rs3931283/PVT1 and rs7158663/MEG3 polymorphisms are associated with diabetic kidney disease and markers of renal function in patients with type 2 diabetes mellitus. Mol Biol Rep. 2023;50:2159-69
36. Brondani LA, Dandolini I, Girardi E, Canani LH, Crispim D, Dieter C. Association between the G/G genotype of the lncRNA MEG3 rs7158663 polymorphism and proliferative diabetic retinopathy. Arch Endocrinol Metab. 2024;68:e240024
37. Kameswaran V, Bramswig NC, McKenna LB, Penn M, Schug J, Hand NJ. et al. Epigenetic regulation of the DLK1-MEG3 microRNA cluster in human type 2 diabetic islets. Cell Metab. 2014;19:135-45
38. Li J, Liu W, Peng F, Cao X, Xie X, Peng C. The multifaceted biology of lncR-Meg3 in cardio-cerebrovascular diseases. Front Genet. 2023;14:1132884
39. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45-63
40. Fan X, Huang H, Ji Z, Mao Q. Long non-coding RNA MEG3 functions as a competing endogenous RNA of miR-93 to regulate bladder cancer progression via PI3K/AKT/mTOR pathway. Transl Cancer Res. 2020;9:1678-88
41. Zha F, Qu X, Tang B, Li J, Wang Y, Zheng P. et al. Long non-coding RNA MEG3 promotes fibrosis and inflammatory response in diabetic nephropathy via miR-181a/Egr-1/TLR4 axis. Aging (Albany NY). 2019;11:3716-30
42. Kaur P, Kotru S, Singh S, Munshi A. Role of miRNAs in diabetic neuropathy: mechanisms and possible interventions. Mol Neurobiol. 2022;59:1836-49
43. Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911-22
44. Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298-305
45. Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet. 2006;38:218-22
46. Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ. et al. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28
47. Higaki J, Baba S, Katsuya T, Sato N, Ishikawa K, Mannami T. et al. Deletion allele of angiotensin-converting enzyme gene increases risk of essential hypertension in Japanese men: the Suita Study. Circulation. 2000;101:2060-5
48. Cai Z, Yang Y, Zhang J. A systematic review and meta-analysis of the serum lipid profile in prediction of diabetic neuropathy. Sci Rep. 2021;11:499
49. Kristensen FPB, Christensen DH, Callaghan BC, Nielsen JS, Hojlund K, Andersen H. et al. Lipid Levels and Risk of Diabetic Polyneuropathy in 2 Danish Type 2 Diabetes Cohorts. Neurology. 2024;103:e209538
50. Grote CW, Wright DE. A Role for Insulin in Diabetic Neuropathy. Front Neurosci. 2016;10:581
51. Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K. et al. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886-98
52. McManus MJ, Murphy MP, Franklin JL. Mitochondria-derived reactive oxygen species mediate caspase-dependent and -independent neuronal deaths. Mol Cell Neurosci. 2014;63:13-23
53. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104-16
54. Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab. 2012;23:169-78
55. Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684-8
56. de Dios C, Abadin X, Roca-Agujetas V, Jimenez-Martinez M, Morales A, Trullas R. et al. Inflammasome activation under high cholesterol load triggers a protective microglial phenotype while promoting neuronal pyroptosis. Transl Neurodegener. 2023;12:10
Author contact
![]() Corresponding authors: Shun-Fa Yang, Ph.D. or Shih-Chi Su, PhD. Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: ssu1org.tw (Shih-Chi Su)
Corresponding authors: Shun-Fa Yang, Ph.D. or Shih-Chi Su, PhD. Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan; Tel: +886-4-24739595 ext. 34253; Fax: +886-4-24723229; E-mail: ysfedu.tw (Shun-Fa Yang); E-mail: ssu1org.tw (Shih-Chi Su)

 Global reach, higher impact
Global reach, higher impact